| Review Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 1-11 Review Article Behavior and management of carp fish: A reviewHesham H. Mohammed1*, Mohamed Ebrahim2, Mohamed I. Youssef1, Al-Sadik Y. Saleem1 and Adel Abdelkhalek31Department of Behavior, Management of Animal, Poultry, and Aquatic, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Department of Animal Wealth Development, Faculty of Veterinary Medicine, Badr University in Cairo (BUC), Cairo, Badr City 11829, Egypt 3Faculty of Veterinary Medicine, Badr University in Cairo (BUC), Cairo, Badr City 11829, Egypt *Corresponding Author: Hesham H. Mohammed. Department of Behavior, Management of Animal, Poultry, and Aquatic, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: heshamvet_hosny [at] yahoo.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
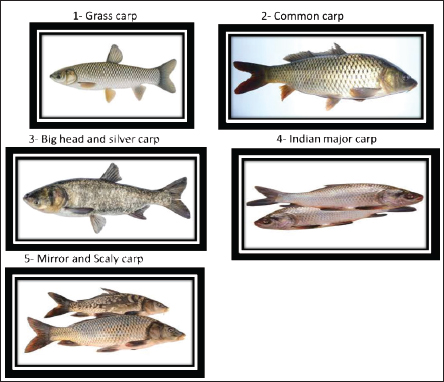
ABSTRACTNowadays, fish production aims to achieve a continuous and immediate generation of top-quality animal protein from the finest sources. Moreover, the aquaculture industry holds a vital position in addressing the rising global appetite for fish and seafood products. In addition, it has played a substantial role in providing affordable animal protein in Egypt in recent years. Therefore, rapid development has occurred in the industrial aquaculture sector in Egypt to compensate for the decrease in red meat production. According to previous studies, Egypt occupied the first rank among African countries and the ninth position globally in the field of fish farming production. This achievement aimed to link up the disparity between fish production and consumption in Egypt. Carp, due to its economic importance in this industry, has expanded worldwide with more evident ecological influences. The carp fish belongs to the Cyprinidae family, which encompasses seven subfamilies, approximately 220 genera, and has been associated with around 20,000 documented species. Given the importance of carp with different species, this work reviews the management, behavior, and different rearing systems of some popular carp species in Egypt. Data search was done on PubMed, SCOPUS, Web of Science, and Google Scholar for the keywords including fish farming, carp fish, management, behavior, rearing systems, Egypt, Africa, and Worldwide. In Egypt, the output of carp is ranked second only to tilapia in aquaculture. A polyculture system is more often used in carp rearing, particularly when raising tilapia, to maximize growth rates, minimize feed conversion ratios, and reduce the amount of fat in the corpses. Furthermore, agro-ecologically valuable agriculture has been linked to integrated carp monoculture. Crop rising was the key to the successful development of pond aquaculture. Keywords: Carp, Polyculture, Monoculture, Extensive and intensive system, Integrate fish farm. IntroductionNumerous African countries were initially exposed to aquaculture at the beginning of the 20th century to meet the recreational fishing demands of the colonial era (Hecht et al., 2006). Aquaculture production worldwide has experienced significant and swift growth in recent years. This can be attributed mainly to a range of innovations that have enhanced control over the production process and competitiveness. These innovations encompass various forms, including groundbreaking concepts and the application of knowledge adapted from terrestrial food production systems. In the wall artwork of an ancient tomb of an Egyptian pharaoh, which dates back to 2500 BC, there is a depiction of a man gathering fish from an earthenware pond, marking the earliest recorded instance of aquaculture in the past (Bardach et al., 1972). Egypt, despite its vast arid land, has a rich history of engaging in aquaculture and holds the top position in Africa, particularly in freshwater aquaculture production (Suloma and Ogata, 2006). Seven types of finfish, including tilapia, various mullet species, different carp species, catfish, bayad, sea bream, and sea bass, constitute the majority of aquaculture production (Sadek et al., 2006, 2013). Aquaculture production is primarily driven by the industry sector which is privately owned, accounting for 98.5% of the whole, while the public sector plays a minor role, contributing just 1.5% (El-Naggar et al., 2008). Fish represent a significant economic protein source when compared to alternative animal protein sources. In developing nations, fish contribute approximately 30% of the whole per capita animal protein consumption (Wang et al., 2015). Fish holds a fundamental place in the traditional Egyptian diet and serves as a crucial and cost-effective source of animal protein (Soliman and Yacout, 2016). The aquaculture sector in Egypt witnessed substantial growth starting in 1998. This growth was the result of continuous and comprehensive efforts by the Egyptian authorities in previous years, coupled with increasing private sector investments (Soliman and Yacout, 2016). As a result, Egypt’s aquaculture industry surged 1,561,457 tons in 2018 compared to 139,389 tons in 1998, constituting 71% of the entire African aquaculture production (FAO, 2003-2020). Nowadays increasingly, there is a heightened recognition of the significance of consuming nourishing foods, and fish is steadily gaining popularity due to its distinct nutritional advantages. Fish is acknowledged as a nutritionally valuable component of the human diet, attributed to its content of both macronutrients (such as proteins, lipids, and ash) and micronutrients (including vitamins and minerals) (Abdel Fattah et al., 2020). The rising demand for meeting the necessary protein production can be met through the implementation of intensive fish farming (Olopade et al., 2015(. Moreover, the objective of carp cultivation extends beyond human consumption; it also encompasses their utilization as biological weed controllers in aquatic environments. This practice is particularly prevalent in the United States, Egypt, and India. Despite, Egypt boasting the most extensive aquaculture industry on the African continent (CAPMAS, 2014), there was a decrease in the source of animal protein. The main goal is to diminish the disparity between fish production and consumption in Egypt. In addition, the predominant practice in freshwater aquaculture involves polyculture, combining Indian major carps such as Rui, Catla, and Mrigal with a variety of other carps, including silver carp, mirror carp, grass carp, common carp, and bighead carp (Dey et al., 2013; Emran et al., 2022), this represents 60% of the time, around overall production of aquaculture in freshwater. Carp production, on the other hand, constitutes approximately 17% of the total aquaculture output in Egypt and holds the second position, following tilapia (Adeleke et al., 2020). Frequently cultivated carp species include the common carp (Cyprinus carpio—L. 1758), silver carp (Hypophthalmichthys molitrix—Valenciennes, 1844), and grass carp (Ctenopharyngodon idella—Steindachner, 1866) (Hagar, 2014; Shaalan et al., 2018). The carp fish belongs to the Cyprinidae family, which encompasses seven subfamilies, approximately 220 genera, and has been associated with around 20,000 documented species (Howes, 1991). Carps are named after their natural geographical occurrence. In China, there are two main categories of carp: Chinese carps, comprising the grass carp (Ctenopharyngodon idella), silver carp (Hypophthalmichthys molitrix), and bighead carp (Aristichthys nobilis), as well as the Indian major carps, which encompass catla (Catla catla), rohu (Labeo rohita), mrigal (Cirrhinus mrigala), and the common carp (Cyprinus carpio) (Aloden, 1996). The elevated expense associated with high-quality fish feed stands as one of the challenges impeding the progress of aquaculture, thus, in Egypt, carp are occasionally raised in composite fish culture alongside tilapia (Mur, 2014). The most distributed species of carp are presented in Figure 1.
