| Research Article | ||
Open Vet J. 2023; 13(9): 1124-1134 Open Veterinary Journal, (2023), Vol. 13(9): 1124–1134 Original Research Evaluation of factors influencing survival time in 77 dogs with lymphomaSoo-Yeon Jeong1,2*1Department of Companion Animal Health Care, Kyung-in Women’s University, Incheon, South Korea 2Department of Veterinary Internal Medicine, College of Veterinary Medicine, Jeonbuk National University, Iksan, South Korea *Corresponding Author: Soo-Yeon Jeong. Department of Companion Animal Health Care, Kyung-in Women’s University, Incheon, South Korea. Email: syjeong [at] kiwu.ac.kr Submitted: 10/07/2023 Accepted: 11/08/2023 Published: 30/09/2023 © 2023 Open Veterinary Journal
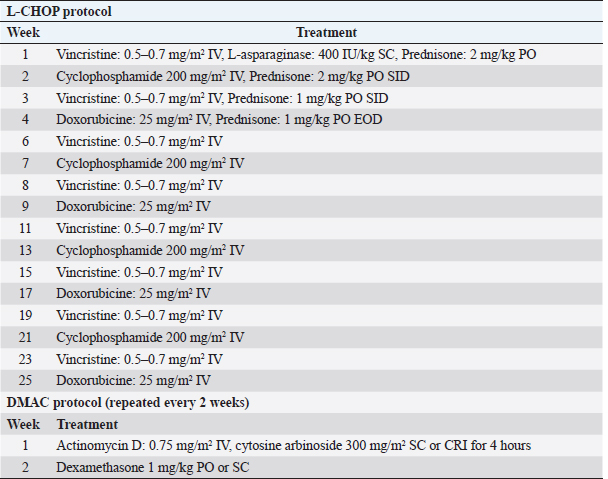
AbstractBackground: Canine lymphoma is one of the most commonly reported hematopoietic tumors. Aim: A few retrospective studies have involved complex evaluations including diagnostic features and treatment protocols, but these studies infrequently demonstrate variable factors that affect survival time, and comparisons among chemotherapeutic protocols are limited. This study aimed to identify prognostic factors that can be simply detected in dogs with lymphoma, such as abnormalities in physical and hematologic findings, and treatment protocols. Methods: Clinical records of 77 dogs diagnosed with lymphoma were retrospectively reviewed. Results: The author newly identified leukocyte and platelet abnormalities as negative prognostic factors. Furthermore, this study suggests that decreased gastrointestinal toxicity and improvements of hematologic abnormalities, such as anemia, thrombocytopenia, and lymphocytosis or lymphoblasts, in peripheral blood during chemotherapy act as positive prognostic factors. Finally, strict adherence to therapeutic protocol and selecting multiple agents as rescue protocol are important to prolong survival time. Conclusion: This study identified indicators to be used as prognostic factors through survival analysis. Keywords: Dog, Chemotherapy, Lymphoma, Prognostic factor, Survival analysis. IntroductionCanine lymphoma is one of the most commonly reported hematopoietic tumors, occurring in 30 per 100,000 dogs (Baskin et al., 2000). It is defined as a clonal proliferation of malignant lymphocytes affecting solid tissues, such as the lymph nodes, spleen, and liver (Gavazza et al., 2009). The cause of lymphoma is reported to be multifactorial. Environmental factors, viral infections, immune-mediated factors, and genetic predisposition (Gavazza et al., 2009; Mortier et al., 2012) are associated with a higher risk of lymphoma occurrence. Based on the World Health Organization (WHO) classification, lymphoma is categorized as clinical stage I, II, III, IV, or V (Vail, 2010). To provide a more accurate depiction, each WHO stage is subdivided into substages a and b. Lymphoma can also be classified according to anatomic form as multicentric, alimentary, mediastinal, cutaneous, or miscellaneous extranodal lymphoma (Dhaliwal et al., 2004; Gavazza et al., 2009), and according to immunologic form as B-cell or T-cell type lymphoma (Vail, 2010). In the early stages of lymphoma, most patients present as relatively healthy dogs with only generalized, superficial lymphadenopathy (Baskin et al., 2000; Vail, 2010). In dogs with parenchymal organ invasions, clinical signs include weight loss, anorexia, and depression (Gorman, 1991; Baskin et al., 2000). Peripheral cytopenias may lead to clinical symptoms relating to sepsis resulting from neutropenia, anemia, or thrombocytopenia in dogs with stage V lymphoma (Vail, 2010). Various multidrug combination chemotherapy protocols are available for the management of lymphoma (Gavazza et al., 2009), and the treatment effects of these protocols have been published. Generally, it is accepted that an L-CHOP (L, L-asparaginase; C, cyclophosphamide; H, doxorubicin; O, vincristine; P, prednisone)-based protocol (Table 1) including the five most active chemotherapeutic drugs is associated with a relatively longer duration of remission than other protocols (Hosoya et al., 2007; Vail, 2010). However, most dogs with lymphoma die because of relapse of chemotherapy-resistant or disseminated disease (Mortier et al., 2012). A shortened survival time is a recognized negative effect related to several factors, such as stage Vb lymphoma according to the WHO guidelines, T-cell immunologic type, and mediastinal anatomic type. Previous studies on prognostic factors of lymphoma are limited to diagnostic features; in addition, comparative studies of protocols are limited. The purpose of this study was to identify factors influencing survival time that can be simply detected in dogs with lymphoma, such as abnormalities of physical and hematologic examinations and treatment protocol. Materials and MethodsThe medical records of 77 dogs