| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 449-458 Original Research Comparative efficacy of difloxacin and nano-emulsion difloxacin as antibacterial agents against Salmonella enterica Serovar enteritidis in chickenssMohammed I. Ashosh1*, Moshira A. El-Abasy2, Ola M. El-Borady3, Ibrahim Elkhaiat4 and Mahmoud M. Ismail21Animal Production Research Institute (APRI), Agriculture Research Center, Dokki, Egypt 2Department of Poultry Diseases, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt 3Institute for Nanoscience and Nanotechnology, Kafrelsheikh University, Kafrelsheikh, Egypt 4Department of Poultry Production, Faculty of Agriculture, Kafrelsheikh University, Kafrelsheikh, Egypt *Corresponding Author: Mohammed I. Ashosh. Animal Production Research Institute (APRI), Agriculture Research Center, Egypt. Email: drashosh14 [at] gmail.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/07/2023 © 2024 Open Veterinary Journal
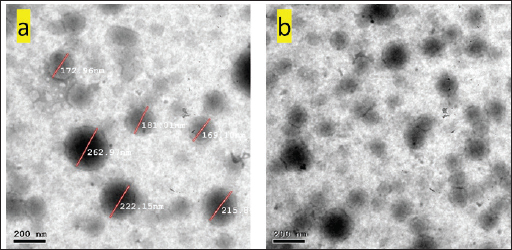
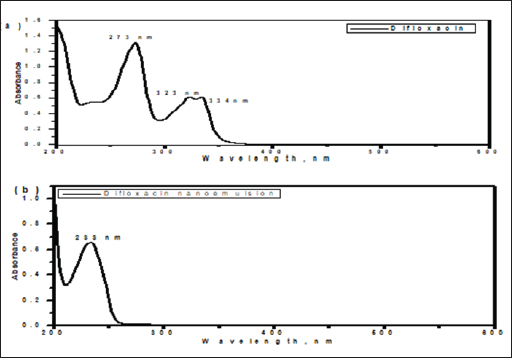
AbstractBackground: Avian salmonellosis is a group of diseases caused by bacteria from the genus Salmonella with a negative impact on poultry, particularly chickens. In addition, salmonellosis is a global food-borne infection. Aim: The aim of this study was to evaluate the effect of nano-emulsion difloxacin (NED) and commercial difloxacin (CD) water supplement on broiler's growth, feed intake, and body weight, weight gain, growth rate, feed conversion ratio (FCR), and mortality rate (MR). The antibiotic sensitivity was determined both in-vivo and in-vitro for NED against Salmonella enterica Serovar enteritidis in chickens. Methods: 1500 one-day of age chicks were grouped into five groups as follows: group 1 (G1) control negative group, G2 control positive group (infected and not treated), G3 (infected and treated with CD, and G4 and G5 (infected and treated with NED at different doses). Samples, including the intestine, liver, and spleen were collected. Agar well diffusion test and minimum inhibitory concentrations were adopted. Histopathological lesions on different tissues were studied. During 35 days of the experiment, the feed intake, growth rate, growth gain, FCR, and MR were recorded daily. In addition, a variety of analytical techniques including transmission electron microscopic analysis, dynamic light scattering, UV-visible spectroscopy, and zeta-potential analysis were applied to characterize NED. Results: The agar well diffusion test indicated that NED was in-vitro effective against S. enteritidis isolates than CD. The minimum inhibitory concentration was recorded as NED inhibited bacterial growth till well 8 at a concentration of 0.78 µg/ml; on the other hand, the CD inhibited bacterial growth till well 6 at a concentration of 0.62 µg/ml. Growth performance and MRs in the groups treated with NED are significantly reduced. Conclusion: Treatment of broiler's drinking water with NED at doses of 0.5 and 1 ml instead of pure CD was able to enforce a new perspective, antibacterial efficacy, enhancing the productive performance, and reducing the MRs of broilers. Keywords: Nano-emulsion difloxacin, Performance, S. Enteritidis. IntroductionThe global poultry industry has significantly grown as a result of raising the consumer demand for chicken meat and eggs. This industrial development has contributed to improving the growth rate, feed efficiency, health status, and reduced pathogen load. Since 1990, Salmonella enterica Serovar enteritidis have been pandemic and a primary factor in food-borne illnesses related to consumption of chicken eggs or other poultry products (Rabsch et al., 2001). New antibiotics are seen as “drugs of last resort” against virulent bacteria (Hafez and Attia, 2020). Difloxacin HCl is a new fluoroquinolone antibiotic, an amphoteric molecule with relatively nonpolar characteristics for a quinolone that difluorinated quinolone, 1-pfluorophenyl-6-fluoro-1,4-dihydro-4-oxo-7-(4-methyl-1-piperazinyl)-3-quinolone-carboxylicacidhydrochloride, marketed for use in veterinary medicine in the United States and Europe. Difloxacin HCl is rapidly absorbed after being orally administered. Difloxacin, like other fluoroquinolones, has a bactericidal effect specifically due to its affinity for the bacterial DNA-gyrase a subunit. The possibility still exists that difloxacin might bind to and block topoisomerase 10. Difloxacin is effective against Gram-negative bacteria. Difloxacin should be therapeutically useful against a variety of Gram-negative and Gram-positive veterinary infections based on its pharmacokinetic characteristics and in vitro activity (Van den Hoven et al., 2000). Despite the development of new antibiotics, treating intracellular infections frequently results in partial bacterial eradication. A major challenge is that many antimicrobials have poor intracellular action and are difficult to transfer through cell membranes, having little to no inhibitory or bactericidal effects on intracellular bacteria (Drulis-Kawa and Dorotkiewicz-Jach, 2010). In addition, antimicrobial toxicity to healthy tissues poses a significant limitation to their use. Therefore, the delivery of the drug to all cells is currently a big challenge to clinicians. Moreover, the reduced membrane permeability of microorganisms has been cited as a key mechanism of resistance to antibiotics (Davin-Regli et al., 2008). Unfortunately, it is a universal fact that new antibiotics ultimately develop resistance (Abu Lila et al., 2022). Consequently, antibiotic-functionalized metallic nanoparticles have recently emerged as an appropriate platform for dealing with bacterial resistance energy loss, and high absorption rates. To avoid