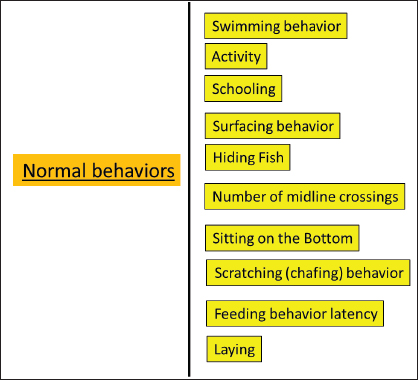
Fig. 1. The most prevalent carp species. Grass carpThe grass carp, scientifically known as C. idella, stands out as one of the most widely cultivated freshwater fish species globally due to its economic significance (FAO, 2016; Shehata et al., 2018). This fish species exhibits notable reproductive capabilities, boasts rapid growth rates, is nutritionally rich, and originally hails from China. It was initially brought to Egypt during the 1980s (Essa et al., 2004; Saleh, 2007). Following the Second World War, it was introduced into numerous countries across all continents. This has garnered growing interest among fishery biologists who see its potential for controlling aquatic organisms (Opuszyński, 1981). After being consistently stocked in government hatcheries for several decades, in 2012, a substantial quantity of approximately a total of 44.226 million grass carp fingerlings was distributed (Gafrd, 2016). Grass carp contribute positively to the environment and public health by enhancing it through the removal of refuge vegetation that conceals snails. This, in turn, enhances the predation process carried out by black carp (Hamouda and Moustafa, 2020). Grass carp typically exhibit incomplete digestion, resulting in roughly 50% of the consumed discharged dietary substances in the form of feces. This supports a substantial number of other fish species’ biomass in the polyculture system (Jhingran and Pullin, 1985). Hamouda and Moustafa (2020) mentioned that grass carp possess an exceptional ability to process a significant amount of aquatic plants, releasing and recycling the nutrients contained within them. This stimulation of preferred plankton communities leads to increased fish production and a reduction in freshwater wastage. The grass carp is originally native to the rivers of China, but it was incorporated into numerous other countries primarily to control biological aquatic weeds and macrophytes in both natural water bodies and artificial ponds (Aloden, 1996). This species is distinguished by its lack of barbels, a terminal and slightly oblique mouth, a moderate to large scale count ranging from 38 to 47, a complete lateral line, and two rows of laterally compressed, pharyngeal teeth with strong serrations featuring prominent parallel grooves (Wu, 1977; Chen, 1998). Grass carp primarily feed on various species of macrophytes in their natural diet (Liu et al., 1963). During the breeding season, secondary sex characteristics are developed in grass carp, such as thicker and serrated 1st rays on their pectoral fins, along with the growth of rough nuptial tubercles on their opercula and scales. However, males tend to lose these tubercles after mating. Grass carp often exhibit incomplete digestion, resulting in approximately 50% of consumed food material being excreted in the form of feces. This supports a substantial of other fish species’ biomass in the polyculture system (Jhingran and Pullin, 1985). Polyculture techniques have been used in rearing grass carp with different species of carp such as silver and common carp, as mentioned before by Chilton and Muoneke (1992). Common carpAmong the freshwater fish, the common carp prefers rivers, lakes, and ponds. It only occasionally inhabits brackish water (Barus, 2002). Moreover, one of the most widely cultivated and significant commercial freshwater fish in the world is the common carp (Cyprinus carpio) (Biro, 1995; Zhou et al., 2003), representing 11% of the global production of freshwater aquaculture (FAO, 2007). Various pond aquaculture systems in Asia are used to cultivate common carp, which accounts for more than 90% of this production (FAO, 2007). Due to its ability to adapt to a variety of ecological conditions, this rapidly growing fish is frequently referred to as an “ecological engineer” or “natural specialist” (Yaqoob, 2021). Farag et al. (2014) mentioned that carp fish had a terminal mouth and no teeth on either of their jaws, but this was made up for by pharyngeal teeth and a well-developed pharyngeal pad. The maxillary barbels are situated at the angle of the mouth and are smaller than the mandibular barbels. The front of the dorsal fin has a powerful, toothed spine and a long, concave base with 17–22 branched rays. Scales on this lateral line range from 32 to 38. There are between 36 and 38 vertebrae (FAO, 2007). The common carp modifies its eating habits, feeding niche, and behavior when food is scarce. The common carp has an impact on other fish species’ eating patterns and behaviors, which may or may not improve the growth and behavior of those species. The common carp is exceptionally adaptable when there is no enough food available. There is proof that common carp consume other fish fry in large quantities when they are deprived of natural food (Weber et al., 2010). Takeuchi et al. (2002) cited that carp fry feed on zooplankton, and by growing up, they become omnivorous. Warm-water fish such as carp thrive in muddy, eutrophic (extremely productive, rich in organic and mineral nutrients) waters. It spawns twice a year, once from January to March and the other between July and August, in pond environments. Common carp can also be raised in substantial, naturally based monoculture production