that were presented with lymphoma were selected and reviewed. All the dogs with lymphoma were classified into a treatment (T) or no-treatment (NT) group. The T group was subdivided into groups A, B, C, D, and E as per the following criteria: Group A, no remission; Group B, partial remission (reduction in size by >50%) and no relapse; Group C, complete remission (no visible disease) and no relapse; Group D, relapse after remission and remission after re-treatment; and Group E (Chun et al., 2007), relapse after remission and no remission after re-treatment. Dogs with other diseases inducing lymphadenopathy, such as general infection, systemic lupus erythematous, and metastasis of malignant neoplasia, except for lymphoma, were excluded. In addition, dogs whose clinical records were incomplete or dogs with a confirmed diagnosis of lymphoblastic leukemia were excluded. A cytological or histopathological diagnosis of lymphoma was required for inclusion in this retrospective study. Dogs were staged according to the WHO guidelines (Owen, 1980; Baskin et al., 2000) based on clinical examination. In addition, the disease was subclassified as either having no clinical signs associated with lymphoma (substage a) or with clinical signs (substage b). Survival times were recorded, based on two standards. First, the data were based on the initial day of diagnosis. To analyze the chemotherapeutic response effect, chemotherapeutic toxicity, and comparisons among protocols, the data used were based on the initial day of the first chemotherapy session. With owner consent, 42 dogs in the T group were initially treated with L-CHOP protocols. In cases that relapse or fail to achieve complete remission, a DMAC (D, dexamethasone; M, melphalan; A, actinomycin D; C, cytosine arabinoside) protocol (Table 1) or CCNU (lomustine) treatment (90–100 mg/m2 body surface area PO every 3 weeks) was applied (Moore et al., 1999; Alvarez et al., 2006) To evaluate the response to anticancer drugs, palpation of the body's lymph nodes and the size of the lymph nodes through ultrasonography were used. Also, if lymph nodes were visible on the X-ray, these data were used. Dogs were evaluated on the day of the hospital visit according to the chemotherapeutic protocol. Remission was evaluated and recorded throughout the survival period. In case of side effects, such as digestive symptoms or neutropenia, the anticancer drug dose was reduced by half or the prescription of anticancer drug was delayed for 2 weeks. Table 1. L-CHOP protocol for dogs with lymphoma (Vail, 2010) and DMAC protocol (Alvarez et al., 2006).
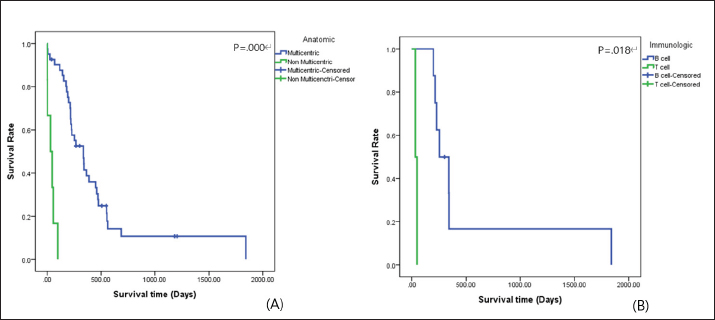
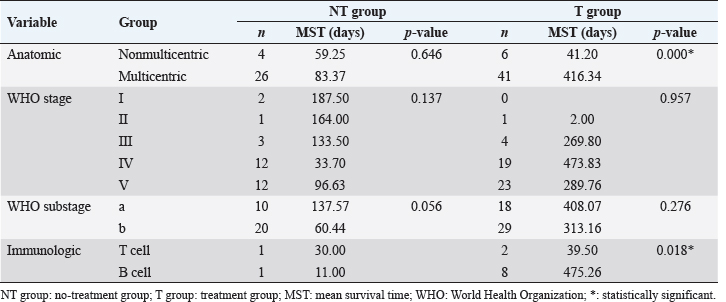
The distribution and survival analysis according to hematologic and gastrointestinal toxicities were classified into the following groups. GI: gastrointestinal; G0: no toxicity; G1: nausea or anorexia; G2: 1–5 times vomiting or 5–7 times diarrhea per day or less than 5% weight loss; G3: 6–10 times vomiting or more than seven times diarrhea per day or from 5% to 10% weight loss; and G4: intractable vomiting or diarrhea or more than 10% weight loss and H0: no toxicity; H1: from 2,000 to 3,000/μl white blood cells or from 1,000 to 1,500/μl neutrophils or from 100,000 to 150,000/μ platelets; H2: from 1,500 to 2,000/μl white blood cells or from 800 to 1,000/μl neutrophils or from 50,000 to 100,000/μ platelets; H3: from 1,000 to 1,500/μl white blood cells or from 500 to 800/μl neutrophils or from 25,000 to 50,000/μ platelets; and H4: less than 1,000/μl white blood cells or less than 500/μl neutrophils or 25,000/μl platelets (Mortier et al., 2012). All the data are reported as the mean and standard deviation of the values, numbers, and percentages. An independent two-sample t-test, a dependent one-sample t-test, a log-rank test, and the Kaplan–Meier method were used for the survival analysis. The Cox proportional hazard method for multivariate analysis was used to identify the effects of diverse variables on survival. The statistical analysis was performed using SPSS. Statistical significance was defined as p < 0.05. This study was a retrospective analysis of past medical charts, and it was confirmed that the study was exempt from review by the Institutional Animal Care and Use Committee of the institution to which the author belongs. ResultsEpidemiologic characteristicsAn analysis of sex revealed that castrated males (37.7%, n=29) were most prevalent, followed by intact females (31.2%, n=24), intact males (18.2%, n=14), and spayed females (12.9%, n=10). The most common breeds were the Maltese and Shih-Tzu (26.0%, n=20) in all groups, followed by the Cocker Spaniel (18.1%, n=14), Miniature Pinscher (6.5%, n=5), Yorkshire Terrier (3.9%, n=3), Schnauzer (3.9%, n=3), Poodle (2.6%, n=2), mixed-breed (2.6%, n=2), Golden Retriever (2.6%, n=2), and other