| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 292–303 Original Research Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in ratsGamal Shams1, Somia Abd Allah2, Raghda Ezzat3* and Mahmoud A. Said41Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Biochemistry Department, Faculty of Medicine, Zagazig University, Zagazig, Egypt 3Faculty of Pharmacy, Zagazig University, Zagazig, Egypt 4Medical Administration, Students' Hospital, Zagazig University, Zagazig, Egypt *Corresponding Author: Raghda Ezzat. Faculty of Pharmacy, Zagazig University, Zagazig, Egypt. Email: raghda_ezzat86 [at] yahoo.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
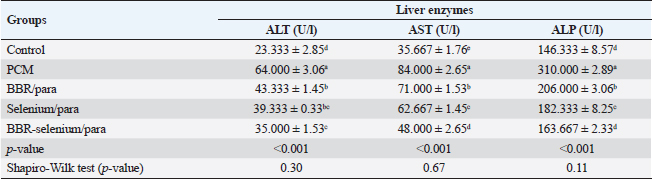
ABSTRACTBackground: Paracetamol (PCM) overdosing induces hepatotoxicity, which can result in death if the dose is high enough and the patients are not given N-acetyl cysteine. Berberine (BBR) has a variety of biological proprieties including anti-inflammatory and antioxidant activities. Aim: Assessment of the potential effect of BBR and selenium when used alone or together on the PCM–induced acute hepatic toxicity in rats. Methods: This research involved 40 clinically healthy mature adult male albino rats, their weights ranged from 150 to 200 g and housed in standard conditions. Our study involved evaluating the potential effect of BBR and selenium when used alone or together on the PCM–induced acute hepatic toxicity via estimation of the liver function tests, determination of the antioxidant enzyme activities, lipid peroxidation markers, immune-modulatory effects, liver histopathological, and immunohistochemical studies. Results: Co-treatment of BBR (150 mg/kg BW) with selenium (5 mg/kg BW) showed significant improvement in the liver function parameters, the antioxidant enzyme activities, reduction in the nitric oxide (NO), lysozyme, malondialdehyde (MDA), TNF-α, and TGF-β1 levels, and marked elevation in the IgM levels. Conclusion: Altogether, BBR, selenium, or both augment antioxidant activity and alleviate PCM-induced hepatic toxicity. Keywords: Protective, Berberine, Selenium, Paracetamol, Induced hepatic toxicity. IntroductionHepatotoxicity refers to liver damage caused by chemicals. Certain pharmacological substances can harm the organ when consumed in excess dosages or even when administered within therapeutic parameters without observation of the withdrawal times. Hepatotoxicity can also be caused by other chemical agents (Feng et al., 2018). Paracetamol (PCM) overdosing induces hepatotoxicity, which can result in death if the dose is high enough and the patients are not given N-acetyl cysteine. The production of the oxidized metabolite of PCM and its interaction with glutathione causes hepatotoxicity in PCM overdosing. For many years, it has been recognized that therapeutic PCM use is linked to subclinical increases in liver damage indicators (Baskar et al., 2014). Berberine (BBR) is an alkaloid isoquinoline derivative identified from numerous plant species (Miwa et al., 1998). It has been shown that this compound has a variety of biological proprieties, involving anti-hyperglycaemic, anti-neoplastic, anti-inflammatory, and antioxidant actions (Ang et al., 2010; Polk et al., 2016; Xu et al., 2019). Production of inflammatory mediators is inhibited by BBR according to previous studies (Lou et al., 2011; Fontanella et al., 2014). In addition, BBR appears to improve the antioxidant defense mechanism within the cell, involving the activity of glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) while decreasing oxidative stress variables such as malondialdehyde (MDA), NO levels, and protein carbonyl content (Zhou and Zhou, 2011). BBR has been found to impact inflammation by reducing IL-6 and TNF-α levels, consequently increasing the synthesis of matrix metalloproteinase in a variety of healthy and pathological contexts (Ehteshamfar et al., 2020; Wang et al., 2020). BBR’s antioxidant impact was equivalent to that of vitamin C, a potent antioxidant. On laboratory animals (mice) with doxorubicin-induced hepatotoxicity, the hepatoprotective effect of BBR was revealed. BBR pre-treatment dramatically decreased both histological injury and functional hepatic tests (Zhao et al., 2012). The mechanism by which BBR lowers CCl4 (carbon tetrachloride)-induced hepatotoxicity was also investigated. BBR has a favorable effect on liver changes via regulation of the inflammatory response and lowering of nitrosamine and oxidative stress in hepatic tissue. BBR contributes to the reduction of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and TNF-α levels, suppresses the decline in SOD activity, and increases lipid peroxidation (Domitrović et al., 2011). Selenium is an effective mineral and micronutrient with anti-inflammatory and antioxidant properties that can alter the immunological and inflammatory responses in the cell. Selenium is an essential component of the enzyme Gpx, which detoxifies free oxygen radicals and other toxic oxygen derivatives (Ringuet et al., 2021). Se deficiency is associated with several diseases, including liver damage, and Se supplementation can recover the hepatic damage (Xu et al., 2022). Furthermore, Se may assist in the treatment of liver diseases including hepatocellular carcinoma (Chi et al., 2021). The aim of this work was to evaluate the potential effect of BBR and selenium when used alone or together on the PCM–induced acute hepatic toxicity. Materials and MethodsMedicationsBBR (wellbetx)®: was obtained from Santa Cruse Chemical Company Inc, Dallas, TX, this product was supplied as tablets (each tablet contains, 500 mg) and was administered at a dose of 150 mg/kg BW orally once daily for successive 28 days (Zhang et al., 2019). Selenium (selenium)®: was obtained from Interpharma UK Company. This product was supplied as tablets; each tablet contains 100 mg administered at a dose of 5 mg/kg orally once daily according to Behne et al. (1991) for 28 days. PCM (Panadol)® was obtained from GlaxoSmithKline. This product was supplied as tablets (each tablet contains, 500 mg) and was administrated according to the