| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 284–291 Original Research Prevalence, molecular characterization, and antimicrobial resistance among Escherichia coli, Salmonella spp., and Staphylococcus aureus strains isolated from Egyptian broiler chicken flocks with omphalitisRania Shaheen*, Moshira El-Abasy, Hanem El-Sharkawy and Mahmoud M. IsmailDepartment of Poultry and Rabbit Diseases, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt *Corresponding Author: Rania Shaheen. Department of Poultry and Rabbit Diseases, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt. Email: raniashaheen220 [at] yahoo.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
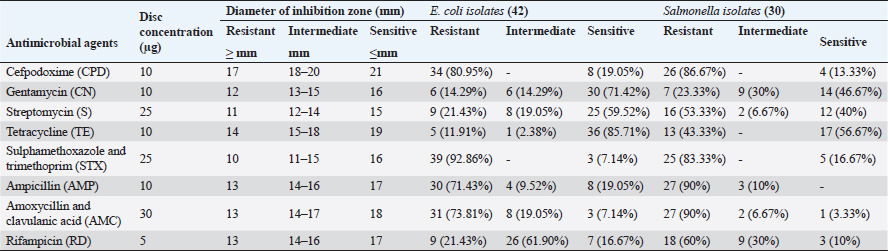
ABSTRACTBackground: Bacterial Omphalitis has been reported as a significant cause of mortalities in newly hatched broiler chicks. Aim: This study aimed to assess the occurrence of omphalitis among broiler chickens in Gharbia governorate in Egypt. In addition, the bacteria associated with the occurrence of omphalitis in broiler chickens were also investigated and characterized. Methods: For this purpose, 43 farms in that area were surveyed. The comparative levels of omphalitis caused by Escherichia coli (E. coli), Salmonella spp., and Staphylococcus aureus (S. aureus) were screened in 129 chicks. The drug resistance to eight commonly used antimicrobials in Egyptian poultry farms was screened using the disk diffusion method. Results: The overall incidence rate of omphalitis was 37.21%. In birds with omphalitis, the co-prevalence of S. aureus, Salmonella spp., and E. coli was 87.5%. When compared to healthy flocks, broiler chicks with omphalitis caused by Salmonella spp., E. coli, and S. aureus had a greater mortality rate in the first week of life. However, there were no significant differences in the mortality cases caused by these pathogens. Eighty-seven percent of the cases of omphalitis were linked to E. coli and 75% to Salmonella spp. and S. aureus. From the yolk sac of broiler chicks with omphalitis, E. coli, Salmonella spp., and S. aureus were isolated at rates of 87.5%, 62.5%, and 45.8%, respectively. The isolates of E. coli and Salmonella spp. exhibited great sensitivity to gentamycin and Tetracycline; however, the strongest drug resistance was observed toward cefpodoxime, sulphamethoxazole and trimethoprim, ampicillin, and amoxycillin and clavulanic acid. The recovered isolates of S. aureus showed susceptibility to chloramphenicol (72.37%), oxytetracycline (81.82%), and erythromycin (81.82%). However, every S. aureus isolate that was found resistant to amoxycillin and clavulanic acid, penicillin G and oxacillin. of blaTEM, blaSHV, and blaCTX-M genes has been proposed as the genetic cause of β-lactam antibiotic resistance in Salmonella spp. and E. coli. MecA and blaZ; however, were found in every strain of S. aureus. Conclusion: The frequency of omphalitis and its associated mortalities was comparatively high in Gharbia governorate. More efforts should be made to adopt strict hygienic standards for controlling and preventing such disease and this will consequently lead to minimizing the use of antimicrobials in poultry farms. Keywords: Omphalitis, Escherichia coli, Salmonella spp., Staphylococcus aureus, Antimicrobial resistant. IntroductionEgypt’s farming of broiler chickens is a significant industry. There are commercial operations in this business that are crucial to the growth of the economy. According to reports, one of the main causes of mortality in recently hatched broiler chicks is bacterial omphalitis (Pattison et al., 2008). Although omphalitis has been linked to mortality rates of 5%–10%, the infection may be responsible for significantly higher deaths in a batch of chicks within their first week of life (Rahman et al., 2007). Although bacterial omphalitis is not communicable, it is mainly caused by unhygienic hatchery equipment (Mosqueda and Lucio, 1985; Saif et al., 2003). It has also been reported that inadequately healed navels can harbor germs, leading to yolk sac infection (Mosqueda and Lucio, 1985). It is noteworthy that following hatching, the incidence of omphalitis and yolk sac infection rises and then falls by the end of the first week of life (Pattison et al., 2008; Yassin et al., 2009). The pathogens that cause omphalitis in newly hatched chicks include Escherichia coli, Enterobacter spp., Pseudomonas spp., Salmonella spp., Proteus spp., Staphylococcus spp., Klebsiella spp., and Clostridium spp. (Shane, 1999; Cortés et al., 2004; Giovanardi et al., 2005; Iqbal et al., 2006). About 70% of freshly hatched chicks with omphalitis had a high prevalence of E. coli in their yolk sacs, according to other investigations (Saif et al., 2003; Ahmed et al., 2009). After E. coli in chicks, Salmonella species and Staphylococcus aureus are thought to be the most significant bacterial drivers of yolk sac infection (Nasrin et al., 2012). The clinical signs of omphalitis in broiler chickens are most commonly associated with depression, gathering around the source of heat, and head drooping. The navel may partially open. During the course of the infection there is a progressive increase in the