breeds (7.8%, n=6). The mean age at the time of the initial medical records was 7.47 ± 2.99 years. Thirty-two (41.5%) dogs were older than 8 years, 26 dogs (33.8%) were 6–7 years old, and 19 dogs (24.7%) were younger than 5 years of age. Clinical signsLymphadenomegaly (98.7%, n=76) with lymphoma was most prevalent, followed by gastrointestinal signs (81.8%, n=63), abnormalities in general condition (49.4%, n=38), respiratory signs (16.9%, n=13), ophthalmic problems (2.6%, n=2), urinary tract problems (1.3%, n=1), skin problems (1.3%, n=1), and neurologic signs (1.3%, n=1). One dog had no clinical signs at presentation (1.3%). Hematologic examinationThe complete blood cell count (VetScan ® HM2 Hematology System; Abaxis, Inc., Union City, CA) showed anemia (reference range of hematocrit: 37%–55% and reference range of hemoglobin: 12–18 g/dl) in 39.0% (n=30) of cases, further classified as nonregenerative in 76.9% (n=20) and regenerative in 23.1% (n=6). Leukocytosis (reference range of white blood cells: 6–17 × 103/μl) was identified in 33.8% (n=26) of cases, and leukopenia (reference range of white blood cells: 6–17 × 103/μl) presented in 2.6% (n=2). Platelet (reference range: 200–500 × 103/μl) estimation was reduced or increased by 48.1% (n=37) and 1.3% (n =1), respectively. The presence of circulating lymphoblasts or lymphocytosis during differential counting was detected in 36.5% (n=27). The most represented abnormalities in the electrolytes analysis were hyponatremia (14.9%, n=11), followed by hypochloremia (9.5%, n=7), hyperkalemia (8.1%, n=6), hypokalemia (6.8%, n=5), hypernatremia (2.7%, n=2), and hyperchloremia (2.7%, n=2). However, 66.2% (n=49) of the cases were within the normal range for electrolytes. The C-reactive protein (CRP) values ranged from below 10 to 77 mg/l (reference range: <10 mg/l), and the mean value was 50.00 ± 16.65 mg/l. High D-dimer values were identified in 61.1% of the dogs, and the mean value was 0.84 ± 1.07 mg/l (reference range: <0.3 mg/l). In addition, high fibrinogen degradation product (FDP) values were observed in 66.0%, and the mean value was 15.00% ± 7.28% (reference range: 0%–10%). The mean values of activated partial thromboplastin time (aPTT) and prothrombin time (PT) were 30.97 ± 61.33 seconds (reference range: 12–16 seconds) and 15.30 ± 27.99 seconds (reference range: 6.2–8.2 seconds), respectively. Diagnostic featuresBased on the WHO clinical staging system, the majority of dogs presented with stage Vb lymphoma (32.5%, n=25), followed by stage IVb (27.3%, n=21), stage IVa (13.0%, n=10), stage Va (13.0%, n=10), stage IIIa (6.5%, n=5), stage Ia (2.6%, n=2), stage IIIb (2.6%, n=2), stage IIa (1.3%, n=1), and stage IIb (1.3%, n=1). The multicentric type was the most common form of lymphoma identified in this study (87.0%, n=67), followed by the alimentary type (7.8%, n=6), the mediastinal type (2.6%, n =2), an equal prevalence of the cutaneous type (1.3%, n=1), and miscellaneous extranodal type (1.3%, n=1). Immunophenotyping was performed for 12 cases and indicated a prevalence of the B-cell type (75.0%, n=9). Survival analysisSurvival analysis according to diagnostic features The 30 dogs from the NT group were analyzed to identify a correlation between survival times and diagnostic features. According to the anatomic type, there was a difference in survival time between the multicentric type and the nonmulticentric type. Dogs with the multicentric type showed a longer mean survival time (83.37 days) than dogs with the nonmulticentric type (59.25 days). However, this difference was not significant (p=0.646). There was no statistical significance demonstrated among the WHO stage (p=0.137) or substage (p=0.056). The mean survival time of the dogs diagnosed with substage a (137.57 days) was longer than that of dogs diagnosed with substage b (60.44 days). The 47 dogs with lymphoma in the T group were also analyzed. Significant differences in survival time were found for both the anatomic type (p=0.000) and the immunologic type (p=0.018). The survival curves for the anatomic type and immunologic type are presented in Figure 1. The mean survival time of dogs with a multicentric type (416.34 days) was about 10 times longer than that of dogs with a nonmulticentric type (41.20 days). Based on the immunologic type, the mean survival time of the B-cell type (475.26 days) was about 12 times longer than that of the T-cell type (39.50 days). There were no statistically significant differences in the WHO stage (p=0.957) or substage (p=0.276). The mean survival time of WHO substage a (408.07 days) was slightly longer than that of substage b (313.16 days).
Fig. 1. Survival curve according to (A) anatomic type and (B) immunologic type in the T group of dogs with lymphoma. Table 2. Survival analysis according to diagnostic features of the dogs with lymphoma.
