| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 32-45 Original Research Protective effectiveness of two vaccination schemes against the prevalent Egyptian strain of Newcastle disease virus genotype VIIMohamed A. Lebdah1, Alaa Abdallah2, Esraa E. Hamouda1, Nora M. Elseddawy3 and Reham M. ElBakrey1*1Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Alwatania Poultry Company, Giza, Egypt 3Department of Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt Corresponding Author: Reham M. ElBakrey. Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: rehamemara3 [at] gmail.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
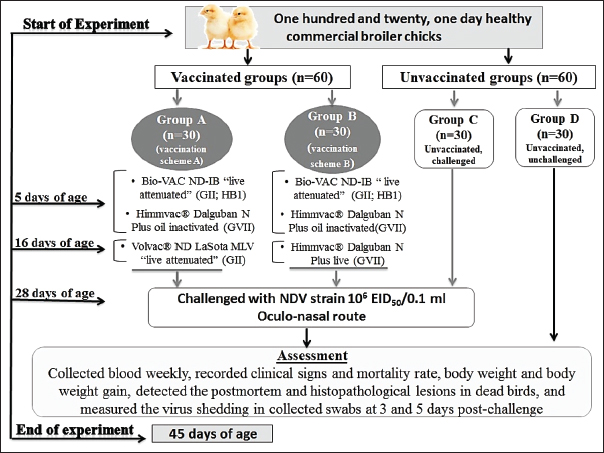
AbstractBackground: Despite the strict preventive immunization used in Egypt, Newcastle disease remained a prospective risk to the commercial and backyard chicken industries. The severe economic losses caused by the Newcastle disease virus (NDV) highlight the importance of the trials for the improvement and development of vaccines and vaccination programs. Aim: In the present study, we evaluated the effectiveness of two vaccination schemes for protection against the velogenic NDV (vNDV) challenge. Methods: Four groups (A–D) of commercial broiler chickens were used. Two groups (G–A and G–B) were vaccinated with priming live HB1 GII simultaneously with inactivated GVII vaccines at 5 days of age, then boosted with live LaSota GII vaccine in group A and live recombinant NDV GVII vaccine in group B on day 16. Groups A to C were challenged with NDV/Chicken/Egypt/ALEX/ZU-NM99/2019 strain (106 Embryo infective dose 50/0.1 ml) at 28 days of age. Results: Two vaccination schemes achieved 93.3% clinical protection against NDV with body gain enhancement; whereas, 80% of the unvaccinated-challenged birds died. On day 28, the mean HI antibody titers were 4.3 ± 0.33 and 5.3 ± 0.33 log2 in groups A and B, respectively. As well as both programs remarkably reduced virus shedding. The two vaccination schemes displayed close protection efficacy against the vNDV challenge. Conclusion: Therefore, using the combination of a live attenuated vaccine with an inactivated genetically matched strain vaccine and then boosting it with one of the available live vaccines could be considered one of the most effective programs against current field vNDV infection in Egypt. Keywords: Antibody dynamic, Genotype VII, Histopathology, Newcastle disease, Vaccine efficacy. IntroductionOne of the most important and greatest sources of animal protein for human consumption is the poultry sector (Ravikumar et al., 2022), and Egypt is among the countries that have a substantial poultry industry (Ali et al., 2019). Occasionally, this sector suffers from economic losses as a result of different pathogens, especially those related to respiratory conditions, and the Newcastle disease (ND) is particular (Roussan et al., 2008). ND is a notifiable, highly contagious viral disease that has a serious clinical impact and huge financial losses for the worldwide poultry industry due to its high mortality rates, which reached 100%, declined productivity, the expenses of disease control and prevention, and trade restrictions (Alexander and Gough, 2003). Newcastle disease virus (NDV), also known as avian paramyxovirus-1 or avian orthoavulavirus-1 (AOaV-1), is the cause of ND. It is a negative-sense, single-stranded, nonsegmented RNA-enveloped virus. It belongs to the Paramyxoviridae family of the genus AOaV-1within the Mononegavirales order (ICTV, 2018; Dimitrov et al., 2019). NDV consists of six genes coding six structural proteins. The six proteins are the fusion (F), hemagglutinin-neuraminidase (HN), RNA polymerase (L), nucleocapsid protein, phosphoprotein (P), and matrix protein (M) (Lamb and Kolakofsky, 1996). The “V and W” are two other proteins coded through P protein mRNA editing (Czeglédi et al., 2006). The HN and F proteins are surface glycoproteins as spike-like projections on the viral envelope, including the neutralizing and protective antigens, and contribute to the pathogenicity and antigenicity of NDV (Alexander, 1988; De Leeuw et al., 2005). Genotypically, AOaV-1 is categorized into two clades, nominated as class I and class II. Class I commonly contains avirulent viruses, while class II includes at least 20 genotypes; genotype XV was excluded from the last analysis because it contains only recombinant sequences and has virulent and avirulent strains (Diel et al., 2012; Snoeck et al., 2013; Dimitrov et al., 2019). The genotypes V, VI, and VII of virulent NDVs are highly mobile, widely disseminated, and conducting the main recent outbreaks globally in domestic and wild birds (Dimitrov et al., 2016). Clinically, there are three main pathotypes of NDV: velogenic, mesogenic, and lentogenic strains (Alexander et al., 1997). The biological characterization of the pathogenicity of NDV strains is ultimately important, and this is applied through some tests that are internationally recognized, such as mean death time (MDT), intracerebral pathogenicity index (ICPI), and intravenous pathogenicity index. The ICPI test is thought to be the most accurate and popular test for determining virulence using 10 1-day-old chicks (Alexander and Senne, 2008; Terregino and Capua, 2009; OIE Terrestrial Manual, 2021). In Egypt, NDV was first discovered in 1947 (Daubney and Mansy, 1948). Since that time, outbreaks of NDV have been recurrently reported and genotypes II, VI, and VII of class II have been commonly recognized (Mohamed et al., 2009, 2011; Radwan et al., 2013; Rohaim et al., 2016; Sabra et al., 2017). NDV sub-genotype VII was first isolated in 2012 (Radwan et al., 2013) and is thought to be accountable for the concurrent outbreaks (Ewies et al., 2017; Amer et al., 2019; Eid et al., 2022a). The control of ND is achieved by applying the compulsory preventive vaccination of flocks using live and inactivated vaccines of NDV with several programs, hygienic measures through culling of infected birds, as well as good biosecurity approaches (Mansour et al., 2021). Many NDV commercial vaccines are obtainable from different genotypes (as genotypes I, II, and recently VII) and are used in the Egyptian poultry field (Ellakany et al., 2019; Mahmoud et al., 2019; Sultan et al., 2021a). Finally, recombinant GVII-matched chimeric vaccines have been developed using reverse genetics, which provides significant defense against the homologous genotype NDV in pathogen-free chicken (Miller et al., 2007). Despite the frequent and intense mandatory vaccine application in Egypt and worldwide, NDV genotype VII continues to pose a serious risk to poultry production (Rui et al., 2010; Diel et al., 2012). Although vaccination approaches are comparatively efficient in the prevention of severe illness and deaths of infected birds, some of them may fail to prevent either infection or virus shedding (Mansour et al., 2021). Nonetheless, compared to nonvaccinated birds, most vaccinations will considerably reduce the virus-shedding amount in the saliva and feces (Miller et al., 2009). Like any country, the reduction of economic burden due to NDV infections is a primary objective of scientists and poultry producers in Egypt. As a result, cutting-edge vaccination techniques were used to improve protection, decrease viral shedding, and probably prevent the spread