method described by Dash et al. (2007) as a single oral dose (750 mg/kg). Animals and the study protocolFrom the Animal House of the Faculty of Veterinary Medicine, Zagazig University, 40 mature male albino rats that are clinically healthy, and their weights ranged between 150 and 200 g were enrolled. They were housed in conventional circumstances in metal cages with wood shaving bedding, at room temperature (about 25℃), and under typical laboratory aeration settings. The animals had full access to standard food and water. The animals were separated equally into five groups. The first group (control): Rats received only physiological saline. The second group (PCM): Rats received a single oral dose of PCM (750 mg/kg p. o). The third group (BBR then PCM): Rats received oral doses of BBR (150 mg/kg) for 28 consecutive days then a single dose of PCM (750 mg/kg). The fourth group (Selenium then PCM): Rats received oral doses of selenium (5 mg/kg) for 28 consecutive days then a single dose of PCM (750 mg/kg). The fifth group (Selenium + BBR then PCM): Rats received oral doses of both selenium (5 mg/kg) and BBR (150 mg/kg) for 28 consecutive days then a single dose of PCM (750 mg/kg). SamplingBlood was drawn from the tail vein on day 30, left to clot, and separated by centrifugation at 3,000 rpm for 15 minutes to determine immuno-modulatory effects (cellular and humoral response, nitric oxide (NO), lysozyme, and IgM), lipid peroxidation marker, antioxidant enzymes (CAT, Gpx, and SOD), liver function parameters (AST, ALT, and ALP), liver histopathological study, and immune histochemical parameter (TNF-α, and TGF-β1). Histopathological determinationA part of the hepatic tissues was fixed in a 10% buffered formalin solution at room temperature for 24 hours, dehydrated in a series of ascending alcohols, and embedded in paraffin wax at the completion of the experiment. For general histological and morphological investigation, H&E was utilized to stain approximately 5 μm thick sections (Malatesta, 2016). Biochemical determinations of liver functionsFollowing the manufacturing guidelines, enzymatic colorimetric kits (ALP Assay Kit Abcam, Catalog No: ab83369) were utilized for assessment of ALP activity according to Busquet et al. (2012), (AST Assay Kit Abcam, Catalog No: ab105135) was utilized for assessment of AST activity, and (ALT Assay Kit Abcam, Catalog No: ab105134) was utilized for assessment of ALT activity according to Reitman and Frankel (1957). Determination of lipid peroxidation marker in serumUsing a Rat Malondialdehyde ELISA Kit (MyBioSource, Catalog No: MBS738685), MDA concentration as a marker of lipid peroxidation was assessed (Esterbauer and Cheeseman, 1990). Estimation of antioxidant enzyme activities in the liver tissuesSOD Activity Assay Kit (Abcam, Cat. No: ab65354) utilized for evaluation of SOD activity (Misra and Fridovich, 1972), rat CAT ELISA Kit (MyBioSource, Catalog No: MBS701908) utilized for measurement of CAT activity (Aebi, 1984), and with GPx ELISA Kit (Biodiagnostic, Catalog No: GP 2524), GPx activity was determined (Paglia and Valentine, 1967). Pathological and immunohistochemical studiesFixation and tissue processing by an automated tissue processor, formalin-preserved rat hepatic tissue specimens were processed. The processing started with a two-step method for fixation and dehydration. Forty-eight hours of tissue immersion in 10% buffered formalin, followed by thirty minutes of fixative removal in distilled water. Subsequently, the tissues were dehydrated using a series of alcohol solutions. The tissue was initially exposed for 120 minutes to 70% alcohol, then for 90 minutes to 90% alcohol, followed by two cycles with absolute alcohol. Following dehydration, the materials were purified using a series of xylene modifications. It involves tissue immersion for 1 hour in a mixture of 50% alcohol and 50% xylene, followed by tissue immersion for 1.5 hours in xylene alone. The samples were implanted, saturated with paraffin wax, and encased in a block. 4–5 um paraffin slices were H&E-treated (Malatesta, 2016). Statistical analysisUsing SPSS version 23, we calculated descriptive statistics as the mean and SE from the collected data. Moreover, the Shapiro–Wilk test verified that the data were normally distributed. The one-way analysis of variance and the Duncan multiple ranges test was used as a post hoc test. At the 0.05 level of significance, there is no difference between the means that are separated by the same letter in the same column. Ethical approvalZU_IACUC Committee approval number (ZU_IACUC/2/F/51/2022) had approved the experimental protocol. ResultsEffect of BBR or selenium and their combination on liver enzyme activities of PCM-treated male ratsData illustrated the effect of BBR (150 mg/Kg b.wt) or selenium (5 mg/Kg b.wt) and their combination on liver enzyme activities of PCM (750 mg/Kg b.wt). The data of all parameters follow a normal distribution (p > 0.05). The ALT (expressed as UL/l) was higher in PCM treatment (64 ± 3.06), while the lowest value of ALT (23.3 ± 2.85) was observed in the controls. In addition, it was noted that there are no statistically significant differences between BBR/para treatment (43.33 ± 1.45) and Selenium/para (39.33 ± 0.33), on the other side, there are no statistically significant differences between selenium/para treatment (39.33 ± 0.33) and BBR-Selenium/para treatment (35 ± 1.53). The addition of BBR or selenium combined with PCM significantly decreased ALT values compared to PCM alone. All treatments had a significant effect on ALT compared to the control (p < 0.05). The percentage of change (increase or decrease) in ALT compared to control was +174%, +86%, +69%, and +50% for PCM, BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. While the percentage of change (increase or decrease) in ALT compared to PCM was −32%, −39%, and −45% for BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively (Table 1). Table 1. Effect of BBR (150 mg/kg b.wt.) or selenium (5 mg/kg b.wt) and their combination on liver enzyme activities of PCM (750 mg/kg b.wt)—treated materials.