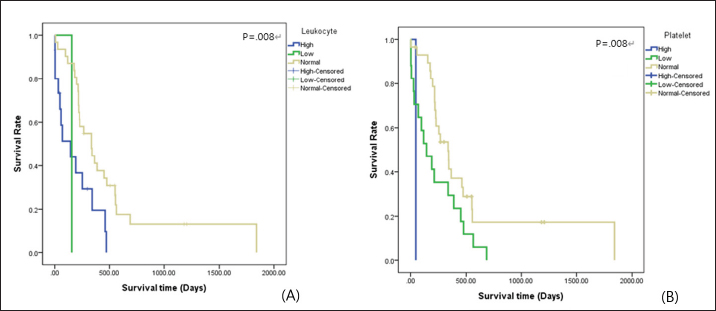
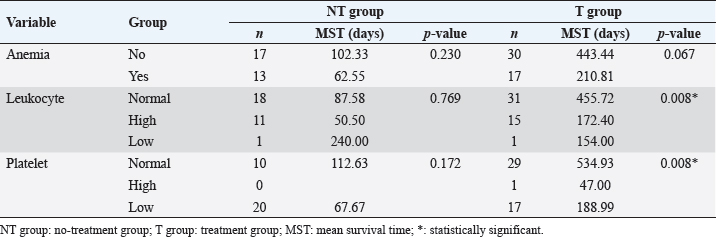
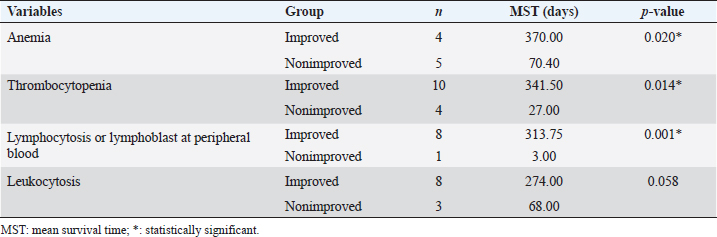
Survival analysis according to diagnostic features of T and NT groups is shown in Table 2. Survival analysis according to hematologic examinations The group without anemia of NT group showed a longer survival time (102.33 days) than the group with anemia (62.55 days). Dogs with platelet counts within the normal range showed a longer survival time (112.63 days) than dogs with thrombocytopenia (67.67 days). However, the presence of anemia, leukocyte, and platelet abnormalities did not show a statistically significant negative association with survival time. The group without anemia of T group demonstrated a longer survival time (443.44 days) than the group with anemia (210.81 days). The mean survival time of dogs with normal leukocyte values (455.72 days) was much longer than that of dogs with leukocytosis (172.40 days) or leukopenia (154.00 days), and the difference was statistically significant (p=0.008) (Fig. 2A). Dogs with normal platelet values showed longer survival times (534.93 days) than dogs with thrombocytopenia (188.99 days) or thrombocytosis (47.00 days), and the associated survival curve is shown in Figure 2B. There was a statistically significant difference among the groups (p=0.008). Survival analysis according to hematologic examinations is listed in Table 3. A comparison of the improved group and nonimproved group regarding hematologic abnormalities after chemotherapy is listed in Table 4. Dogs with anemia showed longer survival times (370.00 days) in the improved group than the nonimproved group (70.40 days), and there was a statistically significant difference (p=0.020). In addition, the mean survival time of the improved group (341.50 days) was longer than that of the nonimproved group (27.00 days) in dogs with thrombocytopenia. There was a statistically significant difference (p=0.014). In dogs with lymphocytosis or lymphoblasts in the peripheral blood, the improved group showed a longer survival time (313.75 days) than the nonimproved group (3.00 days). The improvement of lymphocytosis or lymphoblasts in the peripheral blood showed a statistically significant association with the survival time (p=0.001). Based on leukocytosis, the improved group presented a longer survival time (274.00 days) than the nonimproved group (68.00 days). However, statistical significance was not observed (p=0.058).
Fig. 2. Survival curve according to (A) leukocyte value and (B) platelet value in the T group of dogs with lymphoma. Table 3. Survival analysis according to hematologic examination findings of the dogs with lymphoma.
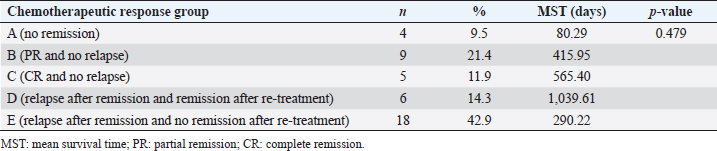
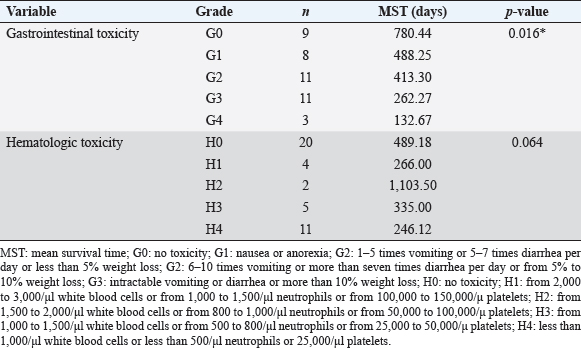
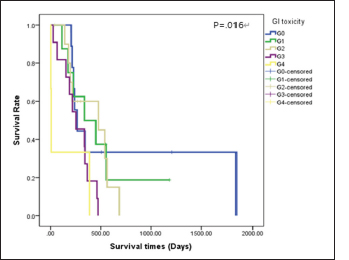
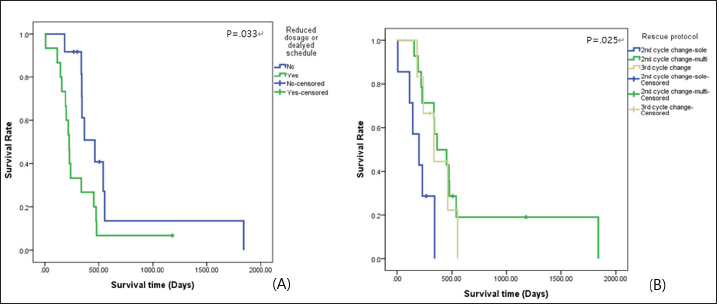
Survival analysis according to chemotherapeutic response groups For the analysis of the response to chemotherapy, 42 dogs with multicentric lymphoma in the T group were reviewed. Among groups A, B, C, D, and E, no statistical differences were evident (p=0.479) for survival time from the first day of chemotherapeutic treatment to the date of death. In the A group, the no remission group, the survival time was shorter than that in the groups responding to chemotherapy, such as the B, C, D, and E groups. Table 5 shows the distribution and survival analysis according to chemotherapeutic response groups. Survival analysis according to chemotherapeutic toxicity grades The distribution and survival analysis according to hematologic and gastrointestinal toxicities of the 42 dogs with lymphoma that were treated with chemotherapeutic agents are listed in Table 6. Also, a survival curve relating survival time and gastrointestinal toxicity grade is presented in Figure 3. The longest survival time was observed in the G0 grade (780.44 days), followed by the G1 grade (488.25 days), G2 grade (413.30 days), G3 grade (262.27 days), and G4 grade (132.67 days). The higher the gastrointestinal grade, the longer the survival time, and there was a significant correlation between survival time and the gastrointestinal toxicity grade (p=0.016). In the hematologic toxicity evaluation, dogs with no hematologic toxicity were dominant (47.6%, n=20). The H4 grade