| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 341–349 Original Research Immunological studies on the effects of toltrazuril and neem extract in broiler chickens suffering from coccidiosisMohamed H. Kairy1, Hosny Abd El Fadel1, Abd El Aleim F. Abd El Aleim1, Gehan N. Gad2, Fatma El Zahra A. Youssef2*, Amira M. Ibrahim3, Walaa F. SaadEldin31Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Department of Biochemistry, Toxicology and Nutritional Deficiencies, Animal Health Research Institute (AHRI), Agriculture Research Center (ARC), Zagazig, Egypt 3Educational Veterinary Hospital, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Fatma El Zahra A. Youssef. Department of Biochemistry, Toxicology and Nutritional Deficiencies, Animal Health Research Institute (AHRI), Agriculture Research Center (ARC), Zagazig, Egypt. Email: karimsalama63 [at] gmail.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
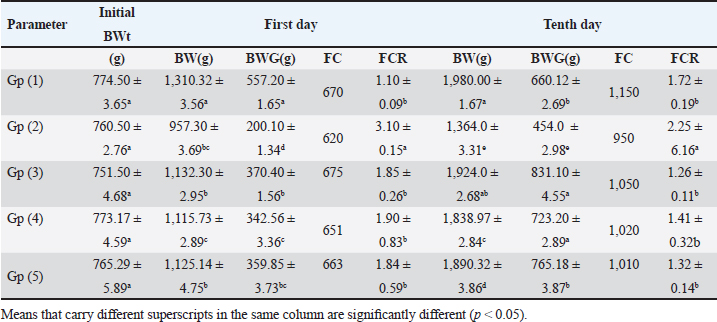
ABSTRACTBackground: The prevalence of avian coccidiosis in the poultry industry has grown, resulting in substantial financial losses from high mortality, stunted growth, reduced productivity, and expensive medical expenses. Aim: The purpose of the current study was to assess the immunological effects of neem leaf extract and toltrazuril on broilers that had contracted coccidiosis. Methods: In this investigation, 100 one-day-old Cobb broiler chicks without sexes were employed. The chicks were divided into five equal groups, with 20 birds in each. On the 14th day of life, the birds in groups 2, 3, 4, and 5 received an oral inoculation with 1 × 105 sporulated oocysts of Eimeria tenella (E. tenella) (field isolate). The first group (Gp), which consists of 20 healthy broilers, served as a negative control. Gp (2) contains experimentally infected broilers and nontreated (served as a positive control). Gp (3) contains experimentally infected broilers treated with toltrazuril (1 ml/l drinking water) for two consecutive days. Gp (4) contains experimentally infected broilers treated with neem leaf extract 4% (50 ml/l drinking water) for 5 successive days, and Gp (5) contains experimentally infected broilers treated with toltrazuril (1 ml/l drinking water) and a half dose of neem leaves extract 4% (25 ml/l drinking water) for 5 successive days. For the purpose of estimating body weight growth and feed conversion ratio, each broiler was weighed separately at the start of the trial and again on the 1st and 10th day after treatment. In addition to obtaining intestinal samples for immunohistochemistry, blood samples were also obtained for immunological examination. Results: As compared to the negative control group, the experimentally infested broilers with E. tenella showed significant decreases in serum nitric oxide, lysosome, phagocytic percent, and phagocytic index, along with significant increases in white blood cells (WBCs), lymphocyte, heterophilis, eosinophilis, basophilis, monocyte, serum total protein, γ globulin, fibrinogen, and haptoglobin. When compared to the control positive group, experimentally infested broilers treated with either neem or toltrazuril alone or in combination demonstrated significant increases in serum total protein, nitric oxide, lysozyme, phagocytic percent, and phagocytic index, but significant decreases in WBCs, lymphocytes, heterophile, eosinophile, basophile, and monocyte. The intestinal peroxidase stain of broilers infected with E. tenella exhibited a significant positive expression for CD4, but the infected broilers treated with toltrazuril and half a dosage of neem displayed a negative expression for CD4, identical to the negative control. Conclusion: The broiler chickens infested with E. tenella may have a variety of negative impacts on their immune systems and immunohistopathological findings. Nonetheless, toltrazuril and neem extract, either separately or in combination, function as anticoccidial medications that may enhance the broiler chicks’ immune state. Keywords: Immunity, Neem extract, Broiler chicks, Coccidiosis, Toltrazuril. IntroductionInfestations of parasites pose a major risk to health and reduce productivity (Nazmiye, 2008). Eimeria spp., are the culprits behind coccidiosis, which is acknowledged as a significant protozoal illness affecting birds (Kitandu and Juranová, 2006). Due to two factors—confined host rearing circumstances and resistance to chemoprophylaxis—avian coccidiosis has grown more common in the poultry sector (Stephen et al., 1997). Due to high mortality, poor growth, decreased output, and high medical costs, the disease has a substantial financial impact on the chicken business (Williams, 1998; Abd El-Maksoud et al., 2014). The amount of coccidians that complete their life cycles should be limited by the ideal anticoccidial (Jain, 1986). According to Doumas et al. (1981) and Mathis et al. (2004), toltrazuril is a triazinone medication with broad-spectrum anticoccidial and antiprotozoal activity. By preventing the nuclear division of schizonts, macrogametes, and the wall-forming bodies of macrogaments, toltrazuril is effective against all species of Eimeria and against all intracellular developmental phases, including schizogony and gametogony (Said et al., 2010). Herbal extracts are an alternative and safe anticoccidial medication because they do not leave tissue residue or produce drug resistance (Ghafouri et al., 2023). Due to their low toxicity and low cost of manufacturing, herbal plants and their byproducts may be used as treatments