| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 70-89 Original Research Impact of Sel-Plex® dietary supplementation on growth performance, physiological response, oxidative status, and immunity-linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophilaEman Moustafa Moustafa1*, Mustafa Shukry2, Mohamed M. Zayed3, Foad A. Farrag4, Wesam E. Abd El-Aziz1 and Amira A. Omar11Fish Diseases and Management Department, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt 2Animal Physiology Department, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt 3Aquaculture Department, Faculty of Aquatic Fisheries Sciences, Kafrelsheikh University, Kafr El-Sheikh, Egypt 4Department of Basic Veterinary Sciences, Faculty of Veterinary Medicine, Delta University for Science and Technology, Dakahlia, Egypt *Corresponding Author: Eman Moustafa Moustafa. Fish Diseases and Management Department, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt. Email: emantarek2002 [at] yahoo.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
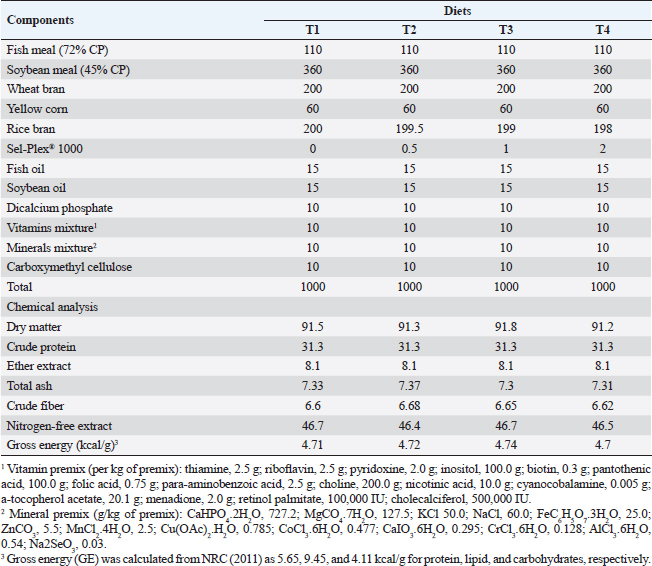
AbstractBackground: Organic selenium (Sel-Plex®) supplementation holds considerable promise for improving the effectiveness of fish production. Aim: This experiment was accomplished to judge the potential benefits of Sel-Plex® nutritional additive on growth outcomes, physiological response, oxidative status, and immunity-linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings exposed to bacterial infection with Aeromonas hydrophila. Methods: Utilizing a basal diet of 30% protein, four experimental diets were prepared, each of which contained Sel-Plex® at concentrations of 0.0, 0.5, 1, and 2 mg/kg, respectively. Three replicates of 20 fish/treatment were used using 240 healthy Nile tilapia fingerlings. Fish were placed in 12 glass aquariums and separated into 4 groups at random. For the entire span of 8 weeks, diets were admitted to fish at a 3% rate of fish biomass/aquarium. After the feeding trial, pathogenic A. hydrophila was intraperitoneally injected into fish of each treatment, and fish were observed for 15 days to track the survival rate (SR) after the challenge. Results: Growth performance, physiological response, immunological parameters (phagocytic activity, phagocytic index, and lysozyme), and antioxidant parameters [catalase, superoxide dismutase (SOD), malondialdehyde, and glutathione peroxidase (GPx)] were noticeably improved in Sel-Plex® treated groups. Moreover, Sel-Plex® increased gene expression linked with the immune system in the liver (tumor necrosis factor-alpha and interleukin 1β), to growth (insulin-like growth factor 1 and growth hormone receptor), and antioxidants (SOD and GPx). Under pathogen-challenge conditions, the employed dietary Sel-Plex® supplementation could successfully lower fish oxidative stress, offering a potential preventive additive for Nile tilapia instead of antibiotics. On the other hand, Sel-Plex® significantly enhanced each of three intestinal morphological measurements (villus width, villus length, and crypt depth), demonstrating the greatest influence on the improvement of intestinal structure overall. In the Nile tilapia control group, the infection with A. hydrophila caused noticeable degenerative alterations in the gut, hepatopancreas, spleen, and posterior kidney. The severity of the lesion was significantly reduced and significantly improved with higher Sel-Plex® concentrations. Sel-Plex® supplemented groups had 100% SRs among the A. hydrophila-challenged groups. Conclusion: It could be advised to enrich the diets of Nile tilapia fingerlings with 1–2 mg.kg−1 of Sel-Plex® to enhance growth rate, physiological response, immunological reaction, and intestinal absorptive capacity Keywords: Aeromonas hydrophila, Immune-related gene expression, Nile tilapia, Organic selenium, Oxidative status. IntroductionAquaculture and fisheries constitute a crucial part of the world’s production of food, health, and nutrient-rich food suppliers (Waite et al., 2014). Seafood helps people consume critical nutrients such as vitamin B, selenium, iodine, zinc, calcium, phosphorus, copper, and iron as well as the fat-soluble D, and A vitamins. It is an effective protein and energy source possessing a significant nutritive value (Jayasekara et al., 2020). In comparison to fast food and manufactured meat byproducts, fish and shellfish products seem to be the healthier option among animal products (Boukid et al., 2022). Consumption of fish is typically recognized as an element of appropriate dietary practices since it is a plentiful supply of essential elements, such as vitamins (A and D3), highly digestible proteins, trace minerals (selenium and iodine), and n-3 long-chain polyunsaturated fatty acids. In aquaculture, exogenous feeding provides the opportunity to modify the nutritional makeup of the fish (Ribeiro et al., 2019). Nile tilapia (Oreochromis niloticus) is a highly valuable freshwater species for commercial aquaculture in Egypt; that may be attributed to its quick development rate, high nutritional value, and disease resilience. However, the latest trend in Egyptian tilapia farming is the direction of further intensification of culture systems, which frequently causes stressful conditions and depressingly disturbs their development and welfare and makes fish more susceptible to stress, and other diseases caused by stress (Dawood et al., 2019). Fish feed is mostly expensive input in aquaculture operations since fish nutrition is a crucial factor in managing the health of farmed finfish and shellfish (Kaur et al., 2017). Fortification is the deliberate addition of one or more micronutrients (such as vitamins and minerals) to a food or condiment to be able to enhance the dietary effect of the food supply and benefit the general public health with little risk to the health. Micronutrients can assist in replacing the micronutrient content lost during processing in addition to enhancing the nutritional value of staple meals (Nölle et al., 2020). As aquaculture production frameworks are expanded, fish are subjected to an increasing number of ecological conditions, such as overcrowding, low water quality, improper handling, and shipping (Sakai, 1999). These stressors might have adverse effects on fish health and development, and they might also make aquaculture systems less effective. The majority of feed supplements used in animal diets including; immunostimulants, antimicrobials, antioxidants, and herbal plants, increase the development performance profitability, survival, and disease resilience of Nile tilapia (O. niloticus); lowering the susceptibility to diseases and protecting fish from stress and diseases especially in winter (Abdelkhalek et al., 2015). Dietary carbohydrates known as prebiotics cannot be digested and do not go through the upper gastrointestinal tract. Prebiotics have a positive effect on the host by selectively encouraging the proliferation and/or stimulating the metabolic activity of beneficial bacteria in the alimentary tract (Manning and Gibson, 2004). Another way they change the composition of gut bacteria is by modifying the substrate type that is available to the current intestinal microbiota (Mei et al., 2011). Aquaculture producers have developed certain technologies that support quick developmental performance through growth promoters and feed additives because this industry is committed to attaining the lowest feed conversion ratio (FCR) and fastest growth (Van Doan et al., 2019). Viruses, parasites, and bacteria are the main disease causes in the aquaculture industry nowadays (Rigos et al., 2021). The largest issue confronting the aquaculture sector is how to maintain fish immune competence and disease resistance while dealing with increased feed costs and treatment limitations (Dawood et al., 2018a). Tilapia has a stronger need for selenium and needs higher dosages of such mineral in their meals to have an impact on their development characteristics. These effects may depend on the chemical composition of the introduced selenium, the fish development stage, or the timing of the treatment (Durigon et al., 2019). Selenium is a vital dietary component that aids in immune system health, promotes a normal cellular immunological response, and strengthens the body’s defenses against infection with viral (Rayman, 2000). According to Sunde (2006), selenium can be found in two different forms: organic (selenocysteine and selenomethionine) and bioavailable inorganic (selenite and selenate). Because the two selenium forms are so similar, the organic selenium (selenomethionine) is taken up as an amino acid by the intestines using comparable procedures to methionine. On the other hand, inorganic selenium is passively absorbed (Shini et al., 2015). Selenium shortage in aquaculture diets can have a number of negative consequences, including slow development, lower performance, weakened immunity, and illnesses that can result in high financial defeats (Naiel et al., 2022). Dietary selenium effectiveness depends on its type. This has caused an industry-wide movement away from the conventional inorganic form of sodium selenite and toward safer, organic forms of selenium. Because of its high toxicity, pro-oxidant qualities, and bioavailability, selenite’s use has been questioned by the industry (Nordberg et al., 2022). Selenium serves a significant element as an immunostimulant, an antioxidant, and a compensatory in the immune system, growth increments, and regular bodily functioning of fish (Lukaszewicz et al., 2013). Antioxidant and immunological responses are two often used metrics to evaluate the health of aquaculture animals (Ekasari et al., 2014). An organic selenium derived from yeast, Sel-Plex, Sel-Plex, provides a remarkable supply of selenium with antioxidant properties. Thus, selenium supplementation holds considerable promise for improving the effectiveness of fish and shrimp production. Organic selenium dietary supplements have been shown to increase the length of harvested tilapia while also improving progeny performance, FCR, weight gain, and fry survival rates (SRs) in tilapia breeders. This investigation was accomplished to judge the potential benefits of Sel-Plex® nutritional additive on the growth outcomes, physiological response, oxidative status, and immunity-linked gene expression in Nile tilapia (O. niloticus) fingerlings exposed to bacterial infection with Aeromonas hydrophila. Materials and MethodsThis investigation was executed for 12 weeks in 2021, at the Fish Diseases Department Laboratory, Faculty of Veterinary Medicine, Kafrelsheikh University, Egypt. Experimental diet preparationFish meal, wheat bran, soybean meal, yellow corn, minerals, vitamins, and fish oil were used to provide a base (control) diet that was isonitrogenous and isocaloric, with 12.6 MJ/kg of digestible energy and 30% crude protein, respectively (made in Kafr El-Sheikh, Egypt; at ALEKHWA® feed factory) (Table 1). Using a feed processor, the dry materials were ground into tiny particles. In the second, third, and fourth diets, rice bran was used as a filler and Sel-Plex® 1000 (Alltech Inc., Nicholasville, KY) was added at a rate of 0.5, 1, and 2 mg/kg to the baseline diet, respectively. Oil, water, dicalcium phosphate, mineral mixture, and vitamins were included in the components after they had been thoroughly combined. The dough was then put through a pelleting extruding machine using a die that was 2–3 mm in diameter. Pellets were allowed to be air-dried before being placed in a 4°C refrigerator for storage. The nutritional profile of test meals was verified using a standardized procedure (AOAC, 2012). The commercial prebiotic product, Sel-Plex®1000 (Alltech Inc., Nicholasville, KY), is an exclusive Alltech organic form of selenium made by yeast. Made to resemble Mother Nature, it is an outstanding nutritional source of selenium. Sel-Plex® selenium is safer and more efficient in meeting the greater demand for livestock raised for quick development, reproductive performance, and health. Table 1. Chemical analysis and composition of the experimental diets (based on dry matter).
