| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 19-24 Original Research Evaluation of the immunization of camels with Brucella abortus vaccine (RB51) in EgyptMousa E. Ahmed1*, Eisa I. Mohamed2, Khoudair M. Ramadan3, Hend E. M. Elsheikh2, Basma M. El-Said4 and Ayman A. Shehata2*1Zagazig Provincial Laboratory, Animal Health Research Institute, Zagazig, Egypt 2Department of Animal Medicine, Infectious Diseases, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 3Brucellosis Research Department, Animal Health Research Institute (AHRI), Giza, Egypt 4Department of Animal Medicine, Internal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Mousa E. Ahmed. Zagazig Provincial Laboratory, Animal Health Research Institute, Zagazig, Egypt. Email: drahmedmosa6 [at] gmail.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
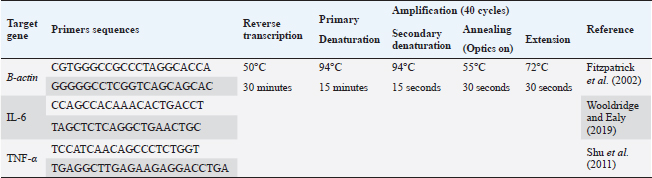
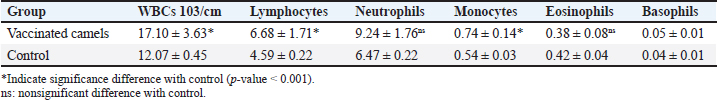
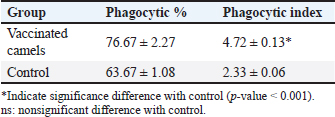
AbstractBackground: Brucellosis is a highly contagious zoonotic disease caused by an intracellular facultative microorganism termed Brucella spp. Control of brucellosis depends on test and slaughter policy as well as vaccination programs. Aim: Estimation of the cell-mediated immunity (CMI) [total leukocytic count (TLC), phagocytic activity, phagocytic index, interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF-α)] in camels after vaccination with RB51 using real-time polymerase chain reaction (PCR). Methods: A total of eight camels were grouped into two groups as follows: group (A): vaccinated with RB51 vaccine [1 dose/2 ml S/C (3 × 1010 CFU)] and group (B): control group. IL-6 and TNF-α were used for estimation of the CMI using real-time PCR on serum samples that were collected at 0, 7, 14, 21, 28, and 60 days after vaccination from each group. In addition, TLC, phagocytic activity, and phagocytic index were evaluated on heparinized blood samples at 0 and 60 days post-vaccination. Results: RB51 vaccine provides a protective immune response which progressively increases from the first week to 60 days after vaccination. Moreover, the levels of TNF-α and IL-6 differed between camels in the vaccinated group. Conclusion: Vaccination of camels with RB51 vaccine (with dose 3 × 1010 CFU) could induce good protective immune responses and this immunological response will be a good indication for a safe field vaccine that can be used for the control of camel brucellosis. Keywords: Brucellosis, Camel, IL-6, RB51, TNF-α. IntroductionBrucella spp. is a Gram-negative, facultative intracellular bacteria that causes severely contagious zoonotic disease and affects domestic and wild mammals. Brucella melitensis, Brucella abortus, and Brucella ovis are the main causes of camel brucellosis (Gwida et al., 2012; Fekadu and Juhar, 2019). Camel brucellosis causes orchitis, epididymitis, stillbirths, infertility, and abortions (Benkirane, 2006). The eradication programs for brucellosis are based mainly on the prevalence of the disease in each country. When the prevalence of brucellosis is low (<1%–2% seropositive animals), the test and slaughter policy as well as the application of hygienic and sanitary measures was admitted. While in highly infected countries (the seroprevalence > 10%) control programs depend mainly on vaccination of all livestock animals of different ages (OIE, 2016). The two most often used vaccines, B. abortus S19 and B. melitensis Rev. 1, interfere with the serological test used to diagnose brucellosis (Refai, 2002). Due to the intracellular nature of the Brucella bacteria, which can survive and proliferate inside the macrophages, cell-mediated immunity (CMI) plays a major part in the protective immune response (Martirosyan et al., 2011). In general, proinflammatory cytokines such as interferon, tumor necrosis factor (TNF), and interleukins (IL-2, IL-4, IL-6, IL-10, IL-12, and IL-1), which promote congenital and acquired immunity and regulate the immune response toward immune-associated cells, play a major role in the infection control and recovery in animals. According to Goenka et al. (2011) and Xavier et al. (2013), the RB51 vaccine was obtained from a serial passage of B. abortus strain 2308 on trypticase soy addition with 1.5% agar and variable engrossment of rifampicin or penicillin. The