for coccidiosis (Abbas et al., 2012). Nimbidin, sodium nimbolide, gedunin, azadirachtin, mahmoodin, epicatechin and catechin, margalone, margolonone and isomargolonone, cyclic trisulphides, polysaccharides GIa, GIIa, GIIIa, GIb, NB-II, and peptidoglycon are among the active components found in neem (Biswas et al., 2002). Immunomodulatory, anti-inflammatory, antihyperglycemic, antimalarial, antifungal, antioxidant, antimutagenic, and anticarcinogenic qualities have been shown for neem leaves and their constituents (Subapriya and Nagini, 2005; Gaurav et al., 2018). Investigating the impact of E. tenella, toltrazuril, and neem leaf extract on certain immunological and immunohistochemical alterations in broilers was the goal of the current study. Materials and MethodsDrugsA: 2.5% toltrazuril (Baycox)® acquired from Bayer Comp. 25 mg/kg bw for two consecutive days is the suggested therapeutic dose, which is equivalent to 1 ml/1 litre of drinking water (Mathis et al., 2003). B. Aqueous neem extract: Leaves were harvested from medium-aged green trees in the agricultural orchards of the Faculty of Agriculture, Zagazig University, Egypt. They were then dried for 24 hours at 37°C in an oven and then pulverized using a metal grinder. Weigh out 40 g of the dried ground leaves, and then transfer them to a nonmetallic container. The method described by Leila (1977) was used to prepare an extract (4%) by pouring 1 l of boiling distilled water over it and letting it sit at room temperature for 5–8 hours. The Eimeria strainIt was kindly acquired from the Parasitology Department, Faculty of Veterinary Medicine, Zagazig University, Egypt. BirdsOne hundred unsexed broiler chicks of a commercial breed (Cobb breed) were employed; they were 1 day old and appeared to be in good health. El-wady Poultry Company supplied them. Following a comprehensive washing and disinfection process, the chicks were kept in a consistently clean and hygienic environment and fed on a specially prepared diet devoid of chemical additives, antibiotics, and anti-coccidial medications. Throughout the 5 weeks of the trial, tap water was available at all times. The chicks were raised on the floor in individual units, with bedding made up of two inches of freshly shredded wood litter. After being adjusted to 32°C for the first week, the temperature was lowered by 2°C each week after that. For the duration of the experiment, ideal light conditions were met every day. The use of routine preventive medication and immunization campaigns against viral and bacterial infections was made. The chicks were divided into five primary groups. Group 1 consisted of 20 healthy broilers (-ve Control). On the 14th day of life, the birds in groups 2–5 received an oral inoculation with 1 × 105 sporulated oocysts of Eimeria tenella (field isolate). Experimentally infested broilers in Gp (2) are not treated (+ve control), while experimentally infested broilers in Gp (3) are treated with toltrazuril (1 ml/l drinking water) for 2 days in a row. In Gp (4), experimentally infested broilers are given a 4% neem leaf extract (50 ml per l of drinking water) for 5 days (Durrani et al., 2008). In Gp (5), experimentally infested broilers are given a half-dose of 4% neem leaf extract (25 ml/l drinking water) and toltrazuril (1 ml/l drinking water) for five consecutive days. Growth performanceTo estimate body weight increase and feed conversion ratio (FCR), each broiler was weighed separately at the start of the research and on the first and 10th day after treatment. Blood samplesThree blood samples were collected from each chick on the first day following therapy. To estimate leukograms, the initial sample was taken on a tube containing EDTA as an anticoagulant (Feldman et al., 2000). For the purpose of estimating serum total protein (Doumas et al., 1981), serum protein fractionation using cellulose acetate electrophoresis (Henry et al., 1974), lysozyme (Schltz, 1987), nitric oxide (Rajarman et al., 1998), the second blood sample was collected in a clean centrifuge tube without anticoagulant and centrifuged at 3,000 rpm for 15 minutes. In accordance with (Lucy and Larry, 1982), the third sample was collected on a tube containing heparin as an anticoagulant for the measurement of phagocytic activity (phagocytic % and phagocytic index). The determination of inflammatory markers including hepatoglobulin (PIT54) and fibrogen (FGB) was done according to Tomas Marques (2017). Tissue specimensOn the first day following treatment, broilers were sacrificed and intestinal tissues were collected for immunohistochemical examination (Kim et al., 2016). Statistical analysisThe automated SPSS program version 16 was used to evaluate the data that was obtained. Ethical approvalThe authors affirm that this work adheres to the highest ethical standards. The authors have ensured the originality of the content, obtained necessary permissions for data and materials used, and followed ethical guidelines for research and publication. ResultsWhen compared to the -ve control, broilers that were experimentally infested with E. tenella exhibited a substantial decrease in weight gain, FC, and FCR on the 1st and 10th day post infection. However, when compared to +ve control, experimentally infested broilers treated with toltrazuril or neem leaf extract, either alone or in a combination, significantly enhanced weight gain and FCR (Table 1). Table 1. Effect of toltrazuril and neem leaves extract 4% on body performances of infected broilers with E. tenella.