of the virus transmission (Mansour et al., 2021). So continuing permutations and combinations to create new and different vaccination programs or schemes using the commercially available traditional and novel vaccines that contain different seed virus strains (homologous or heterologous) is a continuous need. Here, the protective effect of two vaccination schemes, including live attenuated GII (HB1, LaSota) and recombinant NDV (rNDV) vaccine either live or inactivated, was evaluated against velogenic ND GVII challenge in broilers based on reference parameters including, protection from clinical diseases, mortality rate, development of humoral immune response, as well as bird performance. Materials and MethodsCommercial NDV vaccinesThe NDV vaccines used in this study were Bio-VAC ND-IB “live attenuated; ND Genotype II of Hitchner B1 strain” (Fatro S.P.A., Italy), and Volvac® ND LaSota MLV “live attenuated; ND Genotype II” (Boehringer Ingelheim, Ingelheim am Rhein, Germany). Also, the rNDV GVII.1.1 vaccines were used and included Himmvac® Dalguban N Plus live, and Himmvac® Dalguban N Plus oil inactivated vaccines (KBNP, Inc., Chungnam, Korea), which is a chimeric recombinant GVII based on the LaSota strain backbone with the F and HN genes of the GVII 1.1. KBNP-C4152R2L strain (Cho et al., 2008). All the vaccines were applied as stated by the recommendations of the manufacturer. Propagation of challenge field NDV strain and determination of Embryo infective dose 50 (EID50)The ND virus used for the purpose of the challenge was gained from Eid et al. (2022a) and was characterized as velogenic NDV (vNDV) genotype VII.1.1 by sequencing and designated as “NDV/Chicken/Egypt/ALEX/ZU-NM99/2019” with an accession number of (OP219680) on GenBank. The propagation of NDV was carried out using 9-day-old specific pathogen-free embryonated chicken eggs (SPF-ECEs) via the allantoic cavity route. The EID50 of the virus was determined through titration of infected allantoic fluid (AF) in 9-day-old SPF-ECEs, as mentioned in the procedure described by Reed and Muench (1938). The dose of challenge NDV was prepared as 6 Log10 of EID50 per 0.1 ml to inoculate the vaccinated and control birds via the oculo-nasal route (OIE Terrestrial Manual, 2021). Pathogenicity assessment of the NDV/Chicken/Egypt/ALEX/ZU-NM99/2019 strainThe pathogenicity of NDV strain was evaluated using the MDT test, and the ICPI. The MDT was determined through the preparation of 10-fold serial dilutions (10−1 to 10−9) from freshly infected allantoic fluid (AF), and then inoculating 0.1 ml of each dilution in the allantoic cavity of five, 9-day-old SPF-ECEs. The ECEs were examined daily after inoculation for 5–7 days while being incubated at 37°C, with the mortality rate recorded. The embryonic MDT was calculated as described by Alexander and Senne (2008). For the determination of ICPI, the AF with HA titer 9 log2 was tenfold diluted. Subsequently, 10, 1-day-old chicks were inoculated intracerebrally with a volume of 50 µl of diluted AF containing the NDV strain. After inoculation, the chicks were daily observed for 8 days, and the observation scores were recorded. Then, the ICPI value was detected (OIE Terrestrial Manual, 2021). Experimental design for evaluation of the two vaccination schemes against NDV field isolateOne hundred and twenty, 1-day-old healthy commercial broiler chicks (Cobb 500®) with maternally derived antibodies were purchased from the certified local hatchery. The birds were assigned divided into 4 separate groups (G–A to G–D), with 30 birds in each group. Each group was kept separately in experimental units with strict hygienic conditions. The chicks in groups A and B were vaccinated with two vaccination schemes for NDV (A and B), which included live attenuated vaccines of genotype II, and live and inactivated rNDV vaccines of genotype VII, as shown in Figure 1. At 28 days of age, the birds in groups A, B, and C were challenged with “NDV/Chicken/Egypt/ALEX/ZU-NM99/2019” as vNDV genotype VII.1.1 with a dose of 106 EID50/0.1 ml via the oculo-nasal route; in contrast, group C was a control-positive (unvaccinated, challenged) group. At the same time, group D was a control-negative (unvaccinated, unchallenged) group.
Fig. 1. A summarized experimental design for studying the protective effectiveness of vaccines against challenges with the field strain of NDV genotype VII, illustrates the timeline of immunization with vaccines, the challenge with the NDV strain, and the methods of assessment. Clinical samples collectionBlood samples were collected weekly from zero day of age till the experiment ended and sera were separated to monitor the specific NDV antibody titers using the hemagglutination inhibition (HI) test. The average body weight (ABW) and body weight gain (BWG) were calculated every week through the weighting of five live birds from each group. After the challenge, chickens were monitored twice daily to record the clinical signs of disease and mortality rate, as well as postmortem lesions in the examined dead birds. During the inspection of dead birds, tissue samples were immediately collected from different organs for histopathological examination. Moreover, tracheal and cloacal swabs were collected from six randomly selected birds at 3 and 5 days post-challenge (dpc) to detect the virus shedding. Antibody titer against NDVThe monitoring of NDV antibodies was performed via HI test using four haemagglutination (HA) unit (HAU) of the LaSota vaccine strain of NDV (Pestikal® LASOTA SPF) and 1% washed chicken red blood cells (RBCs), consistent with the OIE Terrestrial Manual (2021). The serum samples were thermally inactivated for 30 minutes at 56°C then treated with 1% chicken RBCs. Twofold serial dilutions were carried out and the HI titer was expressed as log2 of the reciprocal of the highest serum dilution, which suppressed the HA activity. Measurement of NDV sheddingThe ND virus shedding was measured in the collected swabs at the 3rd and the 5th dpc using the method described by Eid et al. (2022b), who calculated EID50 based on the HAU quantity by using a formula=(value of HAU with log base 10) + (value of virus particle of one HAU). Whereas, the “value of HAU with log base 10” means the conversion of the HA unit of the examined virus from log base 2 into log base 10, while the “value of virus particle of the one HA unit” means the value of the NDV particle in one HAU, according to Tolba and Eskarous (1962) and Anon (1963). The three pools of tracheal and cloacal swabs were collected from six birds per group (Two birds/pool swab) in 1.5 ml of minimum essential media with antibiotics (Penstrept, Lonza), then clarified by centrifugation at 3,000 rpm for 10 minutes. The clarified swab samples were inoculated into 9-day-old SPF-ECEs (3 SPF-ECEs/swab sample), then incubated at 37°C. The inoculated embryo eggs were monitored every 12 hours to conduct a rapid HA assay of their AF using 10% washed chicken RBCs. The HAU of positive AF was calculated as log base 2 via a hemagglutination (HA) test carried out (OIE Terrestrial Manual, 2021), in which the twofold dilutions of AF were done with an initial dilution of 1/10 and using 1% washed chicken RBCs. The virus titers were calculated by using the previously mentioned formula and reported as EID50/0.1 ml on a log-base 10.
