| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 438–448 Original Research Assessment of antibiotic residues in chicken meatHeba M. Kamouh1*, Reda Abdallah2, Ghada A. Kirrella2, Nader Y. Mostafa2 and Saleh Shafik11Food Hygiene Department, Agricultural Research Center (ARC), Animal Health Research Institute (AHRI), Giza, Egypt 2Food Control Department, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt *Corresponding Author: Heba M. Kamouh. Animal Health Research Institute, Mansoura Branch, Egypt. Email: heba.mostafa2011 [at] gmail.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
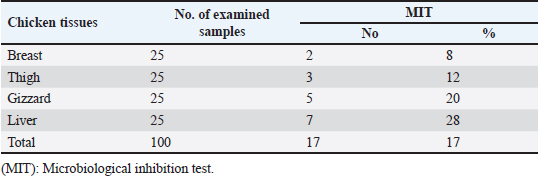
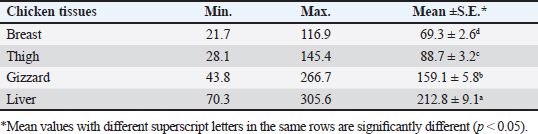
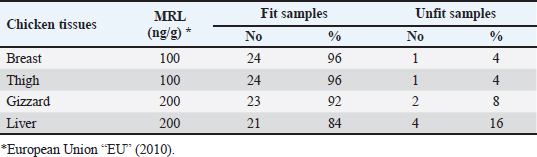
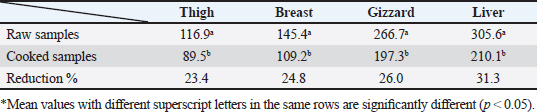
AbstractBackground: Nowadays veterinarians and poultry producers use antibiotics to increase growth rates, bird health, and feed efficiency, egg production, for preventative and therapeutic purposes, and to lessen the prevalence of poultry diseases. Most poultry producers have used a variety of antibiotics, either with or without veterinarian instruction. Although antibiotics are beneficial for the majority of their uses, their unauthorized use has resulted in residues accumulated in poultry products intended for human consumption which represents a serious risk to the general public that could be toxicological, microbiological, or immunological. Aim: This study aimed to the estimation of the residues of three major antimicrobials used in the intensive chicken-rearing systems in Egypt, namely Oxytetracycline (OTC), Gentamicin, and Ciprofloxacin. Moreover, the effect of cooking on such residues was investigated. Methods: A total of 100 chicken meat samples (breast, thigh, gizzard, liver, 25 each) were examined for detection of the aforementioned antimicrobials using the microbial inhibition assay and high-performance liquid chromatography (HPLC). Besides, samples containing the highest antimicrobial residues were examined for the effect of boiling for 30 minutes on such residues. Results: The obtained results revealed that 23%, 21%, and 17% of the examined samples were positive for OTC, gentamicin, and ciprofloxacin residues , respectively . Cooking (boiling) for 30 minutes showed a reduction of the antibiotic residue by 88.2%, 95.2%, and 31.3%, respectively. Conclusion: Antimicrobial residues were detected in the chicken meat parts retailed in Egypt. Cooking can reduce the antimicrobial residues at least in part. Keywords: HPLC, Oxytetracycline, Gentamicin, Ciprofloxacin, Antibiotic residue. IntroductionParticularly in underdeveloped countries, chicken meat provides an affordable, readily accessible, and nutrient-dense source of essential nutrients for diets. It has a considerable amount of B-complex vitamins, minerals, and important polyunsaturated fatty acids, particularly omega (n)-3 fatty acids (Esnaola-Gonzalez et al., 2020). Three main uses of drugs are found in the poultry industry: growth stimulation, prevention, and therapies. Sub-therapeutic dosages of antibiotics are administered to healthy animals as preventative measures thought to be at risk, although the illness has not yet manifested as it should. Conversely, relatively low dosages of antibiotics may be administered, usually as a feed supplement, to suppress gut bacteria and free up more nutrients for animals or birds to ingest (Maharjan and Neupane, 2020). Antibiotic administration leaves residues in animal tissues and consumables produced from them, such as milk, table eggs, red meat, and chicken meat (Al-mashhadany, 2019). Antibiotic residues in animal-derived products, along with noncompliance with usage guidelines (namely, dosage and duration of withdrawal), and subpar cattle farming methods, can have detrimental effects on consumer health (Stella et al., 2020). Antibiotic residues have been linked to allergic reactions, bone marrow abnormalities, disruption of the normal gut microbiome, and direct toxicity. Moreover, extended exposure to antibiotic residues during pregnancy may cause a variety of congenital abnormalities in the offspring (Chen et al., 2019). Antibiotic residues also have the potentially harmful effects of being carcinogenic and mutagenic. Furthermore, the development of microorganisms resistant to antibiotics is linked to medication residues (Stella et al., 2020). Antibiotics such as tetracycline, ciprofloxacin, and gentamicin, are frequently used in underdeveloped nations on poultry feed. Oxytetracycline (OTC) is one of the most widely used antibiotics in veterinary medicine due to its easy oral administration through feed or drinking water, low cost, and broad-spectrum bacteriostatic activity. Due to its high water solubility, this naturally occurring tetracycline molecule is either synthetically synthesized or obtained from the fungus Streptomyces rimosus. It is excreted in its parent form and is not well digested by the body (Slana and Dolenc, 2013). Veterinary professionals typically employ the broad-spectrum aminoglycoside antibiotic gentamicin to treat a variety of bacterial illnesses (Goetting et al., 2011). One of the second-generation fluoroquinolone antibiotics, ciprofloxacin inhibits the enzyme DNA gyrase, which is critical for bacterial chromosome replication. This action provides a broad-spectrum defense against both Gram-positive and Gram-negative bacteria as well as mycoplasma infection (Xiao et al., 2014). In view of public health and food safety issues, it is necessary to identify and quantify the antibiotic residues in various food products generated from animals as well as to implement awareness initiatives regarding the detrimental effects of these residues. Antibiotic residue levels can be assessed using a variety of methods, including biosensor, immunological, enzymatic, microbiological, and mass spectrometric techniques. These methods have poor selectivity and sensitivity. However, it is a fact that measuring and verifying antibiotic residues requires a chemical process. Chromatographic techniques are currently the most accurate way for evaluating antibiotic residues in foods originating from animals. As a result, this study