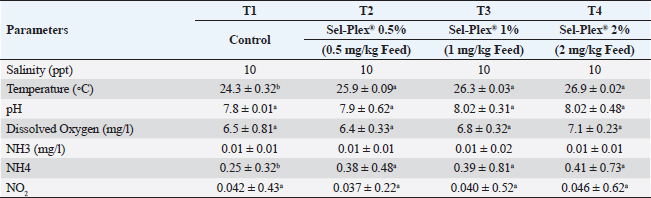
Experimental designThrough a local personal fish hatchery in the Kafr El-Sheikh Governorate, Egypt, 240 healthy Nile tilapia (O. niloticus) fingerlings (beginning body weight, 19.00 ± 3.00 g) were provided and brought to the Fish Diseases Laboratory, Faculty of Veterinary Medicine, Kafrelsheikh University. All gathered fish were accommodated in a fiberglass aquarium. Two weeks before the experiment, a baseline diet was introduced to fish (containing 30% dietary protein content). Following the accommodation time, fish fingerlings were split into 4 groups at random of 60 fingerlings each, and each group was given 3 replicas, each containing 20 fingerlings. Fingerlings were placed in glass tanks that measured 60 × 30 × 40 cm, held 20 fish per aquarium, and had an efficient aeration system. The aquariums also contained 70 l of water. Group 1 (the control group) was given commercial basal diets, whereas Groups 2–4 were fed meals enhanced with 0.5, 1, and 2 mg/kg of Sel-Plex®. Fish were given the prescribed meals for 8 weeks at a rate of 3% of the total biomass in each aquarium. Every 2 weeks over the investigation stage, the fish were weighed, and variations in live body weight were taken into consideration while adjusting the feed amounts. Siphoning was used to remove fish waste and feeding waste, and every day, nearly half of each tank’s water level was replenished with dechlorinated fresh water. Twice a week, water parameters were checked utilizing a water analysis instrument (Lamotte device, USA) according to APHA (1989). During the trial period, the following conditions were observed: temperature between (24°C–27°C), dissolved oxygen 6.5 ± 0.5 mg l−1, pH 7.1 ± 0.8, EC 219 ± 2 μ mho/cm, ammonia adjusted to the typical acceptable limits (˂0.1 mg total ammonia), and day and night photoperiod 12:12 hours (Table 2). Table 2. Water quality metrics were used in this investigation.
Determination of fish growth parametersFish were fully weighed using an electronic balance after 8 weeks (20 fish/each replicate). The following parameters; average daily gain (ADG), specific growth rate (SGR), FCR, total weight gain, protein efficiency ratio, and SR%; were determined according to Magouz et al. (2019). Hematological and biochemical parametersBefore the final sampling, treated fish were starved for 24 hours. A dose of 100 mg/l of tricaine methane-sulfonate was used to anesthetize fish for hematological assessment to minimize stress during sampling (Dawood et al., 2020b). Nine fish from each group were chosen at random and weighed (three fish/replicate). Feldman et al. (2000) report that 5 ml gauge syringes can be utilized to extract blood from the caudal blood vessels. The caudal vein was used to collect blood samples, which were then divided in half. The other blood half was used right away for hematological analysis and differential leukocyte count after being maintained in EDTA-heparinized tubes. According to Urbinate and Carneiro (2006), blood samples were taken from 3 to 4 fish because of the tiny size of the fish. A hemocytometer was used to measure the number of white blood cells (WBCs), and red blood cells (RBCs) in the blood (Houston, 1990). The method of Zinkl (1986) was conducted to determine hemoglobin concentration (Hb g/dl). Decie and Lewis (2006) described the micro-hematocrit method for estimating packed cell volume (PCV%). According to Winthrobe’s (1934) description, the mean corpuscular hemoglobin concentration (MCHC) was calculated. To directly quantify the mean corpuscular volume (MCV), an automated Coulter LH 750 hematology analyzer (Beckman Coulter, Fullerton, CA) was utilized (Dacie and Lewis, 1995). To extract serum, the persisted blood was maintained in non-heparinized tubes. Serum was isolated and maintained at −20°C for subsequent analysis after the blood samples underwent centrifugation at 1,008 g for 15 minutes at 4°C (SCILOGEX, Model: DM0412, USA) after 2 hours. According to Thrall et al. (2012), differential WBC counts were performed. It was air dried, when a thin blood film was obtained, methanol fixed for 3–5 minutes, stained with Geimsa stain for 10–12 minutes, rinsed with purified water and allowed to dry. According to Stoskopf (1993), the WBCs were enumerated among the blood smear’s 100 components. Serum albumins and total proteins were measured following Doumas and Biggs (1972). Globulin is computed as the difference between readings of total protein and albumin. Diagnostic commercial kits (Biodiagnostic Co., Egypt) were utilized in accordance with the manufacturer’s directions to measure alanine aminotransferase (ALT), and serum aspartate aminotransferase (AST) (Reitman and Frankel, 1957), urea (Fawcett and Soctt, 1960), uric acid, and creatinine (Bartels and Bohmer, 1972). Determination of immune parameters
The challenge with A. hydrophilaThe goal of the experiment was to ascertain the impact of adding Sel-Plex® to the diet on O. niloticus fingerlings’ resistance to A. hydrophila bacterial infection. According to Li et al. (2011), an experimental infection was performed via an intra-peritoneal injection of 0.2 ml of fresh A. hydrophila culture suspension (3 × 107 cfu). Along 15 days after infection, everyday mortalities were noted. Following Cruickshank (1975), the concentration of the A. hydrophila strain per 1 ml and the dose for the experimental experiments were estimated utilizing the drop plate technique. The A. hydrophila strain was cultured for 24 hours on Tryptic Soy Agar (TSA) plates; the colonies were taken up and suspended in sterile saline using a 10-fold serial dilution. The next 24 hours were then incubated at 28°C to count the colonies on the TSA plate. The dilutions (102 to 107 CFU) only were just utilized. Every group received an intraperitoneal injection of 0.5 ml/fish of every bacterial dilution. For 2 weeks following injection, all of the fish were maintained for observation. According to Ibrahim et al. (2011), the mortalities were noted two times daily. Dead fish were transported for additional PM inspection. According to Reed and Muench (1938), LD50, which refers to the lethal dose that results in the mortality of 50% of the administered fish, was computed. Gene expression analysisIn accordance with the manufacturer’s guidelines, the extraction of total RNA was carried out using the TRIzol reagent (Life Technologies, Gaithersburg, MD). The cDNA synthesis was performed immediately using the MultiScribe RT enzyme kit (Applied Biosystems, Foster City, CA). The cDNA obtained was subjected to real-time polymerase chain reaction (PCR) analysis, which was conducted in triplicate. Real-time PCR experiments were conducted using Power SYBR Green PCR Master Mix on a 7500 real-time PCR system manufactured by Applied Biosystems in Foster City, CA. Different genes’ relative fold changes in mRNA expression were assessed in relation to the control. The normalization of the fold change in mRNA expression of the measured genes was performed by utilizing the expression of β-actin, which is a widely used housekeeping gene. Table 3 presents the gene accession codes and primer sequences. Histopathological examinationsFor histopathological examinations, tissue samples from the anterior part of the intestine, hepatopancreas, kidney, and spleen were taken immediately after anesthesia using 40% ethyl alcohol from randomly selected five fish from each treatment (0, 0.5, 1, and 2 mg of Sel-Plex®1000) pre and post-challenge with A. hydrophila. The specimens were preserved in 10% formaldehyde solution for 24 hours, and then dehydrated by using ascending concentrations of ethanol (70% to absolute alcohol). The dehydrated samples were cleared in xylene and embedded in paraffin wax. For histopathological and morphometric analysis, thin sections measuring 4–5 µm in thickness were stained utilizing hematoxylin and eosin according to Moustafa et al. (2020). Table 3. Gene primer sequences used in real-time PCR analysis.
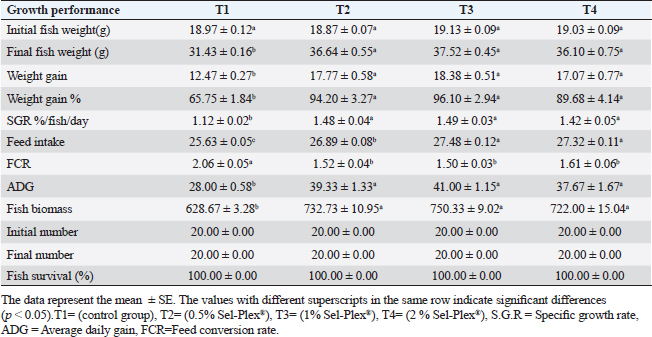
Morphometry of intestinal villiThe dimensions of intestinal villi, including length and width, as well as the depth of crypts and the surface area of villi, were quantified using image analysis software provided by the National Institutes of Health, Bethesda, MD. A selection of 10 random villi and villus-associated crypts were chosen from 5 cross-sections of the intestine. The resulting data was subjected to statistical analysis using a one-way analysis of variance (ANOVA) in SPSS version 22 (SPSS Inc., Chicago, IL). The chosen level of significance was deemed acceptable at a threshold of p < 0.05. The data are reported in the form of means ± standard error (SE). Statistical analysisThe study utilized a two-way ANOVA to examine the impact of dietary Sel-Plex and challenge conditions, as well as their potential interactions. After establishing an interaction between the two factors, a one-way ANOVA was conducted to examine the combined effect of all levels of the two factors on the four groups. The data obtained from each group were subjected to comparison with the control group. Differences within the same group and among the different sample times for each parameter were assessed using the Tukey–Kramer method. Statistical differences were evaluated using one-way ANOVA tests, conducted with SPSS version 22 (SPSS Inc., Chicago, IL). In cases where differences were observed among the experimental groups, the Duncan post-hoc test was employed. The chosen level of significance was deemed acceptable at a threshold of p < 0.05. The data are reported as means ± standard error (SE). Ethical approvalAll fish handling procedures were executed in correspondence to the animal care and use criteria established by the Ethics Committee for scientific purposes, at Kafrelsheikh University, Egypt (KFS-IACUC/138/2023). ResultsThe present investigation examines the physiological responses of O. niloticus fingerlings to Sel-Plex®1000 was evaluated via monitoring of fish growth, and hematological, biochemical, and immunological parameters. Growth performanceIn the present investigation, O. niloticus growth responses to Sel-Plex®1000 were examined. It was found that all groups that received treatment exhibited increases in ADG, weight gain, SGR, final fish weight, weight gain %, feed intake, and fish biomass compared to the control group (T1) (p < 0.05), as shown in Table 4. In addition, the impact of Sel-Plex®1000 on FCR was summarized. It was noticed that, contrasted with the control group (T1), the supplemented groups had the best food conversion rate values (p < 0.05) (Table 4). The rate of SR% was shown to be 100% across all groups. Table 4. The effect of Sel-Plex®1000 on growth performance of O. niloticus.