systems that are supplied in natural waterways and stagnant water ponds, lakes, and temporarily flooded areas. The fry is placed into nursery ponds when they are 4–5 days old. In temperate regions, fish that are one summer old (20–100 g) must be raised to 250–400 g in their second year. A stocking rate of 4,000–6,000 is used per hectare if only cereals are fed, plus roughly 3,000 Chinese carp. Fortunately given that most carp are eaten domestically, the species will continue to be significant where it has historically been cultivated (FAO, 2009). Utilizing extensive and semi-intensive management regimes, the species is frequently raised in earth ponds, allowing for the use of natural resources for development and growth (Adámek et al., 2012). Big head and silver carpKolar et al. (2007) mentioned that large rivers with turbulence are typically where bighead and silver carp spawn, and the current carries the eggs and larvae. Moreover, they are thought to perish if they sink because they are greater in density than water and have a higher density. Silver carp and bighead carp came into existence after the Second World War in numerous nations on all continents, and they assisted in boosting fishery production by raising common carp alongside them in ponds (Opuszyński, 1981). Silver carp is a natural inhabitant in the river systems of China and introduced to numerous nations for aquaculture (Aloden, 1996). Bighead carp are large cyprinids that are native to eastern Asia and are regarded as invasive species in North America (Conover et al., 2007). Silver carp feeds on both phytoplankton and zooplanktons; furthermore, its growth rate depends on stocking density, natural food sources, supplied feeding, and competition with other species in polyculture, feed conversion rate, and environmental conditions (Aloden, 1996). Big head carp is mostly a zooplankton feeder, its food resembles that of the alimentary canal of the Indian major carp Catla, which consumes zooplankton as well, is significantly shorter than that of the silver carp, as mentioned before in Aloden (1996). Murty et al. (1986) noticed that while episodic vertical movements did not start until six hours after hatching in silver carp, occasional whirling started right away. In the aquaculture system, they breed during (July–August) but do not naturally spawn in the still waters of ponds and tanks; instead, it does so in the moving waters of their natural habitat (Aloden, 1996). Indian major carpWeerakoon (2013) mentioned that employing imports from the People’s Republic of China and India of brood stock of Chinese and Indian big carps, respectively, Indian carps were produced through induced spawning. The three major Indian carp originated in the rivers and backwaters of Northern India, Pakistan, and Burma (Deepananda et al., 2014) as natural inhabitants of the freshwater of India and are often mistaken for an allied form that exists in Thailand. It primarily feeds on water column vegetation but also engages in some browsing (Aloden, 1996). It is spawning migration to their various breeding grounds (Tsai and Ali, 1985 and 1986). Rahman (2008) cited that the age of maturity for spawning in Indian major carp is 3–5 years, while Aloden (1996) mentioned that it takes 22 months for it to reach maturity in ponds, where it does so in the second year. Catla’s spawning season lasts from May to August and from June to September in north India and Pakistan (Aloden, 1996). Mirror and scaly carpThere are currently many common carp breeds and strains that have undergone genetic improvement due to centuries of intraspecific hybridization. The two primary phenotypes of carp that are currently cultivated in fish ponds in Central Europe are the scaly and mirror carp, which can be distinguished by the patterns on their scales (Hulák et al., 2010). Mirror carp (C. Carpiovar. specularis), leather carp (C. Carpiovar. nudus), and scale carp (C. Carpiovar. communis) are three varieties of common carp that are primarily confined to the cold upland waters and do not typically breed in the plains. Select ripe brood fish to ensure a successful induced breeding. Fish called scale carp are bottom-dwellers and carnivores. The whole of its body is covered in scales of average size. By using the induced breeding technique, seeds can be produced (Islam and Amin, 2016). Behavior of carpFish behaviors are complicated and frequently alter as they get bigger and react to their environments as well as other living things. Behavior was recorded by using the focal sample technique (Altmann, 1974) after direct or video recording (Abdel Fattah et al., 2020 and Said et al., 2020). The most obvious behaviors in carp fish are presented in Figures 2 and 3. Normal behaviors Normal behaviors are responses to normal or familiar stimuli.
Fig. 2. Normal behaviors of carp fish.
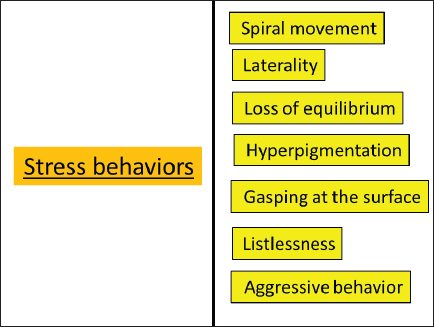
Fig. 3. Abnormal (stress) behavior of carp fish. Stress behaviors (Abnormal behaviors) Stress behaviors (abnormal behaviors) are reactions to upsetting, unsettling, or unexpected stimuli. Stocking density, nutrition strategies, and management practices have the ability to evoke responses to stress, stress tolerance, health parameters, and the development of aggressive behavior (Ashley, 2007).