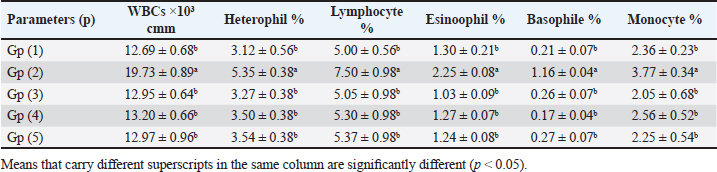
When broilers were experimentally infested with E. tenella, their white blood cell (WBC), lymphocytes, heterophiles, eosinophils, basophils, and monocytes were compared with the (-ve control) significantly increased (Table 2). However, when broilers were experimentally infested and treated with either toltrazuril or neem leaf extract alone or in combination, their WBCs, lymphocytes, heterophiles, eosinophiles, basophiles, and monocytes were significantly decreased when compared with the +ve control. Table 2. Effect of toltrazuril and neem extract 4% on total and differential leukocytic count in infected broilers with E. tenella.
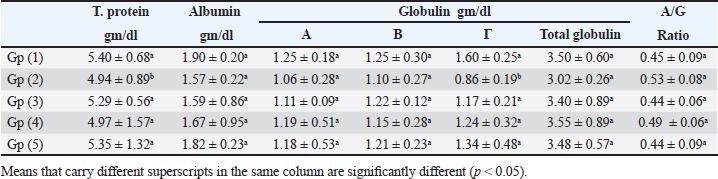
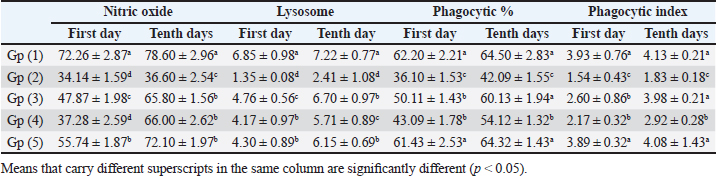
When compared to the -ve control, broilers that were experimentally infested had significant decreases in serum total protein and γ globulin, but only marginal decreases in albumin, α, β, total globulin, and A/G ratio. In addition to a negligible rise in albumin, α, β, γ, total globulin, and A/G ratio as compared to +ve control broilers, experimentally infected broilers treated with toltrazuril or neem alone or in combination showed significant increases in serum total protein (Table 3). When compared to healthy broilers (-ve control), experimentally infested broilers with E. tenella showed significant decreases in serum nitric oxide, lysozyme, phagocytic percent, and phagocytic index. However, when compared to +ve control broilers, broilers treated with toltrazuril or neem extract individually showed significant increases in nitric oxide, lysozyme, phagocytic percent, and phagocytic index (Table 4). Table 3. Effect of toltrazuril and neem extract 4% on protein profile in infested broilers with E. tenella.
Table 4. Effect of toltrazuril and neem extract 4% on nitric oxide, lysosome, phagocytic %, and phagocytic index of infected broilers with E. tenella.
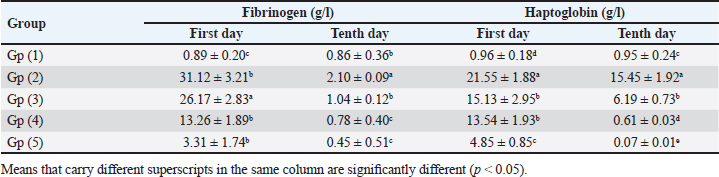
Table 5. Effect of toltrazuril and neem extract on the inflammatory markers (FGB and hepatoglobin) in infected broilers with E. tenella.
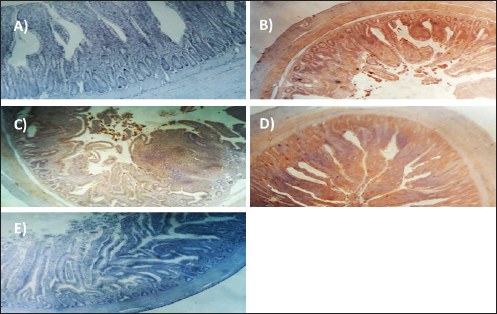
Experimentally infected broilers with E. tenella exhibited a significant increase in fibrinogen and haptoglobin. However, when toltrazuril or neem extract is administered to the broilers, either separately or in combination, the levels of fibrinogen and haptoglobin significantly decrease (Table 5). A healthy broiler’s intestinal peroxidase-stained section revealed -ve expression of CD4. Infested broiler intestines stained with peroxidase demonstrated high positive expression of CD4. The intestinal peroxidase-stained tissue of infested broilers treated with toltrazuril exhibited a moderate positive expression of CD4. Infested broilers treated with neem showed nearly significant +ve expression for CD4 in their peroxidase-stained gut. Peroxidase-stained intestinal tissue of infested broilers given a half-dose of neem and toltrazuril demonstrates -ve expression for CD4 (Fig. 1). DiscussionThe data gathered demonstrated that in comparison to healthy broilers, broilers afflicted with coccidiosis showed a significant reduction in body weight increase, the elevation of feed conversion rate, and feed consumption. The findings of Walk et al. (2011), who reported that coccidiosis causes intestinal disruption that reduces nutrient absorption and feed utilization efficiency and is associated with decreased body weight, weight gain, feed intake, and increased feed conversion rate, corroborated our findings. Besides, El-Banna et al. (2005), noted that broilers with coccidiosis exhibit a decrease in body weight gain and an increase in FCR. The data of the current study is also consistent with that published by James et al. (2022), who reported that broiler coccidiosis resulted in decreased FCR and weight increase.