proposed to estimate antimicrobial residues in chicken meat and giblets (liver, gizzard, and muscles) using the microbial and high-performance liquid chromatography (HPLC) methods. Besides, we investigated how boiling chicken meat affected the amount of antibiotic residue in chicken meat. Materials and MethodsSample collectionA total of 100 samples of chicken liver, gizzard, thigh, and breast, 25 of each were randomly gathered from many chicken slaughterhouses spread around the markets in Kafr El-Sheikh governorate. After being wrapped in a plastic bag and placed in an ice box, each sample was taken to the laboratory of Meat Hygiene, Faculty of Veterinary Medicine, Kafr El-Sheikh University, Egypt, to have the antibiotic residues (ciprofloxacin, gentamicin, and OTC) measured. Antibiotic residue determinationMicrobiological techniques and HPLC were used to achieve this purpose. Microbiological methodThis method was done according to previous approaches (Okerman et al., 2001; Ferrini et al., 2006). Test organismsEscherichia coliThis bacterium was employed on nutritional agar standard II at pH 8.0 to detect the residues of ciprofloxacin. Bacillus subtilisThis bacterium was employed on nutritional agar standard II at pH 7.2 to detect gentamicin residues. Micrococcus luteusThis bacterium was employed on nutrient agar standard II at pH 8.0 to detect the residues of OTC. Setting up the test organismsEscherichia coliEscherichia coli was cultured for 24 hours at 35°C in nutritional broth. After being diluted to a density of 107 cells per milliliter, the culture was refrigerated. Bacillus subtilisBacillus subtilis BGA cells were heavily suspended and injected into nutritional agar standard II (Merk Art. Nr 7883) + 0.1% KH2Po4 (potassium dihydrogen phosphate), pH 7.2 solution. Using a sterile physiological saline solution, the test organism was removed following a 10-day incubation period at 30°C. For 10 minutes, this suspension was centrifuged at 3,000 rpm. After discarding the supernatant, 10 ml of sterile physiological saline was poured into the sediment. Following mixing, the suspension underwent two rounds of centrifugation for 10 minutes each, followed by 30 minutes of heating at 70°C. After diluting the resulting spore suspension to a density of 107 spores/ml, the spores were counted using the pour plate method. The suspension of the prepared spores was refrigerated at 4°C until it was needed. Micrococcus luteusMicrococcus luteus culture was produced by inoculating nutritional broth and incubating it for 48 hours at 37°C. After being diluted to a density of 106 cells per milliliter, the culture was refrigerated. The test plates were prepared according to Ferrini et al., (2006). Then, cylindrical pieces from each sample with a diameter of 9 mm and a thickness of 2 mm were prepared. The test samples were placed on the freshly made cultured plates containing the specific bacteria for each antimicrobial, and incubated for a day at 35°C. The diameter of the zones of inhibition was measured. According to Heitzman (1994), a zone greater than or equal to 2 mm was recorded as a positive result. HPLC-based quantification of antibiotic residuesThe Agilent 1100 HPLC system, manufactured by Agilent Technologies in Waldbronn, Germany, was utilized for the quantitative detection of each antimicrobial in the positive samples from the microbial inhibition assay. It was outfitted with a quaternary pump model G 1311A, UV detector model G 1314A, which was calibrated at a wavelength of 254 nm, auto sampler model G1329A VP-ODS, and a Shim pack (150 × 4.6 mm) column from Shimadzu in Kyoto, Japan. Chemstation software was used to record and combine data. Ciprofloxacin residue determination (McEvoy, 2002; Samanidou et al., 2007)Sample setupFive grams of the homogenate of the material were precisely combined with 10 ml of 0.15 molar cloridrich acid in a polypropylene centrifuge tube, and the mixture was centrifuged at 4,400 rpm for 20 minutes at 4°C. After repeatedly repeating the exaction step, the supernatants were combined and filtered using a 0.2 μm syringe filter. Solid-phase extractionAn Solid-phase extraction (SPE) cartridge (SPE-pack vac 1cc, 100 mg) was preconditioned using 2.5 ml of HPLC-grade water and 2.5 ml of methanol. The cartridge was filled with 14 ml of the last extract. Following the extraction, the cartridge was rinsed three times: once with 3 ml of HPLC grade water, once with 3 ml of 0.2M Na2HPO4 (pH=9), and once again with 5 ml of HPLC grade water. After that, the cartridge was dried using air aspiration, and the antibiotic that had been eluted was evaporated and dried under a nitrogen stream. 200 ml of 0.2 ml Na2HPO4 (pH=9) was used to resolubility the dry residue. The test tube was centrifuged at 4,400 g for 5 minutes at 4°C after being vortex-mixed for 30 seconds. After being moved to an injection vial, the supernatant was introduced into the HPLC apparatus. An HPLC system including a Waters prep LC 4000 (USA) and a Spectroflow 783 UVV detector (WATERS tm 486, tunable absorbance, USA) with a 125 × 4mm i.d. was used to determine the antibiotic. A 5-m-long LiChrospher 100°C 18 HPLC column from the water was utilized. Every piece of data were examined using desktop software (Millennium v 12.15). Conditions for liquid chromatographic operationsWater-HPLC grade acetonitrile (CAN)-triethylamine (TEA) (83:14:0.45 v/v) was the mobile phase employed. However, before introducing CAN, pH was brought down to 2.3 using 85% H3PO4. There was a 1 ml/minute flow rate. The samples were subjected to HPLC analysis to determine the antibiotic level on a ppb basis and to assess the HPLC standards. 