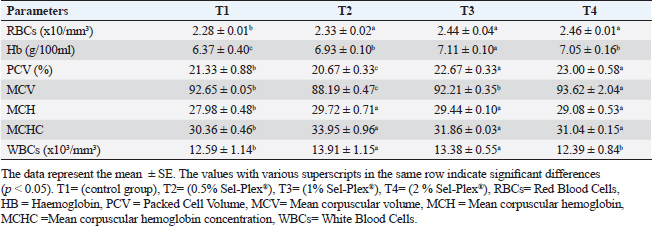
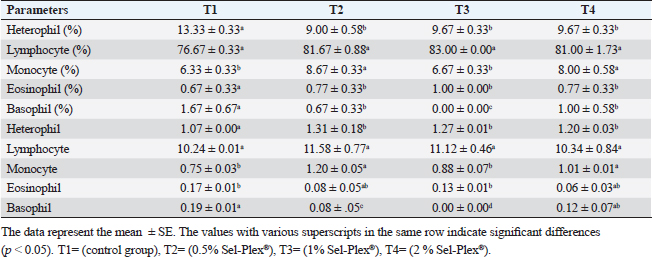
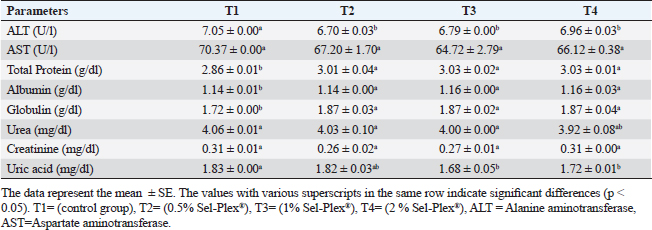
Hematological parametersIn all treatment groups, there was a substantial increase in Hb, RBCs, and MCH (p < 0.05). In comparison to T1, PCV greatly rose in T3 and T4, while it dramatically dropped in T2. MCV significantly increased (p < 0.05) in the T4 group. In comparison with the control group, decreased (p < 0.05) in the T2 group contrasted with T1, and there was no noticeable difference among the T3 and T1 groups. MCHC increased noticeably (p < 0.05) in all treated groups with the greatest value in T2. WBCs considerably (p < 0.05) increased in the T2 and T3 groups contrasted with the T1 group, but not noticeably (p < 0.05) in the T4 group (Table 5). Regarding differential leukocytic count, the observed data revealed that heterophils and eosinophil (%) increased considerably (p < 0.05) in the augmented groups when comparing the control group, while lymphocytes (%) exhibited no changes in the augmented groups contrasted with the control group. Monocyte (٪) was increased significantly in the T2 and T4 groups, while the T3 group showed no observed differences between contrasted with the control group. Basophil (%) was reduced considerably (p < 0.05) in all supplemented groups in comparison to the control group (Table 6). Biochemical analysisAccording to the data shown in Table 7, ALT level expressed a noticeable decline (p < 0.05), in all supplemented groups, while no changes were observed in AST level across all groups. Albumin, total protein, and globulin levels exhibited a noticeable increase in all supplemented groups contrasted with the control group (p < 0.05). The outcomes for uric acid revealed a considerable drop (p < 0.05), in all supplemented groups contrasted with the control group, while urea and creatinine in all groups remained unchanged. Table 5. Sel-Plex®1000 Effect on hematological parameters of O. niloticus.
Table 6. Sel-Plex®1000 Effect on the differential leukocytic count of O. niloticus.
Table 7. Sel-Plex®1000 Effect on biochemical analysis of O. niloticus.
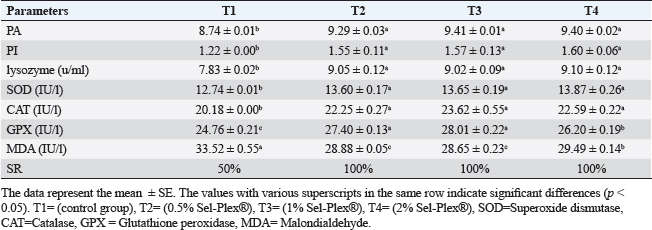
Table 8. Sel-Plex®1000 Effect on immunity and oxidative status of O. niloticus.
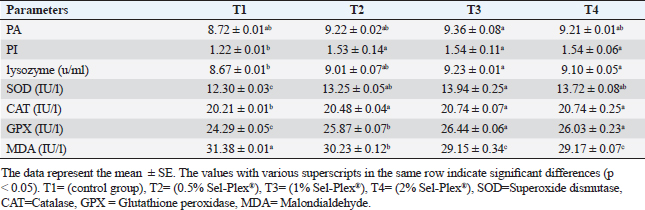
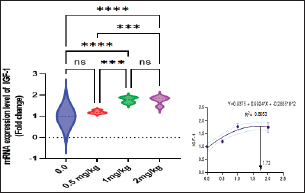
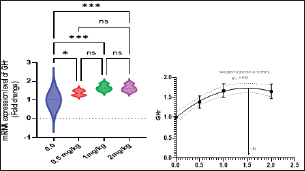
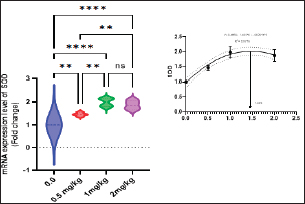
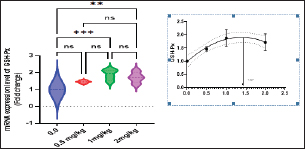
Immunological and antioxidant parametersConcerning the immunological measures, there was no change among the T2, T4 groups, and the control group; however, PA was dramatically increased in the T3 group compared to the control group (p < 0.05). The PI was increased in all supplemented groups in comparison to the control group (p < 0.05). According to Table 8, all supplemented groups’ lysozyme activity significantly increased compared with the control group (p < 0.05). The antioxidant activity showed that CAT, SOD, and GPx activities were raised in all augmented groups with the highest value in the T3 group in comparison to the control group (p < 0.05). MDA levels were noticeably decreased in the T3 and T4 groups (p < 0.05) (Table 8). Antioxidant activity after experimental challenge with A. hydrophilaImmunological measures such as PA, PI, and lysozyme activity were noticeably increased in all supplemented groups in comparison to the control group (p < 0.05). The antioxidant activity showed that CAT, SOD, and GPx activities were raised in all supplemented groups with the highest value in the T3 group contrasted with the control group (p < 0.05). MDA activity was noticeably decreased in all supplemented groups (p < 0.05). The A. hydrophila challenged groups revealed a 100% SR mainly among the Sel-Plex® supplemented groups (Table 9). Relative gene expressionLiver showed upregulation of mRNA expression of insulin-like growth factor 1 (IGF-1), in T3 and T4 groups treated with 1 and 2 mg/kg Sel-Plex®1000 contrasted with other treated groups (p < 0.05), while there were no noticeable differences between T3 and T4 groups (Fig. 1), while liver showed upregulation of mRNA expression of growth hormone receptor (GHR) in all supplemented groups contrasted with the control group (p < 0.05) and no noticeable variation among T2, T3, and T4 groups (Fig. 2). Pairwise comparisons were conducted with the control group to determine the maximum expression values of the aforementioned genes. It was observed that these maximum expression values were noticed at a dietary concentration of 1.7 mg Sel-Plex /kg. Subsequently, a significant reduction in the expression values of these genes was observed (Fig. 1). The present study observed a noticeable (p < 0.05) rise in the mRNA expression of SOD in the liver samples of all groups fed diets supplemented with Sel-Plex®1000 with the highest level in T3 and T4 groups, while there was no noticeable variation between T3 and T4 groups (Fig. 3), while GPx mRNA expression in the liver samples exhibited a substantial difference noticeably (p < 0.05) increased in T3 and T4 groups treated with 1 and 2 mg/kg Sel-Plex®1000 in comparison with other treated groups, while no substantial variation between T3 and T4 groups was observed (Fig. 4). Table 9. Sel-Plex®1000 Effect on immunological and oxidative status of O. niloticus after challenge with A. hydrophila.
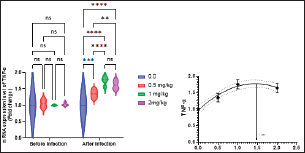
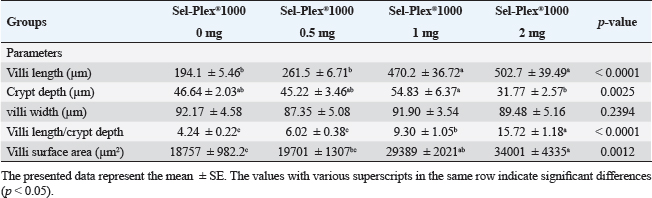
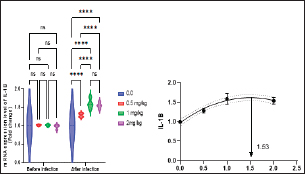
Fig. 1. Gene expression of the liver (IGF-1) of Nile tilapia in different groups fed diets with different doses of Sel-Plex®1000 for 8 weeks. *indicate significant differences between groups (p < 0.05), ns=indicate no significant differences between groups.