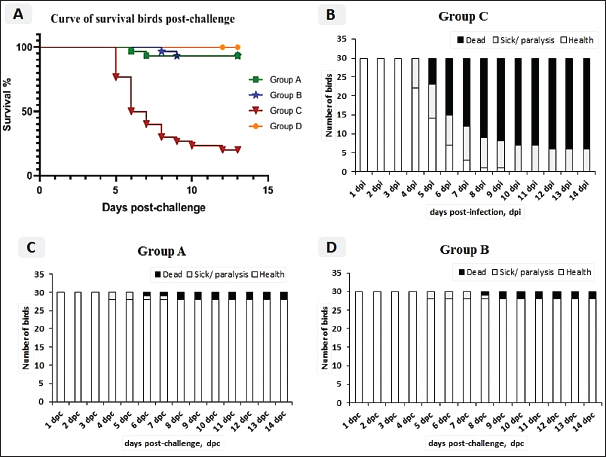
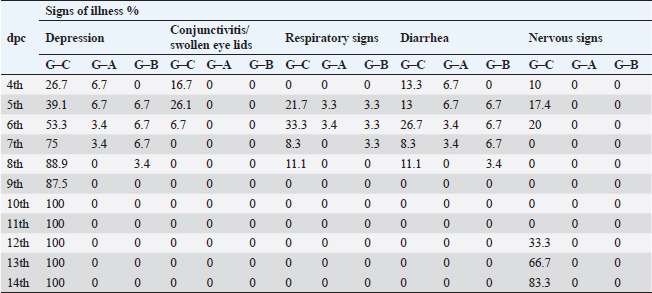
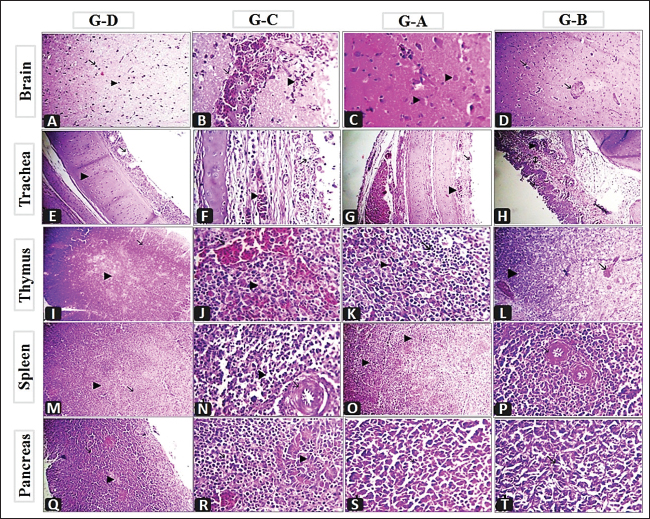
Fig. 2. A) Survival rates in vaccinated and unvaccinated groups post-challenge. B) Numbers of dead, diseased, and healthy birds in the unvaccinated group (G–C) post-challenge. C) Numbers of dead, diseased, and healthy birds in the vaccinated group with the vaccine scheme A (G–A) post-challenge. D) Numbers of dead, diseased, and healthy birds in the vaccinated group with the vaccine scheme B (G–B) post-challenge. Histopathology tissue samplingTissue samples collected from different organs such as the brain, trachea, pancreas, spleen, and thymus during the examination of dead unvaccinated and vaccinated birds post-challenge were fixed in neutral buffered formalin with a concentration of 10%. The paraffin embedding technique was used to process the samples. Sections with a thickness of 4–5 µm were prepared and stained using hematoxylin and eosin, then examined under a light microscope for detection of any histological changes, as described by Suvarna et al. (2018). Statistical analysis of resultsThe data of virus shedding and HI antibody titers were analyzed using GraphPad Prism version 9.00 Software (GraphPad Software, San Diego, CA, www.graphpad.com). Data are displayed as the mean ± standard error of the mean (SEM). A one-way ANOVA followed by a Sidak multiple comparisons test was used to conduct statistical studies. Statistical significance was considered at p-value <0.05. The ABW and BWG were analyzed using SPSS version 21.0, and data are shown as the mean ± SEM. Ethical approvalAll instructions and requirements have been followed in this study for handling and rearing the animals for the purpose of experimental design and research. This was approved by the Animal Care and Use Committee (IACUC), Faculty of Veterinary Medicine, Zagazig University, Egypt, under approval number: ZU-IACUC/2/F/283/2022. ResultsPathogenicity characterization of the NDV/Chicken/Egypt/ALEX/ZU-NM99/2019 field strainThe pathogenicity of the challenged NDV strain was evaluated using the MDT and ICPI tests. The time of embryonic death was recorded at 54.4 ± 9.78 hours post-inoculation. Besides the ICPI value was 1.075. Accordingly, the NDV strain is considered a virulent strain. Clinical protectionThe results of the clinical examination revealed that the level of protection against mortality was 93.3% in the two vaccinated groups (G–A and G–B). Meanwhile, the survival rate in group C, which did not receive any vaccine and was infected with the vNDV field strain, was 20%; in exchange for this, the mortality reached 80% (Fig. 2A). The numbers of dead, diseased, and healthy birds in the different groups (vaccinated and unvaccinated) post-challenge were illustrated in Figure 2B–D. The birds in the unvaccinated-unchallenged group (G–D) showed no clinical signs or mortalities. On the other hand, the control-positive group (unvaccinated-challenge group, G–C) exhibited typical clinical symptoms of vNDV starting from 4-dpc and included depression, recumbence, anorexia, severe respiratory signs such as conjunctivitis, swelling of the eyelid, nasal discharge, and rales, in addition to greenish diarrhea. The nervous manifestations in the form of head tremors, paralysis, and torticollis appeared early at 4 dpc in 10% (3/30) of birds and lately in 83.3% (5/6) of birds (Fig. 3A and B). The two vaccinated groups just exhibited depression, reluctance to move, mild respiratory signs, and diarrhea, which were distinctly less severe and frequent than in control-positive birds (Table 1).
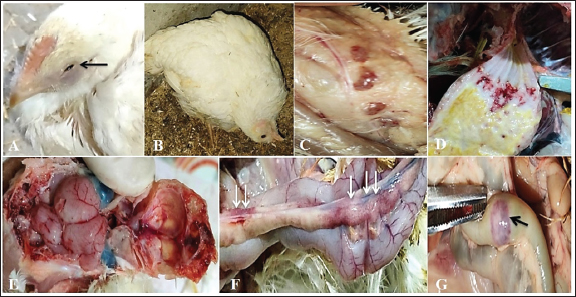
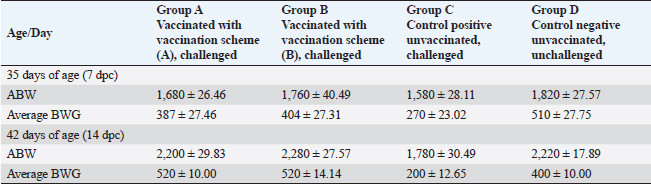
Fig. 3. Clinical findings of unvaccinated birds post-challenged with vNDV field strain. A) Conjunctivitis. B) Nervous signs inform of torticollis. C) Atrophied and congested thymus. D) Haemorrhages at the junction between the proventriculus and esophagus. E) Congested cerebral and cerebellar blood vessels with hemorrhages on the inner aspect of the skull bone. F) Pancreatitis. G) Elliptical ulcers on payer’s patches of the intestine. Body weight and BWG of live unvaccinated and vaccinated birds post-challenge with NDV GVII strainAfter the challenge, the ABW and BWG of the vaccinated birds increased compared to those of the unvaccinated birds. Which, at 7 dpc, the ABW and BWG of birds vaccinated with the vaccination scheme (B) that were primarily vaccinated with live HB1 and inactivated GVII, then boosted with live rNDV GVII, were higher than those vaccinated with the vaccination scheme (A). However, at 14 dpc (end of the experiment), both vaccinated groups gained the same body weight, which was higher than the body gain obtained in the birds of unvaccinated-challenged and unvaccinated-unchallenged groups (positive and negative control groups) (Table 2). Necropsy findingsThe dead birds in group C (control positive; unvaccinated-challenged) exhibited typical gross lesions of vNDV that were severe to moderately marked and included congested cerebral and cerebellar blood vessels, and sometimes hemorrhages on the inner aspect of the skull bone were noted in 70.8% of the dead birds. Also, inflamed cecal tonsils, bluish or reddish elliptical ulcers on payer’s patches of the intestine, and petechial hemorrhages on the proventriculus and/or junction between proventriculus and esophagus were observed in 41.7%, 16.7%, and 29.2%, respectively. 70.8% of dead birds showed pancreatitis, either necrosis or inflammation, and 12.5% showed hemorrhages on the myocardium. Moreover, moderate atrophied lymphoid organs such as the bursa of Fabricius, thymus, and spleen were seen in 95.8%, 50%, and 70.8%, respectively. As well, tracheitis and congested lungs were recorded (Fig. 3C–G). Whereas there were no or mild lesions in vaccinated birds, mild congestion in the cerebral and cerebellar blood vessels was detected in one of two birds that died in group B (vaccinated with vaccine scheme B). Atrophied thymus and spleen were seen in the dead birds of both vaccinated groups (A and B), in addition to mild tracheitis and congested lungs. There were no postmortem lesions recorded in the birds of group D (control negative; unvaccinated-unchallenged) at the end of the experiment. Table 1. Frequency of clinical signs occurrence among vaccinated and unvaccinated groups post-challenge with NDV GVII strain.