levels of inflammation of the umbilicus starting with redness, and then becoming wet and inflamed. At the end, a scab or “navel button” may be visible over the navel. The skin might be wet in severe cases and called “mushy chicks” (Nasrin et al., 2012; Shahjada et al., 2017). Previous studies reported that the lesions associated with omphalitis indicated that some of the acutely infected chicks showed lesions including redness, edema, and sometimes abscess of the navel. Unabsorbed yolk sacs with abnormal color, are also observed (Calnek et al., 1997; Shahjada et al., 2017). The misuse of antibiotics in chicken farming communities led to the spread of antimicrobial-resistant pathogens (Singer et al., 2003; Singer and Hofacre, 2006; Marshall and Levy, 2011; Regmi et al., 2020). Drug resistance is an important phenomenon, that correlates with the mechanisms associated with bacterial tolerance to antibiotics through harboring of resistance genes (Baker-Austin et al., 2006; Cytryn, 2013). Several studies documented that the prevalence of antimicrobial resistance is associated with the massive and abuse of antibiotics in the broiler chicken industry (Baker-Austin et al., 2006; Rizzo et al., 2013; Hu et al., 2017). Although E. coli, Salmonella spp., and S. aureus are very important causes of omphalitis in broiler chicken farms, no information mirrors these bacteria in the Gharbia governorate, Delta Egypt. Therefore, the objective of the current research is to determine the epidemiological data obtained from flocks with omphalitis. The study also provides information about the prevalence and antibiogram of E. coli, Salmonella spp., and S. aureus in cases of omphalitis in chicks. Materials and MethodsSample selectionA random sample of 43 broiler chicken farms was utilized to examine the prevalence of omphalitis in chicks during their initial week of life in the Gharbia governorate of Delta Egypt between April and August 2022. Humane sacrifices were performed on three surviving diseased chicks selected at random from each broiler chicken flock. The yolk sac contents were collected post-mortem in a completely aseptic environment to isolate E. coli, Salmonella, and S. aureus. The specimens were promptly frozen and conveyed to the laboratory within a time frame of 5 hours following their collection. Escherichia coli isolationFive milliliters of tryptic soy broth (TSB) (Oxoid, UK) was used to inoculate an exchange from each yolk sac, which was subsequently incubated at 37℃ for one night. The streaked incubated samples were placed on Oxoid methylene blue agar media (UK) before being incubated at 37℃ overnight. Isolation of SalmonellaOxoid-F broth was inoculated with a transfer from each yolk sac that was collected (Oxoid, UK). The inoculums were subsequently incubated overnight at 37℃. Overnight at 37℃, the colonies were incubated after being streaked onto xylose lysine desoxycholate agar (Oxoid, UK). Subsequent investigations were conducted on the colonies that appeared to be Salmonella spp. based on their coloration. Identification and isolation of S. aureusAs soon as the swabs were obtained, they were transferred to test tubes containing sterile tryptone soya broth (Oxoid, UK). For 24 hours, the bacterial cultures were incubated at 37℃. A loopful was distributed onto the Mannitol salt agar medium (LAB, U K) from each bacterial culture. For 24 hours, the plates were incubated at 37℃ to isolate S. aureus in a selective manner. To corroborate the presence of the suspected colonies, a coagulase test was performed. Biochemical characterization of Salmonella spp., E. coli, and S. aureusBacterial confirmation was achieved through biochemical identification utilizing the API 20E system for E. coli and Salmonella and API 20NE for S. aureus (BioMe’ rieux, Marcy-l’E’ toile, France). Testing for antimicrobial susceptibility (AST)Utilizing the Kirby-Bauer disc diffusion method (Bauer, 1966), the AST was conducted. Each bacterial strain was inoculated with a single colony of bacteria overnight at TSB 24 hours. Diluting the overnight cultures in sterile saline was performed. The suspension was modified in accordance with the 0.5 McFarland standards, which comprised an estimated 1–2 x 108 CFU/ml of E. coli from the American Type Culture Collection 25922. Using a sterile sponge, the saline suspension was applied to the surface of the Mueller–Hinton Agar plate. Using a multidisc dispenser from Oxoid, the antibiotic-containing antimicrobial discs utilized in the Egyptian poultry drug market were dispensed onto the Mueller–Hinton Agar plates at least 24 mm from the center of each disc. Following that, the dishes were incubated at 37℃ overnight for 16–18 hours. Using sliding calipers, the diameters of the inhibited zones, including the diameters of the discs, were determined to the nearest whole millimeter. Standard break points as specified by the Clinical Laboratory Standards Institute (CLSI, 2016) were employed for interpretation (Tables 1 and 2). Table 1. Break-point values of antimicrobial agents according to CLSI (2016) and phenotypic antibiotic sensitivity profiles for E. coli and Salmonella spp., isolates from Broiler chickens with omphalitis.