Management of carpAlthough Egypt has a history of practicing aquaculture for many centuries, modern management techniques only have been implemented recently to increase output (Shaalan et al., 2018). Because of a paradigm change away from conventional broad aquaculture systems and toward semi-intensive and modern intensive aquaculture systems over the past 20 years, the Egyptian aquaculture industry has seen rapid growth (FAO, 2003-2020). Egypt employs a number of aquaculture methods, including circular tanks, excavated floating fish cages, concrete and raceway ponds, pens and enclosures, and earthen ponds (Ghanem and Haggag, 2015). Among the different carp species, the following species are included:
Sharma and Leung (2000) mentioned that Indian farms cultured a number of carp species as well as some noncarp species (such as catfish and tilapia). Studies on carp polyculture using Indian major carp and exotic carp carried out at different stocking levels typically showed that the growth rates of the Indian main carp species were lower than those of the grass carp, common carp, and silver carp (Tripathi et al., 2000). The primary aquaculture methods used in Egypt and other nations involve the cultivation of Nile tilapia, common carp, and silver carp for maximum utilization of food (Abdel-Tawwab et al., 2007). Numerous studies have been conducted on freshwater ponds to examine the growth performance of common carp with tilapia (Zweig, 1989; Milstein, 1995). Khalil et al. (2023) cited that common carp are being raised in polyculture, which has improved their health status and innate immune system. The species percentage and initial body size of carp that are raised alongside tilapia determine the rate at which they grow (Papoutsoglou et al., 1992; Milstein, 1995). Both species achieved the highest growth rates, lowest food conversion ratios, and lowest carcass lipid contents in a proportion of 60% tilapia and 40% carp. Rearing of tilapia as an accompanying fish with carp in ponds is highly recommended for a 1-year-old carp, mullet and adult tilapia with feeding and fertilization can produce 1,218 kg/ha (El Bolock and Labib, 1967). The carp-rearing systems in fish farms can be classified into four main categories as follows. Extensive system With fewer inputs, such as feed, fertilizer, and stocking density (1–2 fish/m2), it is conducted in earthen ponds or in floating cages situated in natural water compounds and produces lower yields than semi-intensive or intensive farming (Sharma and Leung, 2000). Mires (1995) cited the fact that organic and chemical fertilizers are used by fish farmers in extensive systems to increase primary production in ponds, avoid additional feed inputs, and be characterized by low fish production. Nevertheless, El-Gamal (1997) mentioned that because of the rise in salinity, carp rearing in Lake Qarun had ceased. Pond culture is the most widely used method for raising carp in Egypt’s freshwater and brackish water systems (Hamza, 1989). Semi-intensive system According to El-Gamal (1997), it supplies over 75% of Egypt’s total aquaculture output, and the majority of farms are situated in the northern or eastern regions of the Nile Delta. These farms’ water supply is derived from agricultural drainage water. Fertilizers are used in management strategies at lower intensification levels to boost dissolved oxygen levels and promote natural productivity. It has been discovered that crop yields from such methods are greater than those from naturally unfertilized systems (Green, 1992). The fertilizer-feed management technique increases fish yields while also providing the opportunity to use less feed (Teichert-Coddington and Rodeiguez, 1995). Between 5 and 10 fish are stocked per square meter. In government-run farms, polyculture was practiced in smaller ponds of 2 to 6 ha with the use of fertilizers and supplemental feeding, whereas semi-intensive aquaculture was more frequently practiced (FAO, 2003). When natural feed becomes scarce, energy becomes a limiting factor instead of protein, necessitating supplemental feed (Nazish and Mateen, 2011). Intensive system In Egypt, it is not very common; 5 farms produce 500 tons of fish annually, mostly tilapia, using extremely intensive concrete ponds and tank culture (El-Gamal, 1997). Some government and private farms adhere to this culture. In this system, different species were introduced into ponds or cages at high stocking rates that reached 20 fish per square meter. It has been suggested that reduced stress related to fish behavior results in improved growth and physiology in an intensive system (Papoutsoglou et al., 2001). Because of its numerous benefits, including high productivity per unit area, ease of management, harvesting, and net cage culture, which was first used in 1951, accounted for 42% of the carp produced in Japan in 1982. Culture in running water ponds, on the other hand, originated from the technique previously employed for winter carp taken from rice fields (Suzuki, 1986). Integrate fish farm In various countries in Asia and Europe, integrated carp monoculture has been linked to farming (duck, rice, cereals) (Billard et al., 1990). Rice-fish culture is an ancient custom in Egypt; it was conventionally practiced by rice