20 μl of injection volume, 1 ml/minute of flow rate, 220 nm of wave length, 35°C column temperature, 10 minutes of run time, 7 minutes of retention time, 0.1% aqueous formic acid in mobile phase A, and 0.1% formic acid in mobile phase B in acetonitrile (ACN). Determination of gentamicin residues (McGlinchey et al., 2008)Sample setupEach sample was homogenized for one minute in 5 g using the mincer. As a result, a propylene centrifuge tube containing 5 g of homogenate was precisely weighed, 10 ml of acetonitrile was added, and the tube was agitated for 20 minutes. Centrifugation was run at 3,500 rpm for 10 minutes. A 50 ml polypropylene centrifuge tube was used to collect the supernatant. Precisely, 8 ml of acetonitrile and 5 ml of monobasic potassium phosphate buffer were added to the mixed pellet. For 20 minutes, the mixture was shaken. Centrifugation was run at 3,500 rpm for 10 minutes. After combining the supernatants, 40 ml of water was added. For 10 minutes, the mixed solution was centrifuged at 3,500 rpm. SPE cleaning was applied to the supernatant. Solid-phase extractionIn actuality, the SPE cartridge was inserted into the vacuum manifold system, and the solvent trap received the SPE cartridge effluent. Ten milliliters of methanol and 10 ml of water were used to condition the cartridge. The sample was added to the cartridge in three milliliters. At this stage, the cartridge was not allowed to dry out and the flow rate was limited to two drips per second. After flushing the cartridge with 10 ml of deionized water, ten milliliters of acetonitrile were added. The SPE cartridge was vacuum-dried for a minimum of three minutes. 2.5 ml of the 0.1 mol/L ammonium acetate, methanol, and acetonitrile solution was used for each elution step. The gathered elute was dried in a water bath at 30°C using a nitrogen stream. One milliliter of dipotassium hydrogen phosphate buffer was used to reconstitute the sample. Before being injected into the LC system, the sample was combined and passed through 0.45 μm filters for filtering. Conditions for liquid chromatographic operations100 μl of injection volume, 0.7 ml/minutes of flow rate, 287 nm of wave length, ambient column temperature, 30 minutes of stop time, 16 minutes of post time, 0.05% trifluoroacetic acid for mobile phase A, and acetonitrile for mobile phase B. OTC residue determination (Senyuva et al., 2000)Drug extraction from the sampleThe studied samples were trimmed of their exterior fascia and fat, and then finely diced using scissors. Each organ to be examined weighed two grams, which were then divided into extremely tiny pieces and processed into a fine powder using a Sartorius mincer. The measurements were taken using a digital scale. After two minutes of blender homogenization, 0.1 g of citric acid was added. This mixture was supplemented with 1 ml of 30% nitric acid, 4 ml of methanol, and 1 ml of deionized water, in that order. After being well mixed in a vortex for 15 minutes, the mixture containing solid particles was centrifuged for 10 minutes at 5,300 rpm. A 0.45 μm nylon filter was used to filter the solution before 20 μl of it was added to an HPLC for analysis. Chromatographic stateA gradient-method mobile phase containing 0.1% formic acid and methanol at 25°C and 1.5 ml/minutes flow rate. Using the mobile phase as previously mentioned, the separation was carried out on Hypersil gold C18 (10 μm, 100 × 4.6 mm) columns. A photodiode array detector with a 350 nm wave length was used for the detection. Using areas under curves that the program automatically extrapolated, the residue content of the samples was determined (Chromo Quest 5). Calibration curveOTC concentrations in eluent of 10, 20, 30, 40, 50, and 60 μg/L were used to generate the curve. A 100 ml volumetric flask containing 100 mg of OTC standard was precisely weighed and filled with the powder, which was then dissolved in 100 ml of methanol to create a stock solution. These standards were generated using the daily prepared stock solution. OTC had a detection limit of 0.01 ppm and a retention period of 3.9 minutes. In general, the following adjustments were made to the HPLC settings for the measurement of OTC. Antimicrobial residue quantification using peak area analysisTo achieve spiking levels of 1 μg/ml, 500 μl of spiking solution was added to a 10 g control sample that did not contain any antibiotics, such as OTC. To assess the concentrations of these antibiotics, the retention time was estimated. For every antibiotic, a standard calibration curve was applied using five established standard OTC solutions at concentrations of 100, 200, 300, 400, and 500 ppb. Impact of boiling on the Antibiotics residuesChicken thighs were immersed in a water bath that had been heated to 100°C, boiled for 30 minutes, and then removed and allowed to cool. This was done to investigate the effect of boiling on the concentrations of the detected antibiotic residues in the positive samples containing the highest concentrations of the three tested antibiotics (Javadi et al., 2011). The antibiotic residues in each tested sample were determined using HPLC as previously mentioned. Statistical analysisAll data were exposed to statistical analysis using one way analysis of variance, followed by Tukey’s Kramer HSD test where a p-value less than 0.05 is considered significant. Ethical approvalNot needed for this study. ResultsThe findings of this investigation showed that broiler samples included antibiotic residues (gentamicin, ciprofloxacin, and OTC) as identified by both microbiological inhibition and HPLC methods. Ciprofloxacin residues were found in the chicken samples (breast, thigh, gizzard, and liver) at 8%, 12%, 20%, and 28%, respectively, as indicated in Tables 1 and 2. The recorded average residual concentrations (ng/g) were 69.3 ± 2.6, 88.7 ± 3.2, 159.1 ± 5.8, and 212.8 ± 9.1. Table 3 demonstrated that, of the 17 antibiotic-positive samples, only one (4%) sample of breast meat, one (4%), sample of thigh muscle, two (8%) samples from the gizzard, and four (16%) samples from the liver exceeded the maximum residual limit. The findings presented in Table 4 demonstrated how boiling affected the levels of ciprofloxacin residual in chicken samples (breast, thigh, gizzard, and liver). The concentrations of residue ciprofloxacin after boiling were 89.5, 109.2, 197.3, and 210.1, respectively. The percentages of reduction were, in order, 23.4%, 24.8%, 26.0%, and 31.3%. Table 1. Detection of ciprofloxacin residues in the examined samples of chicken meat and offal using MIT (n=25).
Table 2. Concentrations of ciprofloxacin residues (ng/g) in the examined samples of chicken meat and offal using HPLC (n=25).