rapid degradation which can be seen with antibiotics, nanoparticles can facilitate and assist in directly delivering compounds to target organs or systems and so encouraging various health benefits (Gangadoo et al., 2016). Many biological and pharmacological processes carried out at the nano-scale may be studied and controlled through the use of nanotechnology (Khan et al., 2022). The solubility, biocompatibility, stability, as well as ability for molecule binding, might all be improved by properly modifying the nanostructures. The development of hybrid systems composed of biological molecules and nanoparticles opens the door to a variety of applications including, targeted drug delivery and biosensing bioimaging (Barzan et al., 2021). Furthermore, it has been proved that hybrid systems enhance the effectiveness of oral administration by enhancing the bioavailability of drugs that are poorly absorbed (Maincent et al., 1986). The connective tissue can be easily permeated by nanoparticles, which allows the drug or antibiotic to reach its target place without obstructing the capillaries (Couvreur et al., 1977). Tween 80 and Pluronic acid 127 in particular, which are polymeric nanoparticles, have become common antibacterial drug delivery systems (Hagbani et al., 2022). Nanotechnology enables the development of unique carrier systems that improve molecular interactions, so enhance nanoparticles to support the body's reaction against invading pathogens. Overcoming bacterial resistance mechanisms and enhancing the innate and adaptive immune response are two examples of what this involves. Nanoparticle platforms that incorporate conventional antimicrobials or other substances enable tailored medication delivery and reduce treatment resistance. The increased surface-to-volume ratio of nanoparticles in antimicrobial drugs improves drug penetration by rupturing the microbial cell wall or cytoplasmic membrane (Blecher et al., 2011). The present study aimed to assess the effect of nano-emulsion difloxacin (NED) and commercial difloxacin (CD) water supplements at doses of 0.5 and 1 ml per litter of drinking water on performance and protection from mortality. The analytical characterization of NED including transmission, electron microscopic analysis, UV-visible spectroscopy, and zeta-potential analysis were examined. In addition, to study in-vivo and in-vitro antibiotic susceptibility and determine NED activity against S. enterica S. enteritidis. Materials and MethodsChemical and reagentsThe antibiotic difloxacin was purchased from ATCO Pharma. The Pluronic F-127 was obtained from Sigma Aldrich (St. Louis, MO). The tween 80 and absolute ethanol were purchased from MERCK (Germany). All reagents used in the present study were of analytical grade. Nutrient broth, xylose lysine deoxycholate agar (XLD agar), Salmonella Shigella agar (SS agar), and a Motility test medium with triphenyltetraazolium chloride (Oxoid®, UK), Muller Hinton broth and buffered peptone water (HIMedia®, Kennett Square, PA). Synthesis of difloxacin nanoemulsionThe difloxacin nanoemulsion was prepared by the oil-in-water (O/W) nanoemulsion technique, while tween 80 was used as an emulsifier and Pluronic F-127 as a surfactant. Initially, the oil phase was constructed by mixing one ml of difloxacin with 5 ml of absolute ethanol, followed by adding 3 ml of tween 80. Second, the aqueous phase was prepared by dissolving 0.2 gm of Pluronic F-127 in 100 ml distilled water. The nanoemulsion was fabricated by slowly dropping the oil phase into the aqueous phase and the mixture was further pulse-sonicated for 15 minutes in an ice bath (pulse on, 20.0 s; pulse off, 10.0 s), using a Vibra cell™ ultrasonicator (Newton, MA) adjusted at 20,000 Hz, in room temperature. After finishing the sonication period; the organic solvent was evaporated by gentle stirring at room temperature. The final nanoemulsion formed was stored in the refrigerator. Characterization of difloxacin nanoemulsionThe nano-emulsion’s size and surface morphology images were examined via the high-resolution electron microscopy (HR-TEM) model, JOEL JEM-2010, connected with a Gatan digital camera model Erlangshen ES500 and adjusted at an acceleration voltage of 200 kV, a drop of the nanoemulsion was directly deposited on the copper coated film grid and a drop of sodium phosphotungstate (2% w/v) was then placed over the sample after drying the images was taken via the HR-TEM. The zeta potential that reflects the surface charges and the size distribution of the prepared sample was determined by dynamic light scattering using a Malvern Zetasizer model Nano ZS-90, working at 25oC. To estimate the structural composition of the nano-emulsion, Fourier-transform infrared spectra were detected using a JASCO spectrometer in a scanning range of 4000–400 cm-1 using KBr as a reference. The UV-Vis absorption spectra of the free drug and nanoemulsion-containing drug were recorded in the range of 800–200 nm using a UV-visible spectrophotometer (V-630 UV–vis –Jasco, Japan). Examined birds and samplingOne hundred and fifty clinically diseased chicks were collected from 30 broiler farms located in Kafrelsheikh Governorate, Egypt (5 chicks/farm), samples were clinically examined and euthanized humanely to record the postmortem lesions. All samples were transmitted to the Microbiology Laboratory, at the Faculty of Veterinary Medicine at Kafrelsheikh University in buffered peptone water as a transport media. Bacterial isolationThe tissue loopful samples were enriched in selenite F broth (Oxoid, UK) in a sterile tube and incubated statically for an entire night at 37°C. The enrichments were spread with a sterile swab onto selective S.S. agar (Oxoid, UK), which was incubated overnight at 37°C for 24 hours. Smooth and opaque or colorless with black centers colonies were regarded as Salmonella colonies. According to the morphological shape of the suspected Salmonella, colonies were separately picked up and spread again for isolation on XLD agar incubated at 37°C for 24 hours. Confirmed Salmonella isolates were then preserved on semi-solid media for further investigation. Morphological identificationGram staining and bacterial motility testing were performed on a semi-solid agar medium. The morphological characteristics of the bacterial colonies grown on each selective differential media were noted. Biochemical identificationFor the biochemical identification of the bacterial isolates, indole, methyl red, Vogues Proskauer, oxidase and citrate utilization, urease hydrolysis, and H2S production were done (Cheesbrough, 1985). Serological identificationThe biochemically identified Salmonella isolates were serologically typed against known polyvalent and monovalent “O” and “H” (Kauffman, 1974) using Salmonella antiserum (DENKA SEIKEN Company, Japan). Minimal inhibitory concentration (MIC)To evaluate the MIC of CD and synthesized NED against the tested bacterial strains using the broth micro-dilution method (Al Hagbani et al., 2022). NED suspension was used at a final concentration of 100, 50, 25, 6.25 3.125, 1.56, 0.78, 0.39, and 0.19 μg/mg; while CD was used at a final concentration of 100, 10, 5, 2.5, 1.25, 0.625, 0.3125, 0.156, 0.078, and 0.039 μg/mg. Experimental bird's housing and managementA total of 1,500 one-day-old chicks were purchased of Ross chicks from Ismailia's Hatchery. Birds were housed in a caged-system farm and then were divided into five groups each consisting of 300 chicks and each group was subdivided into 100 chicks in the same cage at a different place on the farm. The birds were reared at the optimum temperature, humidity, and ventilation and fed with a previously weighed standard soybean diet (NRC, 1994). The experiment was designed to last for 35 days. Salmonella was orally inoculated directly into the crop through an endo crop tube started on day one and after 4 days the treatment program started for five successive days with 12 hours after clinical signs appeared. NED and CD were provided to broiler groups in the drinking water. Each group was assigned to treatment as follows: G1 control negative group (not infected, and not treated), G2 control positive group (infected and not treated), G3 (infected and treated with CD, and G4 and G5 (infected and treated with NED with different doses). Feed Intake, body weight, weight gain, growth rate, and feed conversion ratio (FCR) were monitored and recorded weekly and the mortality rates (MRs) were recorded daily during the entire experimental period. Preparation of S. enteritidis inoculaSalmonella enteritidis was isolated from broiler chickens and cultured during the night at 37°C in peptone water, to obtain 1 × 109 cfu/ml the next day. Salmonella enteritidis was diluted in sterile phosphate-buffered saline (pH 7.4) before being orally inoculated into the crop of one-day-old throw endocrop tubes (Shi et al., 2018). Histopathology examinationSamples were collected from internal organs including the liver, intestine, and gall bladder of freshly dead or euthanized diseased broiler chickens using a gloved hand and sterile surgical equipment. For histological analysis, the samples were preserved in a 10% formaldehyde solution. Then, processed using the standard paraffin embedding method, and dehydrated using ethanol. After clearing by xylene and processed into 5-m-thick slices followed by staining with hematoxylin-eosin. The observation and measurement of the inflammatory cells, degree of cell degeneration, intestinal villi length, and necrosis under a fluorescence microscope were proceeded (Muna et al., 2016). Bird performance assessmentThe number of weighed birds was optimized using a simple random sampling design by solvin's formula with an expected error of 5% using the following formula: n=N/[1+(N*e2)] where (n) represents the calculated sample size, (N) represents the total population size, and (e) represents error tolerance (0.05 or 0.02); in this study, we have built our calculation based on 0.05 error tolerance. Body weight was estimated by weighing 100 birds from each group at weekly intervals. Feed intake for each bird was calculated by dividing the total amount consumed in each group by the number of birds in this group. Body weight gain, FCR, and MR were further calculated. Statistical analysisStatistical analysis was carried out using SPSS 16.0. Data from the present study were analyzed using one-way ANOVA, investigating the effects of treatment alongside their interactions. Ethical approvalThis study was conducted according to the guidelines of Kafrelsheikh University. ResultsThe morphological studies of the nanoemulsionBy using micrograph images, the TEM images taken for the synthesized nana-emulsion revealed that the most predominant particles for the nanoemulsion possessed spherical shapes and were monodispersed with an average particle size of 170 nm (Fig. 1). The zeta-potential analysisEstimating the surface charge of the prepared difloxacin nanoemulsion was utilized via the calculation of their Zeta potential (El Borady et al., 2020). The zeta potential results obtained revealed a nanoemulsion with a zeta potential value of 32.8 mV. The UV-visible spectroscopy measurementsThe UV-Vis spectrum for the difloxacin crude (Fig. 2a) showed absorption peaks at 273, 323, and 334 nm. While the UV-Vis spectrum for the difloxacin nanoemulsion (Fig. 2b) showed the main absorption peak at 233 nm.