farmers in the northern Nile delta. A price for rice rose, high-yielding varieties were adopted, which increased productivity, and reclaimed land, was turned into a monoculture of rice (Halwart, 1999). El-Bolock and Labib (1967) raised 20–56 g common carp fingerlings in rice fields for 2–3 months at a stocking rate of 750–1,250 fingerlings/ha; the fish yield was 200 kg/ha, and the rice yield increased by 5%–7% as a result. The fish yield that ranged from 91.2 to 104 kg/ha was over a growing period of 153 days when common carp fingerlings were stocked at a stocking rate of 714 fish/ha. In addition, the rice crop increased by 11.4% in comparison to no stocked paddies. Sadek and Moreau (1998) stated that after 90 days of culture, the mean yields of prawns (Macrobrachium rosenbergii) at low and high stocking densities were 429.0 and 844.6 kg/ha, respectively. This was done by stocking the rice fields with 1 and 2 fish per m, respectively. In addition, carp pond farms have a higher agro-ecological value than plow lands and grasslands have a favorable impact on the microclimate of the area around them (Karnai and Szűcs, 2018). ConclusionComparatively speaking to other sources of animal protein, fish is an important economic source of protein, particularly in developing countries; it contributes to about 30% of the total amount of animal protein consumed. One of the most important freshwater fish for commerce and one of the most popularly raised species worldwide is carp. Carp is ranked second after tilapia in aquaculture production in Egypt. Polycuture system is more popular in the rearing of carp, especially with tilapia to achieve the highest growth rates, the lowest feed conversion ratios, and the lowest lipid contents in the carcasses. Moreover, integrated carp monoculture has been associated with agriculture with positive agro-ecological value. The successful development of pond aquaculture was in crop rearing. Conflict of interestThe authors declare that they have no conflict of interest. ReferencesAbdel Fattah, A.F., Fayza, A.A., Al-Sadik, Y.S., Hesham, H.M., Mohamed, Y.I. and Said, N.E. 2020. Effect of the different stocking density on behavior, performance and welfare of the Nile tilapia (Oreochromis niloticus). Egypt. J. Aquat. Biol. Fish. 24(5), 539–560. Abdel-Tawwab, M., Abdelghany, A.E. and Ahmad, M.H. 2007. Effect of diet supplementation on water quality, phytoplankton community structure, and the growth of Nile tilapia, Oreochromis niloticus (L.), Common carp, Cyprinus carpio (L.), and silver carp, Hypophthalmichthys molitrix (V.), polycultured in fertilized earthen ponds. J. Appl. Aquacult. 19(1), 1–24. Adámek, Z., Linhart, O., Kratochvíl, M., Flajšhans, M., Randák, T., Policar, T., Masojídek, J. and Kozák, P. 2012. Aquaculture in the Czech Republic in 2012: modern European prosperous sector based on thousand-year history of pond culture. Aquac. Eur. 37, 5–14. Adeleke, B., Robertson, A.D., Moodley, G. and Taylor, S. 2020. Aquaculture in Africa: a comparative review of Egypt, Nigeria, and Uganda vis-a-vis South Africa. Reviews in fish. Sci. Aquacult. 29(2), 167–197. Aloden, E.T. 1996. Broodstock management: an integral part of hatchery techniques. Aqua. Farm. News. 14(6), 11–17. Altmann, J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–267. Ashley, P.J. 2007. Fish welfare: current issues in aquaculture. Appl. Anim. Behav. Sci. 104, 199–235. Bais, B. 2018. Fish scenario in India with emphasis on Indian major carps. Int. J. Avian. Wild. Biol. 3(6), 409–411. Bardach, J.E., Ryther, J.H. and McLarney, W.O. 1972. Aquaculture: the farming and husbandry of freshwater and marine organisms. New York, NY: John Wiley & Sons, Inc., pp: 351. Barreto, R.E., Carvalho, G.A. and Volpato, G.L. 2011. The aggressive behaviour of Nile tilapia introduced into novel environments with variation in enrichment. Zoology 114(1), 53–57. Barus, V. 2002. Cyprinus carpio (Linnaeus 1758). Freshw. Fish. Eur. 5, 85–179. Billard, R., De Pauw, N., Micha, J.C., Salomoni, C. and Verreth, J. 1990. The impact of aquaculture in rural management. In Business joins science. Eds., De Pauw, N. and Billard, R. Bredene, Belgium: EAS Special Publications, pp: 57–91. Biro, P. 1995. Management of pond ecosystems and trophic webs. Aquaculture 129, 373–386. Calfee, R.D., Puglis, H.J., Little, E.E., Brumbaugh, W.G. and Mebane, C.A. 2016. Quantifying fish swimming behavior in response to acute exposure of aqueous copper using computer assisted video and digital image analysis. JVE 108, e53477. CAPMAS. 2014. Egypt in figures report 2014. Cairo, Egypt: Central Agency for Public Mobilization and Statistics. Ref. No. 71-01112-2014. Chen, Y. 1998. Fauna sinica: osteichthyes cypriniformes II. Beijing, China: Science Press. Chen, T.C., Ormond, R.F.G. and Mok, H.K. 2001. Feeding and territorial behaviour in juveniles of three co-existing triggerfishes. J. Fish. Biol. 59(3), 524–532. Chilton, E.W. and Muoneke, M.I. 1992. Biology and management of grass carp (Ctenopharyngodon idella, Cyprinidae) for vegetation control: a North American perspective. Fish. Boil. Fish. 2, 283–320. Conover, G., Simmonds, R. and Whalen, M. 2007. Management and control plan for Bighead, black, grass, and silver carps in the United States. Washington, DC: Asian Carp Working Group, Aquatic Nuisance Species Task Force, p: 223. Deepananda, K.H., Priyadarshana, T., Lenarolle, W.U. and Udayantha, H.M. 2014. Indian carps reproducing naturally in Udawalawe reservoir, Sri Lanka. Aquacult. Aquar. Conserv. Legis. 