| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 116-135 Original Research Ameliorative effect of BIO-MOS® as a dietary supplementation on growth performance, physiological response, oxidative status, and immunity-linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophilaEman M. Moustafa1*, Foad A. Farrag2, Mustafa Shukry3, Haguer M. Salah El-Din1 and Amira A. Omar11Fish Diseases and Management Department, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt 2Department of Basic Veterinary Sciences, Faculty of Veterinary Medicine, Delta University for Science and Technology, Dakahlia, Egypt 3Animal Physiology Department, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt *Corresponding Author: Eman Moustafa Moustafa. Fish Diseases and Management Department, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt. Email: emantarek2002 [at] yahoo.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
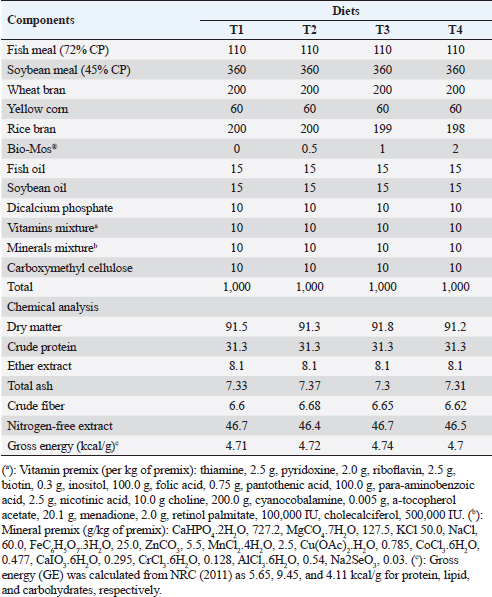
ABSTRACTBackground: Mannanoligosaccharides (MOS) usage in fish production has drawn more attention because of their positive benefits on disease resistance and fish performance. Aim: The ongoing research was executed to assess the potential advantages of Bio-Mos® dietary supplementation regarding the growth outcomes, physiological response, oxidative biomarkers, and immunity-linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings exposed to bacterial infection with Aeromonas hydrophila. Methods: Four experimental diets were developed using a 30% protein baseline diet, with Bio-Mos® added at variable levels; 0.0, 0.5, 1, and 2 g/kg, respectively. 240 healthy Nile tilapia fingerlings were split into 4 groups at random and assigned to 12 glass aquariums (three replicates of 20 fish/treatment). Diets were admitted at a 3% rate of fish biomass/aquarium for 8 weeks. Following the feeding trial, fish from every treatment were intraperitoneally injected with pathogenic A. hydrophila, and then observed for 15 days to record the survival rate percent (SR%) post challenge. Results: Results revealed significant improvement in growth performance, physiological response, immunological parameters (phagocytic index, phagocytic activity, and lysozyme), and antioxidant parameters [catalase, malondialdehyde, glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD)] among Bio-Mos® treated groups. Moreover, Bio-Mos® increased the expression of tumor necrosis factor alpha and Interleukin 1β, genes linked to the liver immune system. Growth-related genes (GHr), antioxidant-related genes (SOD and GSH-Px). In fish subjected to pathogens, dietary MOS supplementation could significantly lower oxidative stress, showing promise as a preventative supplement for Nile tilapia in place of antibiotics. On the other hand, Bio-Mos® considerably improved each of the three intestinal morphological measures (villus width, villus length, and crypt depth), showing the best overall intestinal structure-improving impact. The challenge with A. hydrophila caused marked degenerative alterations in the intestine, hepatopancreas, spleen, and posterior kidney of Nile tilapia, in the control group. However, lesion severity was greatly decreased and showed marked amelioration with an increased concentration of Bio-Mos®. The A. hydrophila-challenged groups revealed a 100% SR% mainly among the Bio-Mos® supplemented groups. Conclusion: It is recommended to enrich the Nile tilapia fingerlings diets with 2 g.kg−1 of MOS for better results on the growth rate, physiological response, immunological response, and intestinal absorptive capacity. Keywords: Aeromonas hydrophila, Immunity linked gene expression, Mannanoligosaccharide, Nile tilapia, Oxidative status. IntroductionIn respect to Egypt, aquaculture serves as the fundamental origin of fish yield. The majority of fish produced in Egypt is produced through aquaculture (Adeleke et al., 2021). It accounts for 77% of the entire production of fish, with pond-based aquaculture in the Nile Delta lakes providing 85% of this supply (Mehrim and Refaey, 2023). According to Al-Wakeel et al. (2019), Egypt produces roughly 73.8% of fish raised in Africa and ranks tenth globally in terms of overall cultured fish production with about a million tons produced annually (FAO, 2022). As a result of the expanded element of escalation and cultivated regions (Nguyen, 2008), tilapia is the dominant fish population in these ponds and records around 65% of complete aquaculture production (Dickson et al., 2016). Oreochromis niloticus, often known as Nile tilapia, continues to be a very important extensively cultivated fish species globally (Prabu et al., 2019) because of their ease of reproduction, resilience to a variety of conditions and illness, quick development, and substantial consumer demand (Elumalai et al., 2019). Merrifield et al. (2010) investigated that nutrition plays a critical part in fish growth, development, and maintenance of fish welfare. Prior recently, the primary protein source in tilapia diets was fish meal. Numerous experiments were compelled to enhance the flourishing exhibition and lower the costs related to the production of cultivated tilapia due to the rising cost and unstable supply of this element. This was accomplished by using probiotics or prebiotics as secure supplements that do not affect consumers or leave residues in farmed fish (Welker and Lim, 2011). Prebiotics are dietary carbohydrates that cannot be digested and bypass the upper gastrointestinal tract. Prebiotics favorably affect the host by increasing the growth and/or initiating the metabolic process of favorable bacteria residing in the alimentary tract (Manning and Gibson, 2004). Another way they modify the composition of gut bacteria is by altering the substrate type that is available to the existing gut microbiome (FAO, 2007). Bio-Mos®, a feed element generated from yeast cell walls (Saccharomyces cerevisiae), is mannanoligosaccharides (MOS) where the primary constituents of yeast cell walls are mannans, glucans as well as chitin (Hady et al., 2012). It improves digestion and gut health in animals by lessening local colonization of pathogenic microorganisms in the alimentary tract. As a prebiotic, MOS promotes healthy bacteria development in the gut, prevents harmful bacteria from conforming to and colonizing in the gut tract, and lessens the negative effects of commensal microflora metabolites which may be attributed to its binding affinity to the intestinal microorganisms through the gastrointestinal tract where the microorganisms are eliminated with excreta and the undigested MOS (Gainza and Romero, 2020). The aquaculture sector still deals with a number of disease-related issues these days, most of which are brought on by bacteria, parasites, and viruses (Rigos et al., 2021). The maintenance of fish immune competence and disease resistance while contending with rising feed prices and treatment constraints is one of the industry’s most significant concerns (Yassien et al., 2022). Because MOS improve fish health and disease resistance, their application in fish farming has drawn great attention in the prior 10 years (Wang et al., 2022). The goal of the ongoing research was to assess the potential advantages of Bio-Mos® nutritional supplementation on the state of oxidative stress, physiological response, development performance, and immunity-linked gene expression in Nile tilapia (O. niloticus) fingerlings rescued by bacterial infection with Aeromonas hydrophila. Materials and MethodsThe ongoing investigation was executed at the fish diseases laboratory, Fac. Vet. Med., Kafrelsheikh Univ., Egypt, for 12 weeks during 2021. Preparation of the experimental dietA baseline (control) diet comprising fish meal, yellow maize, soybean meal, wheat bran, minerals mix, vitamins, and fish oil was prepared using commercial items (Table 1). The diet was designed to be both isonitrogenous and isocaloric, with 300 g/kg of crude protein and 12.6 MJ/kg of digestible energy. Using a feed processor, the dry materials were ground into small particles. In the case of the second, third, and fourth diets, Bio-Mos® was incorporated in the baseline diet at 0.5, 1, and 2 g/kg, respectively, utilizing rice bran as a filler (Bio-Mos® (Alltech Inc., Nicholasville, KY)). After thoroughly combining all components with a mixture of oil, water, dicalcium phosphate, minerals, and vitamins, the dough was extruded through a 2 to 3 mm die using a pelleting apparatus. The pellets were placed in a refrigerator set to 4℃ after air drying. To verify the nutritional profile of the test diets, a conventional procedure was employed (AOAC, 2012). The commercial prebiotic product, Bio-Mos® (Alltech Inc., Nicholasville, KY), is retrieved from S. cerevisiae outer cell wall and contains MOS. Saccharomyces cerevisiae as an entire yeast cell (Probiotic), its extract (MOS-prebiotic), and its pre-probiotic mixture (Synbiotic) act as immune modulators and engines of growth in farmed O. niloticus (Dawson and Pirvulescu, 1999). Experimental designHealthy 240 Nile tilapia (O. niloticus) fingerlings (beginning body weight, 19.00 ± 3,00 g), got from a local private fish farm in Kafr El-Sheikh governorate, Egypt and transferred to Fish Diseases Laboratory, Faculty of Veterinary Medicine, Kafrelsheikh University. All gathered fish were housed in a fiberglass tank. Fish were acclimated to their basic food before the trial [having a level of 30% dietary protein (CP)] for 2 weeks. Following the period of acclimatization, the fingerlings were split into 4 groups of 60, with each group being divided into 3 sets of 20 fingerlings a pond. The fingerlings were held in glass aquariums of 60 × 30 × 40 cm, that can hold 20 fish each, 70 liters of water, and a functional aeration system. First, Groups 1 (the control group) were given a commercial basal meal, while Groups 2 through 4 were given diets enriched with 0.5, 1, and 2 g/kg) of Bio-Mos®. Feeding rates for the experimental diets were 3% of the total stocking biomass in each aquarium for a total of 8 weeks. Over the trial course, fish were weighed every fortnight, and the alteration in live body mass was used to adjust the feed levels. Fish wastes and excreta were removed by siphoning, and each aquarium’s water was gradually refilled with fresh, dechlorinated water to make up about half of its total volume. Table 1. Composition and chemical analysis of experimental diets (on a dry matter basis).