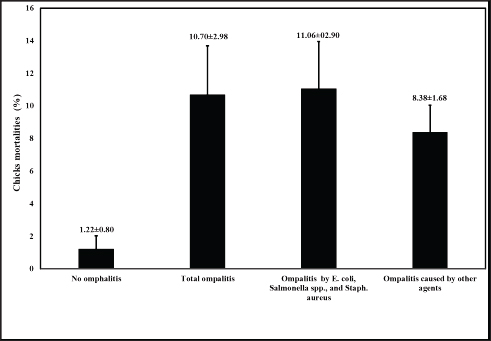
Genomic DNA extraction and purificationA single colony was inoculated into 5 ml of TSB (Oxoid, UK) from each bacterial-specific agar, and then incubated overnight at 37℃. For 10 minutes, the bacterial cultures were centrifuged at 13,000 rpm. After discarding the supernatant, the bacterial granules were reconstituted in sterile nuclease-free water and subjected to boiling for a duration of 10 minutes. Following centrifugation of the boiled bacterial lysates, the supernatant was extracted and stored as DNA templates at −80℃ until further use. Molecular detection of antimicrobial resistance-associated genes and genus-specific markersSpecific primers were utilized to detect Salmonella spp., E. coli, and S. aureus using the inva, phoA, and S. aureus 23S rRNA genes (Oliveira et al., 2003; Hu et al., 2011; Bhati et al., 2016). Isolates of S. aureus, Salmonella spp., and E. coli were screened for three extended spectrum β-lactamase genes that have been linked to resistance to β-lactamase antibiotics. The corresponding genes were blaTEM, blaSHV, and blaCTXM, in accordance with the procedures outlined previously (Colom et al., 2003; Archambault et al., 2006). Furthermore, primers for the oxacillin resistance gene, mecA, and the Penicillin resistance gene, blaZ, were utilized to test S. aureus isolates. The procedure was as previously described (McClure et al., 2006; Bagcigil et al., 2012). The polymerase chain reaction (PCR) was conducted in adherence to the guidelines provided by the manufacturer (Takara, Japan). In summary, 25 µl of the PCR mixture was utilized for each pair of primers. 12.5 µl of EmeraldAmp Max PCR Master Mix (Takara, Japan), 1 µl of each primer (20 pmol), 5.5 µl of molecular grade water, and 5 µl of the extracted DNA template comprised this PCR. PCR reactions were processed using a 2720 thermal cycler from Applied Biosystems. Positive controls consisted of previously validated positive isolates obtained from the Animal Health Research Institute in Egypt. As a control negative, sterile molecular-grade water was utilized. Statistical analysisThe omphalitis-associated mortalities caused by E. coli, Salmonella spp., and S. aureus 1week-old flocks was analyzed by the student t-test. Ethical approvalAll animal procedures and experiments were carried out in adherence to local regulations subsequent to receiving sanction from the Local Ethical Committee of Poultry Diseases of the Faculty of Veterinary Medicine, Kafrelsheikh University, Egypt. ResultsIncidence and identification of the bacterial causes of omphalitisThe total number of broiler chicken farms with positive omphalitis in the first week of life was 16 out of 43 flocks with a prevalence rate of 37.21%. The mortality rate (11.06% ± 2.90%) of the 1-week-old flocks with omphalitis caused by E. coli, Salmonella spp., and S. aureus was significantly higher (p < 0.01) than the mortality rate (1.22% ± 0.80%) in the healthy group. There were no significant differences in the mortality rates between total omphalitis and omphalitis caused by E. coli, Salmonella spp., and S. aureus, and omphalitis caused by other infections (Fig. 1). Table 2. Break-point values of antimicrobial agents according to CLSI (2016) and phenotypic antibiotic sensitivity profiles for S. aureus isolates recovered from broiler chickens with omphalitis.
