| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 25-31 Original Research Clinicopathological studies on ulcerative lymphangitis in cattle: Alterations in serum inflammatory cytokines, anti-microbial, organs functions, and oxidative stress-related biomarkersDina R.S. Gad El-Karim1*, Gamal El-Amrawi2 and Alyaa R. Salama11Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt 2Department of Theriogenology, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt *Corresponding Author: Dina R.S. Gad El-Karim. Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, Alexandria University, Alexandria, Egypt. Email: dina.shabaan [at] alexu.edu.eg Submitted: 01/10/2023 Accepted: 15/12/2023 Published: XX/01/2024 © 2024 Open Veterinary Journal
AbstractBackground: Affection with Corynebacterium pseudotuberculosis (C. pseudotuberculosis) and development of cellulitis and/or abscess formation with cutaneous lymphangitis in cattle is rare to some extent, so literature about the biochemical changes that would accompany this infection is rare. Aim: In this context, the present study was designed to screen the effect of the infection with C. pseudotuberculosis cutaneous lymphangitis on the release of some immune molecules, organ functions, and redox state in Baladi cows. Methods: Fourteen Baladi cows from a small dairy farm in El-Behira, Egypt, were selected to complete this study. After bacteriological culture confirmation, seven of them were found suffering from cutaneous lesions due to infection with C. pseudotuberculosis (Diseased group), while the others were healthy (Healthy group). Serum samples were obtained to evaluate the presumptive changes in some clinicopathological parameters. Results: Serum analysis revealed a significant decrease in the levels of interferon-gamma and interleukin-17 as well as a significant decrement in the concentration of beta-defensin (β-defensin) and lipocalin-2. While serum level of interleukin-10 recorded a significant increase in these animals when compared to healthy control animals. Concurrently, the affected animals recorded a significant elevation in serum levels of hepato-cardiac enzymes, urea, and creatinine in addition to disturbance in the serum redox state. Conclusion: In conclusion, infection with C. pseudotuberculosis cattle may disturb the defensive immune state, body organ function, and redox state of the animals. Keywords: Corynebacterium pseudotuberculosis, Lymphangitis, Cows, Cytokines, Redox state. IntroductionUlcerative lymphangitis (edematous skin disease) is a bacterial disease caused by Corynebacterium pseudotuberculosis (C. pseudotuberculosis) which affects many animal species including, horses, cattle, and sheep (Zavoshti et al., 2009; Cahn and Line, 2010) and even camels (Tejedor-Junco et al., 2008), but it rarely affects human beings (Peel et al., 1997). In cattle, the disease usually appears in the form of cutaneous abscesses and/or cellulitis especially in limbs (Jaiswal et al., 2017; Steerforth and Marutsov, 2017). Corynebacterium pseudotuberculosis is a gram-positive, nonmotile, pleomorphic, facultative anaerobic bacteria that grows sufficiently on blood-agar media and forms opaque whitish colonies (Coyle and Lipsky, 1990). Corynebacterium pseudotuberculosis virulence depends on the excretion of an exo-toxin (phospholipase D toxin) and its ability to survive within macrophages (Yozwiak and Songer, 1993) due to the presence of mycolic acids (MAs) containing outer membrane which serves as permeability barrier (Odhah et al., 2022). Edematous skin disease was reported in cattle and buffaloes from many countries and the infection was confirmed through isolation and biochemical tests (Mohamed and Reda, 2015; Jaiswal et al., 2017). In cattle, infection mainly occurs through skin abrasions