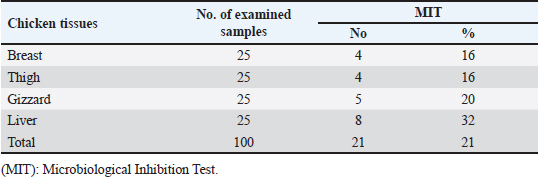
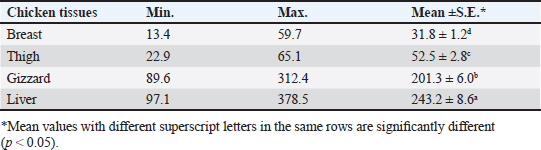
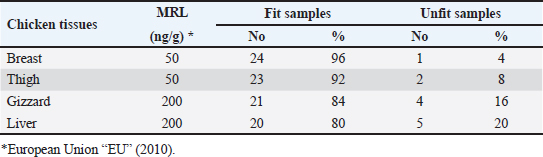
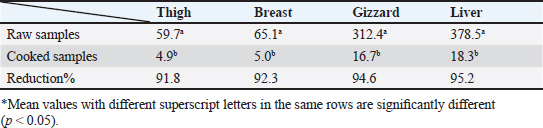
Gentamicin residues were also detected in the chicken samples (breast, thigh, gizzard, and liver) at 16%, 16%, 20%, and 32%, respectively, as shown in Tables 5 and 6. The recorded residual concentrations (ng/g) in these tissues were 31.8 ± 1.2, 52.5 ± 2.8, 201.3 ± 6.0, and 243.2 ± 8.6. Table 7 indicates that out of the 100 samples that were investigated, 25 of the samples (liver, gizzard, thigh, and breast) tested positive for gentamicin residues. One, two, four, and five of these positive samples had gentamicin residue levels over the maximum residue limit (MRL) with percentages of 4%, 8%, 16%, and 20%, respectively. The findings presented in Table 8 demonstrated that gentamicin residual concentrations after boiling were 4.9, 5.0, 16.7, and 18.3 ng/g, respectively, while the corresponding reduction percentages were 91.8%, 92.3%, 94.6%, and 95.2%, respectively. Table 3. Fitness of the examined samples of chicken meat and offal according to their ciprofloxacin residues (n=25).
Table 4. Effect of cooking on ciprofloxacin residues (ng/g) in the chicken meat and offal samples.
Table 5. Detection of gentamicin residues in the examined samples of chicken meat and offal using MIT (n=25).
Table 6. Concentrations of gentamicin residues (ng/g) in the examined samples of chicken meat and offal using HPLC (n=25).
Table 7. Fitness of the examined samples of chicken meat and offal according to their gentamicin residues (n=25).
Table 8. Effect of cooking on gentamicin residues (ng/g) in the chicken meat and offal samples.
Table 9. Detection of OTC residues in the examined samples of chicken meat and offal using MIT (n=25).
Table 10. Concentrations of OTC residues (ng/g) in the examined samples of chicken meat and offal using HPLC (n=25).
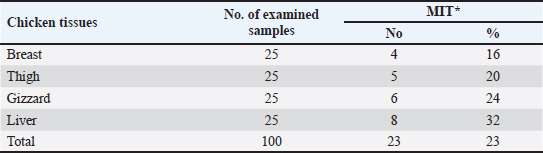
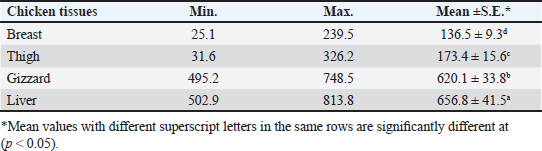
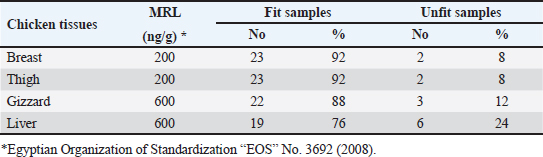
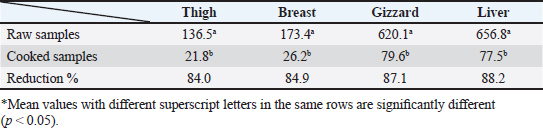
OTC was detected in the chicken samples (breast, thigh, gizzard, and liver) at 16%, 20%, 24%, and 32%, respectively, as indicated in Tables 9 and 10. The recorded average concentrations (ng/g) of OTC were 136.5 ± 9.3, 173.4 ± 15.6, 620.1 ± 33.8, and 656.8 ± 41.5. Table 11 showed that only 8%, 8%, 12%, and 24% of the tested chicken samples were above the MRLs. The findings displayed in Table 12 demonstrated that the levels of OTC residues in the examined samples after boiling were reduced to 21.8, 26.2, 79.6, and 77.5 ng/g, respectively, while the corresponding reduction percentages were 84%, 84.9%, 87.1%, and 88.2%, respectively. Table 11. Fitness of the examined samples of chicken meat and offal according to their OTC residues (n=25).
Table 12. Effect of cooking on OTC residues (ng/g) in the chicken meat and offal samples.