Fig. 2. Gene expression of the liver (GHR) of Nile tilapia in different groups fed diets with different doses of Sel-Plex®1000 for 8 weeks. *indicate significant differences between groups (P < 0.05), ns=indicate no significant differences between groups. It is noticed that the mRNA expression of interleukin 1β (IL-1β), and tumor necrosis factor-alpha (TNF-α) in the liver samples were substantially (p < 0.05) increased in all groups augmented with Sel-Plex®1000, while no substantial variation between T3 and T4 groups before and post challenge with A. hydrophila (Figs. 5 and 6). Histopathological findingsThe morphometric analysis of the intestine of pre-challenged groups showed a marked increase in all measured parameters; villi length, width, crypt depth, villi length/crypt depth, and villi surface area in a dose-dependent pattern especially 1 and 2 mg Sel-Plex®1000 groups, than the other treated groups as illustrated in Table 10 besides, increased branching of intestinal villi in comparison to the other groups (Fig. 7). The histopathological examination of the intestine of fish challenged with A. hydrophila showed severe necrotic changes and sloughing of villi epithelium which include most of the intestinal villi length in the 0.0 mg Sel-Plex®1000 group. The adverse effect diminished with an increase in the concentration of Sel-Plex®1000 (0.5 and 1 mg groups) while the 2 mg Sel-Plex®1000 group showed intact villi with a mild increase in villi length and branching in comparison to the previously mentioned groups. In contrast, the non-challenged groups showed intact villi with an increase in both length and branching in a way dependent on dosage (Fig. 7). The hepatopancreas of pre-challenged groups shows normal architecture of the liver with polyhedral-shaped hepatocytes separated by hepatic sinusoids and intact pancreatic acini while the hepatopancreas of 0 mg Sel-Plex®1000 challenged group show zones of necrosis in both hepatic and pancreatic tissue beside severe vacuolar degeneration and nuclear pyknosis. The other treated groups; 0.5, 1, and 2 mg Sel-Plex®1000 show significant amelioration which ranged from vacuolar degeneration and congestion of blood vessels in the 0.5 mg Sel-Plex®1000 treated group to apparently intact hepatic and pancreatic tissue with mild fatty changes in 2 mg Sel-Plex®1000 treated group (Fig. 8). Histopathological examination of the posterior part of the fish kidney of pre-challenged groups shows normal renal architecture with intact glomeruli, renal tubules, and interstitial tissue. The kidney of the 0.0% Sel-Plex®1000 A. hydrophila-challenged group shows necrosis of some renal tubule, sloughing of the tubular epithelium into the lumen, interstitial edema, and congestion of renal blood vessels. The lesions were markedly diminished with increased Sel-Plex®1000 concentration in a way dependent on dosage from 0.5 to 2 mg (Fig. 9).
Fig. 3. Gene expression of the liver (SOD) of Nile tilapia in different groups fed diets with different doses of Sel-Plex®1000 for 8 weeks. *indicate significant differences between groups (P < 0.05), ns=indicate no significant differences between groups.
Fig. 4. Gene expression of the liver (GSH-Px) of Nile tilapia in different groups fed diets with different doses of Sel-Plex®1000 for 8 weeks. *indicate significant differences between groups (P < 0.05), ns=indicate no significant differences between groups.
Fig. 5. Gene expression of liver (TNF-α) of Nile tilapia pre and post-challenge with A. hydrophila in different groups fed diets with different doses of Sel-Plex®1000 for 8 weeks. *indicate significant differences between groups (P < 0.05), ns=indicate no significant differences between groups.
Fig. 6. Gene expression of the liver (IL-1β) of Nile tilapia pre and post challenge with A. hydrophila in different groups fed diets with different doses of Sel-Plex®1000 for 8 weeks. *indicate significant differences between groups (P < 0.05), ns=indicate no significant differences between groups. Table 10. Morphometric analysis of the intestine of O. niloticus fed on diets containing different levels Sel-Plex®.
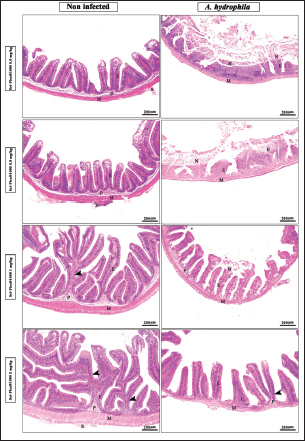
Fig. 7. Photomicrograph of H&E stained panel of the anterior part of the intestine of O. niloticus fed on ration contained 0, 0. 5, 1, and 2 mg of Sel-Plex®1000 pre and post-challenge with A. hydrophila showing intestinal villi lined with simple columnar cells of lamina epithelialis (E), lamina propria (P), muscular layer (M), tunica serosa (S), branched alveoli (arrow head), and areas of necrosis and sloughing of intestinal villi (N).
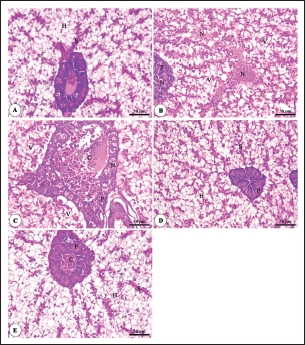
Fig. 8. Photomicrograph of H&E stained panel of hepatopancreas of O. niloticus pre-challenge (A) and postchallenge with A. hydrophila fed on ration contained 0 (B), 0.5 (C), 1 (D) and 2 mg (E) of Sel-Plex®1000 showing hepatocytes (H), blood sinusoids (S), pancreatic acini (P), focal areas of necrosis in hepatic and pancreatic tissue (N), vacuolar degeneration (V), and congestion of blood vessels (C). The histopathological examinations of the spleen of pre-challenged groups show the normal structure of the spleen with intermingled red and white pulp in addition to melanomacrophage centers. The spleen of the 0 mg Sel-Plex®1000 challenged group shows severe degenerative changes and necrosis, depletion of white pulp, and interstitial edema. The 0.5, 1, and 2 mg Sel-Plex®1000 treated group show a remarkable improvement of the spleen with increased melanomacrophage centers in a dose-dependent manner (Fig. 10). DiscussionAquaculture systems can use probiotics as either feed or water additions (Taoka et al., 2006). The kind and duration of probiotic and prebiotic therapy may have an impact on fish health (Welker and Lim, 2011). The efficacy of the probiotic bacterial settlement in the fish digestive system has been demonstrated to be improved by supplementing meals with pre- and probiotics (Mei et al., 2011). The aqua feeds industry is primarily concerned with gaining benefits from the improved growth and resistance response of the great majority of variety of cultivated fish, in addition to preventive healthcare services through a variety of healthy protocols to ensure its manageability in the aquatic farming system (Kiron, 2012). The nutritional health of farmed fish species is the primary factor that affects their immune status and, ultimately, helps to achieve a greater level of assurance (Dawood et al., 2018b).
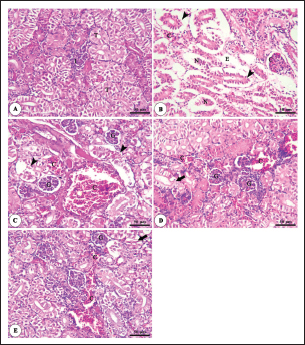
Fig. 9. Photomicrograph of H&E stained panel of kidney of O. niloticus pre-challenge (A) and post-challenge with A. hydrophila fed on ration contained 0 (B), 0.5 (C), 1 (D) and 2 mg (E) of Sel-Plex®1000 showing renal glomeruli (G), renal tubules (T), interstitial tissue (I), congestion of glomerular and interstitial blood vessels (C), degeneration and separation of renal tubular epithelium from its basement membrane (arrow heads), necrosis of some renal tubules (N), mild vacuolar degeneration and nuclear pyknosis (arrows) and edema in the interstitial tissue (E).
Fig. 10. Photomicrograph of H&E stained panel of spleen of O. niloticus pre-challenge (A) and post-challenge with A. hydrophila fed on ration contained 0 (B), 0.5 (C), 1 (D), and 2 mg (E) of Sel-Plex®1000 showing mixed red (R) and white (W) pulp, melanomacrophage centers (MMC), area of necrosis(N), and edema (E). Functional nutritional supplements are a crucial management tool for boosting farmed fish’s resistance to viral illnesses and optimizing the fish’s general growth parameters (Bharathi et al., 2019). While there is growing public concern about antibiotic application in aquaculture due to the increased danger of the discovery of antibiotic-resistant bacteria in these conditions, this is a problem that affects not only aquaculture but also consumers, terrestrial animals, the environment, and other living things (Ghiasi et al., 2021). Prebiotics were added in response to the demand for safe food around the world as organic alternatives to antibiotic therapy as growth promoters, which increased fish resistance and decreased pathogen-related mortality rates (Ahmed et al., 2015). Growth performanceOrganic selenium in the form of selenoyeast or selenomethonine may have advantages over its inorganic counterparts (Mahan, 1999). In the existing study, all investigated growth parameters were raised in all prebiotic-supplemented groups with the greatest values in the T3 group (fed on Sel-Plex® 1 g/kg); these findings may be due to the fish’s improved feed utilization. The obtained growth parameters findings are in accordance with those reported previously; in tilapia (Abdel-Tawwab and Mohapatra, 2008), African catfish (Abdel-Tawwab et al., 2007a), channel catfish (Gatlin and Wilson, 1984), European seabass (Dicentrarchus labrax) (Abd El-Kader et al., 2020) and grouper (Lin and Shiau, 2005) but with un-similar doses of organic selenium. However, the current results conflict with those investigated by Monteiro et al. (2007) who discovered that diets enhanced with organic or inorganic sources of selenium from 1 to 2 mgkg−1 levels had no effect on the growth of fish species. Variations between organic selenium doses used in these studies may be attributed to a variety of selenium origin and sSelenium concentration in the rearing water. The present prebiotic may have had a positive impact on the feed conversion rate improvement by reducing the quantity of feed required to yield one unit of fish, which in turn decreased production costs. The findings concur with those of certain authors (Abdel-Tawwab and Wafeek, 2008). Hematological parametersBlood biochemical measurements are frequently used to detect potential changes in the fishes’ general health state when they consume functional feed additives (Dawood et al., 2019; Moustafa et al., 2021). Regarding the hematological parameters, there was a substantial increase in Hb, RBCs, MCV, and MCH in all supplemented groups, with a dramatic increase of PCV in the T3 and T4 groups. Moreover, a considerable elevation in WBCs, heterophil, eosinophil, and monocyte (%) among treated groups. These