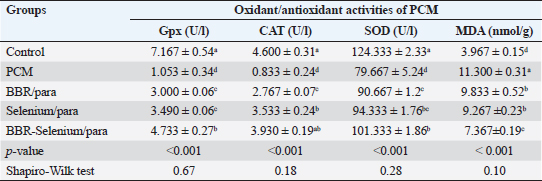
Means within the same column and carrying similar letters are not significantly different from each other at p < 0.05. (ALT): alanine aminotransferase; (AST): aspartate aminotransferase; (ALP): alkaline phosphatase. Regarding AST activity (expressed as U/l), it was on the same trend as ALT, where the highest value of AST (84 ± 2.65) was observed upon PCM treatment, while the lowest value of AST (35.67e ± 1.76) was recorded in the control. The integration between BBR and selenium combined with PCM was the most effective in decreasing AST compared to PCM alone. All treatments had a significant effect on ALT compared to the control (p < 0.05). The percentage of change (increase or decrease) in AST compared to control was +136%, +99%, +76%, and +35% for PCM, BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. While the percentage of change (increase or decrease) in AST compared to PCM was −15%, −25%, and −43% for BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. The same pattern of the former attributes was observed in ALP (expressed as U/l), where the highest value of ALP (310 ± 2.89) was observed with PCM treatment, while the lowest value of ALP (146.33 ± 8.57) was recorded in the control. The addition of both BBR and selenium combined with PCM contributed to a significant decrease in ALP values compared to PCM alone. All treatments had a significant effect on ALP compared to the control (p < 0.05). The percentage of change (increase or decrease) in ALP compared to control was +112%, +41%, +25%, and +12% for PCM, BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. While the percentage of change (increase or decrease) in ALP compared to PCM was −34%, −41%, and −47% for BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively (Table 1). Effect of BBR or selenium and their combination on oxidant/antioxidant activities of PCM-treated male ratsThe effects of BBR or selenium and their combination on oxidant/antioxidant activities (GPX, CAT, SOD, and MDA) of PCM were recorded in Table 2. The data of all parameters follow a normal distribution (p > 0.05). The GPX (expressed as UL/l) was higher in the untreated group treatment (7.17 ± 0.54), while the lowest value of GPX was observed in the PCM treatment (1.05 ± 0.34). The addition of BBR or selenium combined with PCM significantly increased GPX values compared to PCM alone. All treatments had a significant effect on GPX compared to the control (p < 0.05). The percentage of change (increase or decrease) in GPX compared to control was −82.31%, −58.14%, −51.3%, and −33.96% for PCM, BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. The percentage of change (increase or decrease) in GPX compared to PCM was +184.9%, +231.43%, and +349.48% for BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. Regarding CAT, CAT (expressed as U/l) takes the same trend GPX, where the highest value of CAT (4.6 ± 0.31) was remarked with a control group without statistically significant differences between it and BBR-Selenium/PCM (3.93 ± 0.19), while the lowest value of CAT was remarked with PCM (0.83 ± 0.24). The integration between BBR or selenium combined with PCM was the most effective in increasing CAT compared to PCM alone. All treatments had a significant effect on CAT compared to the control (p < 0.05). The percentage of change (increase or decrease) in CAT compared to control was −81.89%, −39.85%, −23.2%, and −14.57% for PCM, BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. While the percentage of change (increase or decrease) in CAT compared to PCM was +184.9%, +231.43%, and +349.48% for BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. The highest value of SOD (124.33 ± 2.33) was observed in the control, while the lowest value of SOD (79.67 ± 5.24) was remarked with PCM. The addition of BBR or selenium combined with PCM significantly increased SOD values compared to PCM alone. All treatments had a significant effect on SOD compared to the control (p < 0.05). On the other hand, there are no statistically significant differences between Selenium/PCM treatment (94.33 ± 1.76) and BBR-Selenium/PCM treatment (101.33 ± 1.86). The percentage of change (increase or decrease) in SOD compared to control was −35.92%, −27.08%, −24.13%, and −15.50% for PCM, BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. While The percentage of change (increase or decrease) in SOD compared to PCM was +13.81, +18.41, and +27.20 for BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. PCM treatment ranked first treatment in MDA level with an average of 11.3 nmol/g. The results showed that there are no significant differences between BBR/PCM (9.83 ± 0.52) and Selenium/PCM (9.27 ± 0.23). The addition of BBR or selenium combined with PCM significantly increased MDA values compared to PCM alone except BBR-Selenium/PCM (7.37 ± 0.19). All treatments had a significant effect on MDA compared to the control (p < 0.05). The percentage of change (increase or decrease) in MDA compared to control was +184.85%, +147.87%, +133.6%, and +85.71% for PCM, BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. The percentage of change (increase or decrease) in MDA compared to PCM was −12.98, −17.99, and −34.81 for BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively (Table 2). Table 2. Effect of BBR (150 mg/kg b.wt) or selenium (5 mg/kg b.wt) and their combination on oxidant/antioxidant activities of PCM (750 mg/kg b.wt)—treated materials.
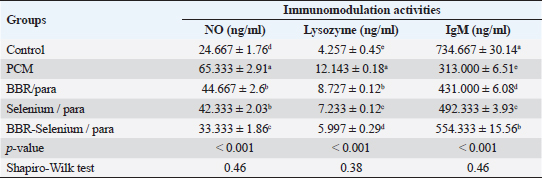
Means within the same column and carrying similar letters are not significantly different from each other at p < 0.05. Effect of BBR or selenium and their combination on immunomodulation activities of PCM-treated male ratsThe impacts of BBR or selenium and their combination on immunomodulation activities (No, Lysozyme, and IgM) of PCM were illustrated in Table 3. The data of all parameters follow a normal distribution (p > 0.05). The NO (expressed as ng/ml) was higher in PCM treatment (65.33 ± 2.91), while the lowest value of NO was observed in the control. Also, there are no significant differences between BBR/PCM treatment (44.67 ± 2.6), and Selenium/PCM treatment (42.33 ± 2.03). The addition of BBR or selenium combined with PCM significantly decreased NO values compared to PCM alone. All treatments had a significant effect on NO compared to the control (p < 0.05). The percentage of change (increase or decrease) in NO compared to control was +164.86%, +81.08%, +71.62%, and +35.13% for PCM, BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. While the percentage of change (increase or decrease) in NO compared to PCM was −31.63%, −35.20%, and −48.98% for BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. The highest value of Lysozyme in Table 3 (12.14 ± 0.18) was observed in the PCM treatment, while the lowest value (4.26 ± 0.45) was recorded in the untreated group. The addition of BBR or selenium combined with PCM significantly decreased Lysozyme values compared to PCM alone. All treatments had a significant effect on Lysozyme compared to the control (p < 0.05). The percentage of change (increase or decrease) in Lysozyme compared to control was +185.25%, +105%, +69.91%, and +40.87% for PCM, BBR/PCM, selenium/PCM, and BBR-Selenium/PCM, respectively. The percentage of change (increase or decrease) in Lysozyme compared to PCM was −31.63, −35.2, and −48.98 for BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. Regarding IgM (expressed as ng/ml) clear that the highest value of IgM (734.67 ± 30.14) was recorded in the control, while the lowest value of IgM (313 ± 6.51) was observed in the PCM. The integration between BBR or selenium combined with PCM was the most effective in increasing IgM compared to PCM alone. All treatments had a significant effect on IgM compared to the control (p < 0.05). The percentage of change (increase or decrease) in IgM compared to control was −57.40%, −41.33%, −32.99%, and −24.55% for PCM, BBR/PCM, Selenium /PCM, and BBR-Selenium /PCM, respectively. While the percentage of change (increase or decrease) in IgM compared to PCM was +37.7%, +57.29%, and +77.1% for BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. Table 3. Effect of BBR (150 mg/kg b.wt) or selenium (5 mg/kg b.wt) and their combination on immunomodulating activities of PCM (750 mg/kg b.wt)—treated materials.
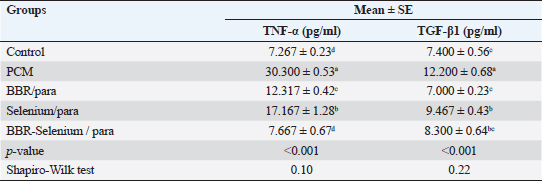
(NO): nitric oxide; (IgM): immunoglobulin M. Table 4. Effect of BBR (150 mg/kg b.wt) or selenium (5 mg/kg b.wt) and their combination on TNF-α and TGF-β1 of PCM (750 mg/kg b.wt)—treated materials.