DiscussionBecause it provides high-quality protein, significant levels of vitamins and minerals, is low in fat, simple to cook, flexible, and perfect for quick service, chicken meat is popular in both developed and developing countries (FAO, 2017). Antibiotic residues are currently the main concern for public health authorities worldwide due to the growing significance of food safety and the WHO’s declaration that antibiotic resistance is a global issue (WHO, 2017). CiprofloxacinOne of the second-generation fluoroquinolone antibiotics, ciprofloxacin inhibits the enzyme DNA gyrase, which is critical for bacterial chromosome replication. This action is seen against a variety of gram-positive and gram-negative bacteria as well as mycoplasma infections (Xiao et al., 2014). Ciprofloxacin was detected in the examined samples at variable rates. Such results were consistent with the findings of Silva and Hollenbach (2010), and Raphaella (2008), who showed that the kidneys and liver serve as the reservoir for ciprofloxacin residues. These findings were also supported by those published by Amjad et al. (2005), who mentioned that, in addition to the liver, kidney, fat, and skin, muscles had the highest rate of ciprofloxacin residues. Ciprofloxacin residues in chicken liver were detected at 44%, thighs at 34%, and breast at 30% (Sattar et al., 2014). Likely, Widiastuti et al. (2022) reported that 67.3% of chicken meat had ciprofloxacin residues, with a mean concentration of 91.22 ± 70.49 ng/g. This result was greater than that of Lee et al. (2018), and Pereira et al. (2018), who identified no samples with residues exceeding the MRL. In 15 (30%) liver samples, Rezaee et al. (2018) discovered ciprofloxacin residues with a mean of 24.8 ± 23.5. Verma et al. (2020) looked for ciprofloxacin residues in the muscle, liver, kidney, and fat of broiler chickens but found none in the gizzard, muscle, or heart. According to the findings, 38.71% of meat samples tested positive for ciprofloxacin in Turkey (Buket et al., 2013). In Egypt, 45.7% of chicken meat samples tested positive for the antibiotic (Amro et al., 2013). The incidence rate was 40% in chicken meat (Chaiba et al., 2017), and 36.15% of chicken meat samples tested positive for antibiotic residues. According to Mohamed (2019), ciprofloxacin residues were found in chicken flesh and its byproducts in high percentages (76%–92%). The MRL for ciprofloxacin and other fluoroquinolones is 100 μg/kg, as set by the European Union (EU, 2010; Tavakoli et al., 2015). In the present study several samples exceeded the established MRL of ciprofloxacin. Similarly, Faten et al. (2016), reported that 42.8% of breast samples have ciprofloxacin residues above the MRL. While, 44.4% of thigh samples had levels higher than the MRL. However, 61.5% of chicken liver samples were rejected because they exceeded the MRL. Heat processing, including boiling, roasting, and frying, is known to cause pH fluctuation, water loss, and denaturation of proteins (Almashhadany, 2020). Food-borne antibiotics may be thermally processed, changing their physicochemical characteristics (Alaboudi, 2017). As a result, some antibiotic residues are unstable chemically and can break down in storage and when exposed to heat (Almashhadany, 2020). In the current investigation, boiling reduced ciprofloxacin residues by 80%. Likely, Javadi et al., (2011) and Fathy et al. (2015), declared that cooking procedures can significantly lower ciprofloxacin residual levels. Furthermore, the majority of the residues from the boiling procedure were released from the tissues into the cooking liquid; therefore, disposing of any fluids derived from the cooked edible tissues may help minimize exposure to antibiotic residues. As a result, boiling produced a higher reduction rate, whereas roasting and grilling, which cause water loss, produced a lower reduction rate (Botsoglou and Fletouris, 2001; Lolo et al., 2006). GentamicinMost of the Gram-negative bacteria are susceptible to the bactericidal effects of gentamicin. It leads to problems with toxicity and resistance when given incorrectly. Worldwide, gentamicin residue in foods derived from animals is very important for public health. Due to the potential health hazards, specifically the nephrotoxic and ototoxic effects, as well as the immune system’s recognized negative impacts and the development of drug-resistant bacteria in both humans and animals, it is not recommended (Tan et al., 2009). The high frequency of gentamicin residue detected in these tissues may be explained by the fact that the liver and kidney are the organs in charge of drug detoxification and excretion of gentamicin and other drugs, respectively. Nevertheless, research suggests that the amount of gentamicin residue differs among tissues (Beyene, 2016), with the liver having a higher residue than other organs. Whereas the samples that met the approved codex of the European Union (MRL) were 96%, 92%, 84%, and 80%. Singh et al. (2019) reported a lower-than-expected result. Of 180 samples, 24 (13.33%) were found to have gentamicin residues, and six samples (3.33%) had gentamicin residues over the maximum residue threshold. In muscle and liver samples, the mean concentration was 53.00 ± 18.70 and 212.00 ± 73.76, respectively. According to Shareef et al. (2009), there are no gentamycin residues found in any of the chicken flesh samples from Iraq. And in 3% of the samples that were analyzed, Thapa (2021) found gentamycin residues. There was no discernible difference in the prevalence of gentamycin residues among tissues (p > 0.05). Gentamycin residues were detected in 4% of the samples from the liver, kidney, and gizzard, but not in any of the samples from the breast meat. Furthermore, Hussein et al. (2016) found that just 2% and 8% of chicken thigh and liver, respectively, had gentamicin residues, with mean values of 0.67 and 0.94 ± 0.17 ppm. The average levels of gentamycin residues in muscle and liver surpass the MRL reported by EU (2010), which is 0.1 and 0.3 ppm, respectively. The findings of Onyeanu et al. (2020) revealed a higher percentage of commercial broilers (65%) that had traces of gentamicin detected at the time of purchase. This suggests that consumers who purchase broilers from the Ikpa slaughterhouse may be exposed to dangerously elevated levels of drug residue. Furthermore, the high frequency of gentamicin residue found is consistent with studies by Adebowale et al. (2016), who found that 100% of chicken production in Ogun State, Nigeria uses gentamicin, either alone or in conjunction with other antibiotics. This has been related to a number of factors, including misuse, self-medication by chicken farmers, and repeated administration of the medication to avoid early mortality linked to Salmonella Typhimurium and E. coli (Doyle, 2006). It has also been linked to failure to follow withdrawal periods. Cooking reduced gentamicin levels in breast, thigh, gizzard, and liver at 91.8%, 92.3%, 94.6%, and 95.2%. This result is almost identical to that of Fathy (2015), who discovered that after boiling and frying, there was no residue of gentamicin left behind and that no residues were found using HPLC (Fathy, 2015). These findings may be explained by the fact that gentamicin is a highly polar, water-soluble chemical that lacks chromophores and fluorophores and is thermally labile (Isoherranen and Soback, 2000). Besides, Singh et al. (2019), noted a drop in the antibiotic’s residual concentration upon boiling, suggesting that heat inactivated the drug and making it largely heat labile. The mean residue content of gentamicin in the liver and muscle samples decreased dramatically to 92.33% and 90.25 %, respectively. However, this is more than that reported by Hussein et al. (2016), who discovered a noteworthy drop in gentamicin residues after boiling, resulting in a reduction percentage of 35.9%. OTCThe observation of OTC dominance may be explained by the fact that OTC is added to feed more frequently than tetracycline and chlortetracycline, either for nutritional or preventative objectives (Cháfer-Pericás et al., 2010). According to “EOS” No. 3692 (2008), the MRLs for OTC in flesh, and giblets are 200 and 600 ug/kg, respectively. Several samples had OTC residues higher than the established limits. Higher results were reached by Hussein and Khalil (2013), while similar levels were detected by Elbayoumi et al. (2018). However, Hussein et al. (2016), and Verma et al. (2021) reported lower results. In contrast, Shahid et al. (2007) discovered that OTC residue was present in 4 out of 7 samples of chicken meat from Pakistan, with concentrations ranging from 30 to 85 ppb. In contrast, the studies conducted by Cetinkaya et al. (2012) found that the samples they examined did not contain any OTC residues. The influence of cooking on OTC residues in chicken breast, thigh, gizzard, and liver samples was demonstrated in this investigation and allowed reduction rates of 84.9%, 84%, 87.1%, and 88.2%, respectively. Elbayoumi et al. (2018) reported a lower result, stating that the mean percentage reduction of OTC residues in broiler meat and liver samples containing the drug after boiling was 85.6% and 79.2%, respectively. According to Shaltout et al. (2019), broiler meat and liver samples had an average reduction percentage of OTC residues of 77.93%. Numerous studies have said that while cooking at the right temperature, for the right amount of time, and with the right amount of force can reduce the presence of some antibiotic residues, it usually does not give customers an extra margin of safety (Fathy et al., 2015; Hussein et al., 2016). Denaturation of the protein was the cause of the whole loss of OTC residues (Nguyen et al., 2013). ConclusionResults showed that ciprofloxacin, gentamicin, and OTC residues were present in chicken samples. Although these residues are not at the levels set by the European Union, they still pose a risk to consumer health because they can trigger allergic reactions or help develop antibiotic-resistant bacterial strains, which have become a global issue in the treatment of infectious diseases in humans. Hence, veterinary authorities ought to regulate and outlaw the use of antibiotics as growth promoters in chicken farms. To prevent veterinary medication residues in poultry and their products, the Ministry of Agriculture and veterinary directorates must establish stringent monitoring programs. To guarantee the appropriate withdrawal times before slaughter and marketing, regulations should be put in place. Furthermore, a policy of monitoring ought to be instituted to guarantee that poultry meat complies with global norms. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingNo external funding has been provided. Authors contributionsAll authors contributed to this study. All authors revised, read, and approved the final manuscript. Data availabilityAll data are provided in the manuscript. Any extra data needed can be provided by the corresponding author upon reasonable request. ReferencesAdebowale, O.O., Adeyemo, O.K., Awoyomi, O., Dada, R., and Adebowale, O., 2016. Antibiotic use and practices in commercial poultry laying hens in Ogun state Nigeria. Rev. Elev. Med. Vet. Pays Trop. 69, 41–45. Alaboudi, A.R. 2017. Antimicrobial residues in table eggs. In egg innovations and strategies for improvements. Eds., Hester, P.Y. San Diego, CA: Academic Press, pp: 447–456. Al-mashhadany, D.A. 2019. Detection of antibiotic residues among raw beef in Erbil City (Iraq) and impact of temperature on antibiotic remains. Ital. J. Food Saf. 8, 6–10. Almashhadany, D. 2020. Detecting antibiotic residues among sheep milk using YCT, DDA, and acidification method in Erbil city, Kurdistan Region, Iraq. Bull. Anim. Sci. Biotechnol. 