Table 2. ABW and BWG in the unvaccinated and vaccinated live birds post-challenged with NDV GVII strain.
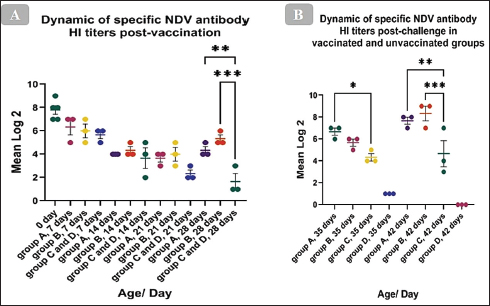
The HI antibody’s dynamicsTo ascertain the dynamics of the HI antibody in birds’ post-vaccination, serum from vaccinated and unvaccinated chickens was collected weekly, and the HI test was carried out using the LaSota GII diagnostic antigen. The mean of the maternally derived anti-NDV antibody titer was 7.8 ± 0.37 log2. The post-vaccination immune response has demonstrated that the antibody titer significantly increased at 28 days of age and just before the challenge when compared with the unvaccinated group. Which, the mean HI antibody titer of group A which was vaccinated with live HB1 GII and inactivated GVII then boosted with live LaSota GII was 4.3 ± 0.33 log2 (p= 0.0075), and in group B, which was vaccinated with live HB1 GII and inactivated GVII then boosted with live rNDV GVII was 5.3 ± 0.33 log2 (p= 0.0002), with no significant difference between them (Fig. 4A). Post-challenge, the HI antibody titers of both vaccinated groups significantly increased compared with the unvaccinated one. At 7 days post-challenge, group A revealed a significant mean HI antibody titer of 6.7 ± 0.33 log2 (p=0.0299) and was 5.7 ± 0.33 log2 in group B. At 14 days post-challenge, the significant mean antibody titers were 6.2 ± 0.87 (p=0.0048) and 8 ± 0.45 (p=0.0008) log2 in groups A and B, respectively, while the unvaccinated group presented 4.3 ± 0.33 and 4.7 ± 1.20 log2 at 7 and 14 days post-challenge, respectively (Fig. 4B).
Fig. 4. Dynamic of specific NDV antibody HI titers (mean log2) post vaccination and challenge with the NDV/Chicken/Egypt/ALEX/ZU-NM99/2019 field strain. The figures display the mean ± SEM (bar), *p < 0.05, **p < 0.01, ***p < 0.001, which represent a significant difference compared to the unvaccinated/unvaccinated-challenged groups.
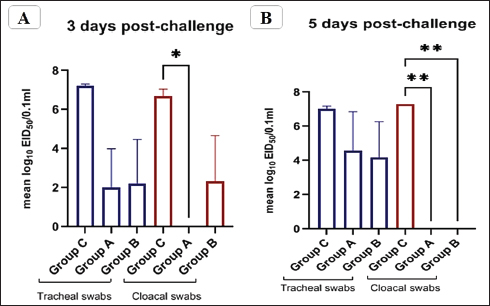
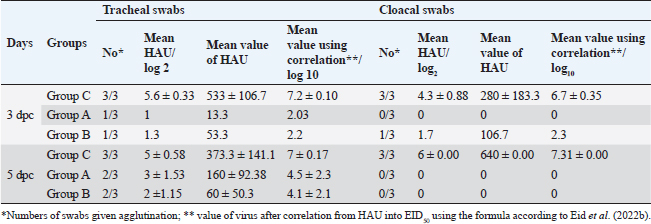
Fig. 5. The titer of tracheal and cloacal virus shedding was expressed as log10 EID50/0.1 ml using the correlation. The mean shedding titers ± SEM, *p < 0.05, **p < 0.01, represent a significant difference compared to the unvaccinated-challenged group (group C). Detection of virus sheddingIn the vaccinated groups, the tracheal virus shedding was reduced in the number of shedders (1–2 out of 3 collected swabs; 33.3%–66.7%), as well as the amount of virus on days 3 and 5 post-challenge. In group A, the reduction of virus shedding was within 2–4.6 log2 HAU/0.1 ml or 2.5–5.2 log10 EID50/0.1 ml, and it was 3–4.3 log2 HAU/0.1 ml or 2.9–5 log10 EID50/0.1 ml in group B when compared to the unvaccinated challenged group (group C). However, no virus shedding was detected in the collected cloacal swabs from the vaccinated group A. While in group B, the virus was detected in 1/3 of collected cloacal swabs at 3 dpc, with a reduction in the amount of virus (2.6 log2 HAU/0.1 ml or 4.4 log10 EID50/0.1 mL reductions), and the shedding was stopped at 5 dpc. In group C, the virus was detected in 100% of the collected tracheal and cloacal swabs at 3 and 5 dpc, as illustrated in Figure 5 and Table 3. Histopathological changesMicroscopic examination of the organs collected from unvaccinated and vaccinated groups post-challenge revealed mild histopathological changes in the two vaccinated groups (G–A and G–B). Group B, which was boosted with live rNDV genotype VII vaccine, achieved more improvement and was able to restore the normal structure of the organs. However, severe deterioration in the histological structure was recorded in all examined organs of unvaccinated birds (G–C), as shown in Figure 6. DiscussionDespite the extensive use of different vaccinations, ND still poses a risk to the poultry industry. Therefore, there are always challenges for scientists to compare the effectiveness of commercially available vaccines against the recent circulating NDV strains. The vaccination programs that depended only on the conventional live-attenuated vaccine did not adequately protect birds against threats from virulent field isolates (Kapczynski and King, 2005; Dewidar et al., 2022; Megahed et al., 2023). Whereas the inactivated vaccine included in the vaccination programs could provide better protection (Hu et al., 2009 and 2011; Roohani et al., 2015; Yang et al., 2017). Furthermore, genotype-matched vaccines could offer superior protection against NDV infection (Liu et al., 2018; Sultan et al., 2021b; Dewidar et al., 2022). Finally, due to molecular biology and reverse genetics developments, the concept of vaccination has been reorganized and given rise to new tools for vaccine development. Recombinant viral vector vaccines are one of these cutting-edge vaccine technologies and are renowned for potent immune responses in the vaccinated host (Bello et al., 2020). Among these available recombinant vaccines that are established on a recombinant LaSota strain backbone, both antigenic genes, the F and HN genes of the LaSota strain, were replaced with other genes of the KBNP-4152 strain (genotype VIId virus). Before the replacement, the recombinant strain was attenuated by inducing a mutation by changing (112) RRQKR (116) to (112) GRQAR (116) at the F cleavage motif (Cho et al., 2008). Table 3. Detection of challenge NDV strain in the collected tracheal and cloacal swabs from vaccinated and unvaccinated groups, the results are shown as mean log2 HAU, Value of titer HAU, and log10 EID50/0.1 ml.