Means within the same column and carrying similar letters are not significantly different from each other at p < 0.05. (TNF): tumor necrosis factor; (TGF-β1): Transforming growth factor beta. Effect of BBR or selenium and their combination on TNF-α, and TGF-β1 of PCM-treated male ratsTable 4 shows the effects of BBR or selenium and their combination on TNF-α, and TGF-β1 of PCM. Results declared that the data of all parameters follow a normal distribution (p > 0.05). The TNF-α (expressed as pg/ml) was higher in PCM treatment (30.3 ± 0.53), while the lowest value of TNF-α observed in the control group (7.27 ± 0.23) without significant differences between it and BBR-Selenium/PCM treatment (7.67 ± 0.67). The addition of BBR or selenium combined with PCM significantly decreased TNF-α level compared to PCM alone. All treatments had a significant effect on TNF-α compared to the control (p < 0.05) except BBR-Selenium/PCM treatment. The percentage of change (increase or decrease) in TNF-α compared to control was +316.95%, +69.49%, +136.23%, and +5.5% for PCM, BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. The percentage of change (increase or decrease) in TNF-α compared to PCM was −59.35, −43.34, and −74.70 for BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively. Regarding TGF-β1 (expressed as pg/ml) followed the same trend of TNF-α, where the highest value of TNF-α (12.2 ± 0.68) was recorded in the PCM treatment, while the lowest value of TNF-α was observed in the BBR/PCM treatment (7 ± 0.23) without significant differences between it and BBR-Selenium/PCM treatment (8.3 ± 0.64). Also, there are no significant differences between Selenium/PCM (9.47 ± 0.43), and BBR-Selenium/PCM (8.3 ± 0.64). The integration between BBR and selenium combined with PCM was the most effective in decreasing TGF-β1 compared to PCM alone. All treatments had a significant effect on TGF-β1 compared to the control (p < 0.05). The percentage of change (increase or decrease) in TGF-β1 compared to control was +62.16%, −5.41%, + 27.03%, and 12.16% for PCM, BBR/PCM, Selenium/PCM, and BBR-Selenium/PCM, respectively.
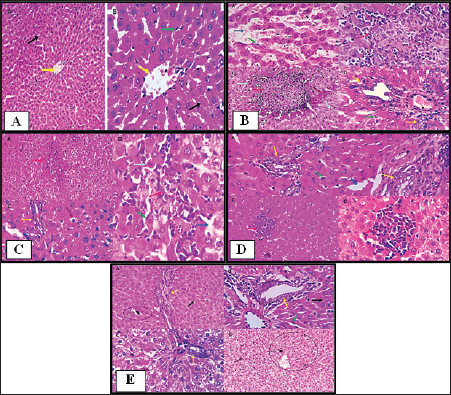
Fig. 1. Histopathological pictures of (A) Group 1: Control group, (B) Group 2: PCM-treated rats, (C) Group 3: BBR and PCM-treated rats, (D) Group 4: Selenium and PCM-treated rats, and (E) Group 5: BBR, Selenium and PCM-treated rats. Histopathological findingsGroup 1 (Control free)Hepatocytes exhibited normal hexagonal plates. The acinus has an oval shape. Its long arm is a fictitious line that connects two adjacent major veins, and the short arm is composed of a common boundary between two adjacent lobules and portal canals. Hepatocytes, which are big polyhedral cells, comprise around 75%–80% of the liver’s total cells. They may include 2–4 spherical nuclei located in the center of the cells. Each nucleus contains a minimum of 2 nucleoli. The lifespan of hepatocyte is of approximately 5 months. The Perisinusoidal space (space of Disse) is generated when the hepatocytes increase the rate of fluid exchange by extending villi into the perisinusoidal vascular space. Disse satellite cells inhabit space. The Biliary tributaries tree had no evidence of inflammatory, degenerative, necrotic, or apoptotic alterations (Figs. 1A and 2).
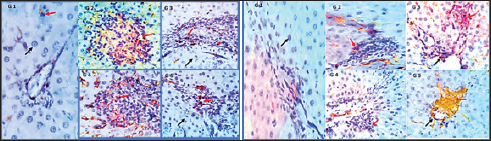
Fig. 2. Photomicrographs showing immunohistochemistry of cytoplasmic reactivity of the hepatic vascular endothelial, Von-Kupffer, and inflammatory cells against TNF-α in the different experimental groups (red arrows), ×400. Photomicrographs showing cytoplasmic reactivity of the hepatic vascular endothelial, Von-Kupffer, and inflammatory cells against TGF-β in the different experimental groups (red arrows) (×400). Group 2 (PCM-treated rats)Examined sections from the liver of this group pointed out multifocal hepatocellular necrosis with complete or partial replacement by mononuclear cells (lymphocytes and macrophages). There were moderate portal and interstitial aggregations of round cells (lymphocytes and macrophages). Vascular congestion, sinusoidal dilatation, and moderate biliary proliferation were seen. Von-Kupffer cells were hypertrophied. Scattered hepatocellular degenerative changes (hydropic degeneration and steatosis), atrophy, and apoptosis were also seen (Figs. 1B and 2). Group 3 (BBR—PCM-treated rats)The hepatic slices displayed what seemed to be normal hepatic parenchyma with maintained cord organization, portal structures, sinusoids, and stroma. A few sections revealed residual hepato-toxic changes as interstitial lymphoplasmacytic infiltration associated with hepato-cellular pressure atrophy. Scattered hepatocellular degeneration and apoptotic changes were observed in a few hepatocytes Mild sinusoidal dilatation and Von-Kupffer cell hypertrophy were also recorded (Figs. 1C and 2). Group 4 (Selenium-PCM-treated rats)The hepatic slices displayed what seemed to be normal hepatic parenchyma with maintained cord organization, portal structures, sinusoids, and stroma. Some sections showed focal interstitial round cells (lymphocytes and macrophages) aggregations, mild portal lymphocytic infiltration, and biliary proliferation in addition to scattered apoptosis. Focal sinusoidal dilatation and Von-Kupffer cell hypertrophy were recorded (Figs. 1D and 2). Group 5 (BBR and Selenium-PCM treated rats)The hepatic slices displayed what seemed to be normal hepatic parenchyma with maintained cord organization, portal structures, sinusoids, and stroma. Mild portal lymphocytic infiltration and minimal biliary proliferation in addition to scattered hepatocellular hydropic degeneration, Von-Kupffer cells hypertrophy, and residual obstructive bile pigment stagnation were recorded in a few sections (Figs. 1E and 2). DiscussionAccording to the findings of the current investigation, BBR and selenium treatment reduced several metabolic alterations caused by PCM toxicity. A comparable elevation in liver enzymes following administering a single dosage of 850 mg/kg PCM to rats was reported by Alipour et al. (2013) confirming PCM’s hepatotoxicity. The same effect was stated by da Silva et al. (2006) following delivery of a single dosage of 650 mg.kg−1 PCM to rats. In addition, Lahouel et al. (2004) and Chandrasekaran et al. (2009) showed a comparable elevation in hepatic MDA content. In line with the current findings, Yousef et al. (2010) and Gupta et al. (2014) demonstrated a decline in CAT activity and hepatic GSH concentration, Also, Gardner et al. (2002) and Şener et al. (2006) reported a PCM-induced NOx