or injuries and transmits to lymph vessels causing multiple abscesses along their courses, but involvement of lymph nodes is rare (Radostits et al., 2004); in addition, metastasis to visceral organs may occur (Yeruham et al., 1997; Steinman et al., 1999). Also, toxemia could occur due to circulation of exo-toxin (Selim, 2001). Infection and outbreaks usually occur in summer as insects may share in disease dissemination (Mohamed and Reda, 2015). Mortality due to affection with edematous skin disease in cattle and buffaloes is low, but disease morbidity is high (Khalil et al., 1995). For any disease, detecting serum biochemical changes may offer sufficient information for disease diagnosis, progression, and establishment of internal organs (liver, kidney, heart, and muscles) affections, in addition to monitoring disease prognosis and treatment efficiency (Agina, 2017). However, there is a paucity of research that discussed serum biochemical changes that accompany affection with edematous skin disease in cattle, so this study aimed to investigate some serum biochemical alterations in Baladi cattle affected with edematous skin disease. Materials and MethodsAnimals and clinical examinationDuring April 2023, this study was conducted on 14 Baladi cows; 2–4 years old and 300–350 kg body weight, belonging to small dairy farms in El-Behira governorate, Egypt. Seven were suffering from cutaneous abscess formation and cellulitis with lymphangitis (diseased group). From the animals’ history, the appearance of lesions was determined about 3 weeks ago, the lesions were present in both forms, either open (ulcerative) or closed along different parts of the body especially hind limbs below the hock. Some animals had ulcerative lesions on the ventral abdomen, pre-scapular region, and neck (Figs. 1–3). Also, the pre-scapular and pre-femoral lymph nodes were enlarged in affected cows. The other seven cows were healthy and enrolled as a healthy control group. Healthy animals were monitored for 2 weeks after sampling to exclude affection of ulcerative lymphangitis. In addition, the animals that received any systemic treatment within the last 7 days before sampling were excluded. Body temperature, pulse rate, and respiratory rate were detected and recorded. Bacteriological sampling and culturingDouble samples were obtained under aseptic conditions for bacteriological examination either directly from ulcerative lesions using swabs or through aspiration from closed lesions. The samples were transported in an ice box for bacteriological analysis. Samples were inoculated into nutrient broth at 37℃ for 24 hours under anaerobic conditions. Then, they were streaked on sheep blood agar media and incubated for 48 hours at 37℃ under aerobic conditions and in a CO2 incubator (Bailey and Scott, 1990). The characteristic colonies of C. pseudotuberculosis bacteria were detected (granular appearance dull colonies) and bacteria were examined with the aid of Gram stain for morphological characterization (Gram-positive, pleomorphic rods arranged in a typical “Chinese letter” arrangement). For confirmation, several biochemical tests were applied including an indole test, gelatin hydrolysis test, urea production, and fermentation of glucose, galactose, lactose, and ribose sugars (Quinn et al., 1994). Blood samplingBlood aliquots were obtained directly from the jugular vein of all the animals in plain vaccutainers, left to clot for 30 minutes at room temperature, and then the samples were centrifuged for 10 minutes at 3,000 r.p.m to separate serum samples which were stored at −20℃ for further biochemical evaluation.
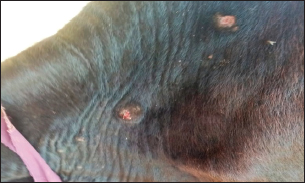
Fig. 1. Presence of abscess on the hind limb of an affected cow.
Fig. 2. Presence of abscess on the neck of affected cow.