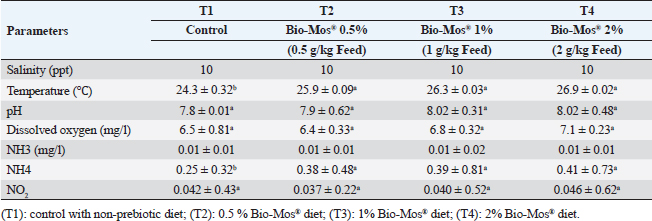
Two times a week, water quality attributes were checked utilizing a water analysis instrument (Lamotte device, USA). Temperature varied between (24℃–27℃), dissolved oxygen 6.5 ± 0.5 mg l−1, pH 7.1 ± 0.8, EC 219 ± 2 μ mho/cm, ammonia amended to the standard allowable limits (˂0.1 mg total ammonia) and during the experimental period, the day and night photoperiods were 12:12 hours. Water quality assessments were conducted in accordance with APHA (1989) (Table 2). Determination of fish growth parametersAfter 8 weeks, an electronic balance was used to weigh each fish (20 fish per replicate). All growth parameters; average daily gain (ADG), specific growth rate (SGR), total weight gain, protein efficiency ratio, feed conversion ratio (FCR), and survival rate percent (SR%) were computed utilizing techniques stated by Magouz et al. (2019). Hematological and biochemical parametersAll fish were starved 24 hours before the final sampling. For hematological examination, a dose of 100 mg/l of tricaine methane sulfonate was used to anesthetize fish to avoid stress during sampling (Dawood et al., 2020). Nine fishes were randomly sampled and weighed from every group (3 fishes/every replicate). Blood was collected, utilizing syringes with a 5 ml gauge, from the caudal blood vessels. Blood samples were split in half. Half of the blood was used right away for differential leukocyte count and hematological analysis after being stored in tubes that had been EDTA-heparinized. According to Urbinate and Carneiro (2006), blood samples taken from three to four fish were combined due to the tiny size of the fish. White blood cells (WBCs × 103/mm), red blood cells (RBCs × 106/mm), hemoglobin concentration (Hb g/dl), packed cell volume (PCV%), mean corpuscular hemoglobin concentration (MCHC), and mean corpuscular volume (MCV) were determined as stated by Magouz et al. (2019). Table 2. Water quality parameters measured in the current study.
To obtain serum, the leftover blood was stored in non-heparinized tubes. Taken samples underwent centrifugation (SCILOGEX, Model: DM0412, USA) at 1,008 × g/15 minutes at 4℃ after 2 hours. The serum was then separated and stored at −20℃ for additional analysis. A thin blood film was created, let to air dry, fixed for 3–5 minutes with methanol, and then stained for 8–10 minutes with Giemsa stain. It was then scrubbed utilizing distilled water and let to dry. Differential WBC counts were done in accordance with Thrall et al. (2012). Albumins, total serum proteins, serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), urea, uric acid, and creatinine analyses were estimated as previously stated (Moustafa et al., 2019). Determination of immune parametersa- Phagocytic index (PI) and phagocytic activity (PA): The PI was estimated following Moustafa et al. (2019). However, PA was estimated with reference to Kawahara et al. (1991). b- Serum lysozyme activity: Serum Lysozyme activity was investigated in light of the Gram-positive lysozyme delicate lyophilized bacterium, Micrococcus lysodeikticus, (Sigma, St. Louis, MO) using the method outlined by Demers and Bayne (1997). c- Superoxide dismutase (SOD) was colorimetrically determined following Nishikimi et al. (1972). d- Catalase (CAT) activity was carried out by spectrophotometric verification of hydrogen peroxide (H2O2), as previously prescribed (Aebi, 1984). e- Glutathione peroxidase (GSH-Px) was ascertained utilizing the technique performed by Prieto et al. (1999). f- Lipid peroxide (Malondialdehyde) (MDA): was colorimetrically detected following Satoh (1978) and Ohkawa et al. (1979). Challenge with A. hydrophilaThe purpose of the experimental infection was to ascertain how Bio-Mos® feed supplementation affected the fingerlings of O. niloticus’ ability to fend off Aeromonas hydrophila bacterial infection. According to Li et al. (2011), an intraperitoneal injection containing 0.2 ml of fresh culture suspension of A. hydrophila (3 × 107 CFU) was used for the experimental infection. Daily mortalities have been documented for 15 days after infection. The concentration of A. hydrophila strain per 1 ml and the dosage for the experimental investigations were estimated using the drop plate method (Cruickshank, 1975). For plate counts on TSA, a 24-hour colony culture of the A. hydrophila strain was employed. After being lifted up and suspended in sterile saline through a 10-fold serial dilution, the colonies were incubated for 24 hours at 28℃. The dilutions (102 to 107 CFU) were only used. Every group received an intraperitoneal injection of 0.5 ml per fish for every bacterial dilution. For 2 weeks following injection, every fish was housed for observation. Ibrahim et al. (2011) stated that the fatalities were documented twice a day. The recently deceased fish were relocated for a more thorough investigation. Per Reed and Muench (1938), the LD50 (the dosage that kills 50% of the inoculated fish) was determined. Table 3. Primers sequences of genes analyzed in real-time PCR.
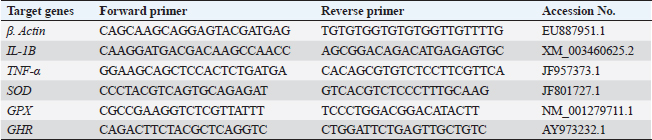
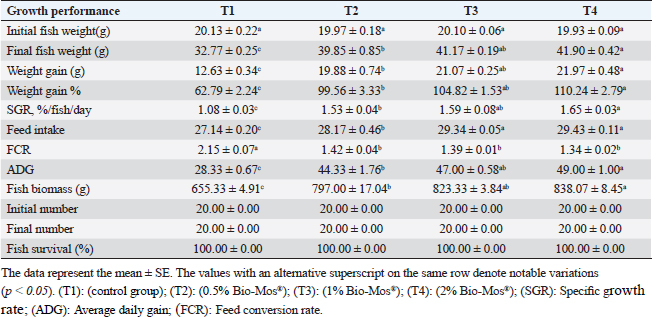
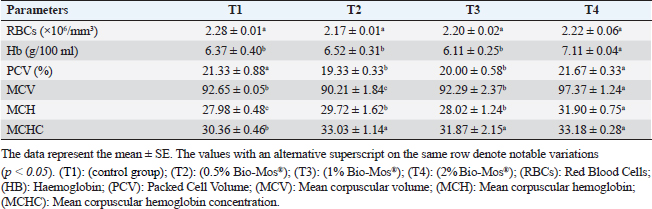
Gene expression analysisFollowing the guidelines provided by the manufacturer, the process of extracting total RNA was carried out utilizing the TRIzol reagent (Life Technologies, Gaithersburg, MD). Using the MultiScribe RT enzyme kit (Applied Biosystems, Foster City, CA), the cDNA was generated right away. Three separate real-time PCR analyses were performed on the resulting cDNA. Power SYBR Green PCR Master Mix (Applied Biosystems, Life Technologies, CA) was used to conduct real-time PCR studies using a 7,500 real-time PCR System (Applied Biosystems, Foster City, CA). Comparing the various genes’ relative fold changes in mRNA expression to the control was done. β-actin, a standard house-keeping gene, expression was utilized to standardize the fold change in mRNA expression of the assessed genes. Table 3 provides the accession codes and primer sequences for genes. Histopathological examinationsFollowing deep anesthesia with 40% ethyl alcohol, tissue samples were collected from the anterior section of the intestine, the hepatopancreas, the kidney, and the spleen from five randomly chosen fish from each treatment (0%, 0.05%, 0.10%, and 0.20% of Bio-Mos®) both before and after the fish were infected with A. hydrophila. Ethyl alcohol was provided in ascending levels of concentrations (70% to 100% alcohol) for dehydration after being fixed for 24 to 48 hours in a 10% neutral buffer formaldehyde solution. After being cleaned in xylene, the dehydrated samples were embedded in paraffin wax. Following Bancroft and Gamble (2007), sections of 4–5 µm thickness were stained with hematoxilin and eosin (HandE), and inspected under a light microscope for histopathological and morphometric examination. Morphometry of intestinal villiWith the aid of image analysis software (NIH, Bethesda, MD), the intestinal villi’s width, length, and crypt depth, along with their surface area, were assessed. A total of ten villi and villus-associated crypts were randomly selected from each of the five intestinal cross-sections. A one-way analysis of variance (ANOVA) was performed on the gathered data using SPSS version 22, SPSS Inc., Chicago, Il. At p 0.05, the accepted level of significance was established. All data are displayed in the form of means ± standard error (SE). Statistical analysisThe effects of dietary MOS, challenge, and their interactions were tested using two-way ANOVA. Once the interaction between the two factors was identified, one-way ANOVA was used to examine each of the four groups for every level of the two factors combined. Tukey-Kramer was used to compare each group’s data to that of the control group, as well as to examine variations within the same group and between sampling periods for every parameter. One-way ANOVA tests (SPSS version 22, SPSS Inc., Chicago, Il) were used to evaluate all statistical differences, and the Duncan post-hoc test was used where there were differences across experimental groups. At p < 0.05, the significance level was deemed acceptable. The presentation of all data is as means ± SE. Ethical approvalThe ongoing research complied with all relevant laws and guidelines of the Animal Ethics Committee of Kafrelsheikh University, Egypt (KFS-IACUC/137/2023) with regard to techniques, animal care, and experimental protocol. ResultsIn the present research, Nile tilapia fingerlings physiological responses to Bio-Mos® were investigated through assurance of fish growth, and hematological, biochemical, and immune parameters. Growth performanceIn the ongoing research, the impact of the utilized prebiotic on O. niloticus growth responses to Bio-Mos® was evaluated. It was found that all treated groups showed increased ADG, SGR, fish final weight, weight gain %, and feed intake with the T4 group showing the highest values in comparison to the control group (T1) (p < 0.05), as shown in Table 4. In addition, the impact of Bio-Mos® on food conversion rate (FCR) was summarized. It was found that, in comparison to the control group (T1), the T3 and T4 groups had the best food conversion rate values (p < 0.05) (Table 4). SR% was 100% in all groups. Hematological parametersRBCs showed no appreciable difference in hematological features across the groups. PCV was noticeably lowered in groups (T2 and T3) as opposed to the control group (p < 0.05), with the exception of the T4 group, where there were no obvious differences. The T4 group experienced a large rise in Hb, MCV, and MHC as opposed to the control group, while in MCHC all treatment groups exhibited a noticeable increase (p < 0.05) (Table 5). Table 4. The effect of Bio-Mos® on growth performance of O. niloticus.