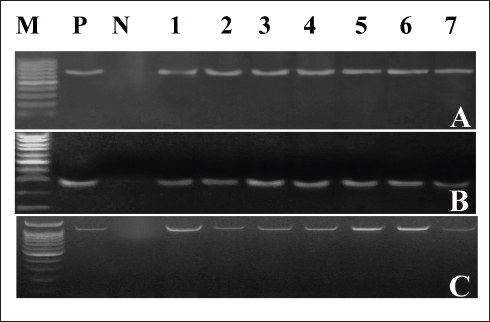
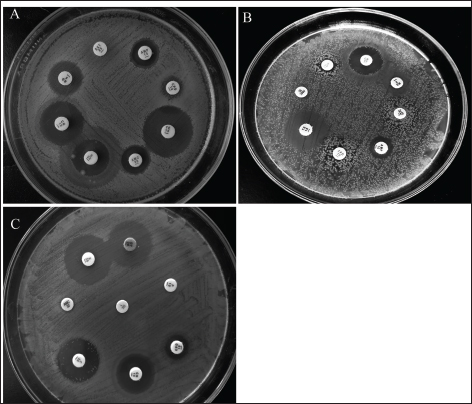
Clinical symptoms of omphalitis observed in the 1-week-old broiler chicks included huddling around the heat source, unhealed and inflamed naval that failed to close with the presence of a scape. There was also diarrhea and a swollen abdomen. We also found wet spots in the abdomen with an increase in the mortalities. The main growth lesions observed were dehydration, unabsorbed yolk sacs, which were severely enlarged, and congested with abnormal yolk contents. Forty-two chicks from 14 broiler flocks had 94 isolates of Enterobacteriaceae, and S. aureus, including 42 E. coli, 30 Salmonella spp., and 22 S. aureus. Seven individual chicks had E. coli only, 13 chicks had co-infected with both E. coli and Salmonella spp., and 17 chicks were infected with E. coli, Salmonella spp., and S. aureus. Whereas two flocks (six chicks) had other causative agents. The isolated bacteria were confirmed by PCR using genus-specific genes including phoA gene for E. coli, invA for Salmonella spp., and 23S rRNA for S. aureus (Fig. 2). The prevalence of omphalitis caused by Enterobacteriaceae and S. aureus represented 87.5% of cases at the flock scale in the study area. Our results indicated that one of the 16 broiler chicken farms with omphalitis was infected with E. coli only. Two broiler flocks were co-infected with either a combination of E. coli and Salmonella spp. or E. coli and S. aureus. Eleven more flocks had mixed infections with E. coli, Salmonella spp., and S. aureus. AST resultsThe overall antimicrobial resistance rate of Salmonella isolates to the tested antibiotics was 66.25%, followed by S. aureus isolates at 57.27%, and then E. coli strains at 48.52%. For Salmonella and E. coli isolates the drug resistance rates were as follows: cefpodoxime (86.67% and 80.95%), gentamycin (23.33% and 13.95%), streptomycin (53.33% and 20.93%), tetracycline (43.33% and 11.63%), sulphamethoxazole and trimethoprim (83.33% and 93.02%), ampicillin (90%% and 71.43%), amoxycillin and clavulanic acid (90% and 73.81%), and rifampicin (60% and 20.93%), respectively (Table 1, Fig. 3). All the 22 tested S. aureus isolates were 100% resistant to three tested β-lactams, namely, oxacillin, penicillin G, amoxycillin and clavulanic acid. In addition, 90% of S. aureus isolates showed resistance to cefpodoxime. While the lowest resistance was to erythromycin (18.18%), oxytetracycline (18.18%), and chloramphenicol (27.27%), while 36.64% of S. aureus isolates were resistant to gentamycin (Table 2, Fig. 3). Molecular characterization of β-lactameses-related genes showed that blaTEM was detected in 20/42 (47.62%) and 18/30 (60%) of E. coli and Salmonella isolates, respectively. While 9/42 (21.43%) of E. coli and 8/30 (26.67%) of Salmonella isolates had blaSHV gene. In addition, blaCTX-M was detected in 28.57% and 30% of the recovered E. coli, and Salmonella isolates, respectively . Furthermore, mecA and blaZ were detected in all S. aureus isolates (Fig. 4). DiscussionThe present study documented 37.21% of omphalitis cases in broiler poultry farms situated in the governorate of Gharbia. The results of this study indicate that veterinarians and farmers in the region did not employ hygienic measures as standard practices at poultry and hatcheries. Prior research has documented a significant incidence of omphalitis (Rosario et al., 2005; Abdel-Tawab et al., 2016). Moreover, we document a significant loss of life among the livestock at 1 week of age due to omphalitis, which was caused by Salmonella spp., E. coli, and S. aureus. Rahman et al. (2007) probably documented a significant number of fatalities among poultry farms that were afflicted with bacterial omphalitis. In comparison to Salmonella spp. and S. aureus, E. coli exhibited the highest prevalence