Fig. 1. Peroxidase-stained intestine of A) healthy broilers showing -ve expression of CD4. B) Infested broilers with E. tenella showed strong +ve expression of CD4. C) Broilers infested with E. tenella treated with toltrazuril showed moderate +ve expression of CD4. D) Infested broilers treated with neem showed almost strong +ve expression of CD4. E) Infested broilers treated with toltrazuril +half dose of neem showed -ve expression of CD4 (×400). The current study found that, as compared to the artificially infected and untreated group (+ve control), broilers with coccidiosis treated with toltrazuril or neem leaf extract, either alone or in combination, showed improvements in body weight, weight gain, and FCR. Toltrazuril’s anticoccidial action could be the cause of these improvements in body weight growth (Rachid et al., 2009). Our findings align with those published by El-Banna et al. (2005), who discovered that toltrazuril effectively treats coccidiosis in broilers and enhances their physical performance. The same outcomes were seen earlier by Sokoł et al. (2014), who reported that toltrazuril enhanced bodily functions and was beneficial in treating quail coccidiosis. In a similar vein, Onyiche et al. (2021) reported that neem extract improves body weight, weight increase, and FCR and is effective against E. tenella. When compared to healthy, noninfected broiler chickens, the current study found that E. tenella significantly increased the levels of WBC, lymphocytes, heterophil, eosinophil, basophil, and monocyte in broiler chickens. Our findings corroborated Coles’s (1997) observation that avian coccidiosis was associated with an increase in eosinophil count. Feldman et al. (2000) previously reported on these results, stating that eosinophilia was seen in birds with coccidiosis. This result was in good agreement with the findings by Razzaq et al. (2003), who found that quail with coccidiosis had a markedly higher WBC count than healthy, noninfected birds. The same findings were published by Seddik and El-Bealawy (2007), who claimed that enteritis brought on by coccidiosis in chicken causes a notable rise in WBCs. Furthermore, according to Chapman (2008), turkey with coccidiosis exhibits elevated WBC, heterophile, and lymphocyte levels. Increased concentrations of heterophils and eosinophils in response to parasite infestation are the cause of elevated WBCs (Wakenell, 2010). This result was consistent with results from Melkamu et al. (2018), who found that, in comparison to healthy, noninfested broilers, broilers infected with coccidiosis had elevated levels of WBCs and heterophil. When compared to infested nontreated broilers (+ve control), experimentally infested broilers treated with toltrazuril or neem leaf extract, either alone or in combination, showed significant decreases in WBCs, lymphocyte, heterophile, eosinophile, basophile, and monocyte. Comparing broilers infected with E. tenella treated with toltrazuril to those infected without treatment, Allam et al. (2008) reported similar outcomes, stating that there was a substantial drop in WBCs. The findings of the present study corroborated those of Sokoł et al. (2014), who found that toltrazuril effectively treated quail coccidiosis and increased the number of WBCs. Toltrazuril treatment for coccidiosis in broiler chickens resulted in a nonsignificant drop in WBCs, lymphocytes, heterophils, eosinophils, and basophils (Rehab, 2017). El-Ghoneimy and El-Shahawy (2017) noted the same alterations in leukograms in broilers with coccidiosis. Comparing broilers infested with E. tenella to those treated with toltrazuril or neem leaf suspension, either separately or in combination, showed improved total leukocyte counts. Similar findings were reported by Roy et al. (2019). Furthermore, in comparison to infected broilers that were not treated for coccidiosis, broilers treated with toltrazuril exhibited noticeably better WBCs, according to Shahira et al. (2021). The same results were observed by Onyiche et al. (2021) who mentioned that neem extract contains natural antioxidants in biological systems against parasites and ameliorative detrimental effects in total and differential leukocytic count. When compared to healthy, noninfected broilers, broilers infested with E. tenella showed substantial decreases in serum total protein, γ globulin, and albumin. There was also an insignificant decrease in α, β, γ, total globulin, and A/G ratio. Our results are confirmed with those reported by El-Banna et al. (2005) who mentioned that coccidiosis in broiler chicks generates a considerable decrease in total protein, γ globulin insignificant decrease in albumin, α, β, γ, globulin, and A/G ratio. Our findings are consistent with those of El-Dakhly et al. (2006), who found that, in comparison to healthy, noninfected broilers, coccidiosis causes a decrease in protein picture. The findings of Patra et al. (2010), who suggested that liver abnormalities and impaired nutritional