| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 144-153 Original Research Effects of the putative probiotics Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis on white leg shrimp, Litopenaeus vannamei, immune response, gut histology, water quality, and growth performanceAmira A. Omar1*, Mohamed S. Marzouk2, Nadia B. Mahfouz1, Ahmed M. Massoud1, Mustafa Shukry3, Foad Farrag4, Mohamed M. Zayed5, Mohamed A. Abd Alaziz1 and Eman M. Moustafa11Department of Fish Diseases and Management, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt 2Department of Aquatic Animal Medicine and Management, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 3Department of Physiology, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt 4Department of Basic Veterinary Sciences, Faculty of Veterinary Medicine, Delta University for Science and Technology, Dakahlia, Egypt 5Department of Aquaculture, Faculty of Aquatic and Fisheries Sciences, Kafrelsheikh University, Kafr El-Sheikh, Egypt *Corresponding Author: Amira A. Omar. Department of Fish Diseases and Management, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt. Email: amira_vet2007 [at] yahoo.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
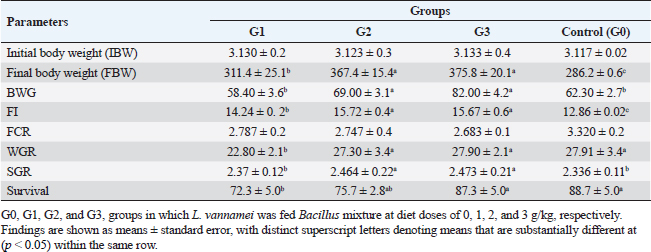
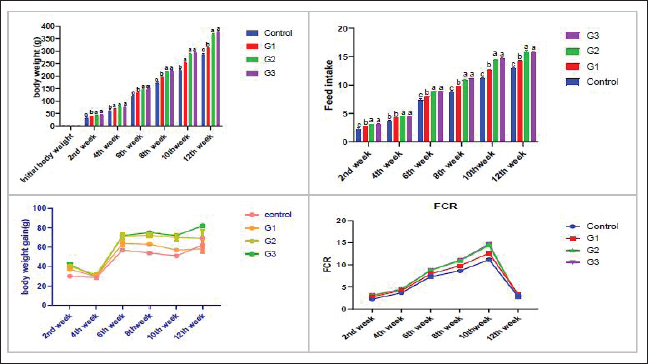
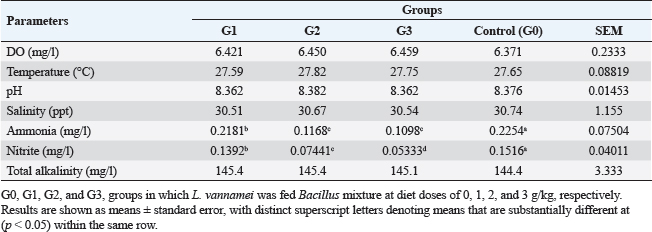
AbstractBackground: A commercially significant species in the aquaculture sector globally, particularly in Egypt, is Litopenaeus vannamei. Aim: The experiment's objective was to ascertain how Sanolife PRO-F impacted the growth, water quality, immunological response, and intestinal morphometry of L. vannamei. Methods: In the current investigation, which lasted 12 weeks, Sanolife PRO-F was administered to shrimp post-larvae at diet doses of 0 (control), 1 (group one), 2 (group two), and 3 (group three) g/kg diet, respectively. Each experimental group had three repetitions. Results: In the current study, shrimp fed on probiotic-treated diets showed a considerable improvement in growth performance measures and survival rate, and the nonspecific immune response was also enhanced. Shrimp fed probiotic diets had longer and more intestinal villi overall. Shrimp fed on the G2 and G3 diets showed no appreciable differences in growth or intestinal morphology. With the G2 and G3 diet, the water had lower concentrations of nitrite and ammonia. Conclusion: The study's findings indicate that Sanolife PRO-F treatment at 2–3 g/kg feed promotes the growth of shrimp, immunological response, gut health and function, and water quality. Keywords: Growth performance, Immune response, Intestinal morphometry, Probiotic diet, Water quality. IntroductionLarge-scale initiatives to produce prawn, mullet, sea bass, and sea bream were established in the Suez Canal region; project Lake Ghalion in Kafr-Elsheikh, and project of East Port Said in 2016 as a result of a national and governmental focus on expanding mariculture over the previous 3 years (USDA, 2016). Egypt produces over 70% of all marine fish produced in the region because the majority of North African countries rely more on fisheries harvests than aquaculture (Shaalan et al., 2018). Litopenaeus vannamei, a crucial economic species, produces 80% of shrimp consumed worldwide. In 2017, there were 445 million metric tons of white leg prawns produced globally, with a total estimated value of 26.7 billion US dollars (Won et al., 2020). White leg prawns have been actively cultured due to the strong market demand for them, which has caused major issues such as infectious disease outbreaks. The most prevalent bacterial pathogen in prawn aquaculture is specifically the Vibrio bacterium, which has a negative influence on immunity, production, and survival (FAO, 2019; El-Saadony et al., 2022). Dietary probiotics’ advantages on prawn aquaculture development, digestive enzyme activity, and disease resistance have been thoroughly documented by several authors. Probiotics are secure methods that are substituted for antibiotics in prawn aquaculture procedures. Regarding innate immune reactions, probiotics actually play a vital part in preventing and maintaining the balance in microbial defense mechanisms between those that are required and those that are excessive (Yan and Polk, 2011). In prawn cultures, numerous bacterial species have been demonstrated to promote growth, immunological responses, and disease resistance. Fish’s digestive tracts naturally include some of these bacteria, some of which have the capacity to create compounds with antibiotic action (Lee et al., 2017). They can thereby improve fish growth, feed efficiency, and immunological responses. Probiotics can be applied in the aquaculture sector to improve feed conversion efficiency and control pathogens. Probiotics are categorized as live microorganisms that, when consumed in appropriate amounts, aid the host. Probiotics function by producing substances that inhibit infections, competing with them for adhesion sites and resources, improving water quality, facilitating enzymatic digestion, and enhancing immune response (Zuo et al., 2019; El-Saadony et al., 2022). One of the most researched and utilized bacteria in