Table 5. Effect of Bio-Mos® on hematological parameters of O. niloticus.
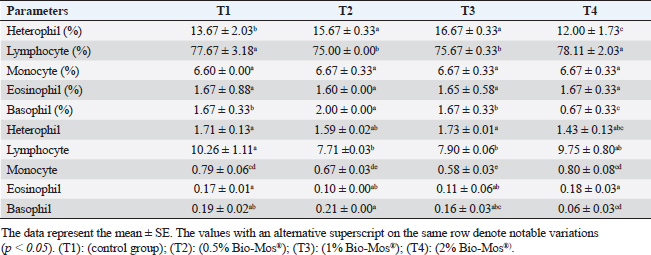
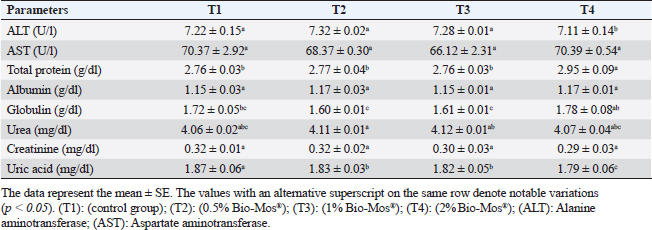
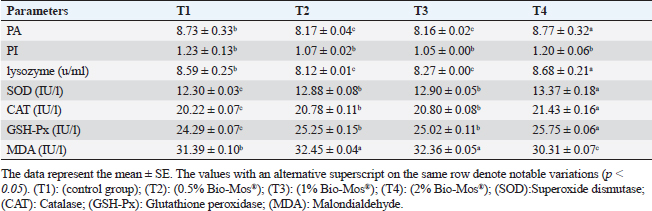
Concerning differential leukocytic count, the findings showed no noticeable change between the T2 and T3 groups and a considerable elevation (p < 0.05) in the heterophil%. In comparison to the T2 and T3 groups, T4 exhibited a substantial (p < 0.05) decline. Lymphocyte% was significantly decreased (p < 0.05) in T2 and T3 groups as opposed to the T1 and there were no noticeable differences between T2 and T3 groups, and no noticeable variation between T4 and T1 was obvious. The results for monocyte and eosinophil% did not reveal any appreciable variations among the treated groups. While there were no significant changes between T3 and T1, basophil% was noticeably greater (p < 0.05) in the T2 group as opposed to T1 while it was noticeably lower (p < 0.05) in the T4 group (Table 6). Biochemical analysisAccording to data presented in Table 7, globulin levels and total protein in the T4 group exhibited a noticeable increase as opposed to the other treatment groups and the control group (p < 0.05), whereas albumin levels in all groups showed no appreciable changes. In contrast to other treated groups, the T4 group showed a noticeable decrease in ALT level (p < 0.05), while there were no changes in AST levels across all groups. When opposed to other treatment groups, the outcomes for urea and uric acid revealed a considerable drop (p < 0.05), especially in the T4 group, and the levels of creatinine in all groups remained unchanged. Immunological and antioxidant parametersRegarding immunological measures, there was a slight increase in the T4 group as opposed to the control group, although PA was dramatically lessened in the T2 and T3 groups as opposed to the control group (p < 0.05). The PI failed to detect any difference between the groups. As shown in Table 8, lysozyme activity noticeably decreased in the T2 and T3 groups in contrast to the T4 group as opposed to the control group (p < 0.05). The antioxidant activity revealed that CAT, GSH-Px, and SOD activities were raised in all treatment groups, with the T4 group having the greatest value when opposed to the control group (p < 0.05). MDA activity increased in the T2 and T3 groups, but it dramatically lessened in the T4 group (p < 0.05). Table 6. Effect of Bio-Mos® on the differential leukocytic count of O. niloticus.
Table 7. Effect of Bio-Mos® on biochemical analysis of O. niloticus.
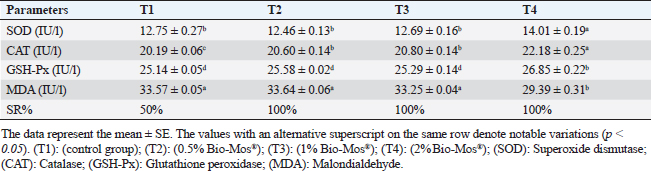
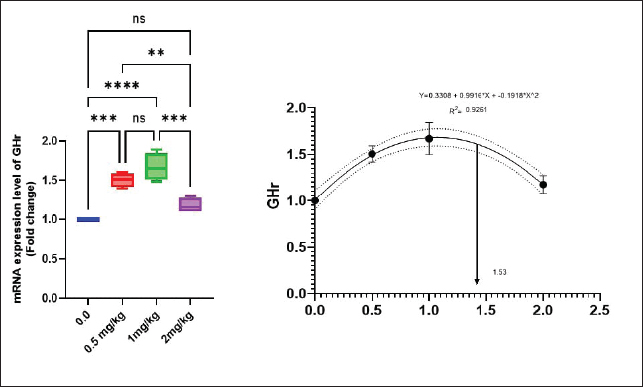
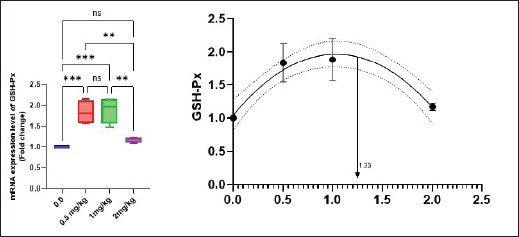
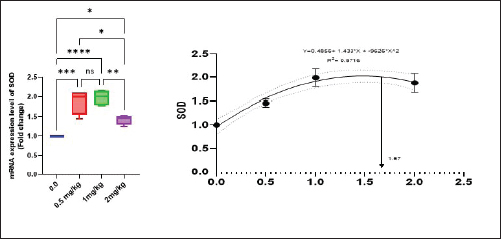
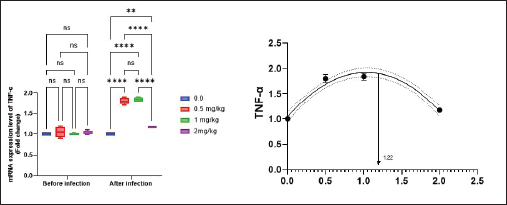
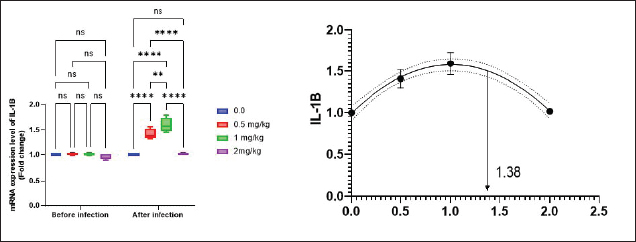
Antioxidant activity after experimental challenge with A. hydrophilaSOD activity was noticeably higher in the T4 group as opposed to the control group, while CAT and GSH-Px activities were up in all treatment groups, with the T4 group having the highest value (p < 0.05). MDA did not differ noticeably between the T2 and T3 groups and the control group; however, the T4 group experienced a sharp decline (p < 0.05). The A. hydrophila challenged groups indicated a 100% SR% mainly among the Bio-Mos® supplemented groups (Table 9). Relative gene expressionLiver showed upregulation of mRNA expression of growth hormone receptor (GHR), GSH-Px and SOD in T2 and T3 groups supplemented with 0.5 and 1 mg/kg Bio-Mos® in relation to other treated groups (p < 0.05), while there is no noticeable differences among T2 and T3 groups supplemented with 0.5 and 1 mg/kg Bio-Mos®, (Figs. 1–3). Liver showed upregulation of mRNA expression of tumor necrosis factor alpha (TNF-α) in T2 and T3 groups supplemented with 0.5 and 1 mg/kg Bio-Mos® in relation to other treated groups (p < 0.05), while there is no noticeable variations between T2 and T3 groups supplemented with 0.5 and 1 mg/kg Bio-Mos®, post challenge with A. hydrophila, while there is no noticeable differences between the all treated groups fed with Bio-Mos® and the control group, before the challenge with A. hydrophila (Fig. 4). Interleukin 1β (IL-1β) was noticeably upregulated in fish of T2 and T3 groups supplemented with 0.5 and 1 mg/kg Bio-Mos®, post challenge with A. hydrophila in relation to other treated groups, with the highest level of (IL-1β) in T3 group supplemented with 1 mg/kg Bio-Mos® (p < 0.05), while there are no noticeable changes among the all treated groups supplemented with Bio-Mos®and the control group, before the challenge with A. hydrophila (Fig. 5). Table 8. Effect of Bio-Mos® on immunity and oxidative status of O. niloticus.