lipopolysaccharide (LPS) extracted from RB51 does not contain an O-chain so it is a rough strain not return to smooth colony morphology in vitro or in vivo. Thus, this vaccine does not interfere with Brucella diagnosis using serological tests so it is valuable in the control and eradication of brucellosis in endemic countries as it produces CMI (Schurig et al., 1991). Finally, quantitative reverse transcription-polymerase chain reaction (Q-RT-PCR) can calculate various genes even with slight physiologic changes in gene activation and can be done with small amounts of the sample so it can be used to detect cytokine gene expression in ruminants. Q-RT-PCR has high specificity, sensitivity, and accuracy. In addition, the high levels of TNF-α produced later reduced bacterial propagation inside the infected cells (Priyanka et al., 2019). Therefore, this study was directed mainly to estimate the efficacy of B. abortus vaccine strain RB51 in camel using Q-RT-PCR as an indicator for TNF-α and IL-6 gene expression about the antigens of Brucella. Materials and MethodsAnimalsEight camels, aged from 1 to 3 years were used in this study. Before vaccination with RB51, deworming of these animals from ecto and endo parasites was applied for four weeks and also they were examined using mRBPT for antibodies against Brucella and proved to be free from brucellosis. SamplesWhole blood samplesFive ml of heparinized blood samples from the jugular vein were collected after vaccination at 0 and 60 days from all camels for measuring total leukocytic count (TLC), phagocytic activity, and phagocytic index. Specimens were directly transported to the Clinical Pathology Laboratory, Clinical Pathology Unit, AHRI—Zagazig Provincial Laboratory within 1 hour. Serum samplesFive milliliters of blood samples from the jugular vein of all camels were collected from the two groups after vaccination at 0, 7, 14, 21 28, and 60 days without anticoagulant then centrifuged at 3,000 rpm for 10 minutes, and all collected sera used for TNF-α and IL-6 examination. Vaccination protocolA total of eight camels were grouped into two groups as follows. Group (A): Five camels injected with RB51 vaccine (1 dose/2 ml (3 × 1010 CFU) subcutaneously). Group (B): Three camels were used as a control group. Brucella abortus RB51 vaccineBrucella abortus strains RB51 a vaccinal strain, lyophilized vaccine, the vaccine was used in camels by a dose of 2 ml by S/C in the neck area, the dose of 2 ml containing (3 × 1010 CFU), serial No. 1472 (Vacuna RB51® Becerras, Tornel Laboratorios, Mexico). Estimation of protection efficacy of vaccination schedulesCellular immunological analysisTotal and differential leukocytic count It was manually applied by using an improved Neubauer hemocytometer (Feldman et al., 2000) with a diluting fluid (1:10 dilution) of a blood sample to damage the RBCs and stain the nuclei of leukocytes. One hundred cells were counted and classified. Phagocytic activity and phagocytic indexPreparation of Candida albicans suspensionMaking a suspension of C. albicans took only an hour after obtaining the strain from the AHRI Provincial Laboratory’s Microbiology Division in Zagazig. The Candida was prepared according to Xiong et al. (2000). The Candida strain was dispersed and grown on Sabouraud’s dextrose agar plates for 24 hours at 37°C. To kill the Candida, several colonies were transferred to a tube covered with 10 ml of physiological normal saline (0.85%), which was then shaken vigorously and vortexed for 10 minutes (Newman and Holly, 2001). After that, it was centrifuged at 1,500 rpm for 8 minutes after being twice washed with phosphate-buffered saline (pH 7.2). The ultimate concentration of Candida (107 cells/ml) was manually adjusted using the hemocytometer. Candida phagocytic activity testSince neutrophils must participate in phagocytic activity, C. albicans must be quickly killed by heat. Next, with minor modifications, Saikia et al. (2003) discussed the staining and counting of intracellular Candida. For each sample’s 1 ml of heparinized blood, a glass tube was filled with 50 µl of fetal calf serum (Sigma) and 50 µl of the Candida solution. After being gently mixed, the samples were allowed to settle at 37°C for 20 minutes. Each tube sample was divided into two blood