increased levels confirm that this rise was the main contributor to the improved growth in treated groups and this clearly indicates that the supplemented fishes were luxuriously endowed with defensive mechanisms presenting them as equipped conquerors against the impending potential pathogenic invasions or any disease as a result of Sel-Plex®feed supplementation. These investigated results concur with those reported by previous authors (Ani et al., 2009; Iqbal et al., 2017). However, this is in contrast to El-Hammady et al. (2007). The hematological parameter results may differ based on the prebiotic kind and dosage, fish species, age, size, physiological status, and eating habits as well as the ecological circumstances (Osuigwe et al., 2005). Biochemical analysisInvestigating blood chemistry analyses mostly supplies important and vital information supporting the management of farmed fish and the diagnosis of their health status (Řehulka et al., 2004). The noticeable decrease in ALT levels in the T4 prebiotic-supplemented group is a similar result to that recorded by Yalçinkaya et al. (2008). This could be clarified by the proof that when liver cells are injured or their membranes are breached, allowing these enzymes to seep out, both ALT and AST levels should rise in the plasma (Abdel-Tawwab et al., 2007a; Kouba et al., 2014). However, there was a decrease in uric acid and AST levels in fish-fed Sel-Plex; the results coincided with Ani et al. (2009) and are in contrast to some researchers (Abdel-Tawwab et al., 2007b; Abdel-Tawwab and Wafeek, 2008). Schaperclaus et al. (1992) clarified that albumin, globulin, and total protein measurement in plasma or serum is a valuable screening tool in fish, as it reflects the overall nutrition state. The significantly increased level of albumin, globulin, and total proteins in all prebiotic-supplemented groups; prebiotic supplementation may reflect the increased inherited body defense and better innate response of fish due to the use of prebiotic supplements (Andrews et al., 2009). These variables were applied as a general symptomatic sign of fish health, immunological capability, stress, and nutritional condition of the fish. The outcomes concur with those that certain authors have reported (Ani et al., 2009; Moustafa et al., 2021); however, as opposed to those stated previously (Abdel-Tawwab et al., 2007a, 2007b; Abdel-Tawwab and Wafeek, 2008). Immunological and antioxidant parametersThe body defense of fish is significantly influenced by nonspecific immunity (Reda et al., 2018). The ability of lysozyme to break down the bond between N-acetyl glucosamine and N-acetyl intracytoic acid and so damage the pathogen microorganisms’ cell wall structure makes it a crucial protective component of the humoral immune reaction (Han et al., 2015). According to a recent study, all groups’ immunological responses to O. niloticus fingerlings were fundamentally altered and improved, with the T3 group showing the greatest levels. This study demonstrated that lysozyme is one of the most physiologically secure mechanisms in fish. It comes from neutrophils and macrophages that produce bacteriolytic substances into mucus and blood to help organisms fight off bacterial, viral, and parasitic diseases (Yano, 1997). One of the main immunological reactions in charge of the ability to directly kill harmful bacteria is bactericidal activity (Guardiola et al., 2014). Lysozyme, whose concentration and activity in serum and skin mucus vary on fish species, ambient conditions, and dietary status, could be used to characterize it (Fast et al., 2002). To boost the lysozyme activity of fish and other animals, which in turn improves the animal’s immunological condition, B-lymphocyte generation is crucial (Kumar et al., 2018). The current findings clarified that Sel-Plex®supplementation substantially altered the dimensions of lysozyme action. Fish that were encouraged to eat diets with 0.1% Sel-Plex®diet showed the highest serum lysozyme activity. The immune-stimulating properties of dietary Sel-Plex®may be the cause of the increased lysozyme action (Dawood et al., 2018b). The results coincided with those reported in goldfish (Carassius auratus) (Choi et al., 2013), juvenile olive flounder (Paralichthys olivaceus) (Lee et al., 2009), yellowtail kingfish (Seriola lalandi) (Le et al., 2013), and juvenile Tor putitora (Khan et al., 2016). Oxidative stress is brought on by an excess of reactive oxygen species (ROS) production (Sinha et al., 2015). Oxidative stress can result from several unfavorable rearing circumstances, including crowding, high ammonia nitrogen levels, and low oxygen levels. This stress can induce a multitude of harms, including cell death through apoptosis or necrosis (Klein and Ackerman, 2003). A principal consequence of lipid peroxidation brought on by ROS is MDA (Xu et al., 2010). The primary enzyme defense system against the negative effects of ROS was generated by the enzymes SOD, CAT, and GSH-Px (Zhou et al., 2020). Fish’s capacity for antioxidants can be determined by analyzing these variables. It is notable that selenium assumes a significant part in actuating cell reinforcement guard frameworks including SOD, CAT, and GPx (Han et al., 2011; Le and Fotedar, 2014). The past emitted catalysts are fundamental for rummaging the ROS (Atencio et al., 2009). The emotional expansion in antioxidant activity (SOD, CAT, and GPx exercises) in all Sel-Plex® treated bunches with the most noteworthy expansion in the T3 bunch explain the oxidative ability of receptive oxygen species (ROS), which are conveyed by phagocytic cells that have been enacted and are answerable for killing or corrupting immersed materials, including microorganisms (Atencio et al., 2009). Selenium is associated with the development of a few kinds of selenoproteins, among which GSH-Px is the main part of the invulnerable arrangement of the body (Rotruck et al., 1973). By promoting the growth of selenium-enzymes and selenioproteins (selenoprotein P, thioredoxin reductase, and GPx framework), it protects the cells from oxidative materials. Hence, a higher concentration of these proteins will function more defensively, enhancing fish and other organisms’ resistance (Kumar et al., 2018), while low groupings of selenoproteins increment oxidative pressure, which then, at that point, prompts viral contamination (Sritunyalucksana et al., 2011). Such selenoproteins partake effectively in the cell reinforcement protection framework and forestall the destructive impacts of receptive oxygen species (Tapiero et al., 2003). They give security to cell films against oxidative pressure by utilizing decreased glutathione, which catalyzes the decrease of hydroperoxides and peroxides (Rider, 2009). The outcomes of the recent investigation are predictable with various other explorations (Abdel-Tawwab et al., 2008; Khan et al., 2016). The prebiotic-enhanced T3 bunches in the ongoing review showed higher CAT activity levels with diminished MDA. The finding proposed that CAT and MDA were not really conversely related. This information recommends that supplementation of 1 mg Sel-Plex kg−1 is by all accounts more powerful in reinforcing the antioxidant system prevention agent framework against oxidative pressure. These results are predictable with various other explorations (Abdel-Tawwab et al., 2007a; Lin and Shiau, 2007; Dawood et al., 2017, 2018b, 2020d). Since CAT is basically one of numerous cell reinforcement catalysts that could stop the blend of MDA, the clarification is fairly straightforward. The last MDA level of fish might be influenced by various cancer prevention agents other than proteins, such as nonenzymatic mixtures (such as L-ascorbic acid and E) and certain prebiotics and probiotics (Wang et al., 2017). Antioxidant activity after experimental challenge with A. hydrophilaPrebiotics in the feeding routine have been demonstrated in various examinations to fundamentally increment fish protection from destructive microorganisms (Zhang et al., 2020). Fish might encounter oxidative pressure due to A. hydrophila, one of the most common pathogenic microscopic organisms linked to aquatic environments (Turutoglu et al., 2012). In the current study, Nile tilapia fingerlings fed Sel-Plex® revealed the highest SOD, CAT, and GPx activities were recorded in the prebiotic supplemented T3 group while, recorded sharp decline in MDA activity after infection with A. hydrophila. As investigated in the present study, A. hydrophila infections are described by the enactment of solid ROS age in the host, which causes oxidative harm (Li et al., 2013; Mir et al., 2017). MDA is known as an indication of ROS-prompted cell harm, and the antioxidant system framework can diminish this harm (Dawood et al., 2018b). The recent findings exhibited that adding a reasonable measure of Sel-Plex® to a feeding regimen smothers unnecessary ROS age and brings down MDA levels. The outcome is comparable to studies by Rathore et al. (2020) which found that Sel-Plex® could increase Nile tilapia resistance to A. hydrophila, Chiu et al. (2010), which found that selenium could improve M. rosenbergii resistance, and Rudy and Fotedar (2013) increase marron, Cherax cainii resistance to Vibrio mimicus. Aeromonas hydrophila challenge was recorded 100% in all treated groups. The recent outcomes demonstrated that the fuse of Sel-Plex® in diet could move along opposition against A. hydrophila disease. The results concur with Rathore et al. (2020). Relative gene expressionLevels of IL-1β, TNF-α, and SOD gene expression are correlated with fish physiological status (Dinarello, 2018), oxidative status (Eˇcimovi´c et al., 2018), and immune system health of the fish (Elbahnaswy and Elshopakey, 2020). IL-1β, and TNF-α, the pro-inflammatory cytokine, had their gene expression levels enhanced in the Sel-Plex® supplemented groups in comparison to the control fish bunch. Simultaneously, those for insulin-like IGF-1, GHR, SOD, and GSH-Px were also improved at the same period. Furthermore, there was no massive distinction somewhere in the range of T3 and T4 bunches enhanced with 1 