Table 9. Effect of Bio-Mos® on oxidative status of O. niloticus after challenge with A. hydrophila.
Fig. 1. Gene expression of the liver (GHR) of Nile tilapia in different groups fed diets with different doses of Bio-Mos® for 8 weeks. * indicate significant differences between groups (p < 0.05), ns=indicate no significant differences between groups.
Fig. 2. Gene expression of the liver (SOD) of Nile tilapia in different groups fed diets with different doses of Bio-Mos® for 8 weeks. * indicate significant differences between groups (p < 0.05), ns=indicate no significant differences between groups.
Fig. 3. Gene expression of liver GSH-Px of Nile tilapia in different groups fed diets with different doses of Bio-Mos® for 8 weeks. * indicate significant differences between groups (p < 0.05), ns= indicate no significant differences between groups.
Fig. 4. Gene expression of liver (TNF-α) of Nile tilapia pre and post challenge with A. hydrophila in different groups fed diets with different doses of Bio-Mos® for 8 weeks. * indicate significant differences between groups (p < 0.05), ns=indicate no significant differences between groups.
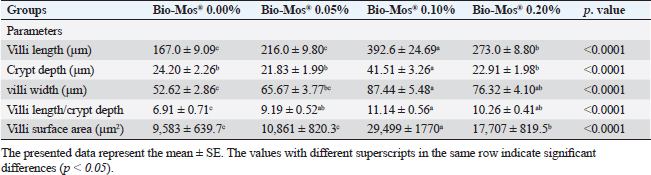
Fig. 5. Gene expression of liver (IL-1β) of Nile tilapia pre and post challenge with A. hydrophila in different groups fed diets with different doses of Bio-Mos® for 8 weeks. * indicate significant differences between groups (p < 0.05), ns=indicate no significant differences between groups. Table 10. Morphometric analysis of the intestine of O. niloticus fed on diets containing different levels Bio-Mos®.
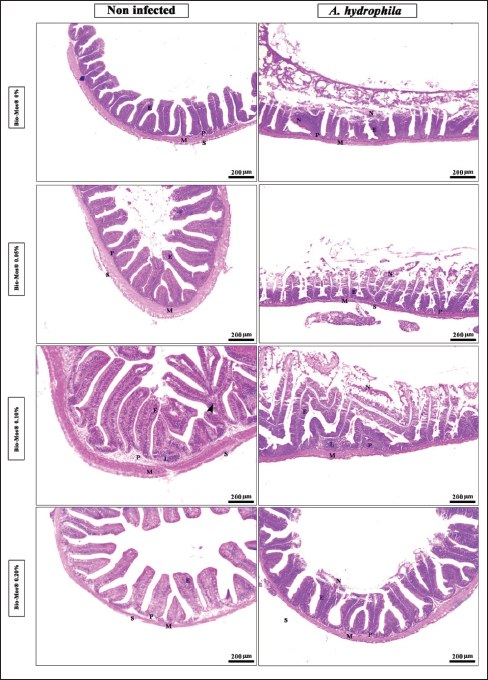
Histopathological findingsIntestinal morphometric analysis of pre-challenged groups revealed a noticeable increase in villi length, width, crypt depth, villi length/crypt depth, and villi surface area in a dose-dependent pattern especially 0.10% Bio-Mos® group than the other treated groups as illustrated in Table 10. Moreover, the 0.10% and 0.20% Bio-Mos® groups showed increased branching of intestinal villi as opposed to the other groups. The histopathological examination of the intestine of fish challenged with A. hydrophila shows marked degenerative changes and necrosis in the intestinal villi and sloughing of the apical epithelium into the intestinal lumen especially 0.0% Bio-Mos® group while the intestine of the other groups shows a marked decrease in the degenerative changes in the intestinal villi especially in 0.10% and 0.20% Bio-Mos® groups compared with 0.05% Bio-Mos® group (Fig. 6). The hepatopancreas of pre-challenged groups shows the normal architecture of the liver with polyhedral-shaped hepatocytes separated by hepatic sinusoids and radiating from a central vein and intact pancreatic acini. On the other hand, the hepatopancreas of the 0.0% Bio-Mos® challenged group shows severe vacuolar degeneration and a large focal area of necrosis in hepatic and pancreatic tissue in addition to severe congestion in hepatic and pancreatic blood vessels. The severity of the lesion shows marked amelioration in both hepatic and pancreatic tissue with increased concentration of Bio-Mos® (Fig. 7). The histopathological examinations of the posterior kidney of pre-challenged groups show the normal architecture of the kidney with intact glomeruli and renal tubules with interstitial hematopoietic tissue. The kidney of the 0.0% Bio-Mos® challenged group shows severe degenerative changes and necrosis of renal glomeruli and tubular epithelium, interstitial edema, and congestion of renal blood vessels. The aforementioned lesions gradually decreased with an increased concentration of Bio-Mos® (Fig. 8).
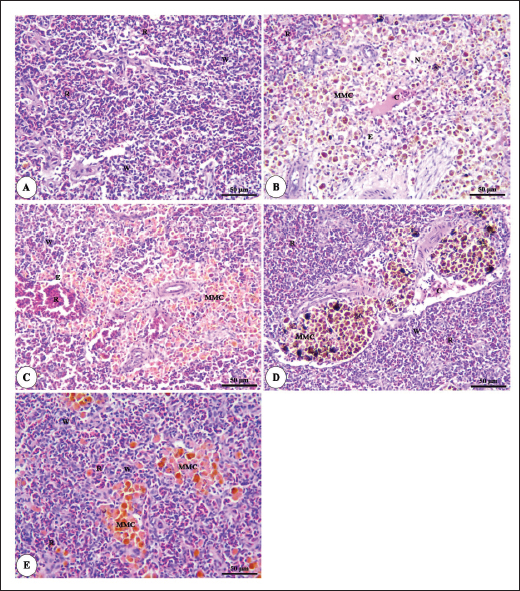
Fig. 6. Photomicrograph of HandE stained panel of anterior part of intestine of O. niloticus fed on ration contained 0%, 0.05%, 0.10%, and 0.20% of Bio-Mos® pre and post-challenge with A. hydrophila showing intestinal villi lined with simple columnar cells of lamina epithelialis (E), lamina propria (P), muscular layer (M), tunica serosa (S), branched alveoli (arrow head), areas of necrosis and sloughing of intestinal villi (N), in addition to lymphocytic aggregation in the lamina propria (L). The histopathological examinations of the spleen of pre-challenged groups show the normal architecture of the spleen with mixed red and white pulp, ellipsoids, and melanomacrophage centers. The spleen of the 0.0% Bio-Mos® challenged group shows severe degenerative changes and necrosis, depletion of white pulp, increase in melanomacrophage centers interstitial edema, and congestion of splenic blood vessels. The previously mentioned degenerative changes were greatly decreased with increased concentration of Bio-Mos® (Fig. 9).
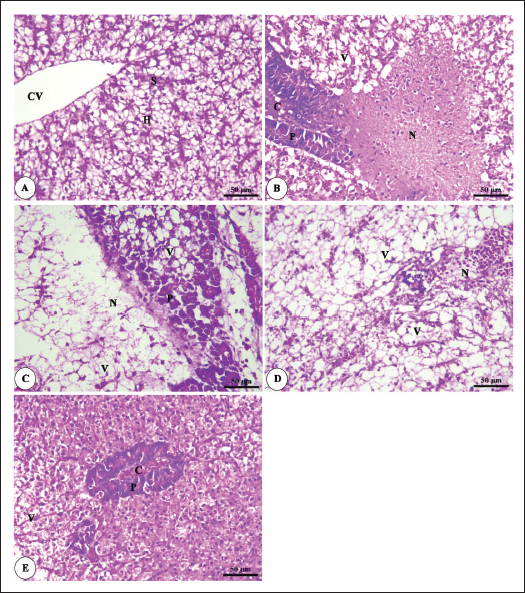
Fig. 7. Photomicrograph of HandE stained panel of hepatopancreas of O. niloticus pre-challenge (A) and post-challenge with A. hydrophila fed on ration contained 0% (B), 0.05% (C), 0.10% (D) and 0.20% (E) of Bio-Mos® showing central vein (CV), hepatocytes (H), blood sinusoids (S), pancreatic acini (P), focal areas of necrosis (N), vacuolar degeneration (V), and congestion of blood vessels (C). DiscussionA functional dietary supplement is a key managemental tool for improving the resistance of farmed fish against infectious diseases, as well as improving the overall growth parameters (Dawood et al., 2017). Antibiotic application in aquaculture has raised public concerns due to the increased danger of antibiotic-resistant bacteria emerging in these ecosystems, which is detrimental to aquaculture as well as consumers, terrestrial animals, and the environment (Ghiasi et al., 2021). Inclusion of prebiotics in response to the worldwide need for safe food as natural substitute growth accelerators to be preferential to antibiotic therapy, which improved fish resistance and reduced mortalities associated with pathogens infection (Ahmad et al., 2015). Growth performanceIn the present research, all evaluated growth parameters were raised in prebiotic-treated groups having the greatest values in the T4 group (fed on Bio-Mos® 2g/kg); these findings may be due to the fish’s improved feed utilization. The obtained growth parameters findings are in accordance with those previously investigated (Samuel et al., 2017). However, they conflict with those published by Peterson et al. (2010) who revealed that the feed conversion ratio and growth outcomes of a number of fish species were not improved by the addition of mannan-oligosaccharide.