Fig. 1. (a) and (b) TEM images taken for the synthesized difloxacin nanoemulsion from different spots.
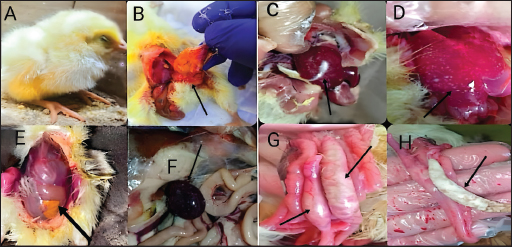
Fig. 2. UV-visible spectroscopy of (a) difloxacin and (b) difloxacin nanoemulsion. Biochemical profile of Salmonella isolates used in current studyOur results showed that salmonella is indole negative; methyl red positive, Vogues–Proskauer test negative, positive for citrate utilization, urea is negative with positive H2S and gas. The biochemical profile indicated the presence of S. enterica S. enteritidis which was confirmed after serological confirmation against known polyvalent and monovalent “O” and “H” Salmonella antisera according to the Kauffmann-white scheme. The antigenic structure of S. enterica S. enteritidisThe antigenic structure of isolated Salmonellae shows that Salmonella isolated were motile containing flagellar antigen “H” with its two phases “H1” and “H2.” Our results indicated that the isolates were serotyped S. enteritidis O 1,9,12 and monophasic H: g, m: -. MICIn a 96-well plate, 100 µL of each bacterial inoculum (1 × 105 CFU/mL) was put into each well. Then, serial dilutions of the synthetic nano-difloxacin in the concentration range of 100–0.195 µg/mL were applied to each well. The CD is also added at the same concentrations for comparative evaluation on a different 96-well plate. Both CD and NED-treated well plates were incubated for a whole night at 37°C. The MIC was recorded as the lowest concentration of CD and NED that successfully inhibited bacterial growth after overnight incubation as NED inhibited bacterial growth tell well 8 at a concentration of 0.78 µg/ml, on the other hand, CD inhibited bacterial growth tell well 6 at concentration of 0.6250 µg/ml. Clinical signsAll groups (except group one) showed typical clinical signs of salmonella infection including depression, shivering, haddling near the heat source, drooped wing, and raffled feather. Also, gasping and loud chirping, inappetance, respiratory manifestation, and conjunctivitis. White Pasty diarrhea Blindness, lameness, and lower body weight. Pathological FindingGross lesionThe freshly dead birds showed discoloration and enlargement of the liver, spleenomegaly, inflammation, and thickening of the intestinal mucosae. Necrotic foci on the surface of the spleen and liver (Fig. 3).
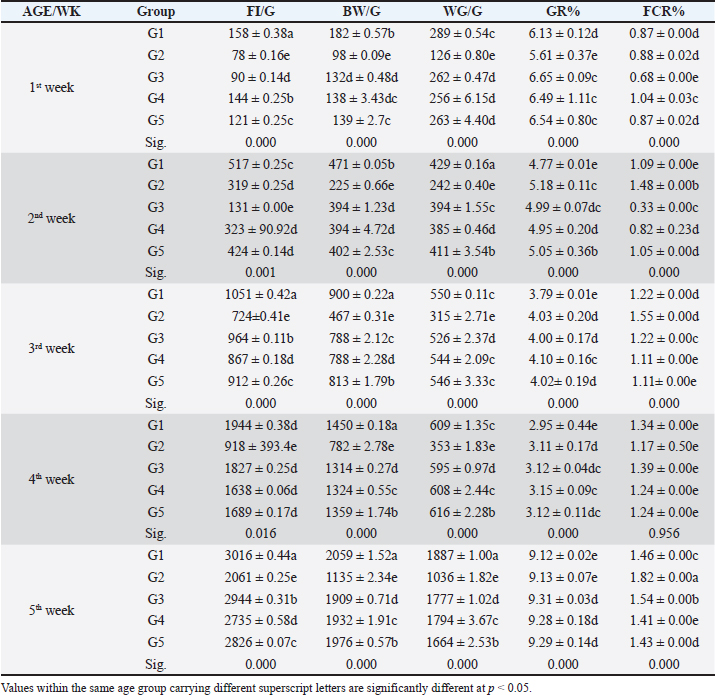
Fig. 3. The gross lesion of the birds infected with S. enterica S. Enteritidis. (A) Depression, inappetance, blindness, and raffled feather. (B) and (E) Unabsorbed yolk sac, enteritis, and severe hepatic congestion. (C) and (D) Congestion, white pinpoint foci of necrosis are scattered throughout the liver parenchyma. Hepatomegaly with gray nodules. (F) Splenomegaly with gray nodules and necrosis. (G) and (H) Catarrhal exudates in the intestine (white cecal core). Small to large firm, cheesy material (usually filled with fibrin) in the cecal core and raised plaques in the mucosa of the lower intestine. Histopathological changesHistopathological changes can be seen in Figure 4. Bird performance outcomesFeed Intake, body weight, weight gain, growth rate, and FCR of chicks treated with CD and NED were summarized in Table 1. Feed intakeIn our study, the feed intake was affected by infection and clinical signs, total feed intake showed that G1 consumed the highest amount of feed than others at week 5, while G2 had the lowest amount at the same week and during 5 weeks of the experiment. G4 was lower than G5 followed by G3, respectively. Body weightOur study displays the effect of infection on body weight during the experiment, at weeks 1, 2, 3, 4, and 5. The table showed that G1 had the highest body weight than others, while G2 was the lowest. G5 was higher than G4 followed by G3 on body weight, respectively. In the first week, no significant variation between G3 and G4. On the other hand, all groups showed significant variation during the period of the experiment.