aquaculture for probiotics or biocontrol is Bacillus spp. Oral treatment of specific strains of Bacillus spp. spores or vegetative cells reduce the mortality of prawns caused by bacterial infections; potential reasons for this effect include stimulation of the host immune system and/or antagonism between the bacteria. Bacillus species release antimicrobial peptides (AMPs) that impede the growth of other microbes, particularly bacteria and fungi. Examples of these AMPs include bacillomycin, fengycin, iturin, surfactin, bacilysin, and subtilin. Since there is little chance that bacteria will develop resistance to such AMPs, it is promising to use AMP-producing Bacillus in aquaculture settings (Proespraiwong et al., 2023). The world’s fastest-expanding industry for producing food is aquaculture. To provide the world’s expanding population’s growing need for protein, production is rising. Aquaculture intensification practices have an adverse effect on the environment, just like those in any other industry by causing the spread of infectious diseases and other pollution issues. According to Bondad-Reantaso et al. (2005), diseases and deteriorating environmental conditions frequently occur and cause significant economic losses. Due to this unproductive condition, chemicals are now being used more frequently to treat pathogenic bacterial diseases. Antibiotic usage led to the emergence of harmful bacteria that are resistant to them, a rise in the risk of bioaccumulation, and the weakening of the immune system in cultured organisms. Alternative methods, such as the use of probiotics, may therefore significantly improve fish farming procedures. Probiotics in water and feed are now crucial components of aquaculture. Probiotic bacteria have been shown to help their aquatic hosts by either enhancing host tolerance or immunity (Uma et al., 1999) or by preventing competition against pathogenic organisms (Austin et al., 1995; Gomez-Gil et al., 2000). These two outcomes both contribute to healthier and greater animal survival rates. In addition to creating essential development elements such as vitamins, amino acids, and gastrointestinal enzymes. During the growing period, probiotics have been demonstrated to promote feed absorption and gut microbial diversity, which in response boosts growth rate and reduces feed conversion ratio (FCR) (Parker, 1974; Fuller, 1989; Rengpipat et al., 1998; Wang et al., 2005). The primary factors affecting shrimp aquaculture are shrimp survival and growth. Higher shrimp production rates are supported by shrimp with good growth and a high survival rate. Due to the animal’s metabolic activity, the water quality of the pond changes as the culture period progresses. For higher survival and growth rates, all of these factors should be kept at their ideal values. Despite the fact that a number of pond probiotics have been introduced to the market, a continuing search is being made to isolate potent probiotic bacteria for use in pond applications. Probiotics are essential for the host immune system’s development and function. According to reports, the gut microbiota performs a variety of functions, including improving the mucosal system, digesting, angiogenesis, and disease defense (Begum et al., 2018; El-Saadony et al., 2022). Shrimp’s innate immune system is crucial to their defense and is actually unable to react to particular vaccines. Thus, vaccine restrictions might be made up for by using Bacillus subtilis as a potential probiotic in prawn farming (Zokaeifar et al., 2012). Maintaining appropriate environmental conditions and physical and chemical characteristics of the rearing water is a significant difficulty in prawn aquaculture. Low water exchange rates and poor water quality may contribute to persistent or reoccurring infections. When culture conditions are taken into account, probiotic bacteria can be useful for managing prawn health; however, little is known about how probiotics affect bioremediation. Previous research has revealed that numerous extracellular enzymes and AMPs are produced by B. subtilis strains (Ochoa-Solano and Olmos-Soto, 2006; Sutyak et al., 2008; Defeng et al., 2013). The purpose of the research was to evaluate the effects of a probiotic combination of different strains of Bacillus on L. vannamei growth performance, immunological response, intestinal morphometry, and water quality cultured in Ghalion Governmental Shrimp Farm, Kafr El-Sheikh Governorate, Egypt. Materials and MethodsConditions and experimental designSanolife PRO-F, INVE Aquaculture, Belgium; total amount of probiotic bacteria utilized for study: 1.0 × 1010 CFU/g; probiotic mixture of three strains (B. subtilis 3.25 × 109 CFU/g, Bacillus licheniformis 3.50 × 109 CFU/g, and Bacillus pumilus 3.25 × 109 CFU/g), was added daily to the basal diet (a commercial prawn pellet diet with 43.79% protein made by the GHALION® factory in Kafr El-Sheikh, Egypt), by using sunflower oil (10 ml/kg feed) (Elsabagh et al., 2018). In the current research, four experimental groups, G0: control, G1: 1 g/kg feed, G2: 2 g/kg feed, and G3: 3 g/kg feed, each with three replicates, were conducted in rectangular concrete ponds measuring 40 