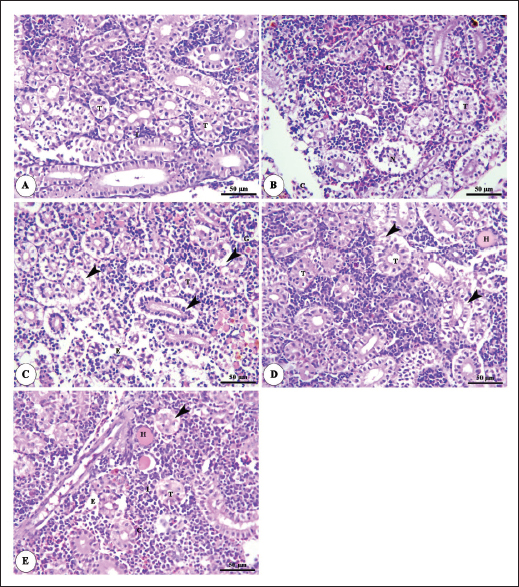
Fig. 8. Photomicrograph of HandE stained panel of kidney of O. niloticus pre-challenge (A) and postchallenge with A. hydrophila fed on ration contained 0% (B), 0.05% (C), 0.10% (D) and 0.20% (E) of Bio-Mos® showing renal tubules (T), interstitial areas of hematopoietic tissue (I), congestion of blood vessels (C), degeneration and separation of renal tubular epithelium from its basement membrane (arrow heads), necrosis of some renal tubules (N) and edema in the interstitial tissue (E) beside the accumulation of hyaline droplets in the lumen of some renal tubules (H). Improvement in feed conversion rate may be attributed to the effect of the currently used prebiotic which decreased the quantity of feed needed to produce one unit of fish and as a result, lowered production cost. The findings concur with those of certain authors (Zhang et al., 2012). Moreover, Bio-Mos® modulated gut microbiota, improving villus integrity and resistance to harmful bacteria, leading to improved intestine growth and an increase in the region where nutrients are absorbed; the outcomes are in accordance with previous records (Gómez and Balcázar 2008). Hematological parametersWhen fish consume functional feed additives, blood biochemical measures are typically employed to detect potential changes in the fishes’ overall health status (Dawood et al., 2019). Concerning the hematological parameters, no appreciable difference in RBCs across the groups was recorded in this current research. The result is in agreement with Sado et al. (2008). While, the T4 group experienced a large rise in Hb, MCV, and MHC. Moreover, a considerable elevation in the heterophil% in Bio-Mos® treated groups; that may be due to the enhanced defense response brought about by Bio-Mos® feed supplementation. The obtained result is similar to Khanjani et al. (2022) and in contrast to Ali et al. (2017). The kind and dosage of probiotics, fish’s physiological state, species, size, age, ecological environments, and feeding schedule may all have an impact on the variance in the results of hematological parameters (Osuigwe et al., 2005).
Fig. 9. Photomicrograph of HandE stained panel of spleen of O. niloticus pre-challenge (A) and postchallenge with A. hydrophila fed on ration contained 0% (B), 0.05% (C), 0.10% (D), and 0.20% (E) of Bio-Mos® showing mixed red (R) and white (W) pulp, melanomacrophage centers (MMC), congestion of splenic blood vessels (C), area of necrosis(N), and edema (E). Biochemical analysisThe noticeable heightened globulin and total protein levels in the T4 prebiotic-treated group might be due to the increased inherited body defense and better innate response of fish in response to prebiotic supplementation (Sahoo and Mukherjee, 2001). These parameters have been employed as a comprehensive clinical measure of fish health, immunological competence, stress, and nutritional status. The outcomes resemble those reported previously Sahoo and Mukherjee (2001); however, opposite to those reported by Sado et al. (2008). Alterations in hematological and serum biochemical variables might be species-related and rely on the amount at which MOS are incorporated into the diet, the components in the diet, and/or the rearing period (Ta’ati et al., 2011). The noticeable decrease in ALT levels in the T4 prebiotic-treated group is a similar result to that recorded by Yalçinkaya et al. (2008). This may be attributed to that both ALT and AST levels should be increasing in the plasma when liver cells sustain an injury or have their membranes broken, allowing these enzymes to seep out (Rehulka, 1996). However, there was a decrease in uric acid, urea, and AST levels in fish-fed Bio-Mos; the outcomes are in agreement with El Mesallamy et al. (2016). Immunological and antioxidant parametersThe current study investigated that O. niloticus fingerlings’ immune responses were fundamentally altered and boosted in all groups, with the highest levels in the T4 group. Fish body defense depends heavily on non specific immunity (Reda et al., 2018). Lysozyme, which can break down the link between N-acetyl intracytoic acid and N-acetyl glucosamine and harm the pathogen microorganisms’ cell wall structure, is an essential protective element of the humoral immune response (Han et al., 2015). This research clarified that one of the most fundamentally safe processes in fish is lysozyme. It comes from macrophages and neutrophils that are released into the mucus and blood to possess bacteriolytic properties, aiding organisms in fighting against bacterial, viral, and parasite illnesses (Yano, 1997). The current findings showed that Bio-Mos® supplementation substantially altered the dimensions of lysozyme action. Fish that were encouraged to eat diets with 0.2% Bio-Mos® diet expressed the highest serum lysozyme activity. The immune-stimulating properties of dietary Bio-Mos® may be the cause of the increased lysozymal action. The outcomes are similar to those reported in hybrid grouper and European eel (Anguilla anguilla), channel catfish (Ictalurus punctatus), Nile tilapia (O. niloticus), and red drum (Ren et al., 2020). Reactive oxygen species (ROS) are produced in excess, which causes oxidative stress (Sinha et al., 2015). Oxidative stress can be ensured by a variety of poor rearing conditions, including low oxygen levels, overcrowding, and high ammonia nitrogen levels, which can lead to a number of damages, including cell death by apoptosis or necrosis (Klein and Ackerman, 2003). MDA is one of the primary byproducts of lipid peroxidation induced by ROS (Xu et al., 2010). CAT together with GSH-Px and SOD developed the main defensive mechanism for enzymes opposing the harmful effects of ROS (Zhou et al., 2020). The evaluation of these variables can reveal information about the antioxidant capacity of fish. The dramatic increase in antioxidant activity (CAT, GSH-Px, and SOD activities) in all Bio-Mos® treated groups with the highest increase in T4 group clarify the oxidative capability of ROS, which are delivered by phagocytic cells that have been activated and are in charge of eliminating or deteriorating ingested items, including microorganisms (Atencio et al., 2009). The prebiotic-supplemented T4 groups in the current study showed higher CAT activity levels with decreased MDA. The finding suggested that CAT and MDA were not necessarily inversely associated. These outcomes are in line with numerous research (Danesh et al., 2022). Since CAT is simply one of many antioxidant enzymes that could stop the synthesis of MDA, the explanation is rather simple. In addition to enzymes, other antioxidants that may affect fish’s final MDA level include non-enzymatic substances like vitamins C and E as well as specific prebiotics and probiotics (Mishra et al., 2015). The mechanisms of these prebiotics’ actions typically included a minimum of one of the subsequent elements: the prebiotic aided in the development of antioxidant bacteria (Cao et al., 2019); the prebiotic itself was a potent antioxidant in vitro (Liu and Huang, 2018); and the prebiotic was able to attach itself to intestinal receptors and use receptor-related pathways to provide antioxidant benefits (Torrecillas et al., 2012). MOS could react with ROS (like hydroxyl radical) (Liu and Huang, 2018). However, MOS can also cause fish to express more of the antioxidant enzyme gene by activating the mannose receptor (Zhu et al., 2023). The enhanced lysozyme, bactericidal, and antioxidant activities shown in this study could be linked to the dietary Bio-Mos® stimulatory effects on resistance. It implies that the activation of antioxidant defense mechanisms such as CAT, GSH-Px, and SOD takes place in the context of both Bio-Mos® (Köhrle et al., 2000). Antioxidant activity after experimental challenge with A. hydrophilaPrebiotics in the diet have been proven in numerous studies to significantly increase fish resistance to harmful microorganisms (Zhao et al., 2011; Poolsawat et al., 2021). Fish may experience oxidative stress due to A. hydrophila, one of the most virulent harmful bacteria connected to aquatic environments (Turutoglu et al., 2012). Based on a recent study, A. hydrophila infections are characterized by the activation of strong ROS generation in the host, which causes oxidative damage (Li et al., 2013). MDA and PC are known as signs of ROS-induced cell damage, and the antioxidant system can lessen this damage (Birben et al., 2012). The current findings demonstrated that adding a suitable amount of MOS to a diet suppresses excessive ROS generation and lowers MDA and PC levels. ROS mostly consist of superoxide anions and hydroxyl radicals, which can harm cells through oxidative stress when present in excess (Reczek and Chandel, 2014). In the ongoing research, Nile tilapia fingerlings fed Bio-Mos® revealed the highest SOD, CAT, and GSH-Px activities were recorded in the prebiotic supplemented T4 group while, recorded sharp decline in MDA