and 2 mg/kg Sel-Plex®, before and post challenge with A. hydrophila, with respect to the expression of IL-1β, and TNF-α genes in the liver. The outcomes are like that announced in previous research (Liu et al., 2005; Cao et al., 2011). Choi et al. (2013) and Khan et al. (2016) likewise noticed a critical connection between the development of chemical level and selenium healthful status and affirmed the idea that supplementing with selenium has a fundamental impact (p < 0.05) on fish growth performance and serum growth hormone levels. Moreover, these outcomes sufficiently upheld the likely impact of Sel-Plex®to improve the resistance and anti-inflammation brought about by A. hydrophila disease in Nile tilapia. Histopathological findingsMorphological respectability is fundamental for keeping up with the ordinary elements of the digestive tract (Gao et al., 2013). Fish having a healthy gut have specific intestinal morphological boundaries (Han et al., 2015). While contrasting different feeding regimes for aquatic animals, a significant morphometry marker to consider is the evaluation of intestinal health, including the ability to absorb nutrients and digestive functions through the length of intestinal villi, thickness of the muscular layer, and number of goblet cells (Yukgehnaish et al., 2020). Prebiotics in the eating routine can assist cultivated fish with consuming their feed all the more successfully and keep up with the strength of their intestinal mucosal epithelium (Pelaseyed et al., 2014). This was likewise affirmed through the upregulation of the growth hormone (GHR). In the recent study, Sel-Plex® considerably ameliorated each of the three intestinal measures (villus width, villus length, and crypt depth), representing the best overall intestinal structure-improving effect. The results are coincided with Ghaniem et al. (2022). According to a histological investigation conducted mostly on Nile tilapia in the control group that was not given Sel-Plex® treatment, the infection with A. hydrophila caused noticeable degenerative alterations in the gut, hepatopancreas, spleen, and posterior kidney. The increase of the inflammatory genes TNF- and IL-1 (interleukin 1) further supports this inflammation. However, with a higher concentration of Sel-Plex®, the severity of the lesion was significantly reduced and showed clear improvement. Improved intestinal absorptive and defensive activities may be related to increased surface area available for absorption and/or produced mucus from many goblet cells, which has a bactericidal effect by covering pathogen receptors and protecting the integrity of the intestinal epithelium (Smirnov et al., 2005; Iqbal et al., 2020). ConclusionOur knowledge of the functional nutritional techniques for using organic selenium (Sel-Plex®) as a prebiotic in freshwater fish diets has been advanced by recent investigation. It is possible to draw the conclusion that prebiotics boosts the antioxidant capacity of fish and raise disease resistance, which is important in modern intensive aquaculture. The dietary Sel-Plex® supplementation is used to help moderate the increased oxidative stress induced by A. hydrophila; they have the potential to be used as a prophylactic additive for Nile tilapia instead of antibiotics when pathogen challenges are present. Due to the improved findings on growth rate, physiological response, immunological reaction, and intestinal absorptive capacity, it may be advised to enrich the diets of Nile tilapia fingerlings with 1–2 g.kg−1 of Sel-Plex®. Author contributionsThe current work’s purpose and design were contributed by all of the authors. The study’s conception and design were created by Eman Moustafa and Amira Omar. Eman Moustafa, Amira Omar, Mohamed Zayed, and Wesam Abd ElAziz handled the preparation of the materials, gathering and analysis of the data. Wesam Abd ElAziz conducted the statistical analysis. The analysis of gene expression was performed by Mustafa Shukry. The histological investigation was conducted by Foad Farrag. Eman Moustafa, Amira Omar, Mohamed Zayed, and Wesam Abd ElAziz wrote the first draft of the work. All authors provided feedback on earlier drafts of the work. Eman Moustafa then reviewed and corrected the final draft. The final version of the manuscript was read and approved by all authors. FundingThere was no additional financial source; this effort was fully supported by the contributions of the authors. Data availabilityThe current manuscript contains all the data supporting the findings of this study. Upon a reasonable request, the corresponding author will provide any further information needed. ReferencesAbd El-Kader, M.F., Fath El-Bab, A.F., Shoukry, M., Abdel-Warith, A.A., Younis, E.M., Moustafa, E.M., El-Sawy, H.B., Ahmed, H.A., Doan, H.V. and Dawood, M.A.O., 2020. Evaluating the possible feeding strategies of selenium nanoparticles on the growth rate and wellbeing of European seabass (Dicentrarchus labrax). Aquacul. Rep. 18, 100539. Abdelkhalek, N.K.M., Ghazy, E.W. and Abdel-Daim, M.M. 2015. Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: impact on lipid peroxidation and oxidative stress. Environ. Sci. Poll. Res. Int. 22, 3023–3031. Abdel-Tawwab, M., Mousa, M.A.A. and Abbass F.E. 2007a. Growth performance and physiological response of African catfish, Clarias gariepinus (B.) fed organic selenium prior to the exposure to environmental copper toxicity. Aquaculture 272(1–4), 335–345. Abdel-Tawwab, M., Mousa, M.A.A., Ahmad, M.H. and Sakr, S.F. 2007b. The use of calcium pre-exposure as a protective agent against environmental copper toxicity for juvenile Nile tilapia, Oreochromis niloticus (L.). Aquaculture 264, 236–246. Abdel-Tawwab, M. and Wafeek, M. 2008. Response of Nile tilapia, Oreochromis niloticus to environmental cadmium toxicity during organic selenium supplementation. In 8th International Symposium on Tilapia in Aquaculture, pp: 415–430. Aebi, H. 1984. Catalase in vitro, methods in enzymology. Elsevier, pp: 121–126. Alexander, J. 2022. Selenium. Handbook on the toxicology of metals. Eds., Nordberg G.A.C. and Washington M. Academic Press, vol. 2, pp: 730–771. Andrews, S.R., Sahu, N.P., Pal, A.K. and Kumar, S. 2009. Hematological modulation and growth of Labeo rohita fingerlings: effect of dietary mannan oligosaccharide, yeast extract, protein hydrolysate and chlorella. Aquac. Res. 41, 61–69. Ani, C., Wallis, S., Kraslawski, A. and Agachi, P.S. 2009. Response of broiler finishers to diets containing graded levels of processed castor oil bean (Ricinus communis L) meal. J. Anim. Physiol. Anim. Nutr. 93(2), 157–164. AOAC. 2012. Official methods of analysis, 18th ed. Washington, DC: Association of Official Analysis of Chemists. APHA. 1989. Standard methods for the examination of water and wastewater. Part 3, determination of metals, 17th ed. Washington DC: American Public Health Association, p: 164. Atencio, L., Moreno, I., Jos, Á., Prieto, A.I., Moyano, R., Blanco, A. and Cameán, A.M. 2009. Effects of dietary selenium on the oxidative stress and pathological changes in tilapia (Oreochromis niloticus) exposed to a microcystin producing cyanobacterial water bloom. Toxicology 53(2), 269–282. Bartels, H. and Bohmer, M. 1972. Quantitative determination of creatinine. Clin. Chim. Acta. 37, 193. Bharathi, S., Cheryl, A., Rajagopalasamy, C.B.T., Uma, A., Ahilan, B., and Aanand, S. 2019. Functional feed additives used in fish feeds. Int. J. Fish. Aquat. Stud. 7(3), 44–52. Boukid, F., Baune, M-C., Gagaoua, M. and Castellari, M. 2022. Seafood alternatives: assessing the nutritional profile of products sold in the global market. Europ. Food Res. Tech. 248, 1777–1786. Cao, H., Zhou, R.J., Wei, Q.W., Li, C.J. and Gui, J.F. 2011. Molecular characterization of the growth hormone in Chinese sturgeon and its expression during embryogenesis and early larval stages. J. Appl. Ichthyol. 27, 501–504. Chiu, S.T., Hsieh, S.L., Yeh, S.P., Jian, S.J., Cheng,W. and Liu, C.H. 2010. The increase of immunity and disease resistance of the giant freshwater prawn: Macrobrachium rosenbergii by feeding with selenium enriched-diet. Fish Shellfish Immunol. 29, 623–629. Choi, Y.J., Kim, N.N., Shin, H.S., Park, M.S., Kil, G.S. and Choi, C.Y. 2013. Effects of waterborne selenium exposure on the antioxidant and immunological activity in the goldfish (Carassius auratus). Mol. Cell Toxicol. 9, 365–373. Cruickshank, R. 1975. Medical microbiology: a guide to diagnosis and control of infection. Edinburgh and London, UK: E and S Livingston Ltd., p: 888. Dacie, J.V. and Lewis, S.M. 1995. Practical haematology, 8th ed. Edinburgh, UK: Churchil Livingstone. Dawood, M.A.O., Koshio, S., El-Sabagh, M., Billah, M.M., Zaineldin, A.I., Zayed, M.M. and Omar, A.A.E-D. 2017. Changes in the growth, humoral and mucosal immune responses following β-glucan and vitamin C administration in red sea bream, Pagrus major. Aquaculture 470, 214–222. Dawood, M.A.O., Koshio, S., Abdel-Daim, M.M. and Van Doan, H. 2018a. Probiotic application for sustainable aquaculture. Rev. Aquac. 11, 907–924; doi:10.1111/raq.12272 Dawood, M.A.O., Koshio, S., Zaineldin, A.I., Doan, H.V., Moustafa, E.M., Abdel-Daim, M.M., Esteban, M.A. and Hassaan, M.S. 2018b. Dietary supplementation of selenium nanoparticles modulated systemic and mucosal immune status and stress resistance of red sea bream (Pagrus major). Fish Physiol. Biochem. 45, 219–230; doi:10.1007/s10695-018-0556-3 Dawood, M.A.O., Magouz, F.I., Salem, M.F.I. and Abdel-Daim, H.A. 2019. Modulation of digestive enzyme activity, blood health, oxidative responses and growth-related gene expression in GIFT by heat-killed Lactobacillus plantarum (L-137). Aquaculture 505, 127–136. Dawood, M.A.O., Abd El-Kader M.F., Moustafa, E.M., Gewaily, M.S. and Abdo, S.E. 2020b. Growth performance and hemato-immunological responses of Nile tilapia (Oreochromis niloticus) exposed to deltamethrin and fed immunobiotics. Environ. Sci. Pollut. Res. 27, 11608–11617. Dawood, M.A.O., Zommara, M., Eweedah, N.M. and Helal, A.I. 2020d. The evaluation of growth performance, blood health, oxidative status and immune-related gene expression in Nile tilapia (Oreochromis niloticus) fed dietary nanoselenium spheres produced by lactic acid bacteria. Aquaculture 515, 734571. Decie, S.I.V. and Lewis, S.M. 2006. Practical haematology, 10th ed. London, UK: Churchill Livingstone, p: 736. Demers, N.E. and Bayne, C.J. 1997. The immediate effect of stress on hormones and plasma lysozyme in rainbow trout. Dev. Comp. Immunol. 