Fig. 6. Histopathological findings of the collected organs from G–D (unvaccinated-unchallenged group), G–C (unvaccinatedchallenged group), G–A (vaccinated with vaccine scheme A-challenged), and G–B (vaccinated with vaccine scheme A-challenged). A) Brain showing normal neuron (arrow) and glia cells (arrow head) X300. B) Brain showing perivascular lymphocytic cuffing around congested blood vessels (arrow) with hemorrhages (arrow head) X1,200. C) Brain showing mild degenerated neuron (arrow head) and neuronophagia (arrow) X1,200. D) Brain showing congestion of blood vessels (arrow) X300. E) Trachea showing normal mucosa (arrow) and submucosa (arrow head) X300. F) Trachea showing congested blood vessels (arrow head) round cells infiltration (arrow), X1,200. G) Congested blood vessels of the trachea (arrow head) with re-epithelization (arrow), X300. H) Congested blood vessels of trachea (arrow head) with re-epithelization (arrow), round cell (arrow with 2 head) X300. I) Thymus showing normal cortex (arrow) and medulla (arrow head) X300. J) Thymus showing congested blood vessels (arrow) depletion of lymphocytes (arrow head) X1,200. K) Few hemorrhages in the thymus (arrow head) with islets of lymphocytes (arrow) X1,200. L) Congested blood vessels of the thymus (arrow) and islets of lymphocytes (arrow head) X300. M) Spleen showing normal white pulp (arrow) and red pulp (arrow head) X300. N) Thickening and fibrinoid necrosis of central arterioles of the spleen with endotheliosis (arrow) and depletion of lymphocytes infiltrated with round cells (arrow head) X1200. O) Spleen showing hyperplasia of lymphocytes in the white pulp (arrow head) X300. P) Thickening and mild hyalinization in the wall of central arterioles of the spleen with endotheliosis (arrow) X1,200. Q) Pancrease showing excorineportion (arrow) and endocrine portion (arrow head) X300. R) Pancrease showing necrosis of acini (arrow head) replaced by round cells (black arrow), congested blood vessels (white arrow) X1,200. S) Re-epithelization of pancreatic acini (arrow), X1,200. T) Re-epithelization of pancreatic acini (arrow), X1,200. In the present study, we designed two vaccination schemes (A and B) using either the available genetic-matched or mismatched NDV vaccines (live and inactivated) and combined those to combat the recently isolated vNDV genotype VII challenge “NDV/Chicken/Egypt/ALEX/ZU-NM99/2019.” The birds in the two vaccination schemes were primarily vaccinated with live HB1 (genotype II) simultaneously with inactivated rNDV genotype VII at 5 days of age, then boosted with live LaSota (genotype II) and live rNDV genotype VII at 16 days for vaccination schemes A and B, respectively. In general, protection against the progress of clinical findings and mortalities as well as improvement in BWG in the two vaccinated-challenged groups (G–A and G–B) was markedly higher than in the unvaccinated-challenged group (G–C). Whereas the unvaccinated-unchallenged birds (G–D) did not show any notable signs or mortalities and this supported the validity of the vaccine challenge experiment. In view of that, the birds in G–C showed severe respiratory signs, conjunctivitis, swelling eyelids, digestive disorders, and weight loss. As well as the nervous manifestations were the main constants and showed up early in 10% of birds at 4 dpc and later on in 83.3% of birds, with an 80% mortality rate. All these reflect the characterization of the virulent challenge virus and its neurotropic, pneumotropic, and viscerotropic affinities in unvaccinated broiler chickens (Ayoub et al., 2019; Butt et al., 2019; Moharam et al., 2019). The two vaccination schemes protected 93.3% of birds with improvement in BWG, and the signs appeared in a low number of birds and were milder with no evidence of conjunctivitis, swollen eyelids, or nervous manifestations compared to the unvaccinated-challenged group; over that, the signs appeared later (from 5 dpc) in group B that was boosted with live rNDV GVII vaccine, and the BWG at 7 dpc was higher. These findings are consistent with the earlier reports by Megahed et al. (2023), who stated a low clinical index and no signs of neurologic symptoms in birds that were immunized with a combination of live and inactivated NDV genotype VII vaccines and a combination of inactivated NDV genotype VII vaccine and Clone 30. Several studies have clarified that vaccines with homology and genetically matched to circulating ND viruses can produce a better defense against the clinical signs development than conventional ones (Cho et al., 2008; Moharam et al., 2019; Megahed et al., 2023). Regarding macro and microscopic findings, the dead birds in the unvaccinated group (G–C) showed typical gross and histological lesions associated with NDV infection (Ewies et al., 2017; Mousa et al., 2020; EL-Morshidy et al., 2021; Sultan et al., 2021b) with noted atrophied lymphoid organs as thymus, bursa, and spleen in 50%–95.8% of birds and associated microscopically with depletion of lymphocytes, which is common with virulent viscerotropic NDV (Wakamatsu et al., 2006; EL-Morshidy et al., 2021). Furthermore, it is worth noting that 70.8% of the dead birds had severe central nervous system lesions as well as pancreatitis, such findings were reported among recent NDV genotype VII isolates (Mousa et al., 2020; EL-Morshidy et al., 2021). The involvement of nervous signs that are explained by severe macro and micro pathological lesions and damage to cerebellar neuronal suggest caudal viral propagation and neurotropism, which are frequent in velogenic neurotropic NDV (Cattoli et al., 2011; Suarez et al., 2020; EL-Morshidy et al., 2021). From our point of view, the tropism and pathogenesis of this strain need more investigation. These changes in the properties of the field virus may play a role in the partial protective effectiveness of vaccination programs (Sarcheshmei et al., 2016). However, there were no or milder macro-and microscopical lesions in the two vaccinated groups as compared with the positive control group. Meanwhile, microscopic findings revealed that group B, which was boosted with the live rNDV genotype VII vaccine, achieved a relative amelioration in its ability to repair the organs to their normal structure. Cardenas-Garcia et al. (2015) and Mansour et al. (2021) stated that the use of vaccine-matched strains will likely provide additional control of NDV with the priming of live lentogenic strains to stimulate specific cell-mediated immunity. Where the birds in group B received two doses of gene-matched vaccine (inactivated and live vaccine). The assessment of NDV vaccinations is not only dependent on the protection against mortality and clinical symptoms related to the disease. The ability of vaccines to reduce the viruses shedding from vaccinated-challenged birds is important for alleviating disease spread (Miller et al., 2009). Nonetheless, the degree of protection is related to the titer of the HI antibodies (Zhao et al., 2017). In the current study, bird serological monitoring just before the challenge highlighted that the mean HI titers in the two vaccinated groups were significantly higher than those in the unvaccinated group and reached 4.3 ± 0.33 and 5.3 ± 0.33 log2 in groups A and B, respectively, with no significant difference between