was the most frequent hematologic toxicity grade (26.2%, n=11), followed by the H3 grade (11.9%, n=5), H1 grade (9.5%, n=4), and H2 grade (4.8%, n=2). The longest survival times were in those presenting with the H2 grade (1,103.50 days), followed by the H0 grade (489.18 days), H3 grade (335.00 days), H1 grade (266.00 days), and H4 grade (246.12 days). However, there was no statistical significance among the grades (p=0.176). The other side effects included dilated cardiomyopathy (2.4%, n=1), acute tumor lysis syndrome (2.4%, n=1), and urinary tract infection (2.4%, n=1). Survival analysis according to the presence of reduced dosage or delated schedule chemotherapeutic protocol The correlation between survival time and the presence of applied modified protocols, which included delayed schedule and reduced chemotherapeutic agent dosage is shown in Figure 4A. The mean survival time of the group that underwent an applied modified protocol was 308.87 days. The mean survival time of the group that strictly followed the protocol was 601.19 days. There was a statistically significant difference in survival time based on the presence of reduced dosage or delayed schedule chemotherapeutic protocol (p=0.033). Table 4. Comparisons between the improved and nonimproved groups of hematologic prognostic factors at postchemotherapy.
Table 5. Distribution and survival analysis according to chemotherapeutic response groups.
Table 6. Survival analysis according to hematologic and gastrointestinal toxicity grades for 42 dogs with lymphoma.
Fig. 3. Survival curve according to gastrointestinal toxicity during chemotherapy in the T group of dogs with lymphoma (n=G0: 9; G1: 8; G2: 11; G3:11; and G4: 3). GI: gastrointestinal; G0: no toxicity; G1: nausea or anorexia; G2: 1–5 times vomiting or 5–7 times diarrhea per day or less than 5% weight loss; G3: 6–10 times vomiting or more than seven times diarrhea per day or from 5% to 10% weight loss; and G4: intractable vomiting or diarrhea or more than 10% weight loss. Survival analysis according to rescue protocols Figure 4B depicts the survival time in relation to rescue protocols. The longest survival time was observed in the group that received a DMAC protocol as the second chemotherapy cycle (633.10 days), followed by the group that received DMAC protocol as the third chemotherapy cycle (370.67 days), and the group that received CCNU treatment as the second chemotherapy cycle (196.43 days). There was a statistically significant difference among the groups (p=0.025). DiscussionA few retrospective studies have performed complex evaluations of survival times and prognostic factors in dogs with lymphoma (Baskin et al., 2000; Jagielski et al., 2002; Gavazza et al., 2009; Mortier et al., 2012). Moreover, several studies have revealed chemotherapeutic treatment effects on survival time and chemotherapeutic toxicities (Alvarez et al., 2006; Hosoya et al., 2007). However, these studies infrequently demonstrate variable prognostic factors, and comparisons among protocols are limited. This study assessed dogs with lymphoma through medical examinations and survival time. The Maltese and Shih-Tzu were the most affected breeds, followed by the Cocker Spaniel. The prevalence of lymphoma in breeds in this study differed from those noted by other authors. Previous studies revealed that lymphoma occurred frequently in Scottish Terriers, Boxers, Beagles, and German shepherd dogs (MacEwen et al., 1987; Keller et al., 1993; Teske, 1993; Jagielski et al., 2002). Data concerning breed prevalence were obtained from the USA and Western Europe. A possible explanation is that breed popularity differs among countries. Lymphoma most frequently affected middle-aged dogs (7.47 ± 2.99 years); this finding concurs with data revealed by other studies, where the average age was between 6.3 and 7.7 years (MacEwen et al., 1987; Teske, 1993; Jagielski et al., 2002). There is no correspondence of sex predilection for lymphoma in dogs (MacEwen et al., 1987; Jagielski et al., 2002). Results obtained in this retrospective study indicated that lymphoma was more frequent in males (55.9%) than in females (44.1%). It is meaningful to note that the ratio of lymphoma is slightly higher in men than women in human medicine (Konopka, 1995; Jagielski et al., 2002).