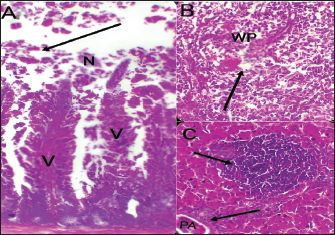
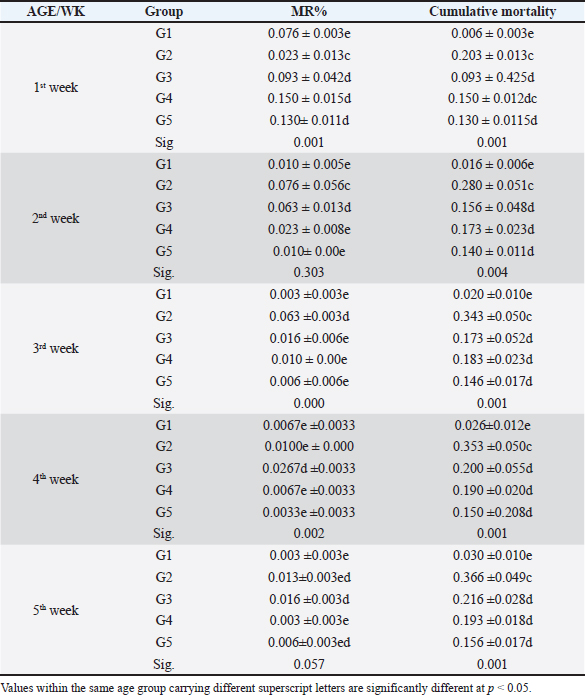
Fig. 4. Histophathological changes in THE experimentally infected chickens with S. enterica S. Enteritidis. (A) Intestine displaying necrosis, sloughing, and desquamation of the intestinal most upper portions of the villi (V), also infiltration of inflammatory cells. Denaturated villi where the lumen is filled with necrotic (N) masses. (B) Spleen showing the marked degree of lymphoid depletion associated with the deposition of fibrin and the degree of lymphoid necrosis within white pulp. (C) Liver having congestion, hemorrhage, focal degeneration, and necrosis associated with mononuclear inflammatory cell infiltration. PA indicates portal area. Weight gainDuring the experiment, body weight gain was recorded weekly. In the first week, all groups were in the same range with no significant variation between G3, G4, and G5. It was also noticed that G1 recorded the highest body weight gain. In the fifth week, G1 had the highest body weight gain followed by G3, G4, G5, and G2. Growth rateThe highest growth rate was observed in the fifth week, G5 had the highest growth rate followed by G3, G4, G2, and then G1. FCRDuring the experiment, at week 5, G4 had the best FCR%, followed by G5, then G1, G3, and G2. MRsThe MR among the different groups over the experimental duration was recorded. The MR in the fifth week showed that G3 had the highest percentage followed by G2, G5, G4, then G1, respectively (Table 2). DiscussionThe TEM results for the prepared difloxacin nanoemulsion are comparable to those obtained for the generation of amoxicillin nanoemulsion using the aslimier approach (Lin et al., 2012). The morphology result featuring no agglomeration for the particles depicting the effectiveness of Pluronic F-127 and Tween 80 used for the nanoemulsion fabrication process stabilized micelles of nanoemulsion. Previously, it was indexed that the zeta potential value shows the strength of electrostatic attraction between nearby charged adjacent particles in a dispersion that are similarly charged, and it serves as a crucial signal for the stability of colloidal dispersions (Rasmussen et al., 2020). The currently prepared nanoemulsion revealed a zeta potential value of 32.8 mV, indicating a significant level of stability of the drug nanoemulsion. These findings point to the increased stability of the particles, as demonstrated by the lack of agglomeration in their TEM images. Antibacterial quinolones derivatives possess the spectral property of absorbing UV light; due to the presence of the quinolone ring has a distinctive UV spectrum. It was found that the absorption peak values for the quinolone ring are located at 227, 270, and 314 nm, which is attributed to the substituted poly-nuclear aromatic compounds. In addition, as reported previously (Zhao et al,. 2020), the short wavelength band between 260 and 280 nm is attributed to the aromatic ring's absorption, while the long wavelength band between 310 and 340 nm is attributed to the n - p* (HOMO-LUMO) electronic transition brought on by the intermolecular H bond formed between the difloxacin antibiotic and the solvent used (H2O). In the present study, the obtained absorption spectrum of the free antibiotic showed a peak at 270 nm, and a second peak was observed in the region from 323 to 334 nm besides, a small shoulder that was recognized at 233 nm, which is matched with literature (Prabhakaran et al., 2009). On the other hand, the absorption spectrum of the drug nanoemulsion showed only a sharp peak at 233 nm and the other peaks were merged with each other and appeared as a very small broad peak centered at 277 nm. The change of the absorption spectrum that occurred for the nanoemulsion may be due to the correlation of the drug's absorption peaks with the other materials (have absorption peaks at the same spectral region (Prabhakaran et al., 2009) used for fabrication of the nanoemulsion. In fact, there are over than 2,500 salmonella serotypes have been identified and more than half of them are related to S. enteritidis which causes enteric diseases that led to death and economic loss in poultry according to (Eng et al., 2015; Wang et al., 2020; Ehuwa et al., 2021). The newly hatched chicks are considered at high risk of infections due to their immature immune systems, undeveloped gut microbiota salmonella easily colonize, interact with the intestinal epithelial cells, then penetrate into the deep tissues, thus causing significant damage to the intestinal mucosal barrier (Neveling et al., 2020; Westrom et al., 2020). The results of the bacterial isolation on differential media have been verified to the genus level by biochemical identification. Also, the serological identification confirmed the identity of the bacterial isolates. Table 1. Feed intake, body weight, weight gain, growth rate, and FCR (Mean ± SE) of chicks treated with CD and NED.