77(2), 29–35. Amjad, H., Naeem, M., Iqbal, J. and Khan, K. 2005. Estimation of selected residual antibiotics in poultry products available in local markets during summer. J. Chem. Soci. Pak. 27, 637–642 Amro, F.H., Hassan, M.A. and Mahmoud, A.H. 2013. Spiramycin residues in chicken meat and giblets. Benha Vet. Med. J. 24, 51–61. Beyene, T. 2016. Veterinary drug residues in food animal products: Its risk factors and potential effects on public health. J. Vet. Sci. Technol. 7(1), 285. Botsoglou, N.A. and Fletouris, D.J. 2001. Stability of residues during food processing. New York, NY: Marcel Dekker, Inc. Buket, E.R., Onurdağ, F.K., Demirhan, B., Özgacar, S.O., Öktem, A.B. and Abbasoğlu, A. 2013. Screening of quinolone antibiotic residues in chicken meat and beef sold in the markets of Ankara, Turkey. Poult. Sci. 92, 2212–2215. Cetinkaya, F., Yibar, A., Soyutemiz, G.E., Okutan, B., Ozcan, A. and Karaca, M.Y. 2012. Determination of tetracycline residues in chicken meat by liquidchromatography tandem mass spectrometry. Food Add. Contam. Part B. 1, 1–5. Cháfer-Pericás, C., Maquieira, Á. and Puchades, R. 2010. Fast screening methods to detect antibiotic residues in food samples. TrAC Trends in Anal. Chem. 29(9), 1038–1049. Chaiba, A., Filali, F.R. and Chebaibi, A. 2017. Investigation of antibiotics residues in poultry products in Meknes-Morocco. J. Advanc. Microbiol. 2(1), 1–8. Chen, J., Ying, G.G. and Deng, W.J. 2019. Antibiotic residues in food: extraction, analysis, and human health concerns. J. Agricul. Food Chem. 67, 7569–7586. Doyle, M.E. 2006. Veterinary drug residues in processed meat: potential health risk? Food research institute briefings. Madison, WI: University of Wisconsin-Madison. European Union (EU). 2010. On pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Commission Regulation (EC) 37-2010. Egyptian Organization of Standardization and Quality Control “EOS” 2008. Maximum residue limits for veterinary drugs in foods, ES: 3692/2008. Elbayoumi, Z., Yuosief, A. and Elbagory, A. 2018. Assessment of doxycycline and oxytetracycline residues in broiler meat. Alex. J. Vet. Sci. 57, 23. Esnaola-Gonzalez, I., Gómez-Omella, M., Ferreiro, S., Fernandez, I., Lázaro, I. and García, E. 2020. An IoT platform towards the enhancement of poultry production chains. Sensors 20(6), 1549. FAO (Food and Agriculture Organization). 2017. Poultry and human nutrition. Agriculture and Consumer Protection Department (AG). Available via http://www.fao.org/contact-us/terms/en/ Faten, S.H., Mousa, M.M., Mahomud, A.H., Wafaa, M.M.H. and Fatma, H.A. 2016. Ciprofloxacin residues in chicken and turkey carcasses Benha Vet. Med. J. 31(2), 136–143. Fathy, F.M., Ahmed, A.M. and Moursi, M.K. 2015. Effect of cooking methods on antibiotic residues in broiler chicken. 2nd Conference of food safety, Suez Canal Univ. Fac. Vet. Med. 1, 76–81. Ferrini, A.M., Mannoni, V. and Aureli, P. 2006. Combined plate microbial assay (CPMA): A 6-plate-method for simultaneous first and second level screening of antibacterial residues in meat. Food Addit. Contam. 23, 16–24. Goetting, V., Lee, K.A. and Tell, L.A. 2011. Pharmacokinetics of veterinary drugs in laying hens and residues in eggs: a review of literature. J. Vet. Pharmacol. Ther. 34(6), 521–556. Heitzman, R.J. 1994. Veterinary drug residues: residues in food-producing animals and their products-reference materials and methods, 2nd ed. Singapore: Wiley-Blackwell, pp: 1–8. Hussein, M.A. and Khalil, S. 2013. Screening of some antibiotics and anabolic steroids residues in broiler fillet marketed in El-Sharkia governorate. Life Sci. J. 10(1), 2111–2118. Hussein, M.A., Mona M.A. and Morshedy, A.M. 2016. Effect of cooking methods on some antibiotic residues in chicken meat. Jpn. J. Vet. Res. 64 (Suppl. 2), S225–231. Isoherranen, N. and Soback, S. 2000. Determination of gentamicin’s C (1), C (1a), and C (2) in plasma and urine by HPLC. Clin. Chem. 46, 837–842. Javadi, A., Mirzaie, H. and Khatibi, S.A. 2011. Effect of roasting, boiling and microwaving cooking methods on enrofloxacin residues in edible tissues of broiler. Afri. J. Pharm. Pharmacol. 5(2), 214–218. Lee, H.J., Cho, S.H., Shin, D. and Kang, H.S. 2018. Prevalence of antibiotic residues and antibiotic resistance in isolates of chicken meat in Korea. Korean J. Food. Sci. Anim. Resour. 38, 1055–1063. Lolo, M., Pedreira, S., Miranda, J.M., Vázquez, B.I., Franco, C.M., Cepeda, A. and Fente, C. 2006. Effect of cooking on enrofloxacin residues in chicken tissue. Food Addit. Contam. 23(10), 988–993. Maharjan, B. and Neupane, R. 2020. Antibiotic residue in marketed broiler meat of Kathmandu Metropolitan City. Arch. Vet. Sci. Med. 3, 1–10. McEvoy, J.D.G. 2002. Contamination of animal feedstuffs as a cause of residues in food. A review of regulatory aspect, incidence and control. Anal. Chim. Acta. 473, 3–26. McGlinchey, T., Rafter, P., Regan, F. and McMahon, G. 2008. A review of analytical methods for determination of aminoglycoside and macrolide residues in food matrices. Anal. Chem. Acta 624, 1–15. Mohamed, K. 2019. HPLC determination of ciprofloxacin residues in chicken meat and its products. Assiut Vet. Med. J. 65(161), 159–163. Nguyen, V., Li, M., Khan, M., Li, C. and Zhou, G. 2013. Effect of cooking methods on tetracycline residues in pig meat. Afri. J. Pharm. Pharmacol. 7, 1448–1454. Okerman, L., Croubels, S., Baere, S., Hoof, J.V., Backer, P.D. and Brabander, H.D. 2001. Inhibition tests for detection and presumptive identification of tetracyclines, beta-lactam antibiotics and quinolones in poultry meat. Food Addit. Contam. 18(5), 385–393. Onyeanu, C.T., Ezenduka, E.V. and Anaga, A.O. 2020. Determination of gentamicin use in poultry farms in Enugu state, Nigeria, and detection of its residue in slaughter commercial broilers. Int. J. One Health 6(1), 6–11. Pereira, A.M., Silva, L.J., Rodrigues, J., Lino, C. and Pena, A. 2018. Risk assessment of fluoroquinolones from poultry muscle consumption: comparing healthy adult and pre-school populations. Food Chem. Toxicol. 