Fig. 4. Survival curve according to (A) presence of reduced dosage or delayed schedule chemotherapeutic protocol and (B) rescue protocols (n=second cycle change-CCNU: 7; second cycle change-DMAC: 14; third cycle change-DMAC: 6). CCNU: lomustine; DMAC: D, dexamethasone; M, melphalan; A, actinomycin D; and C, cytosine arabinoside. Predictably, the most common clinical sign was generalized lymphadenomegaly. This result is similar to that of a previous report (Gavazza et al., 2009). Gastrointestinal signs, such as anorexia, vomiting, and diarrhea, were also common. The presentation and associated gastrointestinal signs of lymphoma reflect nonspecific signs of multicentric lymphoma or the specific form of alimentary lymphoma (Vail, 2010). Thrombocytopenia and anemia were the most common hematological abnormalities observed in lymphoma. The prevalence of thrombocytopenic cases in this study (48.1%) appeared similar to published data (50%). Anemia and thrombocytopenia were found in a large percentage of the dogs in the present study. This result might have been associated with the high percentage of dogs diagnosed with late lymphoma, which has been correlated with bone marrow involvement of neoplastic cells, splenic infiltration of neoplastic cells, and paraneoplastic immune-mediated destruction. The prevalence of lymphocytosis or lymphoblasts in the peripheral blood (36.5%) was slightly higher than that reported in the published data (31.8%) (Mortier et al., 2012), while the prevalence of leukopenia (2.6%) appeared lower than previously reported (7.5%) (Madewell, 1986; Gavazza et al., 2009). Circulating lymphoblasts may also be indicative of bone marrow infiltration (Vail, 2010). The interrelations of electrolyte abnormalities and lymphoma have been rarely reported in veterinary medicine. This study suggested that the majority of dogs (66.2%) with lymphoma show no remarkable findings on electrolyte examination, but from 12.1% to 17.6% of dogs with lymphoma presented abnormalities of each of the electrolyte parameters. In a previous report (Nielsen et al., 2007), the mean serum CRP concentration for dogs with substage a lymphoma was 39.1 mg/l (standard error=14.6), and that for dogs with substage b lymphoma was 34.7 mg/l (standard error=15.7). In this study, the mean value of CRP concentration of dogs with lymphoma was higher than the reference ranges. This finding is associated with hematopoietic neoplasia along with inflammatory and autoimmune disease conditions. However, the CRP concentration might have been falsely elevated, because of concurrent inflammation or infection (Nielsen et al., 2007). Results of coagulation parameters including D-dimer, FDP, aPTT, and PT showed higher concentrations than reference ranges. These results might have reflected hemorrhage from damaged vessels or coagulopathy within dogs with lymphoma (Dewhurst et al., 2008). The large predominance of multicentric lymphoma observed in this retrospective study is another finding in agreement with the findings of a previous study (Gavazza et al., 2009). However, data for the nonmulticentric type might have been underestimated, because these dogs are often misdiagnosed with other cancers, except for lymphoma, and might not have been referred. Usually, lymphoma in dogs is diagnosed late. In over 90% of cases, dogs with lymphoma were classified in class III or higher in the WHO classification at the time of diagnosis. The author suggests that this result was probably attributable to the difficulties in detecting the clinical manifestations of the earlier stages of lymphoma, and the fact that the examined patients mainly presented as referral cases at least a few days following the onset of clinical signs and often after empirical treatment, such as steroids, had been administered (Withrow and Vail, 2007; Gavazza et al., 2009). The frequency of dogs with clinical signs was higher than that of dogs without clinical signs. On the contrary, previous literature reported that most cases did not show clinical signs (Jagielski et al., 2002). As demonstrated in previous reports (Carter et al., 1986; Madewell, 1986; Greenlee et al., 1990; Gavazza et al., 2009), the B-cell type, based on the immunologic type, was dominant in this study. When left untreated without chemotherapy, the expected survival time for dogs with lymphoma is 4–6 weeks (Vail, 2010; Mortier et al., 2012), whereas in dogs treated with multi-agent protocols is 251 days (range, 5–475 days) (Simon et al., 2008; Gavazza et al., 2009). In this study, the mean survival time for dogs in the NT group was 83.30 days, and that of dogs in the T group was 320.41 days. This study suggested multicentric type based on anatomic type and B-cell type based on immunologic type in the T group as positive prognostic factors. Furthermore, the multicentric type in the T group appeared to be related to the prognosis in multivariate analysis. Previous studies (Jagielski et al., 2002; Mortier et al., 2012) revealed that multicentric and B-cell types were relatively responsive to chemotherapy. These suggestions indicate that multicentric type and B-cell type are presented as positive prognostic factors only in the T group. Statistical analysis of immunologic type in the NT group was limited by the number of cases with immunologic diagnoses. There is no association between the WHO stage with prognosis, and this study failed to demonstrate an association between survival time and the WHO stage. Leukocyte and platelet abnormalities in the T