among the bacterial pathogens responsible for omphalitis in that particular region of Egypt. Solely from one of the 16 broiler poultry farms afflicted with omphalitis, E. coli was isolated. Eleven additional flocks were co-infected with E. coli, Salmonella spp., and S. aureus. This study also revealed that two broiler flocks were infected with a combined infection of E. coli and Salmonella spp. or E. coli and S. aureus. This observation is consistent with prior research (Iqbal et al., 2006; Amare et al., 2013; Abdel-Tawab et al., 2016), which documented that E. coli, along with one of the three other bacterial species (S. aureus, Salmonella spp., and Pseudomonas aeruginosa), accounted for the majority of broiler chicken omphalitis cases.
Fig. 1. Mortality rate (%) observed in broiler chicken flocks from the screened farms in Gharbia governorate, Delta Egypt.
Fig. 2. PCR amplification of the causative agents of Omphalitis in 1-week-old broiler chickens. (A) phoA gene-specific genomic markers for E. coli (representative). (B) invA specific marker for Salmonella (representative). (C) S. aureus 23S rRNA genes.
Fig. 3. Antimicrobial sensitivity testing of the recovered A) E. coli, B) Salmonella spp., and C) S. aureus isolates against antimicrobials that are most commonly used in the Egyptian drug market for broiler chickens using the disk diffusion method.
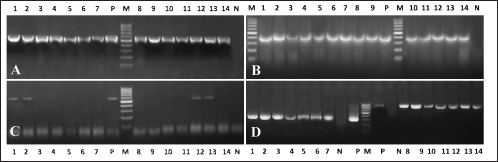
Fig. 4. Electrophoresis of β-lactamases associated with β-lactams resistance in E. coli, Salmonella spp., and S. aureus isolated from Egyptian broiler chicken farms with omphalitis. (A) PCR amplification of blaTEM gives an expected band segment at 516 bp, lane 1–7 representative for Salmonella positive strains while lane 8–14 representative for E. coli positive isolates (B) the expected band segment of blaSHIV at 392 bp, lane 1–7 representative for Salmonella positive strains while lane 8–14 representative for E. coli positive isolates (C) the amplification of blaCTXM gives an expected band at 593 bp lane 1–7 representative for Salmonella positive strains while lane 8–14 representative for E. coli positive isolates (D) lane 1–7 for mecA in S. aureus with expected band size 310 bp while lane 8–14 presentative for 14 for blaZ in S. aureus with expected band size 833 bp. Lane L is a 100bp DNA ladder. Antimicrobial resistance was present in the recovered isolates of Salmonella spp., S. aureus, and E. coli at respective rates of 66.25%, 57.27%, and 40.48%. The relatively high rates observed suggest that certain commercial broiler poultry farms in Egypt make extensive use of antibiotics, resulting in the devolution of the population of antimicrobial-resistant bacteria. The same phenomenon was documented in multiple studies (Akond et al., 2009; Amare et al., 2013; Shahjada et al., 2017). It was noted that resistance phenotypes to β-lactamases, such as ampicillin, amoxycillin and clavulanic acid, and cefpodoxime, which were detected in the AST, were attributable to the existence of blaTEM, blaSHIV, or blaCTXM genes. -lactamase-resistant E. coli and Salmonella spp. were found to contain blaTEM in their entirety, whereas blaCTX-M was detected in 28.57% and 40% of the aforementioned bacteria and spp., respectively. Prior research has documented comparable findings (El-Sharkawy et al., 2017; Gundran et al., 2019). It is noteworthy that each strain of S. aureus that exhibited resistance to Oxacillin and Penicillin G possessed the mecA and BlaZ alleles, which confer resistance to Oxacillin and Penicillin G, respectively. This finding aligns with prior research conducted concurrently (Pinho, 2008; Tahoun et al., 2022). ConclusionThis study threw light on the occurrence of bacterial omphalitis in chicks under intensive rearing systems in the Gharbia governorate in Egypt. The recovered bacterial causes included