21, 363–373. Dinarello, C.A. 2018. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281(1), 8–27. Doumas, B.T. and Biggs, H.G. 1972. Standard methods of clinical chemistry. New York, NY: Academic Press, p: 7. Durigon, E.G., Kunz, D.F., Peixoto, N.C., Uczay, J. and Lazzari, R. 2019. Diet selenium improves the antioxidant defense system of juveniles Nile tilapia (Oreochromis niloticus L.). Braz. J. Biol. 79(3), 527–532. Eˇcimovi´c, S., Velki, M., Vukovi´c, R., ˇCamagajevac, I.Š., Petek, A., Bošnjakovi´c, R., Grgi´c, M., Engelmann, P., Bodó, K. and Filipovi´c-Mariji´c, V. 2018. Acute toxicity of selenate and selenite and their impacts on oxidative status, efflux pump activity, cellular and genetic parameters in earthworm Eisenia andrei. Chemosphere 212, 307–318. Ekasari, J., Azhar, M.H., Surawidjaja, E.H., Nuryati, S., Schryver, P.D. and Bossier, P. 2014. Immune response and disease resistance of shrimp fed biofloc grown on different carbon sources. Fish Shellfish Immunol. 41(2), 332–339. Elbahnaswy, S. and Elshopakey, G.E. 2020. Differential gene expression and immune response of Nile tilapia (Oreochromis niloticus) challenged intraperitoneally with Photobacterium damselae and A. hydrophila demonstrating immunosuppression. Aquaculture 526, 735364. El-Hammady, A.K.I., Ibrahim, S.A. and El-Kasheif, M.A. 2007. Synergistic reactions between vitamin E and selenium in diets of hybrid tilapia (Oreochromis niloticus × Oreochromis aureus) and their effect on the growth and liver histological structure. Egypt. J. Aquat. Biol. Fish. 30, 53–58. Fast, M.D., Ross, N.W., Mustafa, A., Sims, D.E., Johnson, S.C., Conboy, G.A., Speare, D.J., Johnson, G. and Burka, J.F. 2002. Susceptibility of rainbow trout Oncorhynchus mykiss, Atlantic salmon Salmo salar and coho salmon Oncorhynchus kisutch to experimental infection with sea lice Lepeophtheirus salmonis. Dis. Aquat. Org. 52, 57–68. Fawcett, J.K. and Scott, J.E. 1960. A rapid and precise method for the determination of urea. J. Clin. Pathol. 13, 156. Feldman B., Scott T.D., Schier A.F. and Talbot W.S. 2000. Nodal-related signals establish mesendodermal fate and trunk neural identity in zebrafish. Curr. Biol. 10, 531–534. Gao, Y., Han, F., Huang, X., Rong, Y., Yi, H. and Wang, Y. 2013. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: a comparative study. J. Anim. Sci. 91, 5614–5625. Gatlin, D.M. and Wilson, R.P. 1984. Dietary selenium requirement of fingerling channel catfish. J. Nut. Mar. 114(3), 627–633. Ghaniem, S., Nassef, E., Zaineldin, A.I., Bakr, A. and Hegazi, S. 2022. A comparison of the beneficial effects of inorganic, organic, and elemental nano-selenium on Nile tilapia: growth, immunity, oxidative status, gut morphology, and immune gene expression. Biol. Trace Elem. Res. 200, 5226–5241. Ghiasi, M., Nasrollahzadeh, S.H., Fazli, H., Safari, R., Farabi, S.M.V., Binaii, M., Yaghobzadeh, Z., Zare, E., Behrouzi, S., Habibi, F. 2021. Evaluation of some risk factors on the rainbow trout production with emphasis on water microbial indices and heavy metals of farms along the Haraz River. Iran. Sci. Fish. J. 30(6), 25–42. Guardiola, F.A., Gónzalez-Párraga, P., Meseguer, J., Cuesta, A. and Esteban, M.A. 2014. Modulatory effects of deltamethrin-exposure on the immune status, metabolism and oxidative stress in gilthead seabream (Sparus aurata L.). Fish Shellfish Immun. 36(1), 120–129. Han, D., Xie, S., Liu, M., Xiao, X., Liu, H., Zhu, X. and Yang, Y. 2011. The effects of dietary selenium on growth performances, oxidative stress and tissue selenium concentration of gibel carp (Carassius auratus gibelio). Aquacult. Nutr. 17, e741–e749. Han, B., Long, W., He, J., Liu, Y., Si, Y. and Tian, L. 2015. Effects of dietary Bacillus licheniformis on growth performance, immunological parameters, intestinal morphology and resistance of juvenile Nile tilapia (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol. 46, 225–231. Houston, A.H. 1990. Methods for fish biology. Blood and circulation. Chapter 9, pp: 237–334. Ibrahim, M.D., Shahed, I.B., Abo El-Yazeed, H. and Korani, H. 2011. Assessment of the susceptibility of poly culture reared African catfish and Nile Tilapia to Edwardsiella tarda. J. Am. Sci. 7(3), 779–786. Iqbal, S., Atique, U., Mughal, M.S., Khan, N., Haider, M.S., Iqbal, K.J. and Akmal, M. 2017. Effect of selenium incorporated in feed on the hematological profile of Tilapia (Oreochromis niloticus). J. Aquac. Res. Develop. 8, 513. Iqbal, S., Atique, U., Mahboob, S., Haider, M.S., Iqbal, H.S., Al-Ghanim, K.A., Al-Misned, F., Ahmed, Z. and Mughal M.S. 2020. Effect of supplemental selenium in fish feed boosts growth and gut enzyme activity in juvenile tilapia (Oreochromis niloticus). J. King Saud Univ. Sci. 32, 2610–2616. Jayasekara, C., Mendis, E. and Kim, S. 2020. Seafood in the human diet for better nutrition and health. In Encyclopedia of marine biotechnology, 1st ed. Eds., Kim, S.K. New York, NY: John Wiley & Sons Ltd. Kaur, S., Singh, D. and Singh, K. 2017. Effect of selenium application on arsenic uptake in rice (Oryza sativa L.). Environ. Monit. Assess. 189, 430. Kawahara, E., Ueda, T. and Nomura, S. 1991. In vitro phagocytic activity of white spotted shark cells after injection with Aeromonas salmonicida extracelluar products. Gyo. Ken. Japan, 26(4), 213–214. Khan, K.U., Zuberi, A., Nazir, S., Fernandes, J.B.K., Jamil, Z. and Sarwar, H. 2016. Effects of dietary selenium nanoparticles on physiological and biochemical aspects of juvenile Tor putitora. Turk. J. Zool. 40, 704–712. Kiron, V. 2012. Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol. 173(1), 111–133. Klein, J.A. and Ackerman, S.L. 2003. Oxidative stress, cell cycle, and neurodegeneration. J. Clin. Investig. 111, 785–793. Kouba, A., Velíšek, J., Stará, A., Masojídek, J. and Kozák, P. 2014. Supplementation with sodium selenite and selenium-enriched microalgae biomass show varying effects on blood enzymes activities, antioxidant response, and accumulation in common barbel (Barbus barbus). BioMed. Res. Int. 8, 408270. Kumar, N., Krishnani, K.K. and Singh, N.P. 2018. Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ. Sci. Pollut. Res. Int. 25(9), 8914–8927. Le, K.T. and Fotedar, R. 2014. Bioavailability of selenium from different dietary sources in yellowtail kingfish (Seriola lalandi). Aquaculture 420, 57–62. Le, K.T., Fotedar, R. and Partridge, G. 2013. Selenium and vitamin E interaction in the nutrition of yellowtail kingfish (Seriola lalandi): physiological and immune responses. Aquacult. Nutr. 20, 303–313. Lee, J.H., Kim, Y.C., Park, S.L. and Bai, S.C. 2009. Evaluation of the optimum dietary selenium (Se) level to improve immune responses in juvenile olive flounder (Paralichthys olivaceus). J. Korean Fish Soc. 42, 26–33. Li, J., Ni, X.D., Liu, Y.J. and Lu, C.P. 2011. Detection of three virulence genes alt, ahp and aerA in Aeromonas hydrophila and their relationship with actual virulence to zebrafish. J. Appl. Microb. 110(3), 823–830. Li, C., Wang, R., Su, B., Luo, Y., Terhune, J., Beck, B. and Peatman E. 2013. Evasion of mucosal defenses during Aeromonas hydrophila infection of channel catfish (Ictalurus punctatus) skin. Dev. Comp. Immunol. 39(4), 447–455. Lin, Y.H. and Shiau, S.Y. 2005. Dietary selenium requirements of juvenile grouper, Epinephelus malabaricus. Aquaculture 250(1–2), 356–363. Lin, Y.H. and Shiau, S.Y. 2007. The effects of dietary selenium on the oxidative stress of grouper Epinephelus malabaricus, fed high copper. Aquaculture 267(1–4), 38–43. Liu, J., Cao, S., Kim, S., Chung, E.Y., Homma, Y., Guan, X., Jimenez, V. and Ma, X. 2005. Interleukin-12: an update on its immunological activities, signaling and regulation of gene expression. Curr. Immunol. Rev. 1, 119–137. Lukaszewicz, E., Jerysz, A. and Kowalczyk, A. 2013. Effect of dietary selenium and vitamin E on slaughter yield and carcass composition of commercial White Koluda geese. Pak. Vet. J. 33, 462–465. Magouz, F.I., Salem, M.F., Moustafa, E.M. and Elkhamy, S.A. 2019. Impact of biomos and agrimos dietary supplementation on growth performance, feed utilization and immunological parameters of Nile tilapia (Oreochromis Niloticus) fingerlings. Slov. Vet. Res. 56(Suppl 22), 87–98. Mahan, D.C. 1999. Organic selenium: using nature’s model to redefine selenium supplementation for animals. Biotechnology in the feed industry. In Proceedings of the 15th Annual Symposium. Eds., Lyons, T.P. and Jacques, K.A. Nottingham, UK: Nottingham University Press, pp: 523–535. Manning, T. and Gibson, G. 2004. Prebiotics. Best Pract. Res. Clin. Gastroenterol. 18, 287–298. Mei, G.Y., Carey, C.M., Tosh, S. and Kostrzynska, M. 2011. Utilization of different types of dietary fibers by potential probiotics. Can. J. Microbiol. 57, 857–865. Mir, I.N., Sahu, N.P., Pal, A.K. and Makesh, M. 2017. Synergistic effect of L-methionine and fucoidan rich extract in eliciting growth and nonspecific immune response of Labeo rohita fingerlings against Aeromonas hydrophila. Aquaculture 479, 396–403. Monteiro, D.A., Rantin, F.T. and Kalinin, A.L. 2007. Uso do selênio na dieta de matrinxã, Brycon cephalus. Rev. Bras. Saúde Prod. Anim. 8(1), 32–47. Moustafa, E.M., Dawood, M.A.O., Assar, D.H., Omar, A.A., Elbialy, Z.I., Farrag, F.A., Shukry, M. and Zayed, M.M. 2020. Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquaculture 2020, 734589. Moustafa, E.M., Abd El-Kader, M.F., Hassan, M.M., Fath El-Bab, A.F., Omar, A., Farrag, F., Gewida, A.G., Abd-Elghany, M.F., Shukry, M. and Alwakeel, R.A. 2021. Trial for use nanoselenium particle with different dietary regime in Oreochromis niloticus and Mugil cephalus polyculture ponds: growth efficiency, haematological, antioxidant, immunity and transcriptional analysis. Vet. Med. Sci. 2021, 1–12. Naiel, M.A.E., Shehata, A.M., El-Kholy, A.I., El-Naggar, K., Farag, M.R. and Alagawany, M. 2022. The mitigating role of probiotics against the adverse effects of suboptimal temperature in farmed fish: a review. Aquaculture 15, 737877. Nishikimi, M., Roa, N.A. and Yogi, K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Comm. 46, 849–854. Nölle, N., Genschick, S., Schwadorf, K., Hrenn, H., Brandner, S. and Biesalski, H.K. 2020. Fish as a source of (micro) nutrients to combat hidden hunger in Zambia. Food Secur. 