m2 each. Prawn larvae were given the experimental diets for a total of 12 weeks at feeding levels ranging from 7% of body weight for the initial 4 weeks, 6% of total weight for the second 4 weeks, and 5% of total weight for the last 4 weeks. Three times every day, at 8:00, 12:00, and 16:00, the larvae were fed. Experimental feeding trialThe experiment with feeding was conducted during the year 2021 at the Ghalion Governmental Shrimp Hatchery in the Egyptian province of Kafr El-Sheikh. For 1 week, 60,000 wholesome white-leg prawn post larvae (PL18) were acclimated in 12 concrete ponds. Twelve separate concrete ponds each measuring 40 m2 were randomly filled with 5,000 prawn larvae (average weight: 50 g) and kept in an aerated environment for a week while being fed artemia. According to the approach taken by Innes (1966), the ponds received a steady supply of filtered seawater, and all ponds received uniform aeration using air stones and plastic regulators for adjusting air pressure. Growth parametersFor a period of 12 weeks, prawn growth metrics and feed intake (FI) were tracked every 2 weeks. About 30 prawns were randomly selected from each pond every 2 weeks to be weighed. According to Robertson et al. (2000), Felix and Sudharsan (2004), and Venkat et al. (2004), the growth parameters were calculated. Shrimp were caught with a 50-mesh size net, put in a sealed polypropylene container, and transferred to the laboratory. Before weighing, prawn samples were dried to eliminate excess water using clean, sterile filter paper. Using a digital balance (DTF, USA, HZ, and HUAZHI), prawns were weighed. Variables included in the growth assessment were calculated according to Annet (1985) and De Silva and Anderson (1995). Water quality monitoringEach pond’s dissolved oxygen (DO) content was measured at a depth of 50 cm below the water’s surface using a DO meter (AQ 600 Milwaukee, Romania). Water quality indicators were checked on a weekly basis. Each pond’s three water samples were taken by submerging a 250 ml sterilized glass bottle 15 cm beneath the water’s surface. According to Eaton et al. (2005), to assess the pH, temperature, salinity, total alkalinity, and ammonia nitrogen and nitrite using a portable colorimeter (Martini MI 405), physio-chemical examination of water samples was carried out. Analysis of nonspecific immune responsesAccording to Won et al. (2020), at the end of the experiment, six shrimp from each group were sacrificed to provide serum samples for the investigation of nonspecific immune responses, such as lysozyme activity, superoxide dismutase, and myeloperoxidase. Intestinal morphometryFor histomorphometric analysis, a 5 mm transverse fragment from the gut at the third abdominal segment from three shrimps from each group (G0, G1, G2, and G3) was collected. The samples were instantly fixed for 24 hours in a 10% neutral buffer formalin solution. Following consecutive dehydration processes in alcohol (70%–100%), samples were embedded in paraffin blocks. Hematoxylin and eosin (H&E)-stained slices of 4 m thickness were examined under a light microscope, according to Bancroft and Gamble (2007). The length of the intestinal folds was measured using image analysis software (NIH, Bethesda, MD). Statistical analysisThe mean and standard error of all data are shown. p < 0.05 was considered statistically significant for all statistical analyses. One-way variance analysis and Turkey’s group differences detection were also conducted. All analyses were performed using SPSS “Version 22.” Ethical approvalThe guidelines for the study’s conduct were provided by the Kafrelsheikh University in Egypt’s Animal Ethics Committee, which approved the protocol and ethics of the study (KFS-IACUC/143/2023). ResultsGrowth efficiency and biometric measurementsGrowth performance metrics [final weight, FI, weight gain rate (WGR), Body weight gain (BWG), and specific growth rate (SGR)] showed an improvement when G2 and G3 diets were fed compared to G1 and G0 diets (Table 1 and Fig. 1). The G2 and G3 groups did not differ significantly from each other (p > 0.05); however, there were significant differences for each measurement (p < 0.05). All performance metrics showed the highest values in the G2 and G3 groups, then in the G1 and G0 treated groups. The G3 group had the lowest FCR value, followed by the G2, G1, and G0 groups ( p < 0.05), but there were no discernible variations between the various groups. Shrimp in the G2 and G3 groups fared better in terms of survival than shrimp in the G1 and control groups (p < 0.05). Shrimp fed the G2 and G3 diets showed no variation from each other (p > 0.05). Water qualityThe DO concentration was kept constant at 6 mg/l, the water’s salinity ranged from 30 to 31, and all treated groups experienced stable pH values of 8.3 and total alkalinities of 144–145 during the experiment. In comparison to the G1 and control (G0) groups, the ammonia concentration and nitrite levels in the G2 and G3 groups were significantly lower (p < 0.05). Table 2 lists the elements that influence water quality. Table 1. Litopenaeus vannamei growth performance and biometric measurements fed for 12 weeks experimental diets.