the two vaccinated groups. The seroconversion results and correlation with protection against mortality (93.3%) and clinicopathological findings support the herd immunity theory and are consistent with the prior reports of van Boven et al. (2008) and Sultan et al. (2021b). The mean HI antibody titers greater than 3 log2 have been previously mentioned as playing a protective function against NDV (Kapczynski and King, 2005; van Boven et al., 2008). Consequently, the use of two vaccination schemes that depended on live HB1 and inactivated GVII vaccines as priming vaccinations convergently reduced the tracheal virus shedding in terms of the shedders’ numbers and the virus shedding amount compared to the unvaccinated-challenged group. This could be attributed to the dual protective purpose that life created fast local and cell-mediated immunity till pushing the humoral immunity by inactivating one. The high antibody levels in the vaccinated-challenged groups may have been accountable for the reduced virulent virus shedding. This is compatible with the earlier investigations that emphasized the value of inactivated genotype VII ND vaccines in providing better protection and reducing virus shedding (Hu et al., 2011; Roohani et al., 2015; Moharam et al., 2019; Sultan et al., 2020). Also, Miller et al. (2013) suggested that monospecific antibodies are necessary to decrease viral replication. Moreover, group A which was boosted with live LaSota vaccine prevented completely the virus shedding from the cloaca, whereas group B which was boosted with live rNDV GVII vaccine, distinctly reduced the amount of virus shedding from the cloaca (102.3 EID50) at 3 dpc, then the virus shedding stopped at 5 dpc. It is supposed that this amount of virus is not enough to have the ability to cause infection, and Alexander et al. (1999) detected that the amount of NDV between 103 and 104 EID50 must be determined to infect the birds. ConclusionUnder our experimental circumstances, the application of two vaccination schemes revealed close degrees of high protection against mortality and clinicopathological impairment of vNDV. In addition, both schemes distinctly reduced the shedder’s number and virus load. Generally, this study emphasizes the importance of using primarily the live attenuated with inactivated genetically matched vaccinal strains for the best protection of both birds and the environment. Conflict of interestAll authors declare that they have no conflict of interest regarding the publication of this article. FundingThere was no funding source for this study; it was all as a contribution from the authors. Author contributionsAll authors contributed to the aim and design of the work; Mohamed A. Lebdah and Reham M. ElBakrey planned the design and conception of this study. Preparation of the material, data collection, analysis, and investigation were performed by Alaa Abdallah, Esraa E. Hamouda, Reham M. ElBakrey, and Nora M. Elseddawy. The original draft of the manuscript was written by Reham M. ElBakrey and Nora M. Elseddawy. All authors reviewed and edited the prior drafts of the manuscript. The final form of the manuscript was revised by Mohamed A. Lebdah, Reham M. ElBakrey, and Esraa E. Hamouda. All authors read and approved the final version of the manuscript. Data availabilityThe manuscript contains all the data supporting the findings of this study. Any additional information needed is obtainable from the corresponding author upon justifiable request. ReferencesAlexander, D.J. 1988. Newcastle disease diagnosis. In Newcastle disease. Ed., Alexander, D.J. Boston, MA: Springer, pp: 147–160. Alexander, D.J. and Gough, R.E. 2003. Newcastle disease and other avian paramyxovirus infections. In Disease of poultry, 11th ed. Eds., Saif, Y.M., Barnes, H.J., Glisson, J.R., Fadly, A.M., McDougal, L.R. and Swayne, D.E. Ames, IA: Iowa State University Press, pp: 63–87. Alexander, D.J., Manvell, R.J., Banks, J., Collins, M.S., Parsons, G., Cox, B., Frost, K.M., Speidel, E.C., Ashman, S. and Aldous, E.W. 1999. Experimental assessment of the pathogenicity of the Newcastle disease viruses from outbreaks in Great Britain in 1997 for chickens and turkeys, and the protection afforded by vaccination. Avian. Pathol. 28, 501–511. Alexander, D.J., Manvell, R.J., Lowings, J.P., Frost, K.M., Collins, M.S., Russell, P.H. and Smith, J.E. 1997. Antigenic diversity and similarities detected in avian paramyxovirus type 1 (Newcastle disease virus) isolates using monoclonal antibodies. Avian. Pathol. 26(2), 399–418. Alexander, D.J. and Senne, D.A. 2008. Newcastle disease, other avian paramyxoviruses, and pneumovirus infections. In Diseases of poultry. Eds., Saif, Y.M., Fadly, A.M., Glisson, J.R., McDougald, L.R., Nolan, L.K. and Swayne, D. Ames, IA: Blackwell Publishing, pp: 750–798. Ali, A.H.M., Samy, M.M., Fasina, F.O., Hassan, M.K., Kilany, W.H., ElMahdy, S., Saad, A., Lubroth, J. and Jobre, Y. 2019. Field evaluation of common poultry viral vaccines in Egypt: a need for reassessment of the vaccine value chain. Vet. Ital. 55, 231–239. Amer, S.A.M., Ali, M.A., Kandeil, A.M. and Kutkat, M.A. 2019. Advancement in vaccination of broiler chickens with genotype-matched vaccines to currently epidemic Newcastle disease virus genotype VII in Egypt. J. World’s. Poult. Res. 9(3), 117–123. Anon. 1963. Methods for examination of poultry biologics, 2nd ed. Washington, DC: National Academy of Science, National Research Council, pp: 705. Ayoub, M.A., Elfeil, W.K., El Boraey, D., Hammam, H. and Nossair, M.A. 2019. Evaluation of some vaccination programs in protection of experimentally challenged broiler chicken against Newcastle disease virus. Am. J. Anim. Vet. Sci. 14, 197–206. Bello, M.B., Yusoff, K., Ideris, A., Hair-Bejo, M., Jibril, A.H., Peeters, B.P. and Omar, A.R. 2020. Exploring the prospects of engineered Newcastle disease virus in modern vaccinology. Viruses 12(4), 451. Butt, S.L., Moura, V.M.B.D., Susta, L., Miller, P.J., Hutcheson, J.M., Cardenas-Garcia, S., Brown, C.C., West, F.D., Afonso, C.L. and Stanton, J.B. 2019. Tropism of Newcastle disease virus strains for chicken neurons, astrocytes, oligodendrocytes, and microglia. BMC. Vet. Res. 15, 1–10. Cardenas-Garcia, S., Diel, D.G., Susta, L., Lucio-Decanini, E., Yu, Q., Brown, C.C., Miller, P.J. and Afonso, C.L. 2015. Development of an improved vaccine evaluation protocol to compare the efficacy of Newcastle disease vaccines. Biologicals 43, 136–45. Cattoli, G., Susta, L., Terregino, C. and Brown, C. 2011. Newcastle disease: a review of field recognition and current methods of laboratory detection. J. Vet. Diagn. Investig. 23, 637–656. Cho, S.H., Kwon, H.J., Kim, T.E., Kim, J.H., Yoo, H.S., Park, M.H., Park, Y.H. and Kim, S.J. 2008. Characterization of a recombinant Newcastle disease virus vaccine strain. Clin. Vaccine. Immunol. 15, 1572–1579. Czeglédi, A., Ujvári, D., Somogyi, E., Wehmann, E., Werner, O. and Lomniczi, B. 2006. Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus. Res. 120, 36–48. Daubney, R. and Mansy, W. 1948. The occurrence of Newcastle disease in Egypt. J. Comp. Pathol. Ther. 58, 189–200. De Leeuw, O.S., Koch, G., Hartog, L., Ravenshorst, N. and Peeters, B.P. 2005. Virulence of Newcastle disease virus is determined by the cleavage site of the fusion protein and by both the stem region and globular head of the haemagglutinin–neuraminidase protein. J. Gen. Virol. 