absorption from the inflamed gut may be the cause of hypoproteinemia and hypoalbuminemia in broiler hens infected with E. tenella, corroborated our findings. This result was in good agreement with earlier research conducted by Abd El-Aziz et al. (2011), who reported that chicken cecal coccidiosis causes a considerable reduction in the protein picture. The same findings were published by Abd El-Maksoud et al. (2014), who showed that the serum total protein, albumen, globulin level, and A/G ratio of broilers infested with E. tenella decreased. When compared to a broiler infected with E. tenella that was not treated (+ ve control broiler), infested broilers treated with toltrazuril or neem leaves extract, either separately or in combination, showed nonsignificant increases in serum total protein along with insignificant increases in serum albumin, α, β, γ, total globulin, and A/G ratio. Toltrazuril treatment for coccidiosis in broiler hens results in nonsignificant reductions in blood total protein, albumin, and globulin levels (Rehab, 2017). When compared to broilers experimentally infected with E. tenella that were not treated, broilers suffering from coccidiosis treated with toltrazuril exhibited improvement in protein picture producing nonsignificant decreases in serum total protein, albumin, α, β, γ, globulin, and A/G ratio (Mona et al., 2016). The same findings were noted by Shahira et al. (2021), who reported that toltrazuril improves serum protein, albumin, and globulin levels and is useful in the treatment of coccidiosis. The serum total proteins, albumin, and globulin levels of broilers given neem extract improved (Vanessa et al., 2019). The same outcomes were noted by Onyiche et al. (2021), who reported that neem extract has a protective impact on protein images and acts as a natural antioxidant in biological systems against parasites. According to the current study, when compared to noninfested (healthy) broilers, broiler chickens with coccidiosis showed significantly lower serum levels of lysozyme, nitric oxide, phagocytic percent, and phagocytic index. The same results were reported by coccidiosis (Allen, 1997) who indicated that broiler chickens suffering from coccidia showed declines in serum nitric oxide and lysozyme. According to El-Sayed (2002), coccidiosis in broilers causes a considerable drop in phagocytic percentage and phagocytic index. Our findings corroborated those of Wang et al. (2008), who found that coccidiosis significantly reduced lysosome and nitric oxide levels. Our findings are consistent with those of Nasr et al. (2014), who reported that broilers infected with E. tenella exhibited a noteworthy reduction in both phagocytic percentage and phagocytic index. The findings were in line with those of Mohamed et al. (2018), who found that coccidiosis significantly reduced serum nitric oxide, lysosome, phagocytic percentage, and phagocytic index. In comparison to infested broilers that were not treated, broilers that were experimentally infected with E. tenella and treated with either toltrazuril or neem extract alone showed a substantial increase in nitric oxide, lysozyme, phagocytic %, and phagocytic index. ThankGod et al. (2021) reported similar outcomes, stating that neem watery extract contains natural antioxidants in biological systems against parasites and ameliorates negative effects in lysosome phagocytic activity and nitric oxide. The current study has demonstrated that, in comparison to healthy, noninfested broilers, broilers afflicted with coccidiosis have a substantial rise in fibrinogen and haptoglobin. The same findings were reported by Richrds and Augustine (1988), who found that haptoglobin and fibrinogen levels were higher in broilers with coccidiosis. The same findings were reported by Allen (1997), who noted that haptoglobin and fibrinogen levels significantly increased in broiler chicks with coccidiosis. Our findings concurred with those of Georgieva et al. (2010), who reported that an E. tenella infection in broilers causes an increase in the inflammatory markers fibrinogen and haptoglobin in the plasma. This conclusion was consistent with the findings of Wang et al. (2008), who reported that coccidiosis causes a considerable rise in fibrinogen and haptoglobin and a significant decrease in nitric oxide and lysosome. These findings concur with those of Mohamed et al. (2018), who found that in addition to a notable increase in fibrinogen and haptoglobin, coccidiosis also causes a drop in serum nitric oxide and lysosome levels. When compared to experimentally infected broilers that were not treated, broilers that were treated with either toltrazuril or neem alone or in combination had a large rise in nitric oxide and lysozyme along with a significant decrease in fibrinogen and haptoglobin. The same outcomes were noted by Onyiche et al. (2021), who reported that neem watery extract, had a beneficial effect on lysosomes and nitric oxide levels in biological systems by