group were presented as negative prognostic factors. Leukopenia and thrombocytopenia may result from bone marrow suppression or splenic infiltration in dogs with lymphoma (Gavazza et al., 2009). Moreover, chronic inflammation can suppress the correction of blastogenesis mutations or host function for producing healthy lymphocytes. Furthermore, impairment of the immunologic mechanism might lead to resistance to chemotherapeutic agents. This mechanism could explain why leukocyte abnormalities existed as a negative prognostic factor in the T group. Improvements in anemia, thrombocytopenia, and lymphocytosis or lymphoblasts on peripheral blood examination after chemotherapy were associated with longer survival time. This might have represented a responsive effect with chemotherapy, especially in stage V lymphoma. Improvement of leukocytosis was also considered a prognostic factor, although there were no significant differences (p=0.058). In dogs with multicentric lymphoma, the observed response rate was 90.5%, and this value was slightly higher than a previously reported value (81.7%) (Gavazza et al., 2009). The mean survival time of the responding groups (Groups B, C, D, and E) was longer than that of the nonresponding group (Group A). However, many cases (Groups D and E) (60.0%) within the responding groups relapsed within a few weeks. These findings revealed that lymphoma is relatively responsive to chemotherapy treatment, but relapse is a common event, and in the end, the development of resistance to the chemotherapeutic agents causes death. There are many reports (Baskin et al., 2000; Hosoya et al., 2007) about chemotherapeutic toxicity, and a previous report identified that gastrointestinal signs after chemotherapy were observed infrequently. However, studies regarding the association with survival time are limited. This report identified that gastrointestinal toxicity affects survival time. A study (Curran and Thamm, 2016) reported that neutropenia was the most common cause of toxicity, followed by gastrointestinal signs. However, in this study, hematologic toxicity (n=22) was 1.5 times higher than gastrointestinal toxicity (n=22). These differences can take into account differences in breed and genetic distribution by country, and it is thought that limitations can be overcome by extensively examining not only lymphoma but also all diseases for which chemotherapeutic drugs are used. As a result of hematologic toxicity analysis, the group with the longest survival time was H2. The degree of toxicity and the order of survival time were not related and were statistically unrelated, too. Therefore, the hematological toxicity of anticancer drugs was found to be independent of survival time in lymphoma. The clinically positive part is that even if hematological toxicity appears during chemotherapy, it is judged that there is no negative effect on the survival period if appropriate treatment is accompanied. Delayed chemotherapeutic schedule and reduced dosage of chemotherapeutic agents were identified as factors influencing shorter survival time. It has been reported that reducing the dose of chemotherapy by 20% reduces the efficacy by 50% (Mortier et al., 2012). Moreover, a delayed schedule might be associated with a high possibilities of proliferation of neoplastic cells. During chemotherapy in dogs that relapsed or failed to achieve CR, dogs administered rescue protocols of multiple agents revealed longer survival time. In a previous study (Mortier et al., 2012), the mean response day of CCNU as rescue protocol was 86 days, and that of DMAC as rescue protocol was 61 days. This result showed combination chemotherapy is superior to single-agent chemotherapy. This retrospective study had several limitations, such as the number of cases and the accuracy of the analysis. This study included 77 dogs, but most were diagnosed with multicentric lymphoma. A specific analysis of the nonmulticentric types, such as the cutaneous, alimentary, mediastinal, and miscellaneous extranodal types, was not performed. Only 12 dogs underwent diagnosis of immunologic type, and evaluations according to immunologic type were not revealed concretely regarding therapeutic effect and prognostic factors. Furthermore, many combinations of chemotherapy were not used, so it was not possible to conduct an appropriate analysis. Further research needs to complement these limitations, and randomized retrospective studies are warranted to assess factors influencing survival times in dogs with lymphoma. AcknowledgmentNone. Conflict of interestThe author declares that there is no conflict of interest. FundingNone. Data availabilityThe datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. ReferencesAlvarez, F.J., Kisseberth, W.C., Gallant, S.L. and Couto, C.G. 2006. Dexamethasone, melphalan, actinomycin D, cytosine arabinoside (DMAC) protocol for dogs with relapsed lymphoma. J. Vet. Intern. Med. 20, 1178–1183. Baskin, C.R., Couto, C.G. and Wittum, T.E. 2000. Factors influencing first remission and survival in 145 dogs with lymphoma: a retrospective study. J. Am. Anim. Hosp. Assoc. 36, 404–409. Carter, R.F., Valli, V.E. and Lumsden, J.H. 1986. The cytology, histology and prevalence of cell types in canine lymphoma classified according the National Cancer Institute Working Formulation. Can. J. Vet. Res. 50, 154–164. Chun, R. Carrett, L.D. and Vail D. 2007. Cancer chemoterapy. In Withrow & MacEwen’s small animal clinical oncology, 4th ed. Eds., Withrow, S.J. and Vail, D.M. St. Louis, MO: Saunders, Elsevier, pp: 163–193. Curran, K. and Thamm, D.H. 2016. Retrospective analysis for treatment of naïve canine multicentric lymphoma with a 15-week, maintenance-free CHOP protocol. Vet. Comp. Oncol. 