smears, which were then stained with Geimsa stain, fixed with methanol, and examined under a microscope (100×). On each slide, 100 neutrophils were counted, and the positive cells or the number of neutrophils that had been consumed by Candida were determined. Calculation of the phagocytic activity and phagocytic indexThe proportion of phagocytized neutrophils to all neutrophils evaluated was known as phagocytic activity. The average number of particles swallowed by each neutrophil undergoing phagocytosis was used to compute the phagocytic index (Berger and Slapnickova, 2003). Number of positive cells/100 cells is the phagocytic index. Evaluation of protection efficacy of the vaccination schedules through analysis of cytokine-related genes by real-time PCRAnalysis of cytokine-related genes by real-time PCRRNA extraction (according to kit instructions) The experiment was carried out using the QIAamp RNeasy Mini kit (Qiagen, Germany, GmbH). 200 µl of the sample was mixed with 600 µl of RLT buffer, which included 10 µl of β-mercaptoethanol per 1 ml, and the combination was then left to sit for 10 minutes at room temperature. The purification of total RNA procedures was carried out by the instructions for the QIAamp RNeasy Mini kit (Qiagen, Germany, GmbH) using the cleared lysate and 1 l of 70% ethanol. The column underwent DNase digestion to remove any residual DNA. Oligonucleotide primers Primers used were obtained from Metabion (Germany) and are listed in Table 1. SYBR green RT-PCR (according to kit instruction) The efficiency of the primers was evaluated in a 25 µl experiment that contained 10 µl of the 2× HERA SYBR® Green RT-qPCR Master Mix (Willowfort, UK), 1 µl of RT Enzyme Mix (20×), 0.5 µl of each primer at a concentration of 20 pmol, 5 µl of water, and 3 µl of RNA template. The reaction was run on a real-time PCR machine in step one. Analysis of the SYBR green RT-PCR results The normalization of each gene’s expression about the expression of β-actin amplification curves and the computation of CT values was done using the step one software. The variance of gene expression on the RNA of the different samples was evaluated using the “Ct” technique, which Yuan et al. (2006) described, by comparing the CT of each sample with the CT of the positive control group using the following ratio: (2−∆∆ct). Statistical analysisNumbers were given as mean ± SE and count data were expressed as percentages. After the Shapiro-Wilk test, which was successful with a p-value more than 0.05, established that the data were normal, one-way analysis of variance was used to analyze the gene expression data. Dennett’s test was used to evaluate the significance between each group and the control group. Using a chi-square test for association, the link between brucellosis and each camel’s age and sex was evaluated. A one-sample chi-square test was used to examine the significance of different seropositive percentages for different dilutions. At p < 0.05, significance was determined. The data were examined using Graph Pad Prism 8.0.2 (Graph Pad Software, Inc.) and SPSS version 25 (Armonk, NY: IBM Corp.). Ethical approvalThe Faculty of Veterinary Medicine’s ethical council and the Animal Health Research Institute in Dokki, Egypt (Approval No. ZU-IACUC/2/F/308/2022) guided how to handle and care for the animals. ResultsPost-vaccination observationsClinical examination of camels revealed that there were no post-vaccinated reactions. There were nonsignificant variations in body temperature and appetite noticed in any of the vaccinated groups throughout the 14 days after vaccination. Leukogram and phagocytic activity of camels’ post-vaccinationThe results revealed that there was a significant increase in leukocytes till 60 days post-vaccination. The result of differential leukocytic count found a significant increase in lymphocytes and monocytes, whereas there were no significant changes in neutrophil, basophil, and eosinophil post-vaccination compared with control groups (Table 2). The results of phagocytic % and phagocytic index increased in the vaccinated group than control group (Table 3). Table 1. Primers sequences, target genes, amplicon sizes, and cycling conditions for SYBR green real-time PCR.
Table 2. Leukogram of the experimental camels post-vaccination.