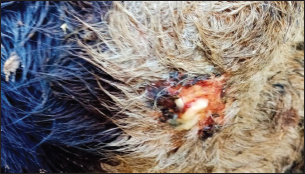
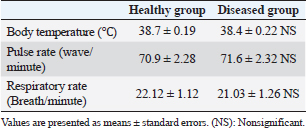
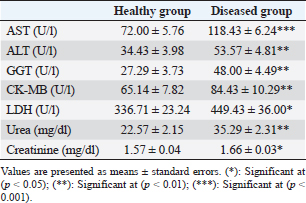
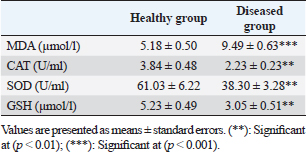
Fig. 3. Ulcerative lesion with the presence of yellowish exudate on the neck of the affected cow. Evaluation of serum inflammatory cytokines and anti-microbial parametersELISA-based serum levels of interferon-γ (IFN-γ) (Cusabio, China), interleukin-17 (IL-17) (Mybiosource, USA), interleukin-10 (IL-10) (Cusabio, China), beta-defensin, and lipocalin-2 (LCN-2) (Mybiosource, USA) were determined. Evaluation of hepatic and cardiac functions related to enzymesSerum activity of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), creatine kinase MB (CK-MB), and lactate dehydrogenase (LDH) enzymes were evaluated using commercially available kits (Diamond Diagnostic, USA). Evaluation of renal-function-associated parametersConcentrations of urea and creatinine were detected in serum samples using commercial kits (Diamond Diagnostic, USA). Evaluation of oxidant/antioxidant biomarkersSerum concentrations of malondialdehyde (MDA) and reduced glutathione (GSH) with serum catalase (CAT) and superoxide dismutase (SOD) activities were determined in serum samples using commercially available kits (Bio-diagnostic, Egypt). Statistical analysisThe comparison between the means of the determined parameters was done using an independent t-test with the aid of the SPSS 16.0 software package for Windows. The values are present as mean ± standard error. Ethical approvalThis study was approved ethically by the institutional animal care and usage committee, at Alexandria University. ResultsChanges in some clinical parameters (body temperature, respiratory rate and pulse rate)Clinical examination of the animals revealed no statistically significant difference in body temperature, pulse rate, and respiratory rate between affected cows and the control group (Table 1). Serum inflammatory cytokines and anti-microbial parametersAs shown in Table 2, serum levels of IFN-γ and IL-17 recorded a significant decrease in animals suffering from edematous skin disease; this decrement was associated with a significant reduction in serum levels of beta-defensin and LCN-2 when compared to mean values of control animals, while the serum level of IL-10 was increased in affected cows as compared to healthy cows. Serum activity of hepatic and cardiac enzymesSerum activity of AST, ALT, GGT, CK-MB, and LDH enzymes recorded a significant increase in animals affected with an edematous skin disease as compared to healthy cows (Table 3). Serum level of urea and creatinineIn comparison with control healthy cows, serum concentration of urea and creatinine recorded a significant increment in animals affected with edematous skin disease (Table 3). Serum oxidant/antioxidant biomarkersThe present data in Table 4 showed that serum level the of MDA was increased significantly in cows with edematous skin disease, while serum activity of antioxidant enzymes (CAT and SOD) and serum GSH levels were decreased in the same group when compared to control healthy animals. Table 1. Mean values of some clinical parameters in healthy and diseased cows.
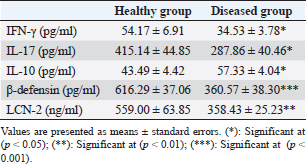
Table 2. Serum concentration of IFN-γ, IL-17, IL-10, β-defensin, and LCN-2 in healthy and diseased animals.
Table 3. Serum concentration of hepato-cardiac enzymes, urea, and creatinine in healthy and diseased animals.
Table 4. Serum concentration of different oxidant/antioxidant parameters in healthy and diseased animals.