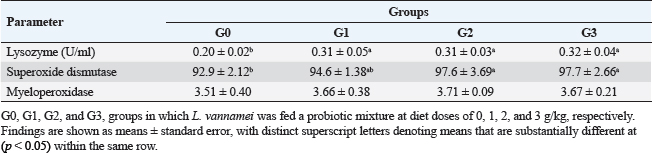
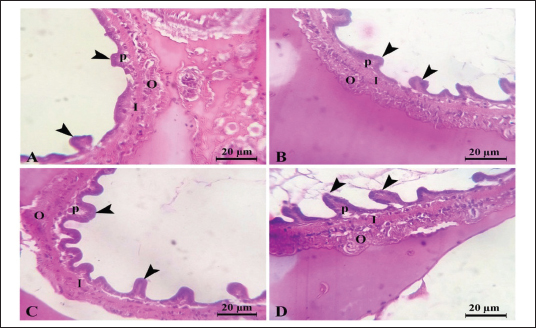
Fig. 1. Litopenaeus vannamei, FI, final body weight, BWG, and FCR fed a probiotic blend of Bacillus strains at diet doses of 0, 1, 2, and 3 g/kg; G0, G1, G2, and G3 respectively for 12 weeks. Nonspecific immune reactionsTable 3 displays the immunological responses overall. When compared to shrimp on the control diet, the lysozyme activity in shrimp-fed probiotic diets was significantly increased (p < 0.05). While myeloperoxidase activity did not differ substantially between the probiotic therapy groups, superoxide dismutase activity was considerably higher in the G2 and G3 groups than in the G1 and control groups (p < 0.05). Gut histologyTable 4 and Figures 2 and 3 provide a summary of the findings of the morphometric investigation. Three layers; epithelial, connective tissue, and muscular, make up the wall of the shrimp’s midgut. The epithelial layer forms numerous intestinal folds which are composed of simple columnar cells (enterocytes) resting on a noncellular basement membrane. Highly vascular loose connective tissue makes up the lamina propria. Figure 3 shows the two smooth muscle layers that make up the visceral muscle of the gut: the inner circular layer and the outer longitudinal layer. Table 2. Effect of Bacillus strains mixture probiotic on water quality in L. vannamei.
Table 3. Nonspecific immune responses of L. vannamei fed the experimental diets for 12 weeks.
Table 4. Morphometry of the intestinal folds in shrimp fed a probiotic blend of Bacillus strains.
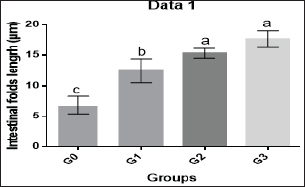
Histological evaluations of the midgut were done 12 weeks after probiotic administration. The length and number of intestinal folds were considerably enhanced in the G1, G2, and G3 groups compared to the G0 group ( p < 0.0001), demonstrating the evident impact of feed supplementation with the Bacillus strains mixed probiotic on this target organ. There was no statistically significant difference between the G2 and G3 groups (Table 4 and Figs. 2 and 3), despite the fact that intestinal fold length grew considerably in the G2 and G3 groups compared to the other groups.
Fig. 2. Histogram of shrimp mid gut of G0, G1, G2, and G3 groups showing intestinal folds’ length. DiscussionProbiotics have a lot of potential for ensuring healthy shrimp with better production (Gomez-Gil et al., 2000). Both the probiotic treatment and control groups kept track of L. vannamei’s growth performance as well as body weight, BWG, FI, FCR, WGR, and SGR every 2 weeks throughout the culture period.
Fig. 3. Photomicrograph of mid gut of shrimp of G0 (A), G 1 (B), G2 (C), and G3 (D) groups showing intestinal folds (arrow head), lamina propria (P), inner circular (I) and outer longitudinal (O) muscular layers. H&E, bar=20µm. The findings demonstrate that over the course of the culture, L. vannamei treated with probiotics outperformed the control group in terms of body weight, BWG, FI, FCR, WGR, and SGR. Similar findings have been reported by Suralikar and Sahu (2001) and Venkat et al. (2004) in Macrobrachium rosenbergii, Ziaei-Nejadz et al. (2006) in Fenneropenaeus indicus, Kai et al. (2014) in probiotics treated L. vannamei. According to Irianto and Austin (2002), it is likely that probiotics have increased feed consumption; completely digested the feed consumed, and stimulated appetite by producing vitamins and breaking down indigestible compounds in the diet. Furthermore, favorable water quality factors would have enhanced shrimp growth and growth-related indicators. Feeding diets supplemented with Bacillus combination considerably improved growth performance metrics, indicating the probiotic’s contribution to reducing stress factors and enhancing fish health; similar results reported by Mandiki et al. (2011) and Won et al. (2020) provide similar results in Eurasian perch Perca fluviatilis L. larvae. Many studies have demonstrated that adding Bacillus probiotics to a diet could increase feed efficiency, while also enhancing nutrient digestion and absorption. In addition, it might establish a colony in the fish gut, promoting the synthesis of fatty and amino acids, the stimulation of digestive enzymes, and the purging of toxic feed components (Balcazar et al., 2007; Avella et al., 2010; Merrifield et al., 2010; Mandiki et al., 2011; Liu et al., 2017; Kewcharoen and Srisapoome, 2019). By improving the host’s gut microbial balance, probiotics administered through feed help the host’s digestion (Gatesoupe, 1999; Moriarty, 1999; Won et al., 2020). When treated in combination, probiotic species should have a stronger impact on shrimp growth than when applied individually. Numerous studies have shown that Bacillus can boost disease resistance, remove harmful bacteria from the gut, and improve fish health (Hostins et al., 2017; Kewcharoen and Srisapoome, 2019). G3 and G2 diets are more effective growth promoters than either G1 or G0 diet, according to the results of this study’s analysis of shrimp body weight, BWG, FI, FCR, WGR, and