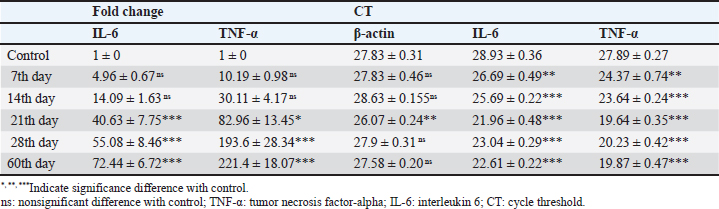
Result of cytokine analysis (CMI response) of vaccinated camelsQ-RT-PCR was used for the detection of the mRNA expressions of TNF-α and IL-6. The fold differences were used to determine the expressing variance in the vaccinated and the control group and there was significant variation in the levels of TNF-α and IL-6 among the vaccinated group that appeared 21 days post-vaccination (Table 4). DiscussionControlling camel brucellosis is a huge problem and needs extensive struggles in developing countries. It is established that vaccination increases the population’s resistance to the disease. Effective vaccine almost eradicates the clinical symptoms of the disease and reduce the contamination of the environment and population at risk to the infectious agent as well. This study aimed to estimate the efficacy of B. abortus vaccine strain RB51 in camels. The efficacy was determined by the cellular immune response. Brucella microorganisms can survive and multiply inside the macrophages because of their facultative intracellular nature so CMI is regarded as essential for immune system protection (Nicoletti and Winter, 1990; Martirosyan et al., 2011). CMI is characterized by the activation of macrophages by antigen-specific T cells leading to the destruction of the microorganism (Oliveira et al., 1998). The ideal vaccine against brucellosis should be easily produced and stored, stable, long immunogenic duration, have minimum interference with serological tests, least undesirable effects in vaccinated animals, and not cause brucellosis in men in case of accidental exposure (Nicoletti and Winter, 1990), eliminate the clinical symptoms of the disease and decrease the contamination of the environment (Olsen, 2013). The TLC was observed to be the highest in camel (1) in comparison to the others and this might be attributed to the microbial pressure (dose 3 × 1010 CFU) (Table 2). Whereas the phagocytic activity and phagocytic index in camel (4) was higher than other camels (Table 3). These results are similar to Higgins et al. (2018) who found an increase in pro-inflammatory response characterized by an increased number of lymphocytes after Rev.1 vaccination in goats. Moreover, CMI is characterized by the stimulation of macrophages through antigen-specific T-cells. The cellular immune response indicated by TLC was shown to be significantly greater in vaccinated camels than in control. In addition, phagocytic activity and phagocytic index in vaccinated camels were significantly greater than control. This may be attributed to the virulence of the RB51 live vaccine. This result agreed with Jonathon (2009). Table 3. The phagocytic activity and phagocytic index post vaccination.
Table 4. Cytokine gene expressions of IL-6 and TNF-a as a comparison between the vaccinating group with the control group, results in the table represent mean ± SE.
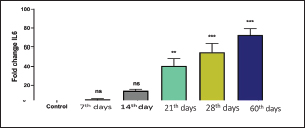
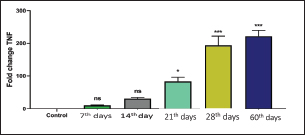
Recently, Q-RT-PCR was used to estimate the cytokine gene expression because it can quantify messenger RNA (mRNA) of any gene and can use a minor amount of sample with high sensitivity, specificity, and accuracy (Giulietti et al., 2001; Priyanka et al., 2019). Cytokine evaluation has a main role in determining the status of the immune response and detecting the severity of the infection. Both TNF-α and IL-6 elevated in acute or chronic brucellosis and had a significant role in the elimination of Brucella microorganisms from the macrophages (David et al., 2018; Lin et al., 2020). Our results revealed that the RB51 vaccine stimulated the production of a highly immune response represented by high levels of IL6 and TNF-α inside the body. There was a significant fold change of IL-6 and TNF-α at 21st, 28th, and 60th day. There was a progressive expression of the IL-6 was found to be increased from the 1st week till the 60th day post-vaccination (Table 4 and Fig. 1). These results revealed a constant release of Th1 cytokine response in RB51 vaccinated camel with a dose of 3 × 1010 CFU, which may be useful for developing long-term immunity against Brucella infections. Our findings agreed with that reported by Hop et al. (2019) who found that great levels of IL-6 stimulate the bactericidal activity of macrophages and differentiation of CD8+ T cells, leading to the Th1 immune response against Brucella infection. In addition, Ramy et al. (2022) found that the level of IL-6 begins to increase from first-week post-vaccination of ewes with RB51. Moreover, Odbileg et al. (2008) found a significant increase in the level of IL-6 from first-week post-vaccination of camel with B. abortus S19. The progressive expression of TNF-α reflected the stimulation of Th1-type immune cells by LPS or endotoxin (Table 4 and Fig. 2). The high production of TNF-α leads to successive reduction of proliferation of the bacteria inside the infected cells (Ottones et al., 2000; Dornand et al., 2002). In addition, Ghalib et al. (1995) and Ko et al. (2002) reported that stimulation of TNF-α cytokine mRNA played a main role in cellular immune response regulation against brucellosis and other intracellular infections.