activity after infection with A. hydrophila. The outcome is comparable to studies by Ren et al. (2020), which found that MOS could increase grouper resistance to Vibrio harvey, Zhu et al. (2023), which found that MOS could increase grouper resistance to A. hydrophila, and Lu et al. (2023) increase grass carp resistance to Aeromonas hydrophila. MOS has been shown to increase the scavenging of free radicals (Lu et al., 2021), indicating that it is a superb natural antioxidant with a wide range of free radical-scavenging characteristics. The principal antioxidant enzymes in fish may also be responsible for their excessive capacity to scavenge free radicals (Atli et al., 2016). Relative gene expressionImmune system health is correlated with TNF-α, IL-1β, and SOD gene expression levels (Elbahnaswy and Elshopakey, 2020), physiological status (Morano, 2007), and oxidative status (Eˇcimovi´c et al., 2018) of fish. When comparing the Bio-Mos® treated fish groups to the control fish group, the proinflammatory IL-1β and cytokines TNF-α had higher levels of gene expression. Concurrently, those for GHR, SOD, and GSH-Px were upregulated. In addition, no noticeable variation was observed between T2 and T3 groups supplemented with 0.5 and 1 mg/kg Bio-Mos®, post challenge with A. hydrophila, regarding TNF-a and IL-1β gene expression in the liver. The outcomes resemble those reported by Jomeh et al. (2021). These findings unequivocally validated Bio-Mos®’s potential impact on boosting immunity and reducing inflammation in Nile tilapia infected with A. hydrophila. Histopathological findingsMorphological consistency is fundamental for keeping up with the ordinary elements of the intestine (Gao et al., 2013). Fish with a healthy gut have certain intestinal morphological parameters (Han et al., 2015). An important morphometry indicator to explore when comparing different feeding strategies for aquatic animals is the healthiness of their intestines. This includes their ability to absorb food and digest it, which can be seen by the number of goblet cells, the thickness of the muscular layer, and the height of the intestinal villi (Khojasteh, 2012). Prebiotics in the diet can help farmed fish consume their feed more effectively and maintain the health of their intestinal mucosal epithelium (Dimitroglou et al., 2009). This was also confirmed through the upregulation of the GHR. In the current investigation, MOS considerably improved each of the three intestinal measures (villus width, villus length, and crypt depth), showing the greatest overall effect on enhancing gut structure. The results are consistent with previous research, in which MOS increased the crypt depth of subadult trout (Staykov et al., 2007), and MOS increased the intestinal villi length of redfish (Zhou et al., 2010). The challenge with A. hydrophila induced marked degenerative changes in the intestine, hepatopancreas, spleen, and posterior kidney of Nile tilapia, as shown in the histopathological study mainly in the control group, not treated with Bio-Mos®. The overexpression of genes linked to inflammation (TNF-α) and IL-1β confirms the presence of inflammation. However, the severity of the lesion was greatly decreased and showed marked amelioration with increased concentration of Bio-Mos®. An increase in the accessible absorption surface area and/or mucus generated by many goblet cells, which has a bactericidal effect by masking pathogen receptors and maintaining intestinal epithelium integrity, may be the cause of increased intestinal absorptive and defensive activities (Smirnov et al., 2005). ConclusionThe current study advances our understanding of the functional nutritional strategies of the use of MOS as a prebiotic in the diets of freshwater fish. It could be concluded that prebiotics in addition to O. niloticus fingerlings diets boost illness resistance and raise fish’s antioxidant capability, which is significant in modern intensive aquaculture. The Dietary MOS supplementation plays a crucial part in moderating the increased oxidative stress caused by A. hydrophila; they could effectively reduce fish oxidative stress under pathogen-challenge circumstances, showing promise as a preventative supplement for Nile tilapia in place of antibiotics. It could be recommended to enrich the Nile tilapia fingerlings diets with 2 g.kg−1 of MOS due to the better results on growth rate, physiological response, immunological response, and intestinal absorptive capacity. AcknowledgmentNone. Conflict of interestThe authors acknowledged no conflict of interest. FundingThis work was entirely supported by the authors’ contributions; there was no outside financial source. Author contributionsAll authors took part in the goal and design of the work. Eman Moustafa and Amira Omar designed the study idea and planning. Data collection, analysis, and material preparation were carried out by Eman Moustafa, Amira Omar, and Haguer Salah El-Din. Statistical analyses were performed by Haguer Salah El-Din. Gene expression analysis was performed by Mustafa Shukry. The histological investigation was conducted by Foad Farrag. The manuscript’s first draft was written by Haguer Salah El-Din, Amira Omar, and Eman Moustafa. Every author offered feedback on earlier drafts of the work. The final manuscript was then edited and revised by Eman Moustafa. The manuscript was read and approved in its final form by all authors. Data availabilityThe current manuscript contains all the information needed to support the study’s conclusions of this study. In response to reasonable demand, the corresponding author will provide any further information needed. ReferencesAdelekea, B., Robertson-Anderssona, D., Moodleya, G. and Taylorb, S. 2021. Aquaculture in Africa: a comparative review of Egypt, Nigeria, and Uganda Vis-A-Vis South Africa. Rev. Fish. Sci. Aquac. 29(2), 167–197. Aebi, H. 1984. Catalase in vitro, methods in enzymology. Amsterdam, The Netherlands: Elsevier, pp: 121–126. Ahmad, M.H., El-Mousallamy, A., Zein, N., Abd El-Naby, A.S. and Mohamed, S.Z. 2015. Evaluation of prebiotic as natural additives on growth performance and blood biochemistry for Nile tilapia (Oreochromis niloticus). Mid. East. J. Appl. Sci. 5(2), 526–533. Ali, S.R., Ambasankar, K., Praveena, E., Nandakumar, S. and Syamadayal, J. 2017. Effect of dietary mannan oligosaccharaide on growth, body composition, hematology and biochemical parameters of Asian seabass (Lates calcarifer). Aquac. Res. 48, 899–908. Al-Wakeel, A.H., Zahran, E., Hafez, E.E., Hamed, M. and Zaki, V.H. 2019. Impacts of mannan oligosaccharides (MOS) on growth performance and gastrointestinal health of Nile tilapia (Oreochromis niloticus). Man. Vet. Med. J. 20(3), 1–7. AOAC. 2012. Official methods of analysis, 18th ed. Washington, DC: Association of Official Analysis of Chemists. APHA. 1989. Standard methods for the examination of water and wastewater, part 3, determination of metals, 17th ed. Washington, DC: American Public Health Association, p: 164. Atencio, L., Moreno, I., Jos, Á., Prieto, A.I., Moyano, R., Blanco, A. and Cameán, A.M. 2009. Effects of dietary selenium on the oxidative stress and pathological changes in tilapia (Oreochromis niloticus) exposed to a microcystin producing cyanobacterial water bloom. Toxicon 53(2), 269–282. Atli, G., Canli, E.G., Eroglu, A. and Canli, M. 2016. Characterization of antioxidant system parameters in four freshwater fish species. Ecotoxicol. Environ. Saf. 126, 30–37. Bancroft, J.D. and Gamble, M. 2007. Theory and practice of histological techniques, 6th ed. London, UK: Churchill Livingstone. Birben, E., Sahiner, U.M., Sackesen, C., Erzurum, S. and Kalayci, O. 2012. Oxidative stress and antioxidant defense. World. Aller. Org. J. 5(1), 9–19. Cao, H., Yu, R., Zhang, Y., Hu, B., Jian, S., Wen, C., Kajbaf, K., Kumar, V. and Yang, G. 2019. Effects of dietary supplementation with β-glucan and Bacillus subtilis on growth, fillet quality, immune capacity, and antioxidant status of Pengze crucian carp (Carassius auratus var. Pengze). Aquaculture 508, 106–112. Cruickshank, R. 1975. Medical microbiology: a guide to diagnosis and control of infection. Edinburgh and London, UK: E and S Livingston Ltd., p: 888. Danesh, H., Ziamajidi, N., Mesbah-Namin, S.A., Nafisi, N. and Abbasalipourkabir, R. 2022. Association between oxidative stress parameters and hematological indices in breast cancer patients. Int. J. Breast. Cancer. 