SGR. Probiotics have been shown to work in aquaculture in a number of different ways, including the production of inhibitory compounds, an increase in host immune response, and an improvement in host nutrition through the production of extra digesting enzymes (Balcázar et al., 2016). According to studies on Penaeus monodon using Bacillus bacteria, growth, survival, as well as immunity were improved (Rengpipat et al., 2000). Supplementation with Bacillus species, B. megaterium, and B. licheniformis boosted L. vannamei’s growth, immunity, and digestive enzyme function as well, this study’s findings also showed that utilizing a probiotic combination sped up shrimp growth. Compared to the G1 and control groups, shrimp survival in the G2 and G3 groups was considerably higher ( p < 0.05). Between shrimp fed the G2 and G3 diets, there were no appreciable differences (p > 0.05). The effects of the probiotic supplement on growth, the immune system, and histomorphological value may be responsible for the shrimp’s higher survival (Kamarudin et al., 1994; Chiu et al., 2007; Zhou et al., 2009; Zhang et al., 2011). In the current investigation, the total alkalinity was 144–145, the pH was 8.3, the water salinity ranged from 30 to 31, and the DO concentration was kept at 6 mg/l. between the control and probiotic-supplemented groups, over various time courses were not statistically different. The ammonia concentration and nitrite levels in the G2 and G3 diet groups were significantly lower than those in the G1 and control groups. Probiotics have probably contributed to better water quality (Moriarty, 1999). These findings could be related to the higher proliferation of helpful bacteria in ponds where shrimp were fed diets enriched with Bacillus, which would then improve the water quality and, as a result, shrimp health and performance (Dalmin et al., 2001; Zhou et al., 2018). These results agreed with Hostins et al. (2017), in which shrimp intestines can be colonized by Bacillus instead of Vibrio. The Sanolife probiotic was added to feed rather than being directly injected into the pond water and our investigation provides no proof that it is present in large quantities. Sanolife probiotic is added to water or applied to feed. We need further comparative studies on the effects of Bacillus probiotics on the bacterial ecology and water quality of ponds. In the current investigation, probiotic-treated groups displayed higher lysozyme activity than the control group (p < 0.05). In addition, shrimp fed the G2 and G3 diets had considerably higher superoxide dismutase activity than shrimp fed the G1 and control diets (p < 0.05), these results agreed with Zoppi et al. (2001) and Salinas et al. (2005). The administration of probiotics that are mutually beneficial and fill different niches in the digestive tract may strengthen or extend the beneficial effects on the host immune system and general health. According to the above results, there was a midgut morphometric study found. Results showed that the use of the Bacillus strains mixture probiotic significantly enhanced the mucosal structure, which influences digestive function, nutrient absorption, and the immune system by forming protective barriers to prevent the colonization of opportunistic pathogens (Wongsasak et al., 2015; Amoah et al., 2019). According to these results, large dosages of the bacillus probiotic mixture lengthened intestinal villi and increased the number of intestinal folds, which considerably increased in the G2 and G3 groups in comparison to the other groups. The large absorptive surface is enhanced by the length and number of additional intestinal folds, allowing the host to absorb more nutrients (Liu et al., 2009). ConclusionIn my conclusion, the current study amply reveals that Bacillus strain supplementation had an interesting effect on L. vannamei growth performance, survival, immunological response, and water quality, all of which increased significantly. It suggests that the interplay of multiple probiotic bacteria may benefit the growth of the prawn. Supplementing with Bacillus probiotics also helped the shrimp’s habitat and lengthened and multiplied the intestinal villi. Therefore, it seems promising to use a probiotic Bacillus mixture in prawn production. This strategy may be a superior one for the expansion of sustainable aquaculture. AcknowledgmentsThe authors would like to acknowledge Ghalion governmental shrimp hatchery, Kafr El-Sheikh Governorate, Egypt, for allocating resources and facilities. Author contributionsConceptualization and experiment design: Amira A. Omar, Mohamed S. Marzouk, Nadia B. Mahfouz, Eman M. Moustafa, Ahmed M. Massoud. Data curation: Amira A. Omar, Ahmed M. Massoud, Mohamed A. Abd Alaziz, Mohamed M. Zayed. Data collection and statistical analysis: Amira A. Omar, Mustafa Shukry. Histological examination: Foad Farrag. Writing the first draft: Amira A. Omar, Eman M. Moustafa. All authors revised and approved the manuscript’s final draught. Data availabilityData is available on request from the authors. ReferencesAmoah, K., Huang, Q.C., Tan, B.P., Zhang, S., Chi, S.Y., Yang, Q.H., Liu, H.Y. and Dong, X.H. 2019. Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 87, 796–808. Annet, C.S. 1985. A model to facilitated optimal aquaculture production by quantitatively relating fish growth to feed and other environmental resources. Doctoral Thesis, Michigan State University, East Lansing, MI. Austin, B., Stuckey, L.E., Robertson, P.A.W., Effendi, I. and Griffith, D.R.W. 1995. A probiotic strain of Vibrio alginolyticus effective in reducing disease caused by Aeromonas salmonicida, Vibrio anguillarum and Vibrio ordalli. J. Fish Dis. 18, 93–96. Avella, M.A., Gioacchini, G., Decamp, O., Makridis, P., Bracciatelli, C. and Carnevali, O. 2010. Application of multi-species of Bacillus in sea bream larviculture. Aquaculture 305, 12–19. Balcazar, J.L., Vendrell, D., De Blas, I., Ruiz-Zarzuela, I., Girona’s, O. and Mu´zquiz, J.L. 2007. In vitro competitive adhesion and production of antagonistic compounds by lactic acid bacteria against fish pathogens. Vet. Microbiol. 122(3–4), 373–380. Balcázar, J.L., Blas, I.D., Ruiz-Zarzuela, I., Cunningham, D., Vendrell, D. and Muzquiz, J.L. 2016. The role of probiotics in aquaculture. Vet. Microbiol. 114, 173–186. Bancroft, J.D. and Gamble, M. 2007. Theory and practice of histological techniques, 5th ed. London, UK: Churchill Livingstone, pp: 125–138. Begum, P.S., Madhavi, G., Rajagopal, S., Viswanath, B., Razak M.A. and Venkataratnamma, V. 2018. Effect of probiotic bacteria on shrimp pond ecosystem and their influence on growth and survival of Litopenaeus vannamei. Int. J. Sci. Res. Sci. Eng. Technol. 4(4), 1158–1165. Bondad-Reantaso, M., Subasinghe, R.P, Arthur, J.R., Ogawa, K., Chinabut, S. and Adlard, R. 2005. Disease and health management in Asian aquaculture. Vet. Parasitol. 132, 249–272. Chiu, C.H., Guu, Y.K., Liu, C.H., Pan, T.M. and Cheng, W. 2007. Immune responses and gene expression in white shrimp, Litopenaeus vannamei, induced by Lactobacillus plantarum. Fish Shellfish Immunol. 23, 364–377. Dalmin, G., Kathiresan, K. and Purushothaman, A. 2001. Effect of probiotics on bacterial population and health status of shrimp in culture pond ecosystem. Indian J. Exp. Biol. 39, 939–942. De Silva, S.S. and Anderson, T.A. 1995. Fish nutrition in aquaculture. London, UK: Champman and Hall. Defeng, X., Yaling, W., Lijun, S., Huanming, L. and Jianrong, L. 2013. Inhibitory activity of a novel antibacterial peptide AMPNT-6 from Bacillus subtilis against Vibrio parahaemolyticus in shrimp. Food Control 30, 58–61. Eaton, A.D., Clesceri, L.S., Rice, E.W., Greenberg, A.E. and Franson, M.A.H. 2005. Standard methods for examination of water and wastewater, 21st ed. Washington, DC: American Water Works Association, Water Pollution Control Federation. Elsabagh, M., Mohamed, R., Moustafa, E.M., Hamza, A., Farrag, F., Decamp, O., Dawood, M.A.O. and Eltholth, M. 2018. Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia, Oreochromis niloticus. Aquac. Nutr. 2018, 1–10. FAO. 2019. Fisheries and aquaculture software. FishStatJ-software for fishery statistical time series. Rome, Italy: FAO Fisheries and Aquaculture Department [Online]. Available via http://www.fao.org/fishery/statistics/software/fishstatj/en (Accessed on 20 February 2023). Felix, N. and Sudharsan, M. 2004. Effect of glycine betaine, a feed attractant affecting growth and feed conversion of juvenile freshwater prawn Macrobrachium rosenbergii. J. Aquac. Nutr. 10, 193–199. Fuller, R. 1989. Probiotics in man and animals. J. Appl. Bacteriol. 66(5), 365–378. Gatesoupe, F.J. 1999. The use of probiotics in aquaculture. Aquaculture 180, 147–165. Gomez-Gil, B., Roque, A. and Turnbull, J.F. 2000. The use and selection of probiotic bacteria for use in the culture of larval aquatic organisms. Aquaculture 191, 259–270. Hostins, B., Laraa, G., Decampc, O., Cesarb, D.E. and Wasielesky, W.J.R. 2017. Efficacy and variations in bacterial density in the gut of Litopenaeus vannamei reared in a BFT system and in clear water supplemented with a commercial probiotic mixture. Aquaculture 480, 58–64. Innes, W.T. 1966. Exotic aquarium fishes, 9th ed. Delran, NJ: Aquarium Incorporated. Irianto, A. and Austin, B. 2002. Use of probiotics to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 25, 110. Kai, H., Jia-Yan, L., Fei, L., Xiao-Lin, L., Lin, L., Lei, X. and Gao-Xue, W. 2014. Effects of dietary administration of Shewanella haliotis D4, Bacillus cereus D7 and Aeromonas bivalviium D15, single or combined, on the growth, innate immunity and disease resistance of shrimp, Litopenaeus vanammei. Aquaculture 428–429, 141–149. Kamarudin, M.S. Jones, D.A., Le Vay, L. and Abidin, A.Z. 1994. Ontogenetic change in digestive enzyme activity during larval development of Macrobrachium rosenbergii. Aquaculture 123, 323–333. Kewcharoen, W. and Srisapoome, P. 2019. Probiotic effects of Bacillus spp. from Pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish Shellfish Immunol. 