14(S1), 147–155. Dewhurst, E., Cue, S., Crawford, E. and Papasouliotis, K. 2008. A retrospective study of canine D-dimer concentrations measured using an immunometric “Point-of-Care” test. J. Small Anim. Pract. 49, 344–348. Dhaliwal, R., Kitchell, B.E. and Messick, J.B. 2004. II linfosarcoma nel cane: carateeristiche cliniche. Veterinaria 18, 39–47. Gavazza, A., Sacchini, F., Lubas, G., Gugliucci, B. and Valori, E. 2009. Clinical, laboratory, diagnostic and prognostic aspects of canine lymphoma: a retrospective study. Comp. Clin. Pathol. 18, 291–299. Gorman, N.T. 1991. The haemolymphatic system. In Manual of small animal oncology. Ed., White RAS. Cheltenham, UK: British Small Animal Veterinary Association. Greenlee, P.G., Filippa, D.A., Quimby, F.W., Patnaik, A.K., Calvano, S.E., Matus, R.E., Kimmel, M., Hurvitz, A.I. and Lieberman, P.H. 1990. Lymphomas in dogs. A morphologic, immunologic and clinical study. Cancer 66, 480–490. Hosoya, K., Kisseberth, W.C., Lord, L.K., Alvarez, F.J., Lara-Garcia, A., Kosarek, C.E., London, C.A. and Couto, C.G. 2007. Comparison of COAP and UW-19 protocols for dogs with multicentric lymphoma. J. Vet. Intern. Med. 21, 1355–1363. Jagielski, D., Lechowski, R., Hoffmann-Jagielska, M. and Winiarczyk, S. 2002. A retrospective study of the incidence and prognostic factors of multicentric lymphoma in dogs (1998-2000). J. Vet. Med. A Physiol. Pathol. Clin. Med. 49, 419–424. Keller, E.T., MacEwen, E.G., Rosenthal, R.C., Helfand, S.C. and Fox, L.E. 1993. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma. J. Vet. Intern. Med. 7, 289–295. Konopka, L. 1995. Chłoniaki złos´liwe. In Choroby Wewneztrzne. Ed., Wojtczak, A. Warszawa, PZWL, Vol. 2, pp: 622–649. MacEwen, E.G., Hayes, A.A., Matus, R.E. and Kurzman, I. 1987. Evaluation of some prognostic factors for advanced multicentric lymphosarcoma in the dogs: 147 cases (1978-1981). J. Am. Vet. Med. Assoc. 190, 564–568. Madewell, B.R. 1986. Hematological and bone marrow cytological abnormalities in 75 dogs with malignant lymphoma. J. Am. Anim. Hosp. Assoc. 22, 235–240. Moore, A.S., London, C.A., Wood, C.A., Williams, L.E., Cotter, S.M., L’Heureux, D.A. and Frimberger, A.E. 1999. Lomustine (CCNU) for the treatment of resistant lymphoma in dogs. J. Vet. Intern. Med. 13, 395–398. Mortier, F., Daminet, S., Vandenabeele, S. and Van de Maele, I. 2012. Canine lymphoma: a retrospective study (2009-2010). Vlaams Diergeneeskd Tijdschr 81, 341–351. Nielsen, L., Toft, N., Eckersall, P.D., Mellor, D.J. and Morris, J.S. 2007. Serum C-reactive protein concentration as an indicator of remission status in dogs with multicentric lymphoma. J. Vet. Intern. Med. 21, 1231–1236. Owen, L. 1980. WHO clinical staging. Document VPH/CMO/80, 20. Geneva, Switzerland: World Health Organization. Simon, D., Moreno, S.N., Hirschberger, J., Moritz, A., Kohn, B., Neumann, S., Jurina, K., Scharvogel, S., Schwedes, C., Reinacher, M., Beyerbach, M. and Nolte, I. 2008. Efficacy of a continuous, multiagent chemotherapeutic protocol versus a short term single-agent protocol in dogs with lymphoma. J. Am. Vet. Med. Assoc. 232, 879–885. Teske, E. 1993. Canine malignant lymphoma: a review and comparison with human non-Hodgkin’s lymphoma. Vet. Q. 16, 209-219. Vail, D.M. 2010. Hematopoietic tumors. In Textbook of veterinary internal medicine: diseases of the cat and dog. Eds., Ettinger, S.J. and Feldman, E.C. St. Louis, Saunders. Withrow, S.J. and Vail, D.M. 2007. Hematopoietic tumors. In Withrow and MacEwen’s small animal clinic oncology. Ed4., St Louis, Saunders/Elsevier. | ||
| How to Cite this Article |
| Pubmed Style SJ, . Evaluation of factors influencing survival time in 77 dogs with lymphoma. Open Vet J. 2023; 13(9): 1124-1134. doi:10.5455/OVJ.2023.v13.i9.8 Web Style SJ, . Evaluation of factors influencing survival time in 77 dogs with lymphoma. https://www.openveterinaryjournal.com/?mno=159722 [Access: May 29, 2024]. doi:10.5455/OVJ.2023.v13.i9.8 AMA (American Medical Association) Style SJ, . Evaluation of factors influencing survival time in 77 dogs with lymphoma. Open Vet J. 2023; 13(9): 1124-1134. doi:10.5455/OVJ.2023.v13.i9.8 Vancouver/ICMJE Style SJ, . Evaluation of factors influencing survival time in 77 dogs with lymphoma. Open Vet J. (2023), [cited May 29, 2024]; 13(9): 1124-1134. doi:10.5455/OVJ.2023.v13.i9.8 Harvard Style , S. J. & (2023) Evaluation of factors influencing survival time in 77 dogs with lymphoma. Open Vet J, 13 (9), 1124-1134. doi:10.5455/OVJ.2023.v13.i9.8 Turabian Style , Soo-Yeon Jeong, and . 2023. Evaluation of factors influencing survival time in 77 dogs with lymphoma. Open Veterinary Journal, 13 (9), 1124-1134. doi:10.5455/OVJ.2023.v13.i9.8 Chicago Style , Soo-Yeon Jeong, and . "Evaluation of factors influencing survival time in 77 dogs with lymphoma." Open Veterinary Journal 13 (2023), 1124-1134. doi:10.5455/OVJ.2023.v13.i9.8 MLA (The Modern Language Association) Style , Soo-Yeon Jeong, and . "Evaluation of factors influencing survival time in 77 dogs with lymphoma." Open Veterinary Journal 13.9 (2023), 1124-1134. Print. doi:10.5455/OVJ.2023.v13.i9.8 APA (American Psychological Association) Style , S. J. & (2023) Evaluation of factors influencing survival time in 77 dogs with lymphoma. Open Veterinary Journal, 13 (9), 1124-1134. doi:10.5455/OVJ.2023.v13.i9.8 |