acting as natural antioxidants against parasites. Furthermore, Liza et al. (2021) report that neem extract-treated coccidiosis-affected rabbits have decreased levels of (haptoglobin and fibrinogen). The intestinal peroxidase stain of a healthy broiler demonstrated -ve expression for CD4, while the intestinal peroxidase of broilers infected with E. tenella showed strong positive expression for CD4. The intestinal peroxidase of broilers infected with E. tenella treated with toltrazuril demonstrated moderate positive expression for CD4, and the intestinal peroxidase of infected broilers treated with neem displayed nearly strong positive expression for CD4, while the intestinal peroxidase-stained intestine of infested broilers treated with toltrazuril +half dose of neem displayed -ve expression for CD4. Broiler infected with E. tenella exhibit positive CD4 in their intestines (Rothwell et al., 1995). Carbonnel et al. (1999) observed the same alteration in intestinal tissue treated with peroxidase dye. ConclusionAlthough broiler hens infected with E. tenella have numerous negative effects on immunity and immunohistopathological picture, toltrazuril and neem leaf extract, either separately or in combination, function as anticoccidial drugs that enhance broiler immunity. AcknowledgmentThe authors would like to thank all people who supported the experimental work. Conflict of interestNo conflict of interest is to be declared. FundingNot applicable. Data availabilityAll data will be made available upon reasonable request. Authors’ contributionsFatma El Zahra Abdel-Hamid Youssef did the experimental work, data analysis, and drafted the manuscript. Amira M. Ibrahim and Walaa Fathy SaadEldin supported the experimental part, sampling, and financial support, Hosny Abd El Fadel, Abd El Aleim F. Abd El Aleim, Gehan N. Gad proposed the research theme, supervised the work, and revised the final manuscript. ReferencesAbbas, R., Iqbal, Z., Blake, D., Khan, M. and Saleemi, M. 2012. Anticoccidial drug resistance in fowl coccidia: the state of play revisited. World’s. Poult. Sci. J. 67(2), 337–350. Abd El-Aziz, M.I., El Shazly, K.A. and El Sherbeney, E.M.E. 2011. The effet of diaclazuril or maduramicin alone or together in ontroling cecal occidosiss in chickens. Kafrelsheikh. Vet. Med. J. 9, 161–187. Abd El-Maksoud, H., Afaf, D. and El-Badry, M. 2014. Biochemical effect of coccidia infestation in laying hen. Benha. Vet. Med. J. 26, 127–133. Allam, H., Bayoumie, H., Seham, M. and Mohamed, I. 2008. Effect of toltrazuril and amprolium plus in broiler chickensinfected with coccidiosis. Assuit. Vet. Med. J. 54, 1–12. Allen, P. 1997. Nitric oxide production during Eimeria tenella infections in chickens. Beltsville, MD: SDA, Agricultural Research Service, vol. 76, pp: 810–813. Biswas, K., Chattopadhyay, I., Banarjee, R.K. and Bandyopadhyay, U. 2002. Biological activities and medicinal properties of neem (Azadirachta indica). Curr. Sci. Rev. 82, 336–345. Carbonnel, H., Lavergne, A., Matuchansky, C., Brouet, J.C., Sigaux, F., Beaugerie, L., Nemeth, J., CoYn, B., Cosnes, J., Gendre, J. and Rambaud, J. 1999. The clinicopathological features of extensive small intestinal CD4 T cell infiltration. Gut 45, 662–667. Chapman, H. 2008. Coccidiosis in the turkey. Avian. Pathol. 37(3), 205–223. Coles, B. 1997. Avian medicine and surgery, 2nd ed. Cambridge, UK: University Press. Doumas, B., Certor, R., Pers, T. and Schaflr, R. 1981. A candidate reference method for determination of total protein in serum. Clin. Chem. 27, 164–167. Durrani, F.R., Chand, N., Jan, M., Sultan, A., Durrani, Z. and Akhtar, S. 2008. Immunomodulatory and growth promoting effects of neem leaves infusion in broiler chicks. Sarhad. J. Agric. 24, 655–659. El-Banna, H., El-Zorba, H. and El-Hady, M. 2005. Anticocci-dial efficacy of drinking water soluble diclazuril on experimental and field coccidiosis in broiler chickens. J. Vet. Med. A. Physiol. Path. Clin. Med. 52, 287–291. El-Dakhly, M., Azza, A., Shalaby, A. and El-Nesr, A. 2006. The efficacy of Lactobacillus acidophilus and/or diclazuril for inhibition and control of Eimeria tenella infection in balady chicks. Kafer El-Sheik. Vet. Med. J. 4, 1–18. El-Ghoneimy, A. and El-Shahawy, I. 2017. Evaluation of amprolium and toltrazuril efficacy in controlling natural intestinal rabbit coccidiosis. Iran. J. Vet. Res. 18, 164–169. El-Sayed, M. 2002. The effect of diclazuril and semduramicin as prophylactic or thera-peutic treatments n broilers infected with Eimeria tenella. Thesis (Pharmacology), Faculty of Veterinary Medicine, Tanta University, Tanta, Egypt. Feldman, B., Zinki, J. and Jain, V. 2000. Schalms veterinary hematology, 5th ed. Philadelp-hia, PA: Lippincott Williams and Wilkins. Gaurav, P., Pramanik, V., Mukesh, K., Sandeep, K., Manoj, K. and Jaswant, S. 2018. Effect of neem (Azadirachta indica) leaves powder against Coccodia in commercial broiler chickens. J. Pharm. Phytochem. 