generation and elevation of the inflammatory biomarkers that agreed with our findings. Compared to the normal group, a considerable elevation of ALP, AST & ALT was found because of PCM supplementation. PCM effect on liver damage in albino Swiss mice was studied by Singh and Mani (2015) and revealed that harmful effects of the medication compromised renal and hepatic function in PCM-treated mice as an elevation in uric acid, Creatinine, urea, Bilirubin, ALP, AST, and ALT levels. Additional testing revealed PCM’s toxicity in the form of liver damage and blood cell abnormalities. Compared to the control, PCM significantly reduced serum uric acid, albumin, Hb and total protein, and elevated LDH activity, AST, ALT, WBCS, and platelet count El Menyiy et al. (2018). AST and ALT increased activity due to their leakage into the circulation because of cellular necrosis or the enhanced plasma membrane permeability, Srinivasa Rao et al. (2014). In the present research, PCM treatment led to a considerable elevation in serum AST, ALT, and ALP activity due to structural damage to hepatocytes as demonstrated by Wang et al. (2008). Evaluation of the severity of acetaminophen-induced liver damage is significantly influenced by AST and ALT enzyme levels. ALT is a superior indicator for detecting liver injury, as it is more specific to the liver (Domitrović et al., 2011). On-going dispute exists on the BBR effects in several experimental models of hepatotoxicity. Janbaz and Gilani (2000) revealed that during CCl4-induced hepatotoxicity, BBR administration (4 mg/kg) had no effect on decreasing liver injury. However, the protective effect of BBR in liver injury as indicated by lower AST and ALT activity in the CCl4 hepatotoxicity model was reported by Sun et al. (2009). Doses may account for the apparent variation between the two studies. Current research indicates that the dosage and duration of BBR treatment may be major variables. In Janbaz and Gilani (2000) study, the application of BBR (4 mg/kg) for 2 days was much lower than the amount described in our study (150 mg/kg), which was decided by our clinical experience and was comparable to the dosage reported by Feng et al. (2008) and the treatment duration in our work was 28 consecutive days. Selenium co-administration suppressed the near-normal increase in hepatic biochemical enzyme levels and reversed the PCM-induced damage to hepatic tissues indicating that selenium provided protection against PCM by maintaining the structural integrity of the hepatic membrane, due to its membrane stabilizing function, which inhibits internal enzyme leakage. Selenium administration provided protection by raising the protein concentration. According to the current investigation, BBR or selenium lowered the quantity of lipid peroxides generated by PCM treatment. BBR or selenium could significantly reduce their high concentrations. When PCM was provided, the antioxidant levels in the liver likewise showed considerable changes. Combined dosing of BBR and selenium could dramatically restore these abnormal readings to near-normal levels. Based on our findings, it is possible that the decrease in GSH, SOD, CAT, and reactive substance levels in PCM-treated rats was attributable to hepatocellular injury and that BBR provided protection against this PCM-induced liver damage. A possible method for PCM-induced BBR protection is via serving as a free radical scavenger and intercepting PCM-metabolizing radicals with microsomal enzymes. BBR and selenium dramatically boosted GSH levels in the liver. The present results suggested that a much-increased GSH content, SOD, and CAT in the liver would provide enhanced protection against oxidative stress, hence lead to the elimination of PCM-induced hepatotoxicity. To normalize PCM-induced deficits, BBR and selenium may be effective agents. Furthermore, BBR was able to scavenge the in vitro-generated free radicals (Pongkittiphan et al., 2015). The current investigation revealed that BBR has hepatoprotective properties against PCM-induced hepatotoxicity. PCM overdose results in oxidative damage, as seen by decreased Gpx, SOD, and CAT activity, and an increase in MDA levels (Soliman et al., 2020). Besides, El-Maddawy and El-Sayed (2018) demonstrated one of the mechanisms by which PCM induces liver damage. Increased N-acetyl benzoquinone imine (NAPQI) synthesis causes hepatotoxicity. In the presence of hepatic GSH, NAPQI can be transformed back to PCM or covalently bound to GSH to form 3-Gpx -S-yl-PCM. However, an overdose of PCM results in extensive NAPQI generation, which depletes hepatic GSH to the point that it can no longer compensate for the massive NAPQI synthesis. In the present research, histological examinations of the livers of PCM-treated rats demonstrated inflammatory cell infiltration associated with necrosis; this conclusion is consistent with that of Papackova et al. (2018). Histopathological findings in hepatocytes of PCM-treated rats were scattered foci of necrosis with nuclear alterations, microvesicular fatty change vacuolation, hydropic degeneration, sinusoids and blood vessel congestion, and eosinophilia. BBR protective effects against PCM-induced hepatotoxicity in our findings are consistent with those described for BBR in liver fibrosis experimental models (Li et al., 2014). Inflammatory cell infiltration initiated by toxic reactive metabolites formation, presumably NAPQI, necrosis, steatosis, and marked hydropic degeneration was induced by chronic low-dose or acute overdose exposure to PCM. APAP-induced toxicity is induced by oxidative stress, DNA damage, endoplasmic reticulum, and mitochondrial stress (Bhushan and Apte, 2019; Wang et al., 2019). In the current study, co-treatment with selenium reduced all PCM-induced changes in toxicity. This protective function may be attributable to the antioxidative activity, which may be mediated by the scavenging or generation of free radicals. TGF-β1 and TNF-α serum levels in PCM-treated rats were significantly higher than those in the control group, according to our findings. In contrast, their levels in rats administered PCM plus BBR or selenium decreased significantly compared to rats administered PCM alone. Compared to PCM alone, a significant reduction of TGF-β1 and TNF-α was found in rats administered PCM, BBR, and selenium. In addition, TGF-β1 and TNF-α levels were comparable between the BBR and selenium-treated rats. Compared to the control, all treatments demonstrated a substantial effect on TGF-β1 and TNF-α. In line with our findings, vitamin E and selenium can prevent