Fig. 1. Comparing the effect of gene expression on IL-6 in vaccinated and control groups. Bars represent mean ± SE. bars carrying ** and *** represent significant differences with the control group, p < 0.001, and bars with ns represent nonsignificant differences with the control.
Fig. 2. Comparing the effect of gene expression on TNF-a in vaccinated and control groups. Bars represent mean ± SE. bars carrying ** and *** represent significant differences with the control group, p < 0.001, and bars wi SE. bars carrying ** and *** represent a highly significant difference with the control group, p < 0.001. These results revealed a constant release of Th1 cytokine (especially TNF-a) response in RB51 vaccinated camel with a dose of 3 X 1010 CFU, which may be useful for developing long-term immunity against Brucella infections. ConclusionTo the best of our knowledge, this is the first report that has evaluated the cell-mediated response post RB51 vaccination in camels in Arabian countries. We found that RB51 (3 × 1010 CFU) could activate a cellular Th1 immune response against camel brucellosis and prove that the control strategy against B. melitensis in Egypt needs rethinking to improve vaccine efficacy. Conflict of interestThe authors declare that they have no conflict of interest. Data availabilityAll data are provided in the manuscript. Any extra data needed can be provided by the corresponding author upon reasonable request. ReferencesBenkirane, A. 2006. Ovine and caprine brucellosis: world distribution and control/eradication strategies in West Asia/North Africa region. Small Rumin. Res. 62(1–2), 19–25. Berger, J. and Slapnickova, M. 2003. Circadian structure of rat neutrophil phagocytosis. Comp. Clin. Pathol. 12, 84–89. David, W., Pascual, Xinghong, Y., Hongbin, W., Zakia, G., Carol, H. and Beata, C. 2018. Alternative strategies for vaccination to brucellosis. Microbes Infect. 20(9–10), 599–605. Dornand, J., Gross, A., Lafont, V., Liautard, J., Oliaro, J. and Liautard, J.P. 2002. The innate immune response against Brucella in humans. Vet. Microbiol. 90, 383–394. Fekadu, G. and Juhar, T. 2019. Review on camel brucellosis: public health importance and status in Ethiopia. Acad. Res. J. Agri. Sci. Res. 7(7), 513–529. Feldman, B.F., Zinkl, J.G., Jain, N.C., Gasper, P.E., Giger, U., De Gopegui, R.R., Grindem, C.B., Kristensen, A.T., Latimer, K.S. and Rogers, K. 2000. Schalm’s veterinary hematology, 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins, pp: 1120–1124. Fitzpatrick, R., Casey, O.M., Morris, D., Smith, T., Powell, R. and Sreenan, J.M. 2002. Postmortem stability of RNA isolated from bovine reproductive tissues. Biochim. Biophys. Acta 1574, 10–14. Ghalib, H.W., Whittle, J.A., Kubin, M., Hashim, F.A., El-Hassan, A.M., Grabstein, K.H., Trinchieri, G. and Reed, S.G. 1995. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J. IMMUNOL. 154(9), 4623–4629. Giulietti, A., Overbergh, L., Valckx, D., Decallonne, B., Bouillon, R. and Mathieu, C. 2001. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25, 386–401. Goenka, R., Parent, M.A., Elzer, P.H. and Cynthia, L.B. 2011. B cell-deficient mice display markedly enhanced resistance to the intracellular bacterium Brucella abortus. J. Infect. Dis. 203(8), 1136–1146. Gwida, M., Adel, E.G., Falk, M., Iahtasham, K., Uwe, R. and Heinrich, N. 2012. Brucellosis in camels. Res. Vet. Sci. 92(3), 351–355. Higgins, J.L., Bowen. R.A. and Gonzalez, J.M. 2018. Cell-mediated immune response in goats after experimental challenge with the virulent Brucella melitensis strain 16 M and the reduced virulence strain Rev. 1. Vet. Immunol. Immunopathol. 