7(1S), 991–993. Georgieva, T., Koinarski, V., Urumova, V., Marutsov, P., Nikolov, J., Chaprazov, T., Walshe, K., Karov, R., Georgiev, I. and Koinarski, Z. 2010. Effects of Escherichia coli infection and Eimeria tenella invasion on blood concentrations of some positive acute phase proteins (haptoglobin (PIT 54), fibrinogen and ceruloplasmin) in chickens. Rev. Méd. Vét. 161(2), 84–89. Ghafouri, S.A., Ghaniei, A., Tavanaee, A., Sadr, S., Charbgoo, A., Ghiassi, S. and Abuali, M. 2023. Evaluation of therapeutic effects of an herbal mixture (Echinacea purpurea and Glycyrrhiza glabra) for treatment of clinical coccidiosis in broilers. Vet. Med. Sci. 9, 829–836. Henry, R., Cannon, D. and Winkelman, J. 1974. Clinical chemistry: principals and techniques. Hagerstown, MD: Harper and Row, pp: 437–440. Jain, N.C. 1986. Schalm’s veterinary hematology, 4th ed. Philadelphia, PA: Lee and Febiger, pp: 55–96. James, T., Micah, S., Oriel, T., Aliyu, A. and David, S. 2022. Field study in the effect of neem aqueous leav extracts in broilers coccidiosis. Poult. Sci. 102, 658–664. Kim, S., Roh, J. and Park, C. 2016. Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J. Pathol. Transl. Med. 50(6), 411–418. Kitandu, A. and Juranová, R. 2006. Progress in control measures for chicken coccidiosis. Acta. Vet. Brno. 75, 265–276. Leila, S.F.M. 1977. A manual on some Philippine medicinal plants (Preparation of drug materials). Bot. Soc. U. P. 20, 78–82. Liza, S., Eman, A., Amany, S., and 2021. Ameliorative effect of neem leaf and pomegranate peel extracts in coccidial infections in New Zealand and V-line rabbits: performance, intestinal health, oocyst shedding, carcass traits, and effect on economic measures. Animals (Basel) 11, 24–41. Lucy, F. and Larry, D. 1982. Ontageny and line difference in mitogenic responses of chicken lymphocyte. Poult. Sci. 62, 579–584. Mathis, G.F., Froyman, R., Irion, T. and Kennedy, T. 2003. Coccidiosis control with toltrazuril in conjugation with anticoccidial medicated or non-medicated feed. Avian. Dis. 47, 463–446. Mathis, G., Froyman, R. and Kennedy, T. 2004. Coccidiosis control by toltrazuril in the drinking water for a 2-day period. Vet. Parasitol. 121, 1–9. Melkamu, S., Mersha, M. and Asrat, M. 2018. Haematological changes caused by coccidiosis in infected chickens. Int. J. Adv. Res. Biol. Sci. 5, 196–202. Mohamed, I., Mosaad, A., Nahed, A. and Asmaa, M. 2018. Effect of high dietary level of some amino acids and coccidial infection on growth performance and health status of broiler chicken. Alexandria. J. Vet. Sci. 58(1), 147–165. Mona, A., Mohamed, H., Abd-El Alim, F. and Ali, M. 2016. Effect of phytobiotics, probiotics and toltrazuril on chicken coccidiosis. Zag. Vet. J. 44(3), 214–223. Nasr, M., Reda, E., Ahmed, O. and Mahmoud, A. 2014, Evaluation of Eimeria oocyst whole antigen vaccine and the enhancive effect of probiotic on broilers. Int. J. Basic. Appl. Sci. 3, 357–362. Nazmiye, A. 2008. Parasitic zoonotic diseases in Turkey. Vet. Ital. 44, 633–646. Onyiche, T.E., Gotep J.G., Tanko J.T., Ochigbo, G.O., Ozoani, H.A., Viyoff, V.Z., Dogonyaro, B.B., Makoshi, M.S., Kinjir, H., Thekisoe, O., Atiku, A.A., Shamaki, D. and Muraina, I.A. Azadirachta indica aqueous leaf extracts ameliorates coccidiosis in broiler chickens experimentally infected with Eimeria oocysts. Sci. Afr. 132, 658–664. Patra, G., Ayoub, M., Chanu, K., Joy, L., Pravo, M., Das, G. and Dvel, K. 2010. Diagnosis of E. tenella infection in chicken. Int. J. Poult. Sci. 9, 813–818. Rachid, M., Akbar, H., Araby, M., Ismae, A., Ashraf, K. and Hashmi, H. 2009. Comparative efficacy of different toltrazuril doses against coccidiosis in broiler chickens. Mansoura. Vet. Med. J. 2, 173–184. Rajarman, V., Nonnecke, B. and Horst, R. 1998. Effect of vitamins E on nitric oxide prod-uction by blood mononuclear leukocytes from neonatal calves fed milk replacer. J. Dairy. Sci. 81, 78–85. Razzaq, A., Hashmi, A., Kamran, H. and Khan, H. 2003. Clinical and haematological effects of coccidiosis and its prophylactic control in quails. Pak. J. Sci. 55, 23–26. Rehab, M. 2017. Evaluation of toltrazuril as anticoccidial drug in broilers. M. Sc Thesis, Submitted to Faculty of Veterinary Medicine (Pharmacology Department), Zagazig University, Zagazig, Egypt. Richrds, P. and Augustine, C. 1988. Serum and liver zinc, copper, and iron in chicks infected with Eimeria acervulina or Eimeria tenella. Biol. Trace. Elem. Res. 17, 207–219. Rothwell, R., Gramzinski, M., Rose, P. and Kaiser, A. 1995. Avian coccidiosis: changes in intestinal lymphocyte populations associated with the development of immunity to Eimeria maxima. Parasite. Immunol. 