86(6), 1759–1769. Dewidar, A.A., Kilany, W.H., El-Sawah, A.A., Shany, S.A., Dahshan, A.H.M., Hisham, I., Elkady, M.F. and Ali, A. 2022. Genotype VII.1.1-based Newcastle disease virus vaccines afford better protection against field isolates in commercial broiler chickens. Animals 12(13), 1696. Diel, D.G., da Silva, L.H., Liu, H., Wang, Z., Miller, P.J. and Afonso, C.L. 2012. Genetic diversity of avian paramyxovirus type 1: proposal for a unified nomenclature and classification system of Newcastle disease virus genotypes. Infect. Genet. Evol. 12(8), 1770–1779. Dimitrov, K.M., Abolnik, C., Afonso, C.L., Albina, E., Bahl, J., Berg, M., Briand, F., Brown, I.H., Choi, K., Chvala, I., Diel, D.G., Durr, P.A., Ferreira, H.L., Fusaro, A., Gil, P., Goujgoulova, G.V., Grund, C., Hicks, J.T., Joannis, T.M., Torchetti, M.K. and Wong, F.Y. 2019. Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. Infect. Genet. Evol. 74, 103917. Dimitrov, K.M., Ramey, A.M., Qiu, X., Bahl, J. and Afonso, C.L. 2016. Temporal, geographic, and host distribution of avian paramyxovirus 1 (Newcastle disease virus). Infect. Genet. Evol. 39, 22–34. Eid, A.A., Hussein, A., Hassanin, O., Elbakrey, R.M., Daines, R., Sadeyen, J.R., Abdien, H.M.F., Chrzastek, K. and Iqbal, M. 2022a. Newcastle disease genotype VII prevalence in poultry and wild birds in Egypt. Viruses 14(10), 2244. Eid, A.A., Ismail, A.E.S.N., Gouda, H.F. and ElBakrey, R.M. 2022b. Rapid reliable EID50 determination for live Newcastle disease viruses and vaccines. Zag. Vet. J. 50(4), 276–286. Ellakany, H.F., Elbestawy, A.R., Abd El-Hamid, H.S., Zedan, R.E., Gado, A.R., Taha, A.E., Soliman, M.A., Abd El-Hack, M.E., Swelum, A.A., Saadeldin, I.M., Ba-Awadh, H. and Hussein, E.O. 2019. Role of pigeons in the transmission of avian avulavirus (Newcastle disease-genotype VIId) to chickens. Animals 9(6), 338. El-Morshidy, Y., Abdo, W., Elmahallawy, E.K., Abd EL-Dayem, G.A., El-Sawak, A., El-Habashi, N., Mosad, S.M., Lokman, M.S., Albrakati, A. and Abou Asa, S. 2021. Pathogenesis of velogenic genotype VII.1.1 Newcastle disease virus isolated from chicken in Egypt via different inoculation routes: molecular, histopathological, and immunohistochemical study. Animals 11(12), 3567. Ewies, S.S., Ali, A., Tamam, S.M. and Madbouly, H.M. 2017. Molecular characterization of Newcastle disease virus (genotype VII) from broiler chickens in Egypt. BJBAS 6, 232–237. Hu, Z., Hu, S., Meng, C., Wang, X., Zhu, J. and Liu, X. 2011. Generation of a genotype VII Newcastle disease virus vaccine candidate with high yield in embryonated chicken eggs. Avian. Dis. 55(3), 91–397. Hu, S., Ma, H., Wu, Y., Liu, W., Wang, X., Liu, Y. and Liu, X. 2009. A vaccine candidate of attenuated genotype VII Newcastle disease virus generated by reverse genetics. Vaccine 27, 904–910. ICTV. 2018. Virus taxonomy. International Committee on Taxonomy of Viruses. Available via https://talk.ictvonline.org/taxonomy/ (Accessed 20 December 2022). Kapczynski, D.R. and King, D.J. 2005. Protection of chickens against overt clinical disease and determination of viral shedding following vaccination with commercially available Newcastle disease virus vaccines upon challenge with highly virulent virus from the California 2002 exotic Newcastle disease outbreak. Vaccine 23, 3424–3433. Lamb, R.A. and Kolakofsky, D. 1996. The paramyxoviruses. In Fields virology. Eds., Fields, B.N., Knipe, D.M. and Howley, P.M. Philadelphia, PA: Lippincott-Raven Publishers, pp: 577–604. Liu, W., Qiu, X., Song, C., Sun, Y., Meng, C., Liao, Y., Tan, L., Ding, Z., Liu, X. and Ding, C. 2018. Deep sequencing-based transcriptome profiling reveals avian interferon-stimulated genes and provides comprehensive insight into Newcastle disease virus-induced host responses. Viruses 10(4), 162. Mahmoud, N.K., El-Deeb, A.H., Emara, M.M., Abd El-Khaleck, M.A. and Hussein, H.A. 2019. Genotypes II and VIId-based inactivated Newcastle disease vaccine reduces virus shedding. Virus. Dis. 30, 453–461. Mansour, S.M., ElBakrey, R.M., Mohamed, F.F., Hamouda, E.E., Abdallah, M.S., Elbestawy, A.R., Ismail, M.M., Abdien, H.M.F. and Eid, A.A. 2021. Avian paramyxovirus type 1 in Egypt: epidemiology, evolutionary perspective, and vaccine approach. Front. Vet. Sci. 8, 647462. Megahed, M.M., Mohamed, W. and Hassanin, O. 2023. Evaluation of different Newcastle disease virus vaccination regimes against challenge with recently isolated genotype Vii virus from Egypt. Slov. Vet. Res. 60, 27-39. Miller, P.J., Afonso, C.L., El Attrache, J., Dorsey, K.M., Courtney, S.C., Guo, Z. and Kapczynski, D.R. 2013. Effects of Newcastle disease virus vaccine antibodies on the shedding and transmission of challenge viruses. Dev. Comp. Immunol. 41, 505–513. Miller, P.J., Estevez, C., Yu, Q., Suarez, D.L. and King, D.J. 2009. Comparison of viral shedding following vaccination with inactivated and live Newcastle disease vaccines formulated with wild-type and recombinant viruses. Avian. Dis. 53, 39–49. Miller, P.J., King, D.J., Afonso, C.L. and Suarez, D.L. 2007. Antigenic differences among Newcastle disease virus strains of different genotypes used in vaccine formulation affect viral shedding after a virulent challenge. Vaccine 25(41), 7238–7246. Mohamed, M.H., Kumar, S., Paldurai, A., Megahed, M.M., Ghanem, I.A., Lebdah, M.A. and Samal, S.K. 2009. Complete genome sequence of a virulent Newcastle disease virus isolated from an outbreak in chickens in Egypt. Virus. Genes. 39, 234–237. Mohamed, M.H., Kumar, S., Paldurai, A. and Samal, S.K. 2011. Sequence analysis of fusion protein gene of Newcastle disease virus isolated from outbreaks in Egypt during 2006. Virol. J. 8, 1–4. Moharam, I., Razik, A.A.E., Sultan, H., Ghezlan, M., Meseko, C., Franzke, K., Harder, T., Beer, M. and Grund, C. 2019. Investigation of suspected Newcastle disease (ND) outbreaks in Egypt uncovers a high virus velogenic ND virus burden in small-scale holdings and the presence of multiple pathogens. Avian. Pathol. 48, 406–415. Mousa, M.R., Mohammed, F.F., El-Deeb, A.H., Khalefa, H.S. and Ahmed, K.A. 2020. Molecular and pathological characterisation of genotype VII Newcastle disease virus on Egyptian chicken farms during 2016–2018. Acta. Vet. Hung. 68, 221–230. OIE Terrestrial Manual. 2021. Newcastle disease (Infection with Newcastle Disease Virus). In Manual of diagnostic tests and vaccines for terrestrial animals (Chapter 3.3.14). Paris, France: OIE. Available via https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.03.14_NEWCASTLE_DIS.pdf/ (Accessed 15 March 2022). Radwan, M.M., Darwish, S.F., El-Sabagh, I.M., El-Sanousi, A.A. and Shalaby, M.A. 2013. Isolation and molecular characterization of Newcastle disease virus genotypes II and VIId in Egypt between 2011 and 2012. Virus. Genes. 47, 311–316. Ravikumar, R., Chan, J. and Prabakaran, M. 2022. Vaccines against major poultry viral diseases: strategies to improve the breadth and protective efficacy. Viruses 14(6), 1195. Reed, L.J. and Muench, H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 27(3), 493–497. Rohaim, M.A., El Naggar, R.F., Helal, A.M., Hussein, H.A. and LeBlanc, N. 2016. Genetic characterization of pigeon paramyxovirus type 1 in Egypt. Br. J. Virol. 