DiscussionCorynebacterium pseudotuberculosis is a substantial member of the Corynebacterium, Mycobacterium, and Nocardia group, and this group possesses several bacterial species of medical, biotechnological, and veterinary interest. Corynebacterium pseudotuberculosis mainly affects ovine causing caseous lymphadenitis, but it can also affect equines, bovines, camels, and humans (Bastos et al., 2012). The ulcerative form of C. pseudotuberculosis is more common in cattle (Al-Gaabary et al., 2005), and usually appears as a pyogenic cutaneous ulceration that is characterized by a sudden onset and lasting for several days (Oreiby et al., 2014) and this would indicate the role of dermo-necrotic exotoxin that is produced from C. pseudotuberculosis (Quinn et al., 1994). Regional lymph nodes were swollen to several times of their normal dimensions indicating that lymphatic tissues are a target for C. pseudotuberculosis as a facultative intracellular micro-organism (Conner et al., 2000). The absence of alteration in temperature (no fever) and other clinical parameters is familiar upon affection of cattle with ulcerative lymphangitis (Steinman et al., 1999). Biochemical changes are a very valuable tool for understanding the pathogenesis of any disease, as they offer the fundamental base for diagnosis and treatment of the disease process (Kaneko et al., 2008). Based on this concept and concerning this study’s findings, the decrease in serum level of IFN-γ, could be explained based on the findings of Rebouças et al. (2011), as their study declared that secretion of IFN-γ is considered a short-lived primary response of the body against infection with C. pseudotuberculosis, so its level was fluctuating and recorded its peak after 5 days from infection induction then declined till day 42 after infection. In addition, the decrease in serum level of IFN-γ could be owed to the ability of MAs immunogen which is present in the outer membrane of C. pseudotuberculosis bacteria (Odhah et al., 2022) to induce depletion of T-lymphocytes (Kim et al., 2014) which produce IFN-γ.IL-17 is a pro-inflammatory cytokine that is produced exclusively by T-cells (Moseley et al., 2003). It is chiefly responsible for the production of anti-microbial peptides (such as defensin and mucin), remodeling proteins, induction of immune defense, and tissue repair (Fossiez et al., 1996). On the other hand, an increased level of IL-17 was proved to be incorporated widely in the occurrence of sepsis (Rendon and Choudhry, 2012), as it may inhibit neutrophils’ microbicidal function and migration (Ye et al., 2001). Therefore, the decrease of IL-17 levels in diseased animals would be a defensive mechanism of the body against the sepsis process. In addition, the decrease in IL-17 production would induce synthesis of IL-10 (anti-inflammatory cytokine) to inhibit excessive inflammatory reaction in case of sepsis (Wu et al., 2015) and this would explain the significant increase in serum level of IL-10 in animals suffering from infection in this study. Unfortunately, the enhanced production of IL-10 would inhibit the production of IFN-γ (Schroder et al., 2003), and decrease antigen presentation (Sabat et al., 2010) which may decrease pathogen clearance. Also, the detected decrement in peripheral blood IL-17 level would be a consequence of the previously mentioned inhibitory effect of C. pseudotuberculosis on T-cells (Kim et al., 2014). β-defensin is one of the most abundant antimicrobial peptides which is produced by epithelial tissues (de Oca, 2010) including skin keratinocytes (Yang et al., 2007). Also, β-defensin comprises an immune-modulatory effect besides its role as a link between adaptive and innate immunity (Yang et al., 1999). Moreover, the decrease in IL-17 production would be reflected in the production of surface anti-microbial proteins (defensins and mucins) (Onishi and Gaffen, 