In the present study, S. enteritidis was the most common prevalent serotype in the collected samples which coordinated with the previous reports (El-Sharkawy et al., 2017). During the period of experimental post-infection, all the groups which infected (except group 1) exhibited gross lesions including hepatomegaly and splenomegaly. The gross lesions clearly indicated an infection with septicemia. The histopathological findings included cellular infiltration of the liver, spleen, and congested liver. These observations were like those detected by previous studies in chickens (Garcia et al., 2010; Nwiyi et al., 2012; Muna et al,. 2016). Salmonella infections have significant negative effects on the production performance, intestinal flora colonization, produce systemic or septicemic disease, and badly affect the gut health of young birds (Bohez et al., 2008; Chalghoumi et al., 2009; Wu et al., 2018). Infection with this bacterium weakens the immune system and causes collateral damage that affects nutrient absorption (He et al., 2005). The poor growth performance observed in the challenged birds can be partly attributed to the decreased appetite and Feed intake (FI) of these birds as a direct consequence of Salmonella infection (Rajani et al., 2016). Preceding studies clearly confirmed the deleterious influence of Salmonella infection on broilers’ growth performance (Okuneye et al., 2016; Rajani et al., 2016; Shao et al., 2016). In the present study, the impact of experimental birds on FI, BW, GR%, and FCR% of broiler chicks are presented. Salmonella enterica S. enteritidis significantly affected the growth performance of chicks so in the positive control group, birds had significantly lowered FI, BW, and higher FCR compared to the negative control birds. The three groups that were treated with CD and NED at different doses had significantly better FI, BW, and FCR in a concentration-dependent manner. However, broiler chicks that received NED had a growth performance close to the negative control group. Besides, the NED groups showed a highly significant decrease in mortality rates. Likely, Gast and Beard (1992) showed that day-old hens orally treated with S. enteritidis had MR that varied significantly (14.5%–89.5%). As a result, this study revealed a decline in the MR, proving the effectiveness of difloxacin nanoemulsion in the treatment of the disease. The reduction of the MRs in the NED-treated groups could be attributed to the effectiveness of NED in reducing the count of S. Enteritidis (Taunde et al., 2021). Table 2. Effect of the different treatments on the MR and cumulative mortality in the experimental groups.
ConclusionThe use of nanotechnology against different infections is one of the potential alternative methods for infection control. Data from this experiment showed that synthetic NED coated by tween 80 and Pluronic F-127 nanoparticles increased the antibacterial efficacy against tested S. Enteritidis and it is more potent and powerful than pure difloxacin. Treatment of broilers in drinking water with NED rather than a pure CD at doses of 0.5 ml and 1 ml/L was able to enforce a new perspective, antibacterial efficacy, and enhancing influences on the productive performance, MR decreases, and body weight gain increase of broilers. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionA.I.M. performed the experiments, analyzed the data, and wrote the manuscript; I.K. worked on measurement and statistical analysis and edited the manuscript; O.M. manufactured nano-emulsion drugs and made up all analytical techniques. M.A., O.M., and M.I. supervised the work. All authors reviewed and approved the manuscript. FundingThis research received no specific grant. Data availabilityAll data will be available upon a reasonable request. ReferencesAbu Lila, A.S., Huwaimel, B., Alobaida, A., Hussain, T., Rafi, Z., Mehmood, K. and Ahmed, A.F. 2022. Delafloxacin-capped gold nanoparticles (DFX-AuNPs): an effective antibacterial nano-formulation of fluoroquinolone antibiotic. Materials 15(16), 5709. Al Hagbani, T., Rizvi, S.M.D., Hussain, T., Mehmood, K., Rafi, Z., Moin, A., Abu Lila, A.S., Alshammari, F., Khafagy, E.S. and Rahamathulla, M. 2022. Cefotaxime mediated synthesis of gold nanoparticles: characterization and antibacterial activity. Polymers 14, 771. Barzan, G., Rocchetti, L., Portesi, C., Pellegrino, F., Taglietti, A., Rossi, A.M. and Giovannozzi, A.M. 2021. Surface minimal bactericidal concentration: a comparative study of active glasses functionalized with different-sized silver nanoparticles. Colloids Surf. B Biointerfaces 204, 111800. Blecher, K., Nasir, A. and Friedman, A. 2011. The growing role of nanotechnology in combating infectious disease. Virulence 2(5), 395–401. Bohez, L., Gantois, I., Ducatelle, R., Pasmans, F., Dewulf, J., Haesebrouck, F. and Van Immerseel, F. 2008. The Salmonella Pathogenicity Island 2 regulator ssrA promotes reproductive tract but not intestinal colonization in chickens. Vet. Microbiol. 