3(2), 27–32. Roohani, K., Tan, S.W., Yeap, S.K., Ideris, A., Bejo, M.H. and Omar, A.R. 2015. Characterization of genotype VII Newcastle disease virus (NDV) isolated from NDV vaccinated chickens, and the efficacy of LaSota and recombinant genotype VII vaccines against challenge with velogenic NDV. J. Vet. Sci. 16(4), 447–457. Roussan, D.A., Haddad, R. and Khawaldeh, G. 2008. Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan. Poult. Sci. 87(3), 444–448. Rui, Z., Juan, P., Jingliang, S., Jixun, Z., Xiaoting, W., Shouping, Z., Xiaojiao, L. and Guozhong, Z. 2010. Phylogenetic characterization of Newcastle disease virus isolated in the mainland of China during 2001–2009. Vet. Microbiol. 141, 246–257. Sabra, M., Dimitrov, K.M., Goraichuk, I.V., Wajid, A., Sharma, P., Williams-Coplin, D., Basharat, A., Rehmani, S.F., Muzyka, D.V., Miller, P.J. and Afonso, C.L. 2017. Phylogenetic assessment reveals continuous evolution and circulation of pigeon-derived virulent avian avulaviruses 1 in Eastern Europe, Asia, and Africa. BMC. Vet. Res. 13(1), 1–13. Sarcheshmei, M., Dadras, H., Mosleh, N. and Mehrabanpour, M.J. 2016. Comparative evaluation of the protective efficacy of different vaccination programs against a virulent field strain of the Newcastle disease virus in broilers. Braz. J. Poult. Sci. 18, 363–370. Snoeck, C.J., Owoade, A.A., Couacy-Hymann, E., Alkali, B.R., Okwen, M.P., Adeyanju, A.T., Komoyo, G.F., Nakouné, E., Faou, A.L. and Muller, C.P. 2013. High genetic diversity of Newcastle disease virus in poultry in West and Central Africa: cocirculation of genotype XIV and newly defined genotypes XVII and XVIII. J. Clin. Microbiol. 51(7), 2250–2260. Suarez, D.L., Miller, P.J., Koch, G., Mundt, E. and Rautenschlein, S. 2020. Newcastle disease, other avian paramyxoviruses, and avian metapneumovirus infections. In Diseases of poultry. Eds., Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., de Witit, S., Grimes, T., Johnson, D., Kromm, M., Prajitno, T.Y., Rubinoff, I. and Zavala, G. Ames, IA: WILEY Blackwell, pp: 109–166. Sultan, H.A., Elfeil, W.K., Nour, A.A., Tantawy, L., Kamel, E.G., Eed, E.M., El Askary, A. and Talaat, S. 2021b. Efficacy of the Newcastle disease virus genotype VII.1.1-matched vaccines in commercial broilers. Vaccines (Basel) 10, 29. Sultan, S., Hamed, M. and Osman, N. 2021a. Evaluation of protection efficiency of different vaccination programs against velogenic Newcastle disease virus in broiler chickens: comparative in field and in laboratory studies. Thai. J. Vet. Med. 51(1), 141–150. Sultan, H.A., Talaat, S., Elfeil, W.K., Selim, K., Kutkat, M.A., Amer, S.A. and Choi, K.S. 2020. Protective efficacy of the Newcastle disease virus genotype VII-matched vaccine in commercial layers. Poult. Sci. 99, 1275–1286. Suvarna, K.S., Layton, C. and Bancroft, J.D. 2018. Bancroft’s theory and practice of histological techniques, 8th ed. Elsevier Health Sciences, England. Terregino, C. and Capua, I. 2009. Conventional diagnosis of Newcastle disease virus infection. In Avian influenza and Newcastle disease: a field and laboratory manual. Eds., Capua, I. and Alexander, D.J. Milano, Italy: Springer Milan, pp: 123–125. Tolba, M.K. and Eskarous, J.K. 1962. Effect of some chemicals on the hemagglutination activities and infectivity to chick embryos of different strains of Newcastle disease and fowl-plague viruses. In Diseases of poultry, 8th ed. Eds., Hofstad, M.S., John, B.H., Calnek, B.W., Reed, W.M. and JR, Y.H.W. Ames, IA: Iowa State University Press. van Boven, M., Bouma, A., Fabri, T.H., Katsma, E., Hartog, L. and Koch, G. 2008. Herd immunity to Newcastle disease virus in poultry by vaccination. Avian. Pathol. 37, 1–5. Wakamatsu, N., King, D.J., Kapczynski, D.R., Seal, B.S. and Brown, C.C. 2006. Experimental pathogenesis for chickens, Turkeys, and pigeons of exotic Newcastle disease virus from an outbreak in California during 2002–2003. Vet. Pathol. 43, 925–933. Yang, H., Zhao, J., Xue, J., Yang, Y. and Zhang, G. 2017. Antigenic variation of LaSota and genotype VII Newcastle disease virus (NDV) and their efficacy against challenge with velogenic NDV. Vaccine 35, 27–32. Zhao, J., Yang, H., Xu, H., Ma, Z. and Zhang, G. 2017. Efficacy of an inactivated bivalent vaccine against the prevalent strains of Newcastle disease and H9N2 avian influenza. Virol. J. 14, 56. | ||
| How to Cite this Article |
| Pubmed Style Lebdah MA, Abdallah A, Hamouda EE, Elseddawy NM, ElBakrey RM. Protective effectiveness of two vaccination schemes against the prevalent Egyptian strain of Newcastle disease virus genotype VII. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 32-45 . doi:10.5455/OVJ.2024.v14.i1.5 Web Style Lebdah MA, Abdallah A, Hamouda EE, Elseddawy NM, ElBakrey RM. Protective effectiveness of two vaccination schemes against the prevalent Egyptian strain of Newcastle disease virus genotype VII. https://www.openveterinaryjournal.com/?mno=174701 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.5 AMA (American Medical Association) Style Lebdah MA, Abdallah A, Hamouda EE, Elseddawy NM, ElBakrey RM. Protective effectiveness of two vaccination schemes against the prevalent Egyptian strain of Newcastle disease virus genotype VII. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 32-45 . doi:10.5455/OVJ.2024.v14.i1.5 Vancouver/ICMJE Style Lebdah MA, Abdallah A, Hamouda EE, Elseddawy NM, ElBakrey RM. Protective effectiveness of two vaccination schemes against the prevalent Egyptian strain of Newcastle disease virus genotype VII. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 32-45 . doi:10.5455/OVJ.2024.v14.i1.5 Harvard Style Lebdah, M. A., Abdallah, . A., Hamouda, . E. E., Elseddawy, . N. M. & ElBakrey, . R. M. (2024) Protective effectiveness of two vaccination schemes against the prevalent Egyptian strain of Newcastle disease virus genotype VII. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 32-45 . doi:10.5455/OVJ.2024.v14.i1.5 Turabian Style Lebdah, Mohamed A., Alaa Abdallah, Esraa E. Hamouda, Nora M. Elseddawy, and Reham M. ElBakrey. 2024. Protective effectiveness of two vaccination schemes against the prevalent Egyptian strain of Newcastle disease virus genotype VII. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 32-45 . doi:10.5455/OVJ.2024.v14.i1.5 Chicago Style Lebdah, Mohamed A., Alaa Abdallah, Esraa E. Hamouda, Nora M. Elseddawy, and Reham M. ElBakrey. "Protective effectiveness of two vaccination schemes against the prevalent Egyptian strain of Newcastle disease virus genotype VII." Open Veterinary Journal 14 (2024), 32-45 . doi:10.5455/OVJ.2024.v14.i1.5 MLA (The Modern Language Association) Style Lebdah, Mohamed A., Alaa Abdallah, Esraa E. Hamouda, Nora M. Elseddawy, and Reham M. ElBakrey. "Protective effectiveness of two vaccination schemes against the prevalent Egyptian strain of Newcastle disease virus genotype VII." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 32-45 . Print. doi:10.5455/OVJ.2024.v14.i1.5 APA (American Psychological Association) Style Lebdah, M. A., Abdallah, . A., Hamouda, . E. E., Elseddawy, . N. M. & ElBakrey, . R. M. (2024) Protective effectiveness of two vaccination schemes against the prevalent Egyptian strain of Newcastle disease virus genotype VII. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 32-45 . doi:10.5455/OVJ.2024.v14.i1.5 |