94, 174–189. Lee, S., Katya, K., Park, Y., Won, S., Seong, M. and Bai, S.C. 2017. Comparative evaluation of dietary probiotics Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol. 61, 201–210. Liu, C.H., Chiu, C.S., Ho, P.L. and Wang, S.W. 2009. Improvement in the growth performance of white shrimp, Litopenaeus vannamei, by a protease-producing probiotic, Bacillus subtilis E20, from natto, J. Appl. Microbiol. 107, 1031–1041. Liu, H., Wang, S., Cai, Y., Guo, X., Cao, Z., Zhang, Y. and Zhou, Y. 2017. Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 60, 326–333. Mandiki, S., Milla, S., Wang, N., Blanchard, G., Djonkack, T., Tanascaux, S. and Kestemont, P. 2011. Effects of probiotic bacteria on growth parameters and immune defence in Eurasian perch Perca fluviatilis L. larvae under intensive culture conditions. Aquaculture Res. 42, 693–703. Merrifield, D.L., Bradley, G., Baker, R.T.M. and Davies, S.J. 2010. Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) II. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria post antibiotic treatment. Aquac. Nutr. 16(5), 496–503. Moriarty, D.J.W. 1999. Disease control in shrimp aquaculture with probiotic bacteria. In Proceedings of the 8th International Symposium on Microbial Ecology. Halifax, Canada: Atlantic Canada Society for Microbial Ecology, pp: 237–243. Ochoa-Solano, L.J. and Olmos-Soto, J. 2006. The functional property of Bacillus for shrimp feeds. Food Microbiol. 23, 519–525. Parker, R.B. 1974. Probiotics, the other half of the antibiotics story. J. Anim. Nutr. Health 29, 4–8. Proespraiwong, P., Mavichak, R., Imaizumi, K., Hirono, I. and Unajak, S. 2023. Evaluation of Bacillus spp. as potent probiotics with reduction in AHPND-related mortality and facilitating growth performance of Pacific white shrimp (Litopenaeus vannamei) farms. Microorganisms 11, 2176. Rengpipat, S., Rukpratanporn, S., Piyatiratitivorakul, S. and Menasaveta, P. 2000. Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquacult. 191, 271-288. Rengpipat, S.W., Phianphak, S., Piyatiratitivorakul, P. and Menasveta 1998. Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth. Aquaculture 167, 301–313. Robertson, P.A.W., O’Dowd, C., Burrells, C., Williams, P. and Austin, B. 2000. Use of Carnobacterium sp. as a probiotic for Alantic salmon, Salmo salar L. and rainbow trout Oncorhynchus myksis, Walbaum. J. Aquac. 185, 235–243. Salinas, I., Cuesta, A., Esteban, M.Á. and Meseguer, J. 2005. Dietary administration of Lactobacillus delbrüeckii and Bacillus subtilis, single or combined, on gilthead seabream cellular innate immune responses. Fish Shellfish Immunol. 19, 67–77. Shaalan, M., El-Mahdy, M., Saleh, M. and El-Matbouli M. 2018. Aquaculture in Egypt: insights on the current trends and future perspectives for sustainable development. Rev. Fish. Sci. Aquac. 26(1), 99–110. Suralikar, V. and Sahu, N.P. 2001. Effect of feeding probiotic (Lactobacillus cremoris) on growth and survival of Macrobrachium rosenbergii postlarvae. J. Appl. Anim. Res. 20, 117–124. Sutyak, K.E., Wirawan, R.E., Aroutcheva, A.A. and Chikindas, M.L. 2008. Isolation of the Bacillus subtilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliquefaciens. J. Appl. Microbiol. 104, 1067–1074. Uma, A., Abraham, T.J. and Sundararaj, V. 1999. Effect of a probiotic bacterium, Lactobacillus plantarum on disease resistance of Penaeus indicus larvae. Indian J. Fish 46(4), 367–373. USDA. 2016. Egypt: the state and development of aquaculture in Egypt. Available via http://www.fas.usda.gov/data/egypt-state-and-development-aquaculture-egypt (Accessed 24 May 2022). Venkat, H.K., Sahu, N.P. and Kamal, K.J. 2004. Effect of feeding Lactobacillus-based probiotic on the gut microflora, growth and survival of post larva of Macrobrachium rosenbergii (de Man). J. Aquac. Res. 35, 501–507. Wang, Y.B., Xu, Z.R. and Mei-Sheng, X. 2005. The effectiveness of commercial probiotics in northern white shrimp Penaeus vannamei ponds. Fish Sci. 71, 1036–1041. Wongsasak, U., Chaijamrus, S. and Kumkhong, S. 2015. Boonanuntanasarn, effects of dietary supplementation with β-glucan and synbiotics on immune gene expression and immune parameters under ammonia stress in Pacific white shrimp. Aquaculture 436, 179–187. Won S., Hamidoghli, A., Choi, W., Bae, J., Jang, W.J., Lee, S. and Bai, S.C. 2020. Evaluation of potential probiotics Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis on growth performance, Litopenaeus vannamei. Microorganisms 8, 281. Yan, F. and Polk, D.B. 2011. Probiotics and immune health. Curr. Opin. Gastroenterol. 27, 496. Zhang, Q., Tan, B.; Mai, K., Zhang, W., Ma, H., Ai, Q. and Liufu, Z. 2011. Dietary administration of Bacillus (B. licheniformis and B. subtilis) and isomaltooligosaccharide influences the intestinal microflora, immunological parameters and resistance against Vibrio alginolyticus in shrimp, Penaeus japonicus (Decapoda: Penaeidae). Aquac. Res. 42, 943–952. Zhou, X.X., Wang, Y.B. and Li, W.F. 2009. Effect of probiotic on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture 287, 349–353. Zhou, S., Xia, Y., Zhu, C. and Chu, W. 2018. Isolation of marine Bacillus sp. with antagonistic and organic-substances-degrading activities and its potential application as a fish probiotic. Mar. Drugs 16, 196. Ziaei-Nejadz, S., Rezaei, M.H., Takami, G.A., Lovett, D.L., Ali-Reza Mirvaghefi, A.R. and Shakouri, M. 2006. The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 252, 516–524. Zokaeifar, H., Balcázar, J.L., Saad, C.R., Kamarudin, M.S., Sijam, K., Arshad, A. and Nejat, N., 2012. Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 33, 683–689. Zoppi, G., Cinquetti, M., Benini, A., Bonamini, E. and Minelli, E.B. 2001. Modulation of the intestinal ecosystem by probiotics and lactulose in children during treatment with ceftriaxone. Curr. Ther. Res. Clin. Exp. 62, 418–435. Zuo, Z., Shang, B., Shao, Y., Li, W. and Sun, J. 2019. Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei. Fish Shellfish Immunol. 86, 160-168. | ||
| How to Cite this Article |
| Pubmed Style Omar AA, Marzouk MS, Mahfouz NB, Massoud AM, Shukry MA, Farrag FA, Zayed MM, Abd-Alaziz MA, Moustafa EM. Effects of the putative probiotics Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis on white leg shrimp, Litopenaeus vannamei, immune response, gut histology, water quality, and growth performance. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 144 -153 . doi:10.5455/OVJ.2024.v14.i1.13 Web Style Omar AA, Marzouk MS, Mahfouz NB, Massoud AM, Shukry MA, Farrag FA, Zayed MM, Abd-Alaziz MA, Moustafa EM. Effects of the putative probiotics Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis on white leg shrimp, Litopenaeus vannamei, immune response, gut histology, water quality, and growth performance. https://www.openveterinaryjournal.com/?mno=175262 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.13 AMA (American Medical Association) Style Omar AA, Marzouk MS, Mahfouz NB, Massoud AM, Shukry MA, Farrag FA, Zayed MM, Abd-Alaziz MA, Moustafa EM. Effects of the putative probiotics Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis on white leg shrimp, Litopenaeus vannamei, immune response, gut histology, water quality, and growth performance. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 144 -153 . doi:10.5455/OVJ.2024.v14.i1.13 Vancouver/ICMJE Style Omar AA, Marzouk MS, Mahfouz NB, Massoud AM, Shukry MA, Farrag FA, Zayed MM, Abd-Alaziz MA, Moustafa EM. Effects of the putative probiotics Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis on white leg shrimp, Litopenaeus vannamei, immune response, gut histology, water quality, and growth performance. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 144 -153 . doi:10.5455/OVJ.2024.v14.i1.13 Harvard Style Omar, A. A., Marzouk, . M. S., Mahfouz, . N. B., Massoud, . A. M., Shukry, . M. A., Farrag, . F. A., Zayed, . M. M., Abd-Alaziz, . M. A. & Moustafa, . E. M. (2024) Effects of the putative probiotics Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis on white leg shrimp, Litopenaeus vannamei, immune response, gut histology, water quality, and growth performance. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 144 -153 . doi:10.5455/OVJ.2024.v14.i1.13 Turabian Style Omar, Amira A., Mohamed S. Marzouk, Nadia B. Mahfouz, Ahmed M. Massoud, Mustafa A. Shukry, Foad A. Farrag, Mohamed M. Zayed, Mohamed A. Abd-Alaziz, and Eman M. Moustafa. 2024. Effects of the putative probiotics Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis on white leg shrimp, Litopenaeus vannamei, immune response, gut histology, water quality, and growth performance. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 144 -153 . doi:10.5455/OVJ.2024.v14.i1.13 Chicago Style Omar, Amira A., Mohamed S. Marzouk, Nadia B. Mahfouz, Ahmed M. Massoud, Mustafa A. Shukry, Foad A. Farrag, Mohamed M. Zayed, Mohamed A. Abd-Alaziz, and Eman M. Moustafa. "Effects of the putative probiotics Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis on white leg shrimp, Litopenaeus vannamei, immune response, gut histology, water quality, and growth performance." Open Veterinary Journal 14 (2024), 144 -153 . doi:10.5455/OVJ.2024.v14.i1.13 MLA (The Modern Language Association) Style Omar, Amira A., Mohamed S. Marzouk, Nadia B. Mahfouz, Ahmed M. Massoud, Mustafa A. Shukry, Foad A. Farrag, Mohamed M. Zayed, Mohamed A. Abd-Alaziz, and Eman M. Moustafa. "Effects of the putative probiotics Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis on white leg shrimp, Litopenaeus vannamei, immune response, gut histology, water quality, and growth performance." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 144 -153 . Print. doi:10.5455/OVJ.2024.v14.i1.13 APA (American Psychological Association) Style Omar, A. A., Marzouk, . M. S., Mahfouz, . N. B., Massoud, . A. M., Shukry, . M. A., Farrag, . F. A., Zayed, . M. M., Abd-Alaziz, . M. A. & Moustafa, . E. M. (2024) Effects of the putative probiotics Bacillus licheniformis, Bacillus pumilus, and Bacillus subtilis on white leg shrimp, Litopenaeus vannamei, immune response, gut histology, water quality, and growth performance. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 144 -153 . doi:10.5455/OVJ.2024.v14.i1.13 |