17, 525–533. Roy, R., Hasan, M.M., Aziz, F.B., Islam, R. and Sarkar, S. 2019. Comparative study of neem leaf (Azadirachta indica) suspension and toltrazuril against coccidiosis in Sonali chicken. Bangladesh. J. Vet. Med. 17, 97–105. Said, A., Khairy, M., Abdel Alim, A. and Zainab, H. 2010. Compatibility of toltrazuril and probiotic in chickens. Zag. Vet. J. 38, 73–81. Schltz, L. 1987. Methods in clinical chemistry. St. Louis, MO: C. V. Mosby Co, pp: 42–46. Seddik, A. and El-Bealawy, M. 2007. The effect of probiotic in broiler chickens infected with E. maxima. Zag. Vet. J. 35, 189–201. Shahira, H., Soad, M., Mervat, A., Gehan, N. and Rania, A. 2021. Comparative biochemical and pathological studies between toltrazuril and garlic supplementation in chickens infected with coccidiosis. Egy. J. Anim. Health. 1, 65–80. Sokoł, R., Gesek, M., Norynska, R. and Michalczyk, M. 2014. Toltrazuril treatment coccidiosis caused by Eimeria sp. in Japanese quails. Polish. J. Vet. Sci. 17(3), 465–468. Stephen, B., Rommel, M., Daugschies, A. and Haberkorn, A. 1997. Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains. Vet. Parasitol. 69(1–2), 19–29 Subapriya, R. and Nagini, S. 2005. Medicinal properties of neem leaves. Curr. Med. Chem. Rev. 5, 149–156. Vanessa, M., Jean, R. and Kissel, N. 2019. Effects of graded levels of Azadirachta indica seed oil on growth performance and biochemical profiles of broiler chickens. Vet. Med. Sci. 5(2), 442–450. Wakenell, P. 2010. Haematology of chickens and Turkeys. In Vet haematology, 6th ed. Eds., Weiss, D.J. and Wardrop, K.J. Ames, IA: John Wiley& Sons, pp: 957–967. Walk, C., Cowieson, A., Remus, J., Novak, C. and McElroy, A. 2011. Effects of dietary enzymes on performance and intestinal goblet cell number of broilers exposed to a live coccidia oocyst vaccine. Poult. Sci. 90, 91–98. Wang, M., Suo, X., Gu, J., Zhang, W., Fang, Q. and Wang, X. 2008. Influence of grape seed proanthocyanidin extract in broiler chickens: effect on chicken coccidiosis and antioxidant status. Poult. Sci. 87, 2273–2280. Williams, R.B. 1998. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int. J. Prasitol. 28, 1089–1098. | ||
| How to Cite this Article |
| Pubmed Style Kairy MH, Abd-El-Fadel H, Abd-El-Aleim AF, Gad GN, Youssef FA, Ibrahim AM, SaadEldin WF. Immunological studies on the effects of toltrazuril and neem extract in broiler chickens suffering from coccidiosis. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 341-349. doi:10.5455/OVJ.2024.v14.i1.31 Web Style Kairy MH, Abd-El-Fadel H, Abd-El-Aleim AF, Gad GN, Youssef FA, Ibrahim AM, SaadEldin WF. Immunological studies on the effects of toltrazuril and neem extract in broiler chickens suffering from coccidiosis. https://www.openveterinaryjournal.com/?mno=178054 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.31 AMA (American Medical Association) Style Kairy MH, Abd-El-Fadel H, Abd-El-Aleim AF, Gad GN, Youssef FA, Ibrahim AM, SaadEldin WF. Immunological studies on the effects of toltrazuril and neem extract in broiler chickens suffering from coccidiosis. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 341-349. doi:10.5455/OVJ.2024.v14.i1.31 Vancouver/ICMJE Style Kairy MH, Abd-El-Fadel H, Abd-El-Aleim AF, Gad GN, Youssef FA, Ibrahim AM, SaadEldin WF. Immunological studies on the effects of toltrazuril and neem extract in broiler chickens suffering from coccidiosis. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 341-349. doi:10.5455/OVJ.2024.v14.i1.31 Harvard Style Kairy, M. H., Abd-El-Fadel, . H., Abd-El-Aleim, . A. F., Gad, . G. N., Youssef, . F. A., Ibrahim, . A. M. & SaadEldin, . W. F. (2024) Immunological studies on the effects of toltrazuril and neem extract in broiler chickens suffering from coccidiosis. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 341-349. doi:10.5455/OVJ.2024.v14.i1.31 Turabian Style Kairy, Mohamed H., Hosny Abd-El-Fadel, Abd-El-Aleim F. Abd-El-Aleim, Gehan N. Gad, Fatma-El-Zahra A. Youssef, Amira M. Ibrahim, and Walaa F. SaadEldin. 2024. Immunological studies on the effects of toltrazuril and neem extract in broiler chickens suffering from coccidiosis. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 341-349. doi:10.5455/OVJ.2024.v14.i1.31 Chicago Style Kairy, Mohamed H., Hosny Abd-El-Fadel, Abd-El-Aleim F. Abd-El-Aleim, Gehan N. Gad, Fatma-El-Zahra A. Youssef, Amira M. Ibrahim, and Walaa F. SaadEldin. "Immunological studies on the effects of toltrazuril and neem extract in broiler chickens suffering from coccidiosis." Open Veterinary Journal 14 (2024), 341-349. doi:10.5455/OVJ.2024.v14.i1.31 MLA (The Modern Language Association) Style Kairy, Mohamed H., Hosny Abd-El-Fadel, Abd-El-Aleim F. Abd-El-Aleim, Gehan N. Gad, Fatma-El-Zahra A. Youssef, Amira M. Ibrahim, and Walaa F. SaadEldin. "Immunological studies on the effects of toltrazuril and neem extract in broiler chickens suffering from coccidiosis." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 341-349. Print. doi:10.5455/OVJ.2024.v14.i1.31 APA (American Psychological Association) Style Kairy, M. H., Abd-El-Fadel, . H., Abd-El-Aleim, . A. F., Gad, . G. N., Youssef, . F. A., Ibrahim, . A. M. & SaadEldin, . W. F. (2024) Immunological studies on the effects of toltrazuril and neem extract in broiler chickens suffering from coccidiosis. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 341-349. doi:10.5455/OVJ.2024.v14.i1.31 |