CCl4-induced liver damage as revealed by Chen et al. (2005). The current study revealed that selenium caused a significant increase in liver GSH and a decrease in liver MDA; therefore, decreasing the PCM-induced severe lipid peroxidation reaction and averting hepatocellular injury. The protective process may also involve the downregulation of TNF-a release, re-equilibration of hepatocyte [Ca2þ] homeostasis, and enhanced immunity (Zhou et al., 2003). The current investigation demonstrated that selenium strongly inhibits the PCM-induced increase in TNF-a, while simultaneously decreasing hepatic NO levels. Rats administered a therapeutic dose of PCM for 30 days had severe glycogen depletion in their hepatocytes. The depletion of glycogen in PCM-treated rats was related to the toxicity of reactive metabolites. These results are comparable to those of a prior study in which mice were treated with PCM (750 mg/kg, I/P). Selenium-treated rats are protected from PCM-induced glycogen depletion by improved detoxification via reactive metabolite scavenging (Hinson et al., 1983). In a mouse model of thioacetamide-induced hepatic fibrosis, Wang et al. (2011) demonstrated the hepatoprotective effects of BBR and its derivative demethylene-BBR, which were related to inhibiting the triggering cell death via the NFB pathway and activation of hepatic stellate cells. In addition, in BBR-fed fish, higher hepatic GSH-Px activity was seen indicating improved liver health and antioxidative condition. Van Doan et al. (2020) revealed that the addition of BBR to Nile tilapia fingerlings (1, 3, 6, and 9 g/kg of diet) boosted skin mucus lysozyme and peroxidase activity, as well as serum lysozyme and peroxidase. Nevertheless, treatment with selenium and its nano form can mitigate the immune-toxic and inflammatory effects of cypermethrin, as observed in groups treated with selenium and nano selenium. Previous investigations have demonstrated that NO, IL-6, and TNF-α were downregulated in these groups (Rupil et al., 2012; Abdou and Sayed, 2019). Selenium may have an anti-inflammatory impact by suppressing the p38 mitogen-activated protein kinase NF- Kb signaling pathways, which reduce the expression of the examined cytokine genes and diminish the inflammatory response (Morris et al., 2003; Kim et al., 2004). In addition, the upregulation of lysozyme by selenium compounds was comparable with the findings of Sheiha et al. (2020), who discovered that selenium supplementation dramatically boosted blood lysozyme activity and the innate immunity of heat-stressed rabbits. This increased lysozyme level may result from selenium’s stimulating influence on immune cells and their lysozyme synthesis. PCM administration to normal rats resulted in a significant increase in hepatic NO levels relative to the normal control group. NO is a signaling molecule that plays a crucial role in inflammation pathophysiology and is abundantly produced under pathological physiological conditions (Gong et al., 2010). After treatment with BBR (150 mg/kg b.wt) or selenium (5 mg/Kg b.wt), PCM-induced increases in NO levels were significantly decreased, according to our findings. Rising ROS levels have a significant role in PCM toxicity. NO is transformed into the toxin Peroxynitrite, which damages mitochondrial cells via superoxide (Ghanem et al., 2016; Guo et al., 2016). Activated Kupffer cells are also essential for the NO and superoxide generation. In the early phase of PCM overdose, NO from Kupffer cells and endothelial cells and superoxide anion from Kupffer cells could generate Peroxynitrite (ONOO-) (Knight et al., 2001). The toxic ONOO- generates nitrated tyrosine, which corresponds with necrosis (Michael et al., 2001). ConclusionThis study is considered among the first to assess the efficacy of the BBR and selenium on hepatic toxicity induced by PCM in rats. The study showed the capability of BBR and selenium to alleviate PCM toxicity by improving liver functions and also ameliorating the histopathological lesions seen in livers. In vitro antioxidant studies showed that BBR and selenium displayed antioxidant activity, BBR and selenium acted as a powerful reducing agent, free radical scavenger, and long-lasting MDA inhibitor. These findings suggest the use of BBR and selenium to decrease toxicity in the therapeutic interest of hepatotoxicity management. AcknowledgmentThe authors would like to thank their supervisors Prof. Dr. Gamal El-Din Amin Shams, Professor of Pharmacology, and Dr. Somia Hassan Abd Allah, Professor of Biochemistry and Head of Clinical Biochemistry and Stem Cell Lab, for their supervision, useful suggestions, and helpful comments throughout their Ph.D. studies. The authors would like to thank all staff members of the Pharmacology department for their support throughout their research. Conflict of interestThe authors declare that they have no conflict of interest. Data availabilityData will be available from the corresponding author upon a reasonable request. Authors’ contributionGS, SA, RE: conceptualization of the study, experimental esign, supervising the experiments. RE, MA: collection of samples, experimental work, drafting of the manuscript. ReferencesAbdou, R.H. and Sayed, N. 2019. Antioxidant and anti-inflammatory effects of nano-selenium against cypermethrin-induced liver toxicity. Cell. Bio. 8, 53. Aebi, H. 1984. Catalase in vitro. In Methods in enzymology. Academic Press. 105, 121–126. Alipour, M., Buonocore, C., Omri, A., Szabo, M., Pucaj, K. and Suntres, Z.E. 2013. Therapeutic effect of liposomal-N-acetylcysteine against acetaminophen-induced hepatotoxicity. J. Drug. Target. 21, 466–473. Ang, C., Kornbluth, M., Thirlwell, M.P. and Rajan, R.D. 2010. Capecitabine-induced cardiotoxicity: case report and review of the literature. Curr. Oncol. 17, 59–63. Baskar, R., Dai, J., Wenlong, N., Yeo, R. and Yeoh, K.W. 2014. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 1, 24. Behne, D., Kyriakopoulos, A., Scheid, S. and Gessner, H. 1991. Effects of chemical form and dosage on the incorporation of selenium into tissue proteins in rats. J. Nutr. 121, 806–814. Bhushan, B. and Apte, U. 2019. Liver regeneration after acetaminophen hepatotoxicity: mechanisms and therapeutic opportunities. Am. J. Pathol. 189, 719–729. Busquet, P., Capurro, V., Cavalli, A., Piomelli, D., Reggiani, A. and Bertorelli, R. 2012. Synergistic effects of galantamine and memantine in attenuating scopolamine-induced amnesia in mice. J. Pharmacol. Sci. 120, 305–309. Chandrasekaran, V.R.M., Hsu, D.Z. and Liu, M.Y. 2009. The protective effect of sesamol against mitochondrial oxidative stress and hepatic injury in acetaminophen-overdosed rats. Shock 32, 89–93. Chen, L., Pan, D.D., Zhou, J. and Jiang, Y.Z. 2005. Protective effect of selenium-enriched Lactobacillus on CCl4-induced liver injury in mice and its possible mechanisms. World. J. Gastroenterol. 