7(5), 333–341. Dey, M.M., Kumar, P., Chen, O.L., Khan, M.A., Barik, N.K., Li, L. and Pham, N.S. 2013. Potential impact of genetically improved carp strains in Asia. Food. Polc. 43, 306–320. DoF. 2018. Fishery statistical yearbook of Bangladesh 2017-18. Fisheries resources survey system. Dhaka, Bangladesh: Department of Fisheries, Ministry of Fisheries and Livestock. El Bolock, A.R. and Labib, W. 1967. Carp culture in the UAR. FAO. Fish. Rep. 44, 165–174. El-Gamal, A.R. Egyptian aquaculture: status and development requirements with special emphasis on tilapias and their potential in aquaculture. In Proceeding of the fourth International Symposium on Tilapia in Aquaculture, Orlando, FL, 1997, pp 9–12. El-Naggar, G.A.M., Nasr-Alla, A.H.M. and Kareem, R.O. Economic analysis of fish farming in Behera Governorate of Egypt. In Proceedings of 8th International Symposium on Tilapia in Aquaculture, Oct 12–14, Cairo, Egypt, 2008, vol. 1, pp 693–707. Emran, H., Akhtaruzzaman, K., Sourav, M. and Madan, M. 2022: Economic assessment of freshwater carp polyculture in Bangladesh: profit sensitivity, economies of scale and liquidity. Aquaculture 548(1), 737552. Essa, A.M., Mabrouk, H.A. and Zaki, M.A. 2004. Growth performance of grass carp, Ctenopharyngodon idella, and hybrid grass carp fingerlings fed on different types of aquatic plants and artificial diet in concrete basins. Egypt. J. Aquat. Res. 30(B), 341–348. Fall, F.M. 2005. Lab exercise: techniques for behavioural research in guppies bowling green state university. Anim. Behav. Boil. 420–543, 1–5. FAO. 2003. Egypt national aquaculture sector overview: characteristics, structure and resources of the sector. Fisheries Aquaculture Department, FAO: Rome, Italy. FAO. 2003-2020. National aquaculture sector overview. Egypt. National aquaculture sector overview fact sheets. In FAO Fisheries and Aquaculture Department [online]. Eds., Salem, A.M. and Saleh, M.A. Rome, Italy: FAO. (Updated 16 November 2010). FAO. 2007. Fishstat plus (v. 2.30). Rome, Italy: FAO. FAO. 2009. In cultured aquatic species fact sheets. In Edited and compiled by Valerio Crespi and Michael New. Ed., Peteri, A. CD-ROM (multilingual), FAO: Rome, Italy. FAO. 2016. FAO-yearbook of fishery statistics: aquaculture production. Rome, Italy: Food and Agriculture Organization of the United Nations, p: 30. Farag, F., Wally, Y.R., Daghash, S.M. and Ibrahim, A.M. 2014. Some gross morphological studies on the internal anatomy of the scaled common carp fish (Cyprinus carpio) in Egypt. J. Vet. Anat. 7(1), 15–29. Ferey, D.F. and Miller, R.J. 1972. The establishment of dominance relationships in the blue gourami. Behaviour 42(1–2), 9–59. Gafrd. 2014. General authority for fish resources development. Fish statistics year book, Ministry of Agriculture and Land Reclamation, Cairo, Egypt. Ghanem, A. and Haggag, M. 2015. Assessment of the feasibility of using filter made of rice straw for treating aquaculture effluents in Egypt. Res. Environ. 5(5), 135–145. Green, B.W. 1992. Substitution of organic manure for pelleted feed in tilapia production. Aquaculture 101(3–4), 213–222. Hagar, D.H.S. 2014. Brief summary about aquaculture in Egypt. Egypt. J. Aquacult. Mar. Biol. 1(1), 00003. Halwart, T. 1999. Fish in rice-based farming systems. In Proceedings of the 19th Session of the International Rice Commission, Cairo, Egypt. 7–9 Sep 1998, FAO, Rome, Italy, pp 130–137. Hamouda, A.H. and Moustafa, E.M. 2020. Insight on prevailing bacterial diseases affecting grass carp (Ctenopharyngodon idella) at Aswan Fish Hatchery, Egypt. AJVS 64(1), 15–29. Hamza, A.K. 1989. Fish culture development in Egypt. Bamidgeh 41(2), 43–49. Hecht, T., Moehl, J.F., Halwart, M. and Subasinghe, R.P. 2006. Regional review on aquaculture development: Sub-Saharan Africa-2005. Rome, Italy: Food and Agriculture Organization of the United Nations, pp: 97, 1017/5. Howes, G.J. 1991. Systematics and biogeography: an overview. In Cyprinid fishes: systematics, biology and exploitation. Eds., Winfield, I.J. and Nelson, J.S. Chapman & Hall London, pp: 1–33. Hulák, M., Kašpar, V., Kohlmann, K., Coward, K., Těšitel, J., Rodina, M., Gela, D., Kocour, M. and Linhart, O. 2010. Microsatellite-based genetic diversity and differentiation of foreign common carp (Cyprinus carpio) strains farmed in the Czech Republic. Aquaculture 298, 194–201. Islam, M.M. and Amin, A.A.M. 2016. The induced breeding of common carps (Cyprinus carpio) in Bangladesh. Ind. J. Sci. 23, 619–632. Ismail, M., Ali, R., Ali, T., Waheed, U. and Khan, Q.M. 2009. Evaluation of the acute toxicity of profenofos and its effects on the behavioral pattern of fingerling common carp ( Cyprinus carpio L., 1758). Bull. Environ. Contam. Toxicol. 