14, 59410. Dawood, M.A.O., AbdEl-kader, M.F., Moustafa, E.M., Gewaily, M.S. and Abdo, S.E. 2020. Growth performance and hemato-immunological responses of Nile tilapia (Oreochromis niloticus) exposed to deltamethrin and fed immunobiotics. Environ. Sci. Pollut. Res. 27, 11608–11617. Dawood, M.A.O., Koshio, S., El-Sabagh, M., Billah, M.M., Zaineldin, A.I., Zayed, M.M. and Omar, A.A.E.D. 2017. Changes in the growth, humoral and mucosal immune responses following β-glucan and vitamin C administration in red sea bream, Pagrus major. Aquaculture 470, 214–222. Dawood, M.A.O., Magouz, F.I., Salem, M.F.I. and Abdel-Daim, H.A. 2019. Modulation of digestive enzyme activity, blood health, oxidative responses and growth-related gene expression in GIFT by heat-killed Lactobacillus plantarum (L-137). Aquaculture 505, 127–136. Dawson, K.A. and Pirvulescu, M. 1999. Mananoligossacarídeos derivados de leveduras como moduladores da resposta imunológica e alternativas aos promotores de crescimento antimicrobianos, 9th ed. Curitiba, Brasil: Ronda Latino Americanada Alltech, pp: 33–41. Demers, N.E. and Bayne, C.J. 1997. The immediate effect of stress on hormones and plasma lysozyme in rainbow trout. Develop. Comp. Immunol. 21, 363–373. Dickson, M., Nasr-Allah, A., Kenawy, D. and Kruijssen, F. 2016. Increasing fish farm profitability through aquaculture best management practice training in Egypt. Aquaculture 465, 172–178. Dimitroglou, A., Merrifield, D.L., Moate, R., Davies, S.J., Spring, P., Sweetman, J. and Bradley, G. 2009. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Anim. Sci. 87, 3226–3234. Eˇcimovi´c, S., Velki, M., Vukovi´c, R., ˇCamagajevac, I.Š., Petek, A., Bošnjakovi´c, R., Grgi´c, M., Engelmann, P., Bodó, K. and Filipovi´c-Mariji´c, V. 2018. Acute toxicity of selenate and selenite and their impacts on oxidative status, efflux pump activity, cellular and genetic parameters in earthworm Eisenia andrei. Chemosphere 212, 307–318. Elbahnaswy, S. and Elshopakey, G.E. 2020. Differential gene expression and immune response of Nile tilapia (Oreochromis niloticus) challenged intraperitoneally with Photobacterium damselae and Aeromonas hydrophila demonstrating immunosuppression. Aquaculture 526, 735364. El Mesallamy, A.M., Ahmad, M.H., Souleman, A.M., El Morsy, A.T. and Abd El-Naby, A.S. 2016. Effects of Roselle calyx (Hibiscus sabdariffa L.)-supplemented diets on growth and disease (Aeromonas hydrophila) resistance in Nile tilapia (Oreochromis niloticus L.). Egypt. Pharm. J. 15, 78. Elumalai, P., Prakash, P., Musthafa, M.S. and Faggio, C. 2019. Effect of alkoxy glycerol on growth performance, immune response and disease resistance in Nile Tilapia (Oreochromis niloticus). Res. Vet. Sci. 123, 298–304. FAO. 2007. FAO technical meeting on prebiotics. Rome, Italy: FAO, pp: 15–16. FAO. 2022. The state of world fisheries and aquaculture 2022. Towards blue transformation. Rome, Italy: FAO. https://doi.org/10.4060/cc0461en Gainza, O. and Romero, J. 2020. Efect of mannan oligosaccharides on the microbiota and productivity parameters of Litopenaeus vannamei shrimp under intensive cultivation in Ecuador. Sci. Rep. 10, 2719. Gao, Y., Han, F., Huang, X., Rong, Y., Yi, H. and Wang, Y. 2013. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: a comparative study. J. Anim. Sci. 91, 5614–5625. Ghiasi, M., Nasrollahzadeh, S.H., Fazli, H., Safari, R., Farabi, S.M.V., Binaii, M., Yaghobzadeh, Z., Zare, E., Behrouzi, S. and Habibi, F. 2021. Evaluation of some risk factors on the rainbow trout production with emphasis on water microbial indices and heavy metals of farms along the Haraz River. Iran. Sci. Fish. J. 30(6), 25–42. Gómez, G.D. and Balcázar, J.L. 2008. A review on the interactions between gut microbiota and innate immunity of fish. FEMS. Immun. Med. Microb. 52, 145–154. Hady, M.M., El-Banna, R.A., Taleb, H.M. and Shimaa, R.A. 2012. Impact of manna oligosaccharide (Bio-Mos®) and esterified glucomannan (MTB-100®) dietary supplementation on performance and health status of Barki Lambs Under Egyptian conditions. Int. J. Chem. Eng. Appl. 3(4), 264–268. Han, B., Long, W., He, J., Liu, Y., Si, Y. and Tian, L. 2015. Effects of dietary Bacillus licheniformis on growth performance, immunological parameters, intestinal morphology and resistance of juvenile Nile tilapia (Oreochromis niloticus) to challenge infections. Fish. Shellfish. Immunol. 46, 225–231. Ibrahim, M.D., Shahed, I.B., Abo El-Yazeed, H. and Korani, H. 2011. Assessment of the susceptibility of poly culture reared African catfish and Nile Tilapia to Edwardsiella tarda. J. Amer. Sci. 7(3), 779–786. Jomeh, R., Chitsaz, H. and Akrami, R. 2021. Effect of anthocyanin extract from Roselle, Hibiscus sabdariffa, calyx on haematological, biochemical and immunological parameters of rainbow trout, Oncorhynchus mykiss. Aquac. Res. 52, 3736–3744. Kawahara, E., Ueda, T. and Nomura, S. 1991. In vitro phagocytic activity of white spotted shark cells after injection with Aeromonas salmonicida extracelluar products. Gyo. Ken. Japan. 26(4), 213–214. Khanjani, M.H., Ghaedi, G. and Sharifinia, M. 2022. Effects of diets containing β-glucan on survival, growth performance, haematological, immunity and biochemical parameters of rainbow trout (Oncorhynchus mykiss) fingerlings. Aquac. Res. 53, 1842–1850. Khojasteh, S.M.B. 2012. The morphology of the post-gastric alimentary canal in teleost fishes: a brief review. Int. J. Aquat. Sci. 3(2), 71–88. Klein, J.A. and Ackerman, S.L. 2003. Oxidative stress, cell cycle, and neurodegeneration. J. Clin. Investig. 111, 785–793. Köhrle, J., Brigelius-Flohé, R., BöckA Gärtner, R., Meyer, O. and Flohé, L. 2000. Selenium in biology: facts and medical perspectives. Biol. Chem. 381(9–10), 849–864. Li, J., Ni, X.D., Liu, Y.J. and Lu, C.P. 2011. Detection of three virulence genes alt, ahp and aerA in Aeromonas hydrophila and their relationship with actual virulence to zebrafish. J. Appl. Microb. 110(3), 823–830. Li, C., Wang, R., Su, B., Luo, Y., Terhune, J., Beck, B. and Peatman, E. 2013. Evasion of mucosal defenses during Aeromonas hydrophila infection of channel catfish (Ictalurus punctatus) skin. Dev. Comp. Immunol. 39(4), 447–455. Liu, Y. and Huang, G. 2018. The derivatization and antioxidant activities of yeast mannan. Int. J. Biol. Macromol. 107, 755–761. Lu, Z.Y., Feng, L., Jiang, W.D., Wu, P., Liu, Y. and Jiang, J. 2021. Mannan oligosaccharides application: multipath restriction from Aeromonas hydrophila infection in the skin barrier of grass carp (Ctenopharyngodon idella). Front. Immunol. 12, 742107. Lu, Z., Feng, L., Jiang, W., Wu, P., Liu, Y., Jiang, J., Kuang, S.H., Tang, L., Li, S.H., Zhong, C.H. and Zhou, X. 2023. Mannan oligosaccharides alleviate oxidative injury in the head kidney and spleen in grass carp (Ctenopharyngodon idella) via the Nrf2 signaling pathway after Aeromonas hydrophila infection. J. Ani. Sci. Biotech. 14, 58. Magouz, F.I., Salem, M.F., Moustafa, E.M. and Elkhamy, S.A. 2019. Impact of biomos and agrimos dietary supplementation on growth performance, feed utilization and immunological parameters of Nile tilapia (Oreochromis Niloticus) fingerlings. Slov. Vet. Res. 56(Suppl 22), 87–98. Manning, T. and Gibson, G. 2004. Prebiotics. Best. Pract. Res. Clin. Gastroenterol. 18, 287–298. Mehrim, A.I. and Refaey, M.M. 2023. An overview of the implication of climate change on fish farming in Egypt. Sustainability 15, 1679. Merrifield, D.L., Dimitroglou, A., Foey, A., Davies, S.J. and Baker, R.T.M. 2010. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302, 1–18. Mishra, V., Shah, C., Mokashe, N., Chavan, R., Yadav, H. and Prajapati, J. 2015. Probiotics as potential antioxidants: a systematic review. J. Agric. Food. Chem. 63, 3615–3626. Morano, K.A. 2007. New tricks for an old dog: the evolving world of Hsp70. Ann. N. Y. Acad. Sci. 1113, 1–14. Moustafa, E.M., Dawood, M.A.O., Assar, D.H., Omar, A.A., Elbialy, Z.I., Farrag, F.A., Shukry, M. and Zayed, M.M. 2019. Modulatory effects of fenugreek seeds powder on the histopathology, oxidative status, and immune related gene expression in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquaculture 515, 734589. Nguyen, T.N. The utilization of soybean products in tilapia feed-a review. In Proceeding of (ISTA), 8th International Symposium on Tilapia in Aquaculture, 2008, vol. 1, pp 53–67. Nishikimi, M., Roa, N.A. and Yogi, K. 1972. The occurrence of supeoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Comm. 46, 849–854. NRC. 2011. Nutrient requirements of fish and shrimp. Washington, DC: Animal Nutrition Series, National Research Council of the National Academies. The National Academies Press, p: 376. Ohkawa, H., Ohishi, N. and Yagi, K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. Osuigwe, D.I., Obiekezie, A.I. and Onuoha, G.C. 2005. Some hematological changes in hybrid catfish (Heterobranchus longifilis x Clarias gariepinus) fed different dietary levels of raw and boiled jack bean (Canavalia ensiformis) seed meal. Afr. J. Biotech. 4, 1017–1021. Peterson, J.A., Schwartz, R.P., Mitchell, S.G., Reisinger, H.S., Kelly, S.M., O’Grady, K.E., Brown, B.S. and Agar, M.H. 2010. Why don’t out-of-treatment individuals enter methadone treatment programes? Int. J. Drug. Policy. 21, 36–42. Poolsawat, L., Li, X., Xu, X., Rahman, M.M., Boonpeng, N. and Leng, X. 2021. Dietary xylooligosaccharide improved growth, nutrient utilization, gut microbiota and disease resistance of tilapia (Oreochromis niloticus X O. aureus). Anim. Feed. Sci. Technol. 