2010), so serum level of β-defensin was decreased in animals affected with edematous skin disease. In the same manner, LCN-2 is considered an acute-phase protein that is secreted mainly from neutrophils besides the majority of body tissue cells during bacterial infection, so it is also called neutrophil gelatinase-associated lipocalin (Goetz et al., 2002). LCN-2 exerts its anti-bacterial action and sharing in innate immunity through binding to iron-laden siderophores which scavenge iron from the host to deliver it to the bacteria for replication (Flo et al., 2004). LCN-2 also contributes to immune-modulating, tissue repair, and epithelial cell regeneration (Santiago-Sánchez et al., 2020). Unfortunately, secretion of LCN-2 is stimulated by IL-17 (Karlsen et al., 2010), so its level would be decreased as a consequence of decrement in IL-17 production as recorded in animals suffering from C. pseudotuberculosis infection. Referring to serum biochemical changes, phospholipase D exo-toxin has the power to degrade mammalian sphingomyelin, especially at the endothelial cells level which increases vascular permeability and allows passage of bacteria into tissue space, then from these sites into the lymphatic system which distributes bacteria from the infection site to other body organs (Guimarães et al., 2011). In this consistency, the elevated level of hepatic enzymes (AST, ALT, ALP, and GGT), renal function-associated biomarkers (urea and creatinine), in addition to cardiac muscle dysfunction-related biomarkers (CK-MB and LDH) could be explained relying on the findings of Al-Gaabary et al. (2010) who reported that infection of sheep with C. pseudotuberculosis was proved to induce hepatic distortion and necrosis with congestion and vacuolar degeneration of hepatocytes. This infection also was implicated in the degeneration of renal tubular epithelial cells and myocardial fibers (Ibtisam, 2008). Our findings are to the results obtained by Mahmood et al. (2015), as their study illuminated that this alteration was recorded in response to the injection of phosplolipase D exotoxin or induced infection with C. pseudotuberculosis itself in goats. The elevated serum level of MDA and depletion of enzymatic and nonenzymatic antioxidants in animals suffering from edematous skin disease would illuminate the evoked oxidative stress due to infection with C. pseudotuberculosis. This redox imbalance was previously investigated upon inoculation of mice with C. pseudotuberculosis as the infection significantly induced a state of oxidative stress which was indicated by the increased production of peroxinitrite and malndialdehyde (lipid peroxides) (Awni et al., 2020). Also, infection in sheep induced a significant elevation in serum level of MDA with a significant decrement in the concentration of GSH and activity of SOD and CAT enzymes (Polat et al., 2023). ConclusionThis study could conclude that infection with C. pseudotuberculosis in cattle did not affect the general clinical parameters of the affected animals, but it could impair hepato-renal and cardiac functions. It also would disturb redox balance and generate a state of oxidative stress. In addition, infection with this bacterium can disturb IFN-γ, inflammatory/ anti-inflammatory cytokines, and tissue anti-microbial molecules production, which would delay recovery and share in the persistence of the infection. AcknowledgmentNone. Conflict of interestThe authors declare that there is no conflict of interest. Data availabilityAll data are provided in the manuscript. Any extra data needed can be provided by the corresponding author upon reasonable request. ReferencesAgina, O.A. 2017. Haematology and clinical biochemistry findings associated with equine diseases—a review. Not. Sci. Biol. 9(1), 1–21. Al-Gaabary, M.H., Ammar, K.M., Osman, S.A. and Badr, A.M.I. Oedematous skin disease in buffaloes: clinical, epidemiological, histopathological and therapeutic studies. In 8th Scientific Congress. Egyptian Society for cattle diseases, Assiut University, Asyut, Egypt, 2005, pp 65–80. Al-Gaabary, M.H., Osman, S.A., Ahmed, M.S. and Oreiby, A.F. 2010. Abattoir survey on caseous lymphadenitis in sheep and goats in Tanta, Egypt. S. Rum. Res. 94, 117–124. Awni, K.J., Abass, R.S., All-Rikabi, R.H. and Nuaman, Z.T. 2020. Antioxidant role of milk thistle in mice infected with a virulent strain of Corynebacterium pseudotuberculosis. Sys. Rev. Pharm. 