126, 216–224. Chalghoumi, R., Théwis, A., Beckers, Y., Marcq, C., Portetelle, D. and Schneider, Y.-J. 2009 Adhesion and growth inhibitory effect of chicken egg yolk antibody (IgY) on Salmonella enterica Serovars enteritidis and TyphimuriumIn vitro. Foodborne Pathog. Dis. 6, 593–604. Cheesbrough, M., 1985. Medical laboratory manual for Tropical Countries. Microbiology 2, 400–480. Couvreur, P., Tulkens, P., Roland, M., Trouet, A. and Speiser, P. 1977. Nanocapsules: a new type of lysosomotropic carrier. FEBS Lett. 84, 323–326. Davin-Regli, A., Bolla, J.M., James, C.E., Lavigne, J.P., Chevalier, J., Garnotel, E., Molitor, A. and Pagès, J.M. 2008. Membrane Permeability and regulation of drug “influx and efflux” in enterobacterial pathogens. Curr. Drug Targets 9, 750–759. Drulis-Kawa, Z. and Dorotkiewicz-Jach, A. 2010. Liposomes as delivery systems for antibiotics, Int. J. Pharm. 387, 187–198. Ehuwa, O., Jaiswal, A.K. and Jaiswal, S. 2021. Salmonella, food safety and food handling practices. Foods 10, 907. El-Borady, O.M., Ayat, M.S., Shabrawy, M.A. and Millet, P. 2020. Green synthesis of gold nanoparticles using Parsley leaves extract and their applications as an alternative catalytic, antioxidant, anticancer, and antibacterial agents. Adv. Powder Technol. 31(10), 4390–4400. El-Sharkawy, H., Tahoun, A. and El-Gohary, A.E.A. 2017. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 9, 8. Eng, S.K., Pusparajah, P., Ab-Mutalib, N.-S., Ser, H.L., Chan, K.G., and Lee, L.H. 2015. Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 8, 284–293. Gangadoo, S., Stanley, D., Hughes, R.J., Moore, R.J. and Chapman, J. 2016. Nanoparticles in feed: progress and prospects in poultry research. Trends Food Sci. Technol., 58, 115–126. Garcia, K.O., Santana, A.M., FreitasNeto, O.C., BerchieriJr, A. and Fagliari, J.J. 2010. Experimental infection of commercial layers using a Salmonella enterica serovar gallinarum strain: blood serum component and histopathological changes. Braz. J. Vet. Pathol. 3, 111–117. Gast, R.K. and Beard, C.W. 1992. Evaluation of a chick mortality model for predicting the consequences of Salmonella enteritidis infections in laying hens. Poult. Sci. 71, 281–287. Hafez, H.M. and Attia, Y.A. 2020. Challenges to the poultry industry: current perspectives and strategic future after the COVID-19 outbreak. F.V.S. 7, 516. Hagbani, T.A., Yadav, H., Moin, A., Lila, A.S.A., Mehmood, K., Alshammari, F., Khan, S., Khafagy, E.-S., Hussain, T. and Rizvi, S.M.D., 2022. Enhancement of vancomycin potential against pathogenic bacterial strains via gold nano-formulations: a nano-antibiotic approach. Materials 15, 1108. He, H., Lowry, V.K., Swaggerty, C.L., Ferro, P.J. and Kogut, M.H. 2005. In vitro activation of chicken leukocytes and in vivo protection against Salmonella enteritidis organ invasion and peritoneal S. enteritidis infection-induced mortality in neonatal chickens by immunostimulatory CpG oligodeoxynucleotide. FEMS Immunol. Med. Microbiol. 43, 81–89. Khan, S., Mansoor, S., Rafi, Z., Kumari, B., Shoaib, A., Saeed, M., Alshehri, S., Ghoneim, M.M., Rahamathulla, and Hani, U.M. 2022. A review on nanotechnology: properties, applications, and mechanistic insights of cellular uptake mechanisms. J. Mol. Liq. 348, 118008. Kauffman, G. 1974. Kauffmann white scheme. J. Acta Pathologica et Microbiologica Scandinavica 61, 385. Lin, Y.H., Chiou, S.F., Lai, C.H., Tsai, S.C., Chou, C.W., Peng, S.F. and He, Z.S. 2012. Formulation and evaluation of water-in-oil amoxicillin-loaded nanoemulsions using for Helicobacter pylori eradication. Process Biochem. 