82, 569–573. Jhingran, V.G. and Pullin, R.S.V. 1985. A hatchery manual for the common, Chinese and Indian major carps. Manila, Philippines: The Asian Development Bank, International Center for Living Aquatic Resources Management, MCC, Philippine. Karnai, L. and Szűcs, I. 2018. Outlooks and perspectives of the common carp production. Rocz. Ann. 1, 64–72. Kestemont, P. 1995. Different systems of carp production and their impacts on the environment. Aquaculture 129(1–4), 347–372. Khalil, A.H., Badrey, A.E., Harabawy, A.S., Ibrahim, A.T.A., Kloas, W. and Osman, A.G. 2023. Impact of polyculture in aquaponics on the hemato-serological and health status of Nile tilapia (Oreochromis niloticus) and carp (Cyprinus carpio). Egypt. J. Basic. Appl. Sci. 10(1), 410–419. Kolar, C.S., Chapman, D.C., Courtenay, W.R., Housel, C.M., Williams, J.D. and Jennings, D.P. 2007. Bigheaded carps: a biological synopsis and environmental risk assessment. Bethesda, MD: American Fisheries Society, p: 33. Liu, W.Y., Li, Y.J., Chen, X.T., Liu, G.X. and Li, S.Y. 1963. The digestion and utilization of grass carp on several herbivorous forages. Act. Hydrobiol. Sin. 3, 112–119. Milstein, A. 1995. Fish-management relationships in Israeli commercial fish farming. Aquacult. Int. 3(4), 292–314. Milstein, A. and Svirsky, F. 1996. Effect of fish species combinations on water chemistry and plankton composition in earthen fish ponds. Aquacult. Res. 27(2), 79–90. Mires, D. 1995. The tilapias. In Production of aquatic animals. Eds., Nash, C.E. and Novotny, A.J. Elsevier, New York, USA, pp: 133–152. Mohamed, A.A., Abdel Rahmanb, A.N., Hesham, H.M., Lamiaa, L.M., Azza, M.A., Sozan A.A. and Walaa, M.E. 2020. Neurobehavioral, apoptotic, and DNA damaging effects of sub-chronic profenofos exposure on the brain tissue of Cyprinus carpio L.: Antagonistic role of Geranium essential oil. Aquatic. Toxicol. 224, 105493. Mur, R. 2014. Development of the aquaculture value chain in Egypt: report of the national innovation platform workshop, Cairo, 19-20 February 2014. Cairo, Egypt: Worldfish. Murty, D.S., Sukramaran, K.K., Reddy, P.V.G.K. and Dey, R.K. 1986. Observations on the life history of silver carp, Hypophthalmichthys molitrix (Valenciennes). J. Inland. Fish. Soc. India. 18(1), 4–14. Nazish, N. and Mateen, A. 2011. Winter growth of carps under different semi-intensive culture conditions. Pak. Vet. J. 31(2), 134–136. Neto, J.F. and Giaquinto, P.C. 2020. Environmental enrichment techniques and tryptophan supplementation used to improve the quality of life and animal welfare of Nile tilapia. Aquacult. Rep. 17, 100354. Olopade, O.A., Mercy, G. and Zabbey, N. 2015. Estimation of length-weight relationship and proximate composition of catfish ( Claria gariepinus burchell, 1822) two different culture facilities. Turk. J. Agric. Food. Sci. Technol. 3(7), 566–570. Opuszyński, K. 1981. Comparison of the usefulness of the silver carp and the bighead carp as additional fish in carp ponds. Aquaculture 25(2–3), 223–233. Papoutsoglou, S.E., Miliou, H., Karakatsouli, N.P., Tzitzinakis, M. and Chadio, S. 2001. Growth and physiological changes in scaled carp and blue tilapia under behavioral stress in mono-and polyculture rearing using a recirculated water system. Aquacult. Int. 9, 509–518. Papoutsoglou, S.E., Petropoulos, G. and Barbieri, R. 1992. Polyculture rearing of Cyprinus carpio (L.) and Oreochromis aureus (St.) using a closed circulated system. Aquaculture 103(3-4), 311–320. Rahman, M.M. 2008. Capture-based aquaculture of wild-caught Indian major carps in the Ganges Region of Bangladesh. In Capturebased aquaculture. Global overview. Eds., Lovatelli, A. and Holthus, P.F. Rome, Italy: FAO Fisheries Technical. FAO. 508, pp: 127–140. Rana, K.J., Siriwardena, S. and Hasan, M.R. 2009. Impact of rising feed ingredient prices on aqua feeds and aquaculture production. Rome, Italy: FAO, p: 541. Sadek, S. 2013. Aquaculture site selection and carrying capacity estimates for inland and coastal aquaculture in the Arab Republic of Egypt. In Site selection and carrying capacities for Inland and coastal aquaculture. Eds., Ross, L.G., Telfer, T.C., Falconer, L., Soto, D. and Aguuilar-Manjarrez, J. pp: 183–196. Sadek, S. and Moreau, J. 1998. Culture of Macrobrachium rosenbergii in monoculture and polyculture with Oreochromis niloticus in paddies in Egypt. Bamidgeh 50(1), 33–42. Sadek, S., Osman, M.F. and Mezayen, A. Aquaculture in Egypt: a fragile colossus? In AQUA 2006 International Conference and Exhibition May 9–13, 2006, Firenze Florence, Italy. Said, E.N., Ahmed, F.A., Saleem, A.S., Mohammed, H.H., Youssef, M.Y. and Fattah, A.F.A. 2020. Behavioural response, welfare, and performance of Nile tilapia (Oreochromis niloticus) under different water temperatures. Int. J. Fish. Aquat. Stud. 8, 1–11. Saleh, M.A. 2007. Freshwater fish seed resources. Assessment of freshwater fish seed resources for sustainable aquaculture. vol. 501, pp: 241. Scott, G.R., Sloman, K.A., Rouleau, C. and Wood, C.M. 2003. Cadmium disrupts behavioural and physiological responses to alarm substance in juvenile rainbow trout (Oncorhynchus mykiss). J. Experim. Boil. 206(11), 1779–1790. Shaalan, M., El-Mahdy, M., Saleh, M. and El-Matbouli, M. 2018. Aquaculture in Egypt: insights on the current trends and future perspectives for sustainable development. Rev. Fish. Sci. Aquacult. 