118, 340–347. Raphaella, W. 2008. enrofloxacin and ciprofloxacin residues in broiler chicken post administration of enrofloxacin. Indones. J. Anim. Vet. Sci. 13, 150–154. Rezaee, M.N., Arefhosseini, S.R., Javadi, A., Lotfipur, F., Ansarin, M., Tamizi, E. and Nemati, M. 2018. Determination of enrofloxacin and ciprofloxacin residues in five different kinds of chicken tissues by dispersive liquid-liquid microextraction coupled with HPLC. Iran. J. Pharm. Res. 17(4), 1182–1190. Samanidou, V.F., Nikolaidou, K.I. and Papadoyannis, I.N. 2007. Advances in chromatographic analysis of tetracyclines in foodstuffs of animal origin—a review. Sep. Purif. Rev. 36, 1–69. Sattar, S., Hassan, M.M., Islam, S.K.M.A., Alam, M., Faruk, M.S.A., Chowdhury, S. and Saifuddin, A.K.M. 2014. Antibiotic residues in broiler and layer meat in Chittagong district of Bangladesh. Vet. World 7(9), 738–743. Senyuva, H., O’den, T. and Sarica, D.Y. 2000. High performance liquid chromatographic determination of oxytetracycline residue in cured meat products. Instrumental Analysis Center. Scientific and Technical Research Council of Turkey. (TUBITAK) 06530. Ankara-Turkey. J. Chem. 24, 395–400. Shahid, M.A, Siddique, M., Abubakar, M., Arshed, M.J., Asif, M. and Ahmad. A. 2007. Status of oxytetracycline residues in chicken meat in Rawalpindi/Islamabad area of Pakistan. Asian J. Poult. Sci. 1(1), 8–15. Shaltout, F.A.E., Shatter, M.A.E. and Sayed, N.F. 2019. Impacts of different types of cooking and freezing on antibiotic residues in chicken meat. J. Food Sci. Nuter. 5, 045. Shareef, A., Jamel, Z. and Yonis, K. 2009. Detection of antibiotic residues in stored poultry products. Iraqi. J. Vet. Sci. 23, 45–48. Silva, J.M.B. and Hollenbach, C.B. 2010. Fluoroquinolones × Resistência bacteriana na medicina veterinária. Arq Inst Biol. São Paulo 77, 363–369. Singh, T., Singh, A., Meena, N. and Kumar, A. 2019. Determination of gentamicin residues in chicken meat by high performance liquid. Indian J. Poult, Sci. 54, 155. Slana, M. and Dolenc, M.S. 2013. Environmental risk assessment of antimicrobials applied in veterinary medicine-A field study and laboratory approach. Environ. Toxic. Pharm. 35, 131–141. Stella, O.I.O., Ezenduka, E.V. and Anaelom, N.J. 2020. Screening for tylosin and other antimicrobial residues in fresh and fermented (nono) cow milk in Delta state, South-South, Nigeria. Vet. World 13, 458–464. Tan, X., Jiang, Y.W., Huang, Y.J. and Hu, S.H. 2009. Persistence of gentamicin residues in milk after the intramammary treatment of lactating cows for mastitis. J. Zhejiang Univ. Sci. B. 10(4), 280–284. Tavakoli, H.R., Firouzabadi, M.S., Afsharfarnia, M., Joneidi, N. and Sa’adat, S. 2015. Detecting antibiotic residues by HPLC method in chicken and calves’ meat in diet of a military center in Tehran. Acta Medica Mediterranea 31, 1427. Thapa, S. 2021. Antibiotic residues in broiler meat sold in dharan, Thesis, Department of Food Technology Central Campus of Technology Institute of Science and Technology Tribhuvan University, Nepal. Available via http://202.45.146.37:8080/jspui/handle/123456789/203 Verma, M.K., Ahmad, A., Pant, D., Rawat P., Sharma, S. and Arya, N. 2020. Screening of enrofloxacin and ciprofloxacin residues in chicken meat by high-performance liquid chromatography. J. Pharm. Res. Inter. 32, 64–69. Verma, M.K., Ahmad, A.H., Pant, D. and Patwal, P.C. 2021. Evaluation of oxytetracycline residues in chicken meat samples by HPLC. Pharma Innov. J. 10(4), 155–157. WHO (World Health Organization). 2017. Pesticide residues in food. Fact sheet July. Widiastuti, R., Martindah., E. and Anastasia, Y. 2022. Detection and dietary exposure assessment of fluoroquinolones residues in chicken meat from the Districts of Malang and Blitar, Indonesia. Trop. Anim. Sci, J. 45(1), 98–103. Xiao, R., He, Z., Diaz-Rivera, D., Pee, G.Y. and Weavers, L.K. 2014. Sono chemical degradation of ciprofloxacin and ibuprofen in the presence of matrix organic compounds. Ultrasonic Sonochem. 21(1), 428–435. | ||
| How to Cite this Article |
| Pubmed Style Kamouh HM, Abdallah R, Kirrella GA, Mostafa NY, Shafik S. Assessment of antibiotic residues in chicken meat. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 438-448. doi:10.5455/OVJ.2024.v14.i1.40 Web Style Kamouh HM, Abdallah R, Kirrella GA, Mostafa NY, Shafik S. Assessment of antibiotic residues in chicken meat. https://www.openveterinaryjournal.com/?mno=181962 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.40 AMA (American Medical Association) Style Kamouh HM, Abdallah R, Kirrella GA, Mostafa NY, Shafik S. Assessment of antibiotic residues in chicken meat. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 438-448. doi:10.5455/OVJ.2024.v14.i1.40 Vancouver/ICMJE Style Kamouh HM, Abdallah R, Kirrella GA, Mostafa NY, Shafik S. Assessment of antibiotic residues in chicken meat. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 438-448. doi:10.5455/OVJ.2024.v14.i1.40 Harvard Style Kamouh, H. M., Abdallah, . R., Kirrella, . G. A., Mostafa, . N. Y. & Shafik, . S. (2024) Assessment of antibiotic residues in chicken meat. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 438-448. doi:10.5455/OVJ.2024.v14.i1.40 Turabian Style Kamouh, Heba M., Reda Abdallah, Ghada A. Kirrella, Nader Y. Mostafa, and Saleh Shafik. 2024. Assessment of antibiotic residues in chicken meat. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 438-448. doi:10.5455/OVJ.2024.v14.i1.40 Chicago Style Kamouh, Heba M., Reda Abdallah, Ghada A. Kirrella, Nader Y. Mostafa, and Saleh Shafik. "Assessment of antibiotic residues in chicken meat." Open Veterinary Journal 14 (2024), 438-448. doi:10.5455/OVJ.2024.v14.i1.40 MLA (The Modern Language Association) Style Kamouh, Heba M., Reda Abdallah, Ghada A. Kirrella, Nader Y. Mostafa, and Saleh Shafik. "Assessment of antibiotic residues in chicken meat." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 438-448. Print. doi:10.5455/OVJ.2024.v14.i1.40 APA (American Psychological Association) Style Kamouh, H. M., Abdallah, . R., Kirrella, . G. A., Mostafa, . N. Y. & Shafik, . S. (2024) Assessment of antibiotic residues in chicken meat. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 438-448. doi:10.5455/OVJ.2024.v14.i1.40 |