47(10), 1469–1478. Maincent, P., Le Verge, R., Sado, P., Couvreur, P. and Devissaguet, J.P. 1986. Disposition kinetics and oral bioavailability of vincamine-loaded polyalkyl cyanoacrylate nanoparticles. J. Pharm. Sci. 75, 955–958. Muna, E.A., Salih, M.H., Zakia, A.M., Halima, M.O., Abeer, A.M., Ameera, M.M. and Idris, S.B. 2016. Pathology of broiler chicks naturally infected with Salmonella enteritidis (S. enteritidis) and Salmonella typhimurium (S. typhimurium) during an outbreak in Sudan. J. Sci. Res. Rep. 10(1), 1–8. National Research Council (NRC). (1994). Nutrient requirements of poultry: 1994. National Academies Press. Neveling, D.P., van Emmenes, L.J., Ahire, E., Pieterse, C. and Dicks L.M.T. 2020. Effect of a multi-species probiotic on the colonisation of salmonella in broilers. Probiotics Antimicrob. Proteins 12, 896–905. Nwiyi, P. and Omadamiro, O. 2012. Seroprevalence and isolation of chicken infected with Salmonella: haematological and pathological evaluation. J. Anim. Feed Res. 2(6), 483–487. Okuneye, O.J., Ogunfolabo, L.A., Fasanmi, O.G., Adekunle, O.F. and Oloso, N.O. 2016. Performance and physiological responses of Salmonella enteritidis challenged broilers fed diets containing antibiotic, probiotic and aromabiotic. J. Dairy Vet. Anim. Res. 3, 00081. Prabhakaran, D., Sukul, P., Lamshöft, M., Maheswari, M.A., Zühlke, S. and Spiteller, M. 2009. Photolysis of difloxacin and sarafloxacin in aqueous systems. Chemosphere 77(6), 739-746. Rabsch, W., Tschape, H. and Baumler, J.A., 2001. Non-typhoidal salmonellosis: emerging problems. Microb. Infect. 3, 237–247. Rajani, J., Dastar, B., Samadi, F., Torshizi, M.A., Abdulkhani, A. and Esfandyarpour, S. 2016. Effect of extracted galactoglucomannan oligosaccharides from Pine wood (Pinus brutia) on Salmonella typhimurium colonisation, growth performance and intestinal morphology in broiler chicks. Br. Poult. Sci. 57, 682–692. Rasmussen, M.K., Pedersen, J.N. and Marie, R. 2020. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 11, 2337. Shao, Y., Wang, Z., Tian, X., Guo, Y. and Zhang, H., 2016. Yeast b-d-glucans induced antimicrobial peptide expressions against Salmonella infection in broiler chickens. Int. J. Biol. Macromol. 85, 573–584. Shi, S., Wu, S., Shen, Y., Zhang, S., Xiao, Y., He, X., Gong, J., Farnell, Y., Tang, Y., Huang, Y. and Gao, L. 2018. Iron oxide nanozyme suppresses intracellular Salmonella enteritidis growth and alleviates infection in vivo. Theranostics 8, 6149–6162. Taunde, P.A., Bianchi, M.V. and Mathai, V.M. 2021. Pathological microbiological and immunohistochemical characterization of avian colibacillosis in broiler chickens of Mozambique. Pesqui. Vet. Bras. 41, e03831. Van den Hoven, R., Wagenaar, J.A. and Walker, R.D. 2000. In vitro activity of difloxacin against canine bacterial isolates. J. Vet. Diagn. Invest. 12(3), 218–223. Wang, X., Wang, H.T., Liu, L.F., Cheng, Z.X., Guo, G., Wen, Q., Luo, H., Shao, Z. and Zhang. T., 2020. Characterization of Salmonella spp. isolated from chickens in central China. BMC Vet. Res. 16, 299. Westrom, B.E., Arevalo Sureda, K., Pierzynowska, S.G. and Perez-Cano, F.J. 2020. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front. Immunol. 11, 1153. Wu, Q.J., Zheng, X.C., Wang, T. and Zhang, T.Y. 2018 Effects of oridonin on immune cells, Th1/Th2 balance and the expression of BLys in the spleens of broiler chickens challenged with Salmonella Pullorum. Res. Vet. Sci. 119, 262–267. Zhao, J.F., Liu, Y.C., Xu, Y.L. and Wang, W.F. 2020. Effects of N-1 substituent on the phototoxicity of fluoroquinolone antibiotics: comparison of pefloxacin and difloxacin. Nuclear Sci. Techniq. 31, 1–12. | ||
| How to Cite this Article |
| Pubmed Style Ashosh MI, El-Abasy MA, El-Borady OM, El-khaiat I, Ismail MM. Comparative efficacy of difloxacin and nano-emulsion difloxacin as antibacterial agents against Salmonella Enterica Serovar Enteritidis in chickens. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 449-458. doi:10.5455/OVJ.2024.v14.i1.41 Web Style Ashosh MI, El-Abasy MA, El-Borady OM, El-khaiat I, Ismail MM. Comparative efficacy of difloxacin and nano-emulsion difloxacin as antibacterial agents against Salmonella Enterica Serovar Enteritidis in chickens. https://www.openveterinaryjournal.com/?mno=181964 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.41 AMA (American Medical Association) Style Ashosh MI, El-Abasy MA, El-Borady OM, El-khaiat I, Ismail MM. Comparative efficacy of difloxacin and nano-emulsion difloxacin as antibacterial agents against Salmonella Enterica Serovar Enteritidis in chickens. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 449-458. doi:10.5455/OVJ.2024.v14.i1.41 Vancouver/ICMJE Style Ashosh MI, El-Abasy MA, El-Borady OM, El-khaiat I, Ismail MM. Comparative efficacy of difloxacin and nano-emulsion difloxacin as antibacterial agents against Salmonella Enterica Serovar Enteritidis in chickens. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 449-458. doi:10.5455/OVJ.2024.v14.i1.41 Harvard Style Ashosh, M. I., El-Abasy