26(1), 99–110. Sharma, K.R. and Leung, P. 2000. Technical efficiency of carp production in India: a stochastic frontier production function analysis. Aquacult. Res. 31(12), 937–947. Shehata, S.M.A., Talab, A.S.A., Ghanem, M.H.M. and Abbas, M.M. 2018. Production and quality evaluation of hot smoked grass carp (Ctenopharyngodon idella) fillets stored at 4±1°C. Egypt. J. Aquatic. Biol. Fish. 22(5), 351–361. Soliman, N.F. and Yacout, D.M. 2016. Aquaculture in Egypt: status, constraints and potentials. Aquacult. Int. 24, 1201–1227. Suloma, A. and Ogata, H.Y. 2006. Future of rice-fish culture, desert aquaculture and feed development in Africa: the case of Egypt as the leading country in Africa. JARQ 40(4), 351–360. Suzuki, R. 1986. Intensive carp rearing in Japan. In Aquaculture of cyprinids. Eds., Billard, R. and Marcel, J. Paris, France: INRA, pp: 327–333. Takeuchi, T., Satoh, S. and Kiron, V (2002). Common carp, Cyprinus carpio. In Nutrient requirements and feeding of finfish for aquaculture. Wallingford, UK: CABI Publishing, pp: 245–261. Teichert-Coddington, D.R. and Rodriguez, R. 1995. Inorganic fertilization and feed reduction in commercial production of Penaeus vannamei during wet dry seasons in Honduras. In Twelfth annual technical report. Eds., Egna, H., Bowman, J., Goetze, B. and Weidner, N. Corvallis, OR; Pond Dynamics/Aquaculture CRSP, Oregon State University, pp: 136–146. Tripathi, S.D., Aravindakshan, P.K., Ayyappan, S., Jena, J.K., Muduli, H.K., Chandra, S. and Pani, K.C.I. 2000. New high in carp production in India through intensive polyculture. J. Aquacult. Trop. 15(2), 119–128. Tsai, C. and Ali, L. 1985. Open water fisheries (Carp) management programme in Bangladesh. Bgd. Fish. Inf. Bull. 2(4), 51. Tsai, C. and Ali, L. 1986. Carp spawn fishery in the Padma (Ganges)-Brahmaputra river system, Bangladesh. Indian. J. Fish. 33(4), 386–401. Wang, Q., Cheng, L., Liu, J., Li, Z., Xie, S. and De Silva, S.S. 2015. Freshwater aquaculture in PR China: trends and prospects. Rev. Aquacult. 7(4), 283–302. Weber, M.J., Brown, M.L. and Willis, D.W. 2010. Spatial variability of common carp populations in relation to lake morphology and physicochemical parameters in the upper Midwest United States. Ecol. Freshw. Fish. 19(4), 555–565. Weerakoon, D.E.M. 2013. Four decades of inland fisheries and aquaculture development in Sri Lanka. Res. J. Univ. Ruhuna. Sri. Lanka. Rohana. 9, 203–224. Woynarovich, A., Moth-Poulsen, T. and Péteri, A. 2010. Carp polyculture in Central and Eastern Europe, the Caucasus and Central Asia: a manual. Rome, Italy: Food and Agriculture Organization of the United Nations. Wu, X.W. 1977. The cyprinid fishes in China, part II. Shanghai, China: Shanghai Scientific and Technical Press. Yaqoob, S. 2021. A review of structure, origin, purpose & impact of common carp (Cyprinus carpio) in India. Ann. Rom. Soc. Cell. Biol. 25(6), 34–47. Zhou, J.F., Wu, Q.J., Ye, Y.Z. and Tong, J.G. 2003. Genetic divergence between Cyprinus carpio carpio and Cyprinus 305 carpio haematopterus as assessed by mitochondrial DNA analysis, with emphasis on origin of European 306 domestic Carp. Genetica 119, 93–97. Zweig, R.D. 1989. Evolving water quality in a common carp and blue tilapia high production pond. Hydrobiologia 171, 11–21. | ||
| How to Cite this Article |
| Pubmed Style Mohammed HH, Ebrahim MA, Youssef MI, Saleem AY, Abdelkhalek AA. Behavior and management of Carp fish: A review. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 1-11. doi:10.5455/OVJ.2024.v14.i1.1 Web Style Mohammed HH, Ebrahim MA, Youssef MI, Saleem AY, Abdelkhalek AA. Behavior and management of Carp fish: A review. https://www.openveterinaryjournal.com/?mno=175624 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.1 AMA (American Medical Association) Style Mohammed HH, Ebrahim MA, Youssef MI, Saleem AY, Abdelkhalek AA. Behavior and management of Carp fish: A review. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 1-11. doi:10.5455/OVJ.2024.v14.i1.1 Vancouver/ICMJE Style Mohammed HH, Ebrahim MA, Youssef MI, Saleem AY, Abdelkhalek AA. Behavior and management of Carp fish: A review. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 1-11. doi:10.5455/OVJ.2024.v14.i1.1 Harvard Style Mohammed, H. H., Ebrahim, . M. A., Youssef, . M. I., Saleem, . A. Y. & Abdelkhalek, . A. A. (2024) Behavior and management of Carp fish: A review. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 1-11. doi:10.5455/OVJ.2024.v14.i1.1 Turabian Style Mohammed, Hesham H., Mohamed A. Ebrahim, Mohamed I. Youssef, Al-Sadik Y. Saleem, and Adel A. Abdelkhalek. 2024. Behavior and management of Carp fish: A review. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 1-11. doi:10.5455/OVJ.2024.v14.i1.1 Chicago Style Mohammed, Hesham H., Mohamed A. Ebrahim, Mohamed I. Youssef, Al-Sadik Y. Saleem, and Adel A. Abdelkhalek. "Behavior and management of Carp fish: A review." Open Veterinary Journal 14 (2024), 1-11. doi:10.5455/OVJ.2024.v14.i1.1 MLA (The Modern Language Association) Style Mohammed, Hesham H., Mohamed A. Ebrahim, Mohamed I. Youssef, Al-Sadik Y. Saleem, and Adel A. Abdelkhalek. "Behavior and management of Carp fish: A review." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 1-11. Print. doi:10.5455/OVJ.2024.v14.i1.1 APA (American Psychological Association) Style Mohammed, H. H., Ebrahim, . M. A., Youssef, . M. I., Saleem, . A. Y. & Abdelkhalek, . A. A. (2024) Behavior and management of Carp fish: A review. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 1-11. doi:10.5455/OVJ.2024.v14.i1.1 |