12, 1385–1406. NRC. 2011. Nutrient requirements of fish and shrimp. Animal nutrition series. Washington, DC: The National Academies Press, pp: 376. Ohkawa, H., Ohishi, N. and Yagi, K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. Osuigwe, D.I., Obiekezie, A.I. and Onuoha, G.C. 2005. Some hematological changes in hybrid catfish (Heterobranchus longifilis x Clarias gariepinus) fed different dietary levels of raw and boiled jack bean (Canavalia ensiformis) seed meal. Afr. J. Biotech. 4, 1017–1021. Pelaseyed, T., Bergström, J.H., Gustafsson, J.K., Ermund, A., Birchenough, G.M.H., Schütte, A., van der Post, S., Svensson, F., Rodríguez-Piñeiro, A.M., Nyström, E.E.L., Wising, C., Johansson, M.E.V. and Hansson, G.C. 2014. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 260(1), 8–20. Prieto, P., Pineda, M. and Aguilar, M. 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269, 337–341. Rathore, S.S., Murthy, H.S., Mamun, M.A.A., Nasren, S., Rakesh, K., Kumar, B.T.N. Abhiman, P.B. and Khandagale, A.S. 2020. Nano-selenium supplementation to ameliorate nutrition physiology, immune response, antioxidant system and disease resistance against Aeromonas hydrophila in monosex Nile tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 199(8), 3073–3088. Rayman, M.P. 2000. The importance of selenium to human health. Lancet 356, 233–241. Ribeiro, A.R., Altintzoglou, Th., Mendes, J., Nunes, M.L., Dinis, M.T. and Dias, J. 2019. Farmed fish as a functional food: perception of fish fortification and the influence of origin—insights from Portugal. Aquaculture 501, 22–31. Reda, R.M., El-Hady, M.A., Selim, K.M. and El-Sayed, H.M. 2018. Comparative study of three predominant gut Bacillus strains and a commercial B. amyloliquefaciens as probiotics on the performance of Clarias gariepinus. Fish Shellfish Immunol. 80, 416–425. Reed, L. and Muench, H. 1938. A simple method of estimating fifty percent end points. Am. J. Trop. Med. Hyg. 27, 493–497. Řehulka, J., Minařík, B. and Řehulková, E. 2004. Red blood cell indices of rainbow trout Oncorhynchus mykiss (Walbaum) in aquaculture. Aquac. Res. 35, 529–546. Reitman, S. and Frankel, S. 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Path. 28, 56–63. Rider, S.A.B. 2009. The roles of organic and inorganic zinc and selenium sources in the nutrition and promotion of health in rainbow trout (Oncorhynchus mykiss). Doctoral thesis. Rigos, G., Katharios, P., Kogiannou, D. and Cascarano, C.M. 2021. Infectious diseases and treatment solutions of farmed greater amberjack Seriola dumerili with particular emphasis in Mediterranean region. Rev. Aquac. 13, 301–323. Rotruck, J.T., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.G. and Hoekstra, W.G. 1973. Selenium biochemical role as a component of glutathione peroxidase. Science 179(4073), 588–590. Rudy, A.N. and Fotedar, R. 2013. Dietary organic selenium improves growth, survival and resistance to Vibrio mimicus in cultured marron, Cherax cainii (Austin, 2002). Fish Shellfish Immunol. 35, 79–85. Sakai, M. 1999. Current research status of fish immunostimulants. Aquacutlure 172, 63–92. Schaperclaus, W., Kulow, H. and Schreckenbach, K. 1992. Fish disease. Rotterdam, The Netherlands: A.A. Balkema. Shini, S., Sultan, A. and Bryden W.L. 2015. Selenium biochemistry and bioavailability: implications for animal agriculture. Agriculture 5, 1277–1288. Sinha, A.K., Zinta, G., AbdElgawad, H., Asard, H., Blust, R. and De Boeck, G. 2015. High environmental ammonia elicits differential oxidative stress and antioxidant responses in five different organs of a model estuarine teleost (Dicentrarchus labrax). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 174–175, 21–31. Smirnov, A., Perez, R., Amit-Romach, E., Sklan, D. and Uni, Z. 2005. Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. J. Nutr. 135(2), 187–192. Sritunyalucksana, K., Intaraprasong, A., Sa-nguanrut, P., Filer, K. and Fegan, D.F. 2011. Organic selenium supplementation promotes shrimp growth and disease resistance to Taura syndrome virus. Sci. Asia. 37, 24–30. Stoskopf, M.K. 1993. Fish medicine. London, UK: W.B. Sainders Company. Sunde, R.A. 2006. Selenium. In Present knowledge in nutrition, 9th ed. Eds., Bowman, B. and Russell, R. Washington, DC: International Life Sciences Institute, pp: 480–497. Taoka, Y., Maeda, H., Jo, J.Y., Kim, S.M., Park, S., Yoshikawa, T. and Sakata, T. 2006. Use of live and dead probiotic cells in tilapia, Oreochromis niloticus. Fish Sci. 72, 755–766. Tapiero, H., Townsend, D.M. and Tew, K.D. 2003. The antioxidant role of selenium and seleno-compounds. J. Pharm. Biomed. Anal. 57, 134–144. Thrall, M.A., Weiser, G., Allison, R.W. and Campbell, T.W. 2012. Veterinary hematology and clinical chemistry, 2nd ed. Turutoglu, H., Ercelik, S. and Corlu, M. 2012. Aeromonas hydrophila-associated skin lesions and septicaemia in a Nile crocodile (Crocodylus niloticus). J. S. Afr. Vet. Assoc. 76(1), 40–42. Urbinate, E.C. and Carneiro, P.C.F. 2006. Sodium chloride added to transport water and physiological responses of Matrinxã Brycon amazonicus (Teleost: Caracidae). Acta Amaz. 36, 569–572. Van-Doan, H., Hoseinifar, S.H., Naraballobh, W., Jaturasitha, S., Tongsiri, S., Chanagun, C. and Ringoe, E. 2019. Dietary inclusion of orange peels derived pectin and Lactobacillus plantarum for Nile tilapia (Oreochromis niloticus) cultured under indoor biofloc systems. Aquaculture 10, 1016. Waite, R, Beveridge, M., Brummett, R., Castine, S., Chaiyawannakarn, N., Kaushik, S., Mungkung, R., Nawapakpilai, S. and Phillips, M. 2014. Increasing productivity of aquaculture. In Working Paper, Installment 6 of Creating a Sustainable Food Future. Washington, DC: World Resources Institute. Wang, Y., Wu, Y., Wang, Y., Xu, H., Mei, X., Yu, D., Wang, Y. and Li, W. 2017. Antioxidant properties of probiotic bacteria. Nutrients 9, 521. Welker, T.L. and Lim, C. 2011. Use of probiotics in diets of tilapia. J. Aquac. Res. Dev. S1, 014. Winthrobe, M.M. 1934. Variations in the size and hemoglobin content of erythrocytes in the blood of various vertebrates. Folia Haematol. 51, 32–49. Xu, J., Zhang, J., Xie, H., Li, C., Bao, N., Zhang, C. and Shi, Q. 2010. Physiological responses of Phragmites australis to wastewater with different chemical oxygen demands. Ecol. Eng. 36, 1341–1347. Yalçinkaya, l., Güngör, T., Bafialan, M. and Erdem, E. 2008. Mannan oligosaccharides (MOS) from Saccharomyces cerevisiae in broilers: effects on performance and blood biochemistry. Turk. J. Vet. Anim. Sci. 32(1), 43–48. Yano, T. 1997. The nonspecific immune system humoral defense. Fish Physiol. 15, 105–157. Yukgehnaish, K., Kumar, P., Sivachandran, P., Marimuthu, K., Arshad, A., Paray, B.A. and Arockiaraj, J. 2020. Gut microbiota metagenomics in aquaculture: factors influencing gut microbiome and its physiological role in fish. Rev. Aquac. 12(3), 1903–1927. Zhang, Z.H., Chen, M., Xie, S.W., Chen, X.Q., Liu, Y.J., Tian, L.X. and Niu, J. 2020. Effects of dietary xylooligosaccharide on growth performance, enzyme activity and immunity of juvenile grass carp, Ctenopharyngodon idellus. Aquac. Rep. 18, 100519.v Zhou, L., Li, H., Qin, J.G., Wang, X., Chen, L., Xu, C. and Li, E. 2020. Dietary prebiotic inulin benefits on growth performance, antioxidant capacity, immune response and intestinal microbiota in Pacific white shrimp (Litopenaeus vannamei) at low salinity. Aquaculture 518, 734847. Zinkle, J.G. 1986. Avian hematology. In Veterinary hematology. Eds., Jain, N.C. Philadelphia, PA: Pai hea and Febiger, pp: 256–260. | ||
| How to Cite this Article |
| Pubmed Style Moustafa EM, Shukry M, Zayed MM, Farrag FA, El-Aziz WEA, Omar AA. Impact of Sel-Plex® dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 70 -89 . doi:10.5455/OVJ.2024.v14.i1.8 Web Style Moustafa EM, Shukry M, Zayed MM, Farrag FA, El-Aziz WEA, Omar AA. Impact of Sel-Plex® dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. https://www.openveterinaryjournal.com/?mno=175107 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.8 AMA (American Medical Association) Style Moustafa EM, Shukry M, Zayed MM, Farrag FA, El-Aziz WEA, Omar AA. Impact of Sel-Plex® dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 70 -89 . doi:10.5455/OVJ.2024.v14.i1.8 Vancouver/ICMJE Style Moustafa EM, Shukry M, Zayed MM, Farrag FA, El-Aziz WEA, Omar AA. Impact of Sel-Plex® dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 70 -89 . doi:10.5455/OVJ.2024.v14.i1.8 Harvard Style Moustafa, E. M., Shukry, . M., Zayed, . M. M., Farrag, . F. A., El-Aziz, . W. E. A. & Omar, . A. A. (2024) Impact of Sel-Plex® dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 70 -89 . doi:10.5455/OVJ.2024.v14.i1.8 Turabian Style Moustafa, Eman M., Mostafa Shukry, Mohamed M. Zayed, Foad A. Farrag, Wesam E. Abd El-Aziz, and Amira A. Omar. 2024. Impact of Sel-Plex® dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 70 -89 . doi:10.5455/OVJ.2024.v14.i1.8 Chicago Style Moustafa, Eman M., Mostafa Shukry, Mohamed M. Zayed, Foad A. Farrag, Wesam E. Abd El-Aziz, and Amira A. Omar. "Impact of Sel-Plex® dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila." Open Veterinary Journal 14 (2024), 70 -89 . doi:10.5455/OVJ.2024.v14.i1.8 MLA (The Modern Language Association) Style Moustafa, Eman M., Mostafa Shukry, Mohamed M. Zayed, Foad A. Farrag, Wesam E. Abd El-Aziz, and Amira A. Omar. "Impact of Sel-Plex® dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 70 -89 . Print. doi:10.5455/OVJ.2024.v14.i1.8 APA (American Psychological Association) Style Moustafa, E. M., Shukry, . M., Zayed, . M. M., Farrag, . F. A., El-Aziz, . W. E. A. & Omar, . A. A. (2024) Impact of Sel-Plex® dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 70 -89 . doi:10.5455/OVJ.2024.v14.i1.8 |