202, 74–84. Hop, H.T., Huy, T.X.N., Reyes, A.W.B., Arayan, L.T., Vu, S.H., Min, W., Lee, H.J., Kang, C.K., Kim, D.H., Tark, D.S. and Kim, S. 2019. Interleukin 6 promotes Brucella abortus clearance by controlling the bactericidal activity of macrophages and CD8(+) T cell differentiation. Infect. Immun. 87, e00431–e00519. Jonathon, A. 2009. Nitroblue tetrazolium tests an assay of neutrophil function in diabetes mellitus. Doctoral dissertation, Madras Medical College, Chennai, India. Ko, J., Gendron-Fitzpatrick, A. and Splitter, G.A. 2002. Susceptibility of interferon regulatory factor (IRF-1) and interferon consensus sequence binding protein (ICSBP) deficient mice to brucellosis. J. Immunol. 168, 2433–2440. Lin, Z., Guo-Yue, L., Wen-Wen, H., Chi, Z., Rui, Z., Yuan-Da, L., Fan, W., Ying, Q., Li, D., Dou-Dou, Z., Xiao-Juan, Q., Hui, G. and Hai, J. 2020. IL-6 and INF-γ levels in patients with brucellosis in the severe epidemic region, Xinjiang, China. Infect. Dis. Poverty 9, 47. Martirosyan, A., Moreno, E. and Gorvel, J.P. 2011. An evolutionary strategy for a stealthy intracellular Brucella pathogen. Immunol. Rev. 240, 211–234. Newman, S.L. and Holly, A. 2001. Candida albicans are phagocytosed, killed, and processed for antigen presentation by human dendritic cells. Infect. Immun. 69, 6813–6822. Nicoletti, P. and Winter, A.J. 1990. Response to Brucella abortus. In Animal brucellosis. Eds., Nielsen K. and Duncan J.R. BocaRaton, FL: CRC Press, pp 83–95. Odbileg, R., Purevtseren, B., Dorj, G., Bazartseren, B., Tumurjav, B., Zagd, G., Janchivdorj, E., Satoru, K., Misao, O. and Kazuhiko, O. 2008. Cytokine responses in camels (Camelus bactrianus) vaccinated with Brucella abortus strain 19 vaccine. J. Vet. Med. Sci. 70(2), 197–201. OIE. 2016. Brucellosis. Chapter 3.1.4. Paris, France: OIE, pp: 355–398. Oliveira, S.C., Harms, J.S., Rech, E.L., Rodarte, R.S., Bocca, A.L., Goes, A.M. and Splitter, G.A. 1998. The role of T cell subsets and cytokines in the regulation of intracellular bacterial infection. Brazilian J. Med. Biol. Res. 31, 77–84. Olsen, S.C. 2013. Recent development in livestock and wildlife brucellosis vaccination. Rev. Sci. Tech. 32(1), 207–217. Ottones, F., Liautard, J., Gross, A., Rabenoclina, F., Liautard, J.P. and Favcro, J. 2000. Activation of human Vgamrna9Vdelta2 Tcclls by a Brucella suis non-peptidic fraction impairs bacterial intracellular multiplication in monocytic infected cells. Immunology 100(2), 252–258. Priyanka, C., Brij, N.S., Om, P.C. and Sudhir, K.K. 2019. Cytokines in brucellosis: biological rhythm at the interface of innate and adaptive immunity. Biol. Rhythm Res. 50(4), 1–13. Ramy, F.G., Samar, M.A., Mohamed, B., Verginia, M.F., Eisa, M., Doaa, N. and Mohamed, E. 2023. Comparative immune responses and cytokine gene expressions in sheep vaccinated with Brucella abortus RB51 vaccine and Brucella melitensis Rev. 1 vaccine. J. Adv. Vet. Res. 13(1), 1–8. Refai, M. 2002. Incidence and control of brucellosis in the Near East region. Vet. Microbiol. 90, 81–110. Saikia, T.C., Pramanik, T. and Thapa, M. 2003. Phagocytic activities of neutrophilic leukocytes in women in various phases of the menstrual cycle, and pregnancy. Southeast Asian J. Trop. Med. Public Health 34, 877–880. Schurig, G.G., Roop, R.M., Bagchi, T., Boyle, S., Buhrman, D. and Sriranganathan, N. 1991. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet. Microbiol. 28(2), 171–188. Shu, D., Subharat, S., Wedlock, D.N., Luo, D., de Lisle, G.W. and Buddle, B.M. 2011. Diverse cytokine profile from mesenteric lymph node cells of cull cows severely affected with Johne’s disease. Clin. Vaccine Immunol. 