275, 114872. Prabu, E., Rajagopalsamy, C.B.T., Ahilan, B., Jeevagan, I.J.M.A. and Renuhadevi, M. 2019. Tilapia—an excellent candidate species for world aquaculture: a review. Ann. Res. Rev. Biol. 31(3), 1–14. Prieto, P., Pineda, M. and Aguilar, M. 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269, 337–341. Reczek, C.R. and Chandel, N.S. 2014. ROS-dependent signal transduction. Curr. Opin. Cell. Biol. 33, 8–13. Reda, R.M., El-Hady, M.A., Selim, K.M. and El-Sayed, H.M. 2018. Comparative study of three predominant gut Bacillus strains and a commercial B. amyloliquefaciens as probiotics on the performance of Clarias gariepinus. Fish. Shellfish. Immunol. 80, 416–425. Reed, L. and Muench, H. 1938. A simple method of estimating fifty percent end points. Am. J. Trop. Med. Hyg. 27, 493–497. Rehulka, J. 1996. Blood parameters in common carp with spontaneous spring viremia (SVC). Aquac. Int. 4, 175–182. Ren, Z., Wang, S., Cai, Y., Wu, Y., Tian, L., Wang, S., Jiang, L., Guo, W., Sun, Y. and Zhou, Y. 2020. Effects of dietary mannan oligosaccharide supplementation on growth performance, antioxidant capacity, non-specifc immunity and immune-related gene expression of juvenile hybrid grouper (Epinephelus lanceolatus ♀ X Epinephelus fuscoguttatus ♂). Aquaculture 523, 735195. Rigos, G., Katharios, P., Kogiannou, D. and Cascarano, C.M. 2021. Infectious diseases and treatment solutions of farmed greater amberjack Seriola dumerili with particular emphasis in Mediterranean region. Rev. Aquac. 13, 301–323. Sado, R.Y., Bicudo, Á.J.D.A. and Cyrino, J.E.P. 2008. Feeding dietary mannan oligosaccharides to juvenile Nile tilapia, Oreochromis niloticus, has no effect on hematological parameters and showed decreased feed consumption. J. World. Aquac. Soc. 39, 821–826. Sahoo, P.K. and Mukherjee, S.C. 2001. Effect of dietary 1,3 β-glucan on immune responses and disease resistance of healthy and aflatoxin B1-induced immune compromised rohu (Labeo rohita Hamilton). Fish. Shellfish. Immunol. 11, 683–695. Samuel, A., Adel, A.C., Malachi, A.W. and Donald, A.D. 2017. Effects of Bacillus subtilis strain and the prebiotic previda on growth, immune parameters and susceptibility to Aeromonas hydrophila infection in Nile Tilapia. Aquac. Res. 2017, 1–13. Satoh, K. 1978. Serum lipid peroxide in cerebrovascular disorders de-termined by a new colorimetric method. Clin. Chim. Acta, 90, 37–43. Sinha, A.K., Zinta, G., AbdElgawad, H., Asard, H., Blust, R. and De Boeck, G. 2015. High environmental ammonia elicits differential oxidative stress and antioxidant responses in five different organs of a model estuarine teleost (Dicentrarchus labrax). Comp. Biochem. Physiol. Part C. Toxicol. Pharmacol. 174–175, 21–31. Smirnov, A., Perez, R., Amit-Romach, E., Sklan, D. and Uni, Z. 2005. Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. J. Nut. 135(2), 187–192. Staykov, Y., Spring, P., Denev, S. and Sweetman, J. 2007. Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout (Oncorhynchus mykiss). Aquac. Int. 15, 153–161. Ta’ati, R., Soltani, M., Bahmani, M. and Zamini, A.A. 2011. Growth performance, carcass composition and immune physiological indices in juvenile great sturgeon (Huso huso) fed on commercial prebiotic, immunoster. Iran. J. Fish. Sci. 10, 324–335. Thrall, M.A., Weiser, G., Allison, R.W. and Campbell, T.W. 2012. Veterinary hematology and clinical chemistry, 2nd ed. Hoboken, NJ: Wiley-Blackwell. Torrecillas, S., Makol, A., Caballero, M.J., Montero, D., Dhanasiri, A.K.S., Sweetman, J. and Izquierdo, M. 2012. Effects on mortality and stress response in European sea bass, Dicentrarchus labrax (L.), fed mannan oligosaccharides (MOS) after Vibrio anguillarum exposure. J. Fish. Dis. 35, 591–602. Turutoglu, H., Ercelik, S. and Corlu, M. 2012. Aeromonas hydrophila-associated skin lesions and septicaemia in a Nile crocodile (Crocodylus niloticus). J. S. Afr. Vet. Assoc. 76(1), 40–42. Urbinate, E.C. and Carneiro, P.C.F. 2006. Sodium chloride added to transport water and physiological responses of Matrinxã Brycon amazonicus (Teleost: Caracidae). Acta. Amaz. 36, 569–572. Wang, S., Tian, L., Wu, Y., Zhou, Y., Guan, B., Li, J. and Cai, Y. 2022. An accidental discovery of mannan-oligosaccharide’s protection effect against air exposure and its potential mechanism in hybrid grouper (Epinephelus lanceolatus X Epinephelus fuscoguttatus ♂). Fish. Physiol. Biochem. 48, 1285–1297. Welker, T.L. and Lim, C. 2011. Use of probiotics in diets of tilapia. J. Aquac. Res. Develop. S1, 014. Xu, J., Zhang, J., Xie, H., Li, C., Bao, N., Zhang, C. and Shi, Q. 2010. Physiological responses of Phragmites australis to wastewater with different chemical oxygen demands. Ecol. Eng. 36, 1341–1347. Yalçinkaya, l., Güngör, T., Bafialan, M. and Erdem, E. 2008. Mannan oligosaccharides (MOS) from Saccharomyces cerevisiae in broilers: effects on performance and blood biochemistry. Turk. J. Vet. Anim. Sci. 32(1), 43–48. Yano, T. 1997. The nonspecific immune system:humoral defense. Fish. Physiol. 15, 105–157. Yassien, S.H.A., Abd El-Rahim, S.A., Osman, M.F., Hamouda, R.E., Soliman, M.A.M. and Nageib, R.M. 2022. Factors affecting aquaculture farms’ profitability and constraints facing fish farmers in Egypt. Egypt. J. Aquat. Biol. Fish. 26(2), 519–527. Zhang, J., Liu, Y., Tian, L., Yang, H., Liang, G. and Xu, D. 2012. Effects of dietary mannan oligosaccharide on growth performance, gut morphology and stress tolerance of juvenile Pacific white shrimp (Litopenaeus vannamei). Fish. Shellfish. Immunol. 33, 1027–1032. Zhao, Y., Ma, H., Zhang, W., Ai, Q., Mai, K., Xu, W., Wang, X. and Liufu, Z. 2011. Effects of dietary β-glucan on the growth, immune responses and resistance of sea cucumber, Apostichopus japonicus against Vibrio splendidus infection. Aquaculture 315, 269–274. Zhou, Q., Buentello, J.A. and Gatlin, D.M. 2010. Effects of dietary prebiotics on growth performance, immune response and intestinal morphology of red drum (Sciaenops ocellatus). Aquaculture 309, 253–257. Zhou, L., Li, H., Qin, J.G., Wang, X., Chen, L., Xu, C. and Li, E. 2020. Dietary prebiotic inulin benefits on growth performance, antioxidant capacity, immune response and intestinal microbiota in Pacific white shrimp (Litopenaeus vannamei) at low salinity. Aquaculture 518, 734847. Zhu, L., Wang, S.H., Cai, Y., Shi, H., Zhou, Y., Zhang, D., Guo, W. and Wang, SH. 2023. Effects of five prebiotics on growth, antioxidant capacity, non-specific immunity, stress resistance, and disease resistance of juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ X Epinephelus lanceolatus ♂). Animals 13, 754 | ||
| How to Cite this Article |
| Pubmed Style Moustafa EM, Farrag FA, Shukry M, Din HMSE, Omar AA. Ameliorative effect of BIO-MOS® as a dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 116 -135 . doi:10.5455/OVJ.2024.v14.i1.11 Web Style Moustafa EM, Farrag FA, Shukry M, Din HMSE, Omar AA. Ameliorative effect of BIO-MOS® as a dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. https://www.openveterinaryjournal.com/?mno=175312 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.11 AMA (American Medical Association) Style Moustafa EM, Farrag FA, Shukry M, Din HMSE, Omar AA. Ameliorative effect of BIO-MOS® as a dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 116 -135 . doi:10.5455/OVJ.2024.v14.i1.11 Vancouver/ICMJE Style Moustafa EM, Farrag FA, Shukry M, Din HMSE, Omar AA. Ameliorative effect of BIO-MOS® as a dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 116 -135 . doi:10.5455/OVJ.2024.v14.i1.11 Harvard Style Moustafa, E. M., Farrag, . F. A., Shukry, . M., Din, . H. M. S. E. & Omar, . A. A. (2024) Ameliorative effect of BIO-MOS® as a dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 116 -135 . doi:10.5455/OVJ.2024.v14.i1.11 Turabian Style Moustafa, Eman M., Foad A. Farrag, Mostafa Shukry, Haguer M. Salah El Din, and Amira A. Omar. 2024. Ameliorative effect of BIO-MOS® as a dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 116 -135 . doi:10.5455/OVJ.2024.v14.i1.11 Chicago Style Moustafa, Eman M., Foad A. Farrag, Mostafa Shukry, Haguer M. Salah El Din, and Amira A. Omar. "Ameliorative effect of BIO-MOS® as a dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila." Open Veterinary Journal 14 (2024), 116 -135 . doi:10.5455/OVJ.2024.v14.i1.11 MLA (The Modern Language Association) Style Moustafa, Eman M., Foad A. Farrag, Mostafa Shukry, Haguer M. Salah El Din, and Amira A. Omar. "Ameliorative effect of BIO-MOS® as a dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 116 -135 . Print. doi:10.5455/OVJ.2024.v14.i1.11 APA (American Psychological Association) Style Moustafa, E. M., Farrag, . F. A., Shukry, . M., Din, . H. M. S. E. & Omar, . A. A. (2024) Ameliorative effect of BIO-MOS® as a dietary supplementation on growth performance, physiological response, oxidative status, and immunity linked gene expression in Nile tilapia (Oreochromis niloticus) fingerlings challenged with Aeromonas hydrophila. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 116 -135 . doi:10.5455/OVJ.2024.v14.i1.11 |