11, 5795. Chi, X., Liu, Z., Wei, W., Hu, X., Wang, Y., Wang, H. and Xu, B., 2021. Selenium-rich royal jelly inhibits hepatocellular carcinoma through PI3K/AKT and VEGF pathways in H22 tumor-bearing mice. Food. Fun. 1, 9111–9127. da Silva Melo, D.A., Saciura, V.C., Poloni, J.A.T., Oliveira, C.S.A., Alves Filho, J.C.F., Padilha, R.Z., Reichel, C.L., Neto, E.J., Oliveira, R.M., D’avila, L.C. and Kessler, A. 2006. Evaluation of renal enzymuria and cellular excretion as an marker of acute nephrotoxicity due to an overdose of paracetamol in Wistar rats. Clin. Chim. Acta. 373, 88–91. Dash, D.K., Yeligar, V.C., Nayak, S.S., Ghosh, T., Rajalingam, R., Sengupta, P., Maiti, B.C. and Maity, T.K. 2007. Evaluation of hepatoprotective and antioxidant activity of Ichnocarpus frutescens (Linn.) R. Br. on paracetamol-induced hepatotoxicity in rats. Trop. J. Pharm. Res. 6, 755–765. Domitrović, R., Jakovac, H. and Blagojević, G. 2011. Hepatoprotective activity of berberine is mediated by inhibition of TNF-α, COX-2, and iNOS expression in CCl4-intoxicated mice. Toxicology 280, 33–43. Ehteshamfar, S.M., Akhbari, M., Afshari, J.T., Seyedi, M., Nikfar, B., Shapouri-Moghaddam, A., Ghanbarzadeh, E. and Momtazi-Borojeni, A.A. 2020. Anti-inflammatory and immune-modulatory impacts of berberine on activation of autoreactive T cells in autoimmune inflammation. J. Cell. Mol. Med. 24, 13573–13588. El-Maddawy, Z.K. and El-Sayed, Y.S. 2018. Comparative analysis of the protective effects of curcumin and N-acetyl cysteine against paracetamol-induced hepatic, renal, and testicular toxicity in Wistar rats. Environ. Sci. Pollut. Res. 25, 3468–3479. El Menyiy, N., Al-Waili, N., El Ghouizi, A., Al-Waili, W. and Lyoussi, B. 2018. Evaluation of antiproteinuric and hepato-renal protective activities of propolis in paracetamol toxicity in rats. Nutr. Res. Pract. 12, 535–540. Esterbauer, H. and Cheeseman, K.H. 1990. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods. Enzymol. 186, 407–421. Feng, Y.B., Luo, W.Q. and Zhu, S.Q. 2008. Explore new clinical application of Huanglian and corresponding compound prescriptions from their traditional use. Zhongguo. Zhong. Yao. Za. Zhi. 33, 1221–1225. Feng, Y., Spezia, M., Huang, S., Yuan, C., Zeng, Z., Zhang, L., Ji, X., Liu, W., Huang, B., Luo, W. and Liu, B. 2018. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes. Dis. 5, 77–106. Fontanella, C., Aita, M., Cinausero, M., Aprile, G., Baldin, M.G., Dusi, V., Lestuzzi, C., Fasola, G. and Puglisi, F. 2014. Capecitabine-induced cardiotoxicity: more evidence or clinical approaches to protect the patients’ heart? Onco. Targets. Ther. 7, 1783–1791. Gardner, C.R., Laskin, J.D., Dambach, D.M., Sacco, M., Durham, S.K., Bruno, M.K., Cohen, S.D., Gordon, M.K., Gerecke, D.R., Zhou, P. and Laskin, D.L. 2002. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: potential role of tumor necrosis factor-alpha and interleukin-10. Toxicol. Appl. Pharmacol. 184, 27–36. Ghanem, C.I., Pérez, M.J., Manautou, J.E. and Mottino, A.D. 2016. Acetaminophen from liver to brain: new insights into drug pharmacological action and toxicity. Pharmacol. Res. 109, 119–131. Gong, G., Qin, Y., Huang, W., Zhou, S., Yang, X. and Li, D. 2010. Rutin inhibits hydrogen peroxide-induced apoptosis through regulating reactive oxygen species mediated mitochondrial dysfunction pathway in human umbilical vein endothelial cells. Eur. J. Pharmacol. 628, 27–35. Guo, C., Xie, G., Su, M., Wu, X., Lu, X., Wu, K. and Wei, C. 2016. Protective effect of pioglitazone, a PPARγ agonist against acetaminophen-induced hepatotoxicity in rats. Mol. Cell. Biochem. 393, 223–228. Gupta, R.K., Patel, A.K., Shah, N., Choudhary, A.K., Jha, U.K., Yadav, U.C., Gupta, P.K. and Pakuwal, U. 2014. Oxidative stress and antioxidants in disease and cancer: a review. Asian Pacif. J. Cancer Prev. 15(11), 4405-4409. Hinson, J.A., Mays, J.B. and Cameron, A.M. 1983. Acetaminophen-induced hepatic glycogen depletion and hyperglycemia in mice. Biochem. Pharmacol. 32, 1979–1988. Janbaz, K.H. and Gilani, A.H. 2000. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia 71, 25–33. Kim, S.H., Johnson, V.J., Shin, T.Y. and Sharma, R.P. 2004. Selenium attenuates lipopolysaccharide-induced oxidative stress responses through modulation of p38 MAPK and NF-kappaB signaling pathways. Exp. Biol. Med. (Maywood). 229, 203–213. Knight, T.R., Kurtz, A., Bajt, M.L., Hinson, J.A. and Jaeschke, H. 2001. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol. Sci. 62, 212–220. Lahouel, M., Boulkour, S., Segueni, N. and Fillastre, J.P. 2004. The flavonoids effect against vinblastine, cyclophosphamide and paracetamol toxicity by inhibition of lipid-peroxydation and increasing liver glutathione concentration. Pathol. Biol. (Paris). 52, 314–322. Li, J., Pan, Y., Kan, M., Xiao, X., Wang, Y., Guan, F., Zhang, X. and Chen, L., 2014. Hepatoprotective effects of berberine on liver fibrosis via activation of AMP-activated protein kinase. Life. Sci. 98, 24–30. Lou, T., Zhang, Z., Xi, Z., Liu, K., Li, L., Liu, B. and Huang, F. 2011. Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes. Inflammation 34, 659–667. Malatesta, M. 2016. Histological and histochemical methods—theory and practice. Eur. J. Histochem. 2016, 25–29. Michael, S.L., Mayeux, P.R., Bucci, T.J., Warbritton, A.R., Irwin, L.K., Pumford, N.R. and Hinson, J.A. 2001. Acetaminophen-induced hepatotoxicity in mice lacking inducible nitric oxide synthase activity. Nitric. Oxide. 5, 432–441. Misra, H.P. and Fridovich, I. 1972. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 247, 3170–3175. Miwa, M., Ura, M., Nishida, M., Sawada, N., Ishikawa, T., Mori, K., Shimma, N., Umeda, I. and Ishitsuka, H. 1998. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Europ. J. Cancer. 34, 1274–1281. Morris, K.R., Lutz, R.D., Choi, H.S., Kamitani, T., Chmura, K. and Chan, E.D. 2003. Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Infect. Immun. 71, 1442–1452. Paglia, D.E. and Valentine, W.N. 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70, 158–169. Papackova, Z., Heczkova, M., Dankova, H., Sticova, E., Lodererova, A., Bartonova, L., Poruba, M. and Cahova, M. 2018. Silymarin prevents acetaminophen-induced hepatotoxicity in mice. PLoS One 13, 191–203. Polk, A., Shahmarvand, N., Vistisen, K., Vaage-Nilsen, M., Larsen, F.O., Schou, M. and Nielsen, D.L. 2016. Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ. Open. 6, e012798. Pongkittiphan, V., Chavasiri, W. and Supabphol, R. 2015. Antioxidant effect of berberine and its phenolic derivatives against human fibrosarcoma cells. Asian. Pac. J. Cancer. Prev. 16, 5371–5376. Reitman, S. and Frankel, S. 1957. Colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28, 56–63. Ringuet, M.T., Hunne, B., Lenz, M., Bravo, D.M. and Furness, J.B. 2021. Analysis of bioavailability and induction of glutathione peroxidase by dietary nanoelemental, organic and inorganic selenium. Nutrients 13, 5–9. Rupil, L.L., de Bem, A.F. and Roth, G.A. 2012. Diphenyl diselenide-modulation of macrophage activation: down-regulation of classical and alternative activation markers. Innate. Immun. 18, 627–637. Şener, G., Omurtag, G.Z., Sehirli, Ö., Tozan, A., Yüksel, M., Ercan, F. and Gedik, N., 2006. Protective effects of ginkgo biloba against acetaminophen-induced toxicity in mice. Mol. Cell. Biochem. 283, 39–45. Sheiha, A.M., Abdelnour, S.A., Abd El-Hack, M.E., Khafaga, A.F., Metwally, K.A., Ajarem, J.S., Maodaa, S.N., Allam, A.A. and El-Saadony, M.T. 2020. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals (Basel) 10, 50–58. Singh, D.P. and Mani, D. 2015. Protective effect of Triphala Rasayana against paracetamol-induced hepato-renal toxicity in mice. J. Ayurveda. Integr. Med. 6, 181–186. Soliman, A.M., Rizk, H.A., Shalaby, M.A. and Elkomy, A.A. 2020. Mechanisms of hepato-renal protective activity of Ocimum basilicum leaf extract against paracetamol toxicity in rat model. Adv. Anim. Vet. Sci. 8, 385–391. Srinivasa Rao, V., Wilkin Einstein, J. and Das, K. 2014. Hepatoprotective and antioxidant activity of Lannea coromandelica Linn. on thioacetamide induced hepatotoxicity in rats. Int. Lett. Nat. Sci. 3, 45–49. Sun, Y., Xun, K., Wang, Y. and Chen, X., 2009. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anticancer. Drugs. 20, 757–769. Van Doan, H., Hoseinifar, S.H., Jaturasitha, S., Dawood, M.A. and Harikrishnan, R. 2020. The effects of berberine powder supplementation on growth performance, skin mucus immune response, serum immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 520, 734–739. Wang, H.J., Tashiro, S.I., Onodera, S. and Ikejima, T. 2008. Inhibition of insulin-like growth factor 1 receptor signaling enhanced silibinin-induced activation of death receptor and mitochondrial apoptotic pathways in human breast cancer MCF-7 cells. J. Pharmacol. Sci. 107, 260–269. Wang, L., Wei, W., Xiao, Q., Yang, H. and Ci, X., 2019. Farrerol ameliorates APAP-induced hepatotoxicity via activation of Nrf2 and autophagy. Int. J. Biol. Sci. 15, 788–799. Wang, Q., Zhang, M., Liang, B., Shirwany, N., Zhu, Y. and Zou, M.H. 2011. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS One 6, 254–256. Wang, Y., Zhou, X., Zhao, D., Wang, X., Gurley, E.C., Liu, R., Li, X., Hylemon, P.B., Chen, W. and Zhou, H. 2020. Berberine inhibits free fatty acid and LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes. PLoS One 15, 232–239. Xu, D., Chen, X., Li, X., Mao, Z., Tang, W., Zhang, W., Ding, L. and Tang, J. 2019. Addition of capecitabine in breast cancer first-line chemotherapy improves survival of breast cancer patients. J. Cancer. 10, 418–429. Xu, L., Lu, Y., Wang, N. and Feng, Y. 2022. The role and mechanisms of selenium supplementation on fatty liver-associated disorder. Antioxidants (Basel) 11, 50–53. Yousef, M.I., Omar, S.A., El-Guendi, M.I. and Abdelmegid, L.A. 2010. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food. Chem. Toxicol. 48, 3246–3261. Zhang, N., Sheng, M., Wu, M., Zhang, X., Ding, Y., Lin, Y., Yu, W., Wang, S. and Du, H. 2019. Berberine protects steatotic donor undergoing liver transplantation via inhibiting endoplasmic reticulum stress-mediated reticulophagy. Exp. Biol. Med. (Maywood). 244, 1695–1704. Zhao, X., Zhang, J., Tong, N., Chen, Y. and Luo, Y. 2012. Protective effects of berberine on doxorubicin-induced hepatotoxicity in mice. Biol. Pharm. Bull. 35, 796–800. Zhou, J., Chen, L., Chen, L.J., Pan, D.D. and Jiang, Y.Z. 2003. Effects of Lactobacillus rich in selenium on RBC lipid peroxidation and immune function in mice with CCl4–injected liver injury. Yingyang Xuebao 25, 405–408 Zhou, J.Y. and Zhou, S.W. 2011. Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b expression in diabetic rat liver. Fitoterapia 82, 184–189. | ||
| How to Cite this Article |
| Pubmed Style Shams G, Allah SA, Ezzat R, Said MA, . Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in rats. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 292-303. doi:10.5455/OVJ.2024.v14.i1.26 Web Style Shams G, Allah SA, Ezzat R, Said MA, . Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in rats. https://www.openveterinaryjournal.com/?mno=177393 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.26 AMA (American Medical Association) Style Shams G, Allah SA, Ezzat R, Said MA, . Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in rats. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 292-303. doi:10.5455/OVJ.2024.v14.i1.26 Vancouver/ICMJE Style Shams G, Allah SA, Ezzat R, Said MA, . Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in rats. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 292-303. doi:10.5455/OVJ.2024.v14.i1.26 Harvard Style Shams, G., Allah, S. A., Ezzat, R., Said, M. A. & (2024) Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in rats. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 292-303. doi:10.5455/OVJ.2024.v14.i1.26 Turabian Style Shams, Gamal, Somia Abd Allah, Raghda Ezzat, Mahmoud A. Said, and . 2024. Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in rats. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 292-303. doi:10.5455/OVJ.2024.v14.i1.26 Chicago Style Shams, Gamal, Somia Abd Allah, Raghda Ezzat, Mahmoud A. Said, and . "Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in rats." Open Veterinary Journal 14 (2024), 292-303. doi:10.5455/OVJ.2024.v14.i1.26 MLA (The Modern Language Association) Style Shams, Gamal, Somia Abd Allah, Raghda Ezzat, Mahmoud A. Said, and . "Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in rats." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 292-303. Print. doi:10.5455/OVJ.2024.v14.i1.26 APA (American Psychological Association) Style Shams, G., Allah, S. A., Ezzat, R., Said, M. A. & (2024) Ameliorative effects of berberine and selenium against paracetamol-induced hepatic toxicity in rats. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 292-303. doi:10.5455/OVJ.2024.v14.i1.26 |