18(9), 1467–1476. Wooldridge, L.K. and Ealy, A.D. 2019. Interleukin-6 increases inner cell mass numbers in bovine embryos. BMC Dev. Biol. 19(1), 1–11. Xavier, M.N., Winter, M.G., Spees, A.M., Nguyen, K., Atluri, V.L., Silva, T.M.A., Bäumler, A.J., Müller, W., Santos, R.L. and Tsolis, R.M. 2013. CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLoS Pathog. 9(6), e1003454. Xiong, J., Kang, K., Liu, L., Yoshida, Y., Cooper, K.D. and Ghannoum, M.A., 2000. Candida albicans and Candida krusei differentially induce human blood mononuclear cell interleukin-12 and gamma interferon production. Infect. Immun. 68, 2464–2469. Yuan, J.S., Reed, A., Chen, F. and Stewart, C.N. 2006. Statistical analysis of real-time PCR data. BMC Bioinform. 7, 85. | ||
| How to Cite this Article |
| Pubmed Style MEA, Mohamed EI, Ramadan KM, Elsheikh HE, El-said BM, Shehata AA. Evaluation of the immunization of camels with Brucella abortus vaccine (RB51) in Egypt. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 19-24. doi:10.5455/OVJ.2024.v14.i1.3 Web Style MEA, Mohamed EI, Ramadan KM, Elsheikh HE, El-said BM, Shehata AA. Evaluation of the immunization of camels with Brucella abortus vaccine (RB51) in Egypt. https://www.openveterinaryjournal.com/?mno=174658 [Access: May 09, 2024]. doi:10.5455/OVJ.2024.v14.i1.3 AMA (American Medical Association) Style MEA, Mohamed EI, Ramadan KM, Elsheikh HE, El-said BM, Shehata AA. Evaluation of the immunization of camels with Brucella abortus vaccine (RB51) in Egypt. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 19-24. doi:10.5455/OVJ.2024.v14.i1.3 Vancouver/ICMJE Style MEA, Mohamed EI, Ramadan KM, Elsheikh HE, El-said BM, Shehata AA. Evaluation of the immunization of camels with Brucella abortus vaccine (RB51) in Egypt. Open Vet J. (2024), [cited May 09, 2024]; 14((1) (Zagazig Veterinary Conference)): 19-24. doi:10.5455/OVJ.2024.v14.i1.3 Harvard Style , M. E. A., Mohamed, . E. I., Ramadan, . K. M., Elsheikh, . H. E., El-said, . B. M. & Shehata, . A. A. (2024) Evaluation of the immunization of camels with Brucella abortus vaccine (RB51) in Egypt. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 19-24. doi:10.5455/OVJ.2024.v14.i1.3 Turabian Style , Mousa E. Ahmed, Eisa I. Mohamed, Khoudair M. Ramadan, Hend E.M. Elsheikh, Basma M. El-said, and Ayman A. Shehata. 2024. Evaluation of the immunization of camels with Brucella abortus vaccine (RB51) in Egypt. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 19-24. doi:10.5455/OVJ.2024.v14.i1.3 Chicago Style , Mousa E. Ahmed, Eisa I. Mohamed, Khoudair M. Ramadan, Hend E.M. Elsheikh, Basma M. El-said, and Ayman A. Shehata. "Evaluation of the immunization of camels with Brucella abortus vaccine (RB51) in Egypt." Open Veterinary Journal 14 (2024), 19-24. doi:10.5455/OVJ.2024.v14.i1.3 MLA (The Modern Language Association) Style , Mousa E. Ahmed, Eisa I. Mohamed, Khoudair M. Ramadan, Hend E.M. Elsheikh, Basma M. El-said, and Ayman A. Shehata. "Evaluation of the immunization of camels with Brucella abortus vaccine (RB51) in Egypt." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 19-24. Print. doi:10.5455/OVJ.2024.v14.i1.3 APA (American Psychological Association) Style , M. E. A., Mohamed, . E. I., Ramadan, . K. M., Elsheikh, . H. E., El-said, . B. M. & Shehata, . A. A. (2024) Evaluation of the immunization of camels with Brucella abortus vaccine (RB51) in Egypt. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 19-24. doi:10.5455/OVJ.2024.v14.i1.3 |