11(12), 1833–1837. Bailey, A. and Scott, S. 1990. Diagnostic microbiology, 8th ed. St. Louis, MO: Mosby Company. Bastos, B.L., Portela, R.W.D., Dorella, F.A., Ribeiro, D., Seyffert, N., Castro, T.L.D., Miyoshi, A., Oliveira, S.C., Meyer, R. and Azevedo, V. 2012. Immunological responses in animal models and zoonotic potential. J. Clin. Cell. Immunol. S4, 005. Cahn, C.M. and Line, S. 2010. The Merck veterinary manual, 9th ed. Whitehouse Station, NJ: John Wiley & Sons, Merck & Co. Conner, K.H., Quirie, M.M., Baird, G. and Donachie, W. 2000. Characterization of United Kingdom isolates of Corynebacterium pseudotuberculosis using pulsed-field gel electrophoresis. J. Clin. Microbiol. 38 (7 ), 2633–2637. Coyle, M.B. and Lipsky, B.A. 1990. Coryneform bacteria in infectious diseases: clinical and laboratory aspects. Clin. Micro. Rev. 3, 227–246. de Oca, E.P.M. 2010. Human β-defensin 1: a restless warrior against allergies, infections, and cancer. Int. J. Biochem. Cell. Biol. 42, 800–804. Flo, T.H., Smith, K.D., Sato, S., Rodriguez, D.J., Holmes, M.A., Strong, R.K., Akira, S. and Aderem, A. 2004. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921. Fossiez, F., Djossou, O., Chomarat, P., Flores-Romo, L., Ait-Yahia, S., Maat, C., Pin, J.J., Garrone, P., Garcia, E., Saeland, S. and Blanchard, D. 1996. T-cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593–2603. Goetz, D.H., Holmes, M.A., Borregaard, N., Bluhm, M.E., Raymond, K.N. and Strong, R.K. 2002. The neutrophilipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell. 10, 1033–1043. Guimarães, A., Carmo, F.B., Paulett, R.B., Seyffert, N., Ribeiro, D., Lage, A.P., Heinemann, M.B., Miyoshi, M., Azevedo, V. and Gouveia, A.M.G. 2011. Caseous lymphadenitis: epidemiology, diagnosis, and control. IIOAB. J. 2(2), 33–43. Ibtisam, M.A. 2008. Someclinicopathological and pathological studies of C. ovis infection in sheep. Egypt. J. Comp. Pathol. Clin. Pathol. 21, 327–343. Jaiswal, V., Verma, H. and Rawat, S. 2017. Ulcerative lymphangitis in Sahiwal cattle. Intas. Polivet. 18, 83–84. Kaneko, J.J., Harvey, J.W. and Bruss, M.L. 2008. Clinical biochemistry of domestic animals, 6th ed. Cambridge, MA: Academic Press. Karlsen, J.R., Borregaard, N. and Cowland, J.B. 2010. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-α is controlled by IҡB- ζ but neither by C/EBP-β nor C/EBP-δ. J. Biol. Chem. 285(19), 14088–14100. Khalil, N.G., Seddek, S.R. and Nashed, S.M. 1995. Studies on ulcerative lymphangitis in buffaloes in Assiut. Assiut. Vet. Med. J. 33(65), 93–99. Kim, Y.J., Kim, H.J., Jeong, S.K., Lee, S.H., Kang, M.J., Yu, H.S. and Yu, J. 2014. A novel synthetic mycolic acid inhibits bronchial hyperresponsiveness and allergic inflammation in a mouse model of asthma. Aller. Asth. Immunol. Res. 6, 83–88. Mahmood, Z.K.H., Jesse, F.F., Saharee, A.A., Jasni, S., Yusoff, R. and Wahid, H. 2015. Assessment of blood changes post-challenge with Corynebacterium pseudotuberculosis and its exotoxin (phospholipase D): a comprehensive study in goat. Vet. World. 8, 1105–1117. Mohamed, S.R. and Reda, L.M. 2015. Oedematousskin disease in buffaloes and cows. Egypt. J. Vet. Sci. 45, 1–10. Moseley, T.A., Haudenschild, D.R., Rose, L. and Reddi, A.H. 2003. Interleukin-17 family and IL-17 receptors. Cytokine. Growth. Factor. Rev. 14, 155–174. Odhah, M.N., Jesse, F.F.A., Paul P.T., Chung, E.L.T., Faeza, M.N.N., Norsidin M.J., Garba, B. and Lila, M.A.M. 2022. A current review on mycolic acid immunogen of Corynebacterium pseudotuberculosis. J. Adv. Vet. Res. 12(2), 177–186. Onishi, R.M. and Gaffen, S.L. 2010. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 129, 311–321. Oreiby, A.F., Abo El-Wafa, S.A., Hegazy, Y.M. and Al-Gaabary, M.H. 2014. Oedematous skin disease, a comparison of changeable clinical nature in cattle and buffalo. Kafrelsheikh. Vet. Med. J. 12(2), 91–108. Peel, M.M., Palmer, G.G., Stacpoole, A.M. and Kerr, T.G. 1997. Human lymphadenitis due to Corynebacterium pseudotuberculosis: report of ten cases from Australia and review. Clin. Infect. Dis. 24, 185–191. Polat, E., Kaya, E., Karagülle, B. and Akin, H. 2023. Effects of caseous lymphadenitis agent (Corynebacterium pseudotuberculosis) isolate from superficial abscesses of sheep on oxidative stress factors: oxidative stress in sheep with caseous lymphadenitis. J. Hell. Vet. Med. Soc. 74(3), 6213–6221. Quinn, S.G., Carter, H.E., Markey, B.K. and Carter, G.R. 1994. Clinical veterinary microbiology, 1st ed. Mosby, Yearbook Europe Limited, London, UK. Radostits, O.M., Gay, C.C., Blood, D.C. and Hinchcliffe, K.W. 2004. Veterinary medicine, 9th ed. Philadelphia, PA: Saunders Co. Rebouças, M.F., Portela, R.W., Lima, D.D., Loureiro, D., Bastos, B.L., Moura-Costa, L.F., Vale, V.L., Miyoshi, A., Azevedo, V. and Meyer, R. 2011. Corynebacterium pseudotuberculosis secreted antigen-induced specific gamma-interferon production by peripheral blood leukocytes: a potential diagnostic marker for caseous lymphadenitis in sheep and goats. J. Vet. Diag. Invest. 23, 213–220. Rendon, J.L. and Choudhry, M.A. 2012. Th17 cells: critical mediators of host responses to burn injury and sepsis. J. Leukoc. Biol. 92, 529–538. Sabat, R., Grütz, G., Warszawska, K., Kirsch, S., Witte, E., Wolk, K. and Geginat, J. 2010. Biology of interleukin-10. Cytokine. Growth. Factor. Rev. 21, 331–344. Santiago-Sánchez, G.S., Pita-Grisanti, V., Quiñones-Díaz, B., Gumpper, K., Cruz-Monserrate, Z. and Vivas-Mejía, B.E. 2020. Biological functions and therapeutic potential of lipocalin 2 in cancer. Int. J. Mol. Sci. 21, 4365–4379. Schroder, M., Meisel, C., Buhl, K., Profanter, N., Sievert, N., Volk, H.D. and Grütz, G., 2003. Different modes of IL-10 and TGF-beta to inhibit cytokine-dependent IFN-gamma production: consequences for reversal of lipopolysaccharide desensitization. J. Immunol. 170, 5260–5267. Selim, S.A. 2001. Review: edematous skin disease of buffaloes in Egypt. Vet. Med. J. B. 48, 241–258. Steerforth, D. and Marutsov, P. 2017. Ulcerative lymphangitis due to Corynebacterium pseudotuberculosis in Bulgarian Holstein dairy cows. Vet. Rec. Case. Rep. 5, e000454. Steinman, A., Elad, D. and Shpigel, N.Y. 1999.Ulcerative lymphangitis and coronet lesions in an Israeli dairy herd infected with Corynebacterium pseudotuberculosis. Vet. Rec. 145, 604–606. Tejedor-Junco, M.T., Lupiola, M.T., Schulz, U. and Gutierrez, C. 2008. Isolation of nitrate-reductase positive Corynebacterium pseudotuberculosis from dromedary camels. Trop. Anim. Health. Prod. 40, 165–167. Wu, H.P., Shih, C.C., Chu, C.M., Huang, C.Y., Hua, C.C., Liu, Y.C. and Chuang, D.Y. 2015. Effect of interleukin-17 on in vitro cytokine production in healthy controls and patients with severe sepsis. J. Formos. Med. Assoc. 114, 1250–1257. Yang, D., Chertov, O., Bykovskaia, S.N., Chen, Q., Buffo, M.J., Shogan, J., Anderson, M., Schroder, J.M., Wang, J.M., Howard, O.M. and Oppenheim, J.J. 1999. β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286, 525–528. Yang, D., Liu, Z.H., Tewary, P., Chen, Q., de la Rosa, G. and Oppenheim, J.J. 2007. Defensin participation in innate and adaptive immunity. Curr. Pharm. Dis. 13, 3131–3139. Ye, P., Rodriguez, F.H., Kanaly, S., Stocking, K.L., Schurr, J., Schwarzenberger, P., Olive, P., Huang, W., Zhang, J. and Shellito, J.E. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194, 519–527. Yeruham, I., Elad, D., Van-Ham, M., Shpigel, N.Y. and Perl, S. 1997. Corynebacterium pseudotuberculosis infection in Israeli cattle: clinical and epidemiological studies. Vet. Rec. 140, 423–427. Yozwiak, M.S. and Songer, J.G. 1993. Effect of Corynebacterium pseudotuberculosis phospholipase D on viability and chemotactic responses of ovine neutrophils. Am. J. Vet. Res. 54, 392–397. Zavoshti, F.R., Sioofy-khojine, A.B. and Mahpeikar H.A. 2009. A case report of ulcerative lymphangitis (a mini-review of causes and current therapies). Turk. J. Vet. Anim. Sci. 33(6), 525–528. | ||
| How to Cite this Article |
| Pubmed Style El-Karim DRG, El-Amrawi GM, Salama AR. Clinicopathological studies on ulcerative lymphangitis in cattle: Alterations in serum inflammatory cytokines, anti-microbial, organs functions and oxidative stress-related biomarkers. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 25-31. doi:10.5455/OVJ.2024.v14.i1.4 Web Style El-Karim DRG, El-Amrawi GM, Salama AR. Clinicopathological studies on ulcerative lymphangitis in cattle: Alterations in serum inflammatory cytokines, anti-microbial, organs functions and oxidative stress-related biomarkers. https://www.openveterinaryjournal.com/?mno=174659 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.4 AMA (American Medical Association) Style El-Karim DRG, El-Amrawi GM, Salama AR. Clinicopathological studies on ulcerative lymphangitis in cattle: Alterations in serum inflammatory cytokines, anti-microbial, organs functions and oxidative stress-related biomarkers. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 25-31. doi:10.5455/OVJ.2024.v14.i1.4 Vancouver/ICMJE Style El-Karim DRG, El-Amrawi GM, Salama AR. Clinicopathological studies on ulcerative lymphangitis in cattle: Alterations in serum inflammatory cytokines, anti-microbial, organs functions and oxidative stress-related biomarkers. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 25-31. doi:10.5455/OVJ.2024.v14.i1.4 Harvard Style El-Karim, D. R. G., El-Amrawi, . G. M. & Salama, . A. R. (2024) Clinicopathological studies on ulcerative lymphangitis in cattle: Alterations in serum inflammatory cytokines, anti-microbial, organs functions and oxidative stress-related biomarkers. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 25-31. doi:10.5455/OVJ.2024.v14.i1.4 Turabian Style El-Karim, Dina R.S. Gad, Gamal M. El-Amrawi, and Alyaa R. Salama. 2024. Clinicopathological studies on ulcerative lymphangitis in cattle: Alterations in serum inflammatory cytokines, anti-microbial, organs functions and oxidative stress-related biomarkers. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 25-31. doi:10.5455/OVJ.2024.v14.i1.4 Chicago Style El-Karim, Dina R.S. Gad, Gamal M. El-Amrawi, and Alyaa R. Salama. "Clinicopathological studies on ulcerative lymphangitis in cattle: Alterations in serum inflammatory cytokines, anti-microbial, organs functions and oxidative stress-related biomarkers." Open Veterinary Journal 14 (2024), 25-31. doi:10.5455/OVJ.2024.v14.i1.4 MLA (The Modern Language Association) Style El-Karim, Dina R.S. Gad, Gamal M. El-Amrawi, and Alyaa R. Salama. "Clinicopathological studies on ulcerative lymphangitis in cattle: Alterations in serum inflammatory cytokines, anti-microbial, organs functions and oxidative stress-related biomarkers." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 25-31. Print. doi:10.5455/OVJ.2024.v14.i1.4 APA (American Psychological Association) Style El-Karim, D. R. G., El-Amrawi, . G. M. & Salama, . A. R. (2024) Clinicopathological studies on ulcerative lymphangitis in cattle: Alterations in serum inflammatory cytokines, anti-microbial, organs functions and oxidative stress-related biomarkers. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 25-31. doi:10.5455/OVJ.2024.v14.i1.4 |