E. coli, Salmonella spp., and S. aureus had clear antimicrobial resistance toward the most commonly used drugs in poultry farms in Egypt. Therefore, national monitoring and educational programs are strongly recommended among poultry farmers to reduce such a challenging issue. AcknowledgmentsThe authors also thank the veterinarians for their kind support during sampling procedures. Author contributionsConceptualization: H.E., M.M., and M.E., Methodology: H.E. and R.S., Investigation: H.E., R.S., M.M., and M.E. Data analysis: H.E. and R.S., Writing original draft: H.E. and R.S., Writing-review and editing: H.E., R.S., M.M., and M.E., Supervision: H.E., M.M., and M.E.; All authors have read and agreed to the published version of the manuscript. Conflict of interestsThe authors declare that they have no conflict of interest. FundingNot applicable. Data availabilityIf you have a valid request for further information, you can get it from the corresponding author of this paper. ReferencesAbdel-Tawab, A.A., Nasef, S.A. and Ibrahim, O.A. 2016. Bacteriological and molecular studies on bacteria causing omphalitis in chicks with regard to disinfectant resistance. Glob. Vet. 17(6), 539–545. Ahmed, M., Sarker, A. and Rahman, M. 2009. Prevalence of infectious diseases of broiler chickens in Gazipur district. Bangl. J. Vet. Med. 7(2), 326–331. Akond, M.A., Alam, S., Hassan, S. and Shirin, M. 2009. Antibiotic resistance of Escherichia coli isolated from poultry and poultry environment of Bangladesh. Int. J. Food. Saf. 11, 19–23. Amare, A., Amin, A.M., Shiferaw, A., Nazir, S. and Negussie, H. 2013. Yolk sac infection (omphalitis) in Kombolcha poultry farm, Ethiopia. Am. Euras. J. Sci. Res. 8(1), 10–14. Archambault, M., Petrov, P., Hendriksen, R.S., Asseva, G., Bangtrakulnonth, A., Hasman, H. and Aarestrup, F.M. 2006. Molecular characterization and occurrence of extended-spectrum β-lactamase resistance genes among Salmonella enterica serovar Corvallis from Thailand, Bulgaria, and Denmark. Microb. Drug. Resist. 12(3), 192–198. Bagcigil, A.F., Taponen, S., Koort, J., Bengtsson, B., Myllyniemi, A.-L. and Pyörälä, S. 2012. Genetic basis of penicillin resistance of S. aureus isolated in bovine mastitis. Acta. Vet. Scand. 54(1), 1–7. Baker-Austin, C., Wright, M.S., Stepanauskas, R. and McArthur, J. 2006. Co-selection of antibiotic and metal resistance. Trends. Microbiol. 14(4), 176–182. Bauer, A. 1966. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 45, 149–158. Bhati, T., Nathawat, P., Sharma, S.K., Yadav, R., Bishnoi, J., and Kataria, A.K. 2016. Polymorphism in spa gene of Staphylococcus aureus from bovine subclinical mastitis. Vet. World. 9(4), 421–424. Calnek, B., Barnes, H., Beard, C., McDougald, L. and Saif, Y. 1997. Diseases of poultry 10th ed. Poult. Sci. 87, 1643–1648. CLSI. 2016. Performance standards for antimicrobial susceptibility testing, 26th ed. Wayne, PA: CLSI Supplement M100S. Colom, K., Pérez, J., Alonso, R., Fernández-Aranguiz, A., Lariño, E. and Cisterna, R. 2003. Simple and reliable multiplex PCR assay for detection of bla TEM, bla SHV and bla OXA–1 genes in Enterobacteriaceae. FEMS. Microbiol. Lett. 223(2), 147–151. Cortés, C.R., Isaías, G.T., Cuello, C.L., Flores, J.M.V., Anderson, R.C. and Campos, C.E. 2004. Bacterial isolation rate from fertile eggs, hatching eggs, and neonatal broilers with yolk sac infection. Revista. Latinoamericana. De. Microbiologia. 46(1-2), 12–16. Cytryn, E. 2013. The soil resistome: the anthropogenic, the native, and the unknown. Soil. Biol. Biochem. 63, 18–23. El-Sharkawy, H., Tahoun, A., El-Gohary, A.E.-G.A., El-Abasy, M., El-Khayat, F., Gillespie, T. and El-Adawy, H. 2017. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut. Pathogens. 9(1), 1–12. Giovanardi, D., Campagnari, E., Ruffoni, L.S., Pesente, P., Ortali, G. and Furlattini, V. 2005. Avian pathogenic Escherichia coli transmission from broiler breeders to their progeny in an integrated poultry production chain. Avian. Pathol. 34(4), 313–318. Gundran, R.S., Cardenio, P.A., Villanueva, M.A., Sison, F.B., Benigno, C.C., Kreausukon, K. and Punyapornwithaya, V. 2019. Prevalence and distribution of blaCTX-M, blaSHV, blaTEM genes in extended-spectrum β-lactamase-producing E. coli isolates from broiler farms in the Philippines. BMC. Vet. Res. 15(1), 1–8. Hu, H.-W., Wang, J.-T., Li, J., Shi, X.-Z., Ma, Y.-B., Chen, D. and He, J.-Z. 2017. Long-term nickel contamination increases the occurrence of antibiotic resistance genes in agricultural soils. Environ. Sci. Technol. 51(2), 790–800. Hu, Q., Tu, J., Han, X., Zhu, Y., Ding, C. and Yu, S. 2011. Development of multiplex PCR assay for rapid detection of Riemerella anatipestifer, Escherichia coli, and Salmonella enterica simultaneously from ducks. J. Microbiol. Methods. 87(1), 64–69. Iqbal, M., Shah, I., Ali, A., Khan, M. and Jan, S. 2006. Prevalence and in vitro antibiogram of bacteria associated with omphalitis in chicks. Proteus 13(5), 8. Marshall, B.M. and Levy, S.B. 2011. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 24(4), 718–733. McClure, J.-A., Conly, J.M., Lau, V., Elsayed, S., Louie, T., Hutchins, W. and Zhang, K. 2006. Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton-Valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from-resistant staphylococci. J. Clin. Microbiol. 44(3), 1141–1144. Mosqueda, T.A. and Lucio, M.B. 1985. Enfermedades communes de la saves domesticas. Universidad Nacional Autonoma de Mexico, pp: 377–384. Nasrin, S., Islam, M., Khatun, M., Akhter, L. and Sultana, S. 2012. Characterization of bacteria associated with omphalitis in chicks. Bangl. Vet. 29(2), 63–68. Oliveira, S., Rodenbusch, C., Ce, M., Rocha, S. and Canal, C. 2003. Evaluation of selective and non‐selective enrichment PCR procedures for Salmonella detection. Lett. Appl. Microbiol. 36(4), 217–221. Pattison, M., McMullin, P., Bradbury, J.M., and Alexander, D. 2008. Poultry disease, 6th ed. London, UK: Saunders Elsevier, pp: 141–142. Pinho, M.G. 2008. Mechanisms of beta-lactam and glycopeptide resistance in Staphylococcus aureus. Staphy. Mol. Genet. pp: 207–226. Rahman, M., Rahman, A. and Islam, M. 2007. Bacterial diseases of poultry prevailing in Bangladesh. J. Poult. Sci. 1(1), 1–6. Regmi, B., Dhakal, I., Shah, M. and Pande, K. 2020. A retrospective study of poultry diseases diagnosed at the Veterinary Laboratory, Pokhara, Nepal. Int. J. Food Sci. Agricult. 4(4), 398–402. Rizzo, L., Manaia, C., Merlin, C., Schwartz, T., Dagot, C., Ploy, M. and Fatta-Kassinos, D. 2013. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci. Total. Environ. 447, 345–360. Rosario, C., Puente, J., Verdugo-Rodriguez, A., Anderson, R., and Eslava, C. 2005. Phenotypic characterization of ipaH+ Escherichia coli strains associated with yolk sac infection. Avian. Dis. 49(3), 409–417. Saif, Y., Barnes, H., Glisson, J., Mcdougald, L., Fadly, A. and Swayne, D. 2003. Diseases of poultry, 11th ed. Ames, IA: Iowa State Press, 2121, pp: 1103–1113. Shahjada, Z., Hussain, K., Islam, M.M., Majumder, S., Hasan, I., Rahman, M. and Saha, S. 2017. Bacteria causing omphalitis in newly hatched chicks from broiler and layer flocks and their antibiotic profiles. Int. J. Natl. Soc. Sci. 4(2), 73–81. Shane, S. 1999. Promoting chick quality and livability. Zootecnica. Int. 22, 46–49. Singer, R.S., Finch, R., Wegener, H.C., Bywater, R., Walters, J. and Lipsitch, M. 2003. Antibiotic resistance—the interplay between antibiotic use in animals and human beings. Lancet. Infect. Dis. 3(1), 47–51. Singer, R.S. and Hofacre, C.L. 2006. Potential impacts of antibiotic use in poultry production. Avian. Dis. 50(2), 161–172. Tahoun, A., Elnafarawy, H.K., El-Sharkawy, H., Rizk, A.M., Alorabi, M., El-Shehawi, A.M. and El-Khodery, S. 2022. The prevalence and molecular biology of Staphylococcus aureus isolated from healthy and diseased equine eyes in Egypt. Antibiotics 11(2), 221. Yassin, H., Velthuis, A.G., Boerjan, M. and van Riel, J. (2009). Field study on broilers’ first-week mortality. Poult. Sci. 88(4), 798–804. | ||
| How to Cite this Article |
| Pubmed Style Shaheen R, El-Abasy M, El-Sharkawy H, Ismail MM. Prevalence, molecular characterization and antimicrobial resistance among Escherichia coli, Salmonella spp. and Staphylococcus aureus strains isolated from Egyptian broiler chicken flocks with omphalitis. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 284-291. doi:10.5455/OVJ.2024.v14.i1.25 Web Style Shaheen R, El-Abasy M, El-Sharkawy H, Ismail MM. Prevalence, molecular characterization and antimicrobial resistance among Escherichia coli, Salmonella spp. and Staphylococcus aureus strains isolated from Egyptian broiler chicken flocks with omphalitis. https://www.openveterinaryjournal.com/?mno=177378 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.25 AMA (American Medical Association) Style Shaheen R, El-Abasy M, El-Sharkawy H, Ismail MM. Prevalence, molecular characterization and antimicrobial resistance among Escherichia coli, Salmonella spp. and Staphylococcus aureus strains isolated from Egyptian broiler chicken flocks with omphalitis. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 284-291. doi:10.5455/OVJ.2024.v14.i1.25 Vancouver/ICMJE Style Shaheen R, El-Abasy M, El-Sharkawy H, Ismail MM. Prevalence, molecular characterization and antimicrobial resistance among Escherichia coli, Salmonella spp. and Staphylococcus aureus strains isolated from Egyptian broiler chicken flocks with omphalitis. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 284-291. doi:10.5455/OVJ.2024.v14.i1.25 Harvard Style Shaheen, R., El-Abasy, . M., El-Sharkawy, . H. & Ismail, . M. M. (2024) Prevalence, molecular characterization and antimicrobial resistance among Escherichia coli, Salmonella spp. and Staphylococcus aureus strains isolated from Egyptian broiler chicken flocks with omphalitis. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 284-291. doi:10.5455/OVJ.2024.v14.i1.25 Turabian Style Shaheen, Rania, Moshira El-Abasy, Hanem El-Sharkawy, and Mahmoud M. Ismail. 2024. Prevalence, molecular characterization and antimicrobial resistance among Escherichia coli, Salmonella spp. and Staphylococcus aureus strains isolated from Egyptian broiler chicken flocks with omphalitis. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 284-291. doi:10.5455/OVJ.2024.v14.i1.25 Chicago Style Shaheen, Rania, Moshira El-Abasy, Hanem El-Sharkawy, and Mahmoud M. Ismail. "Prevalence, molecular characterization and antimicrobial resistance among Escherichia coli, Salmonella spp. and Staphylococcus aureus strains isolated from Egyptian broiler chicken flocks with omphalitis." Open Veterinary Journal 14 (2024), 284-291. doi:10.5455/OVJ.2024.v14.i1.25 MLA (The Modern Language Association) Style Shaheen, Rania, Moshira El-Abasy, Hanem El-Sharkawy, and Mahmoud M. Ismail. "Prevalence, molecular characterization and antimicrobial resistance among Escherichia coli, Salmonella spp. and Staphylococcus aureus strains isolated from Egyptian broiler chicken flocks with omphalitis." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 284-291. Print. doi:10.5455/OVJ.2024.v14.i1.25 APA (American Psychological Association) Style Shaheen, R., El-Abasy, . M., El-Sharkawy, . H. & Ismail, . M. M. (2024) Prevalence, molecular characterization and antimicrobial resistance among Escherichia coli, Salmonella spp. and Staphylococcus aureus strains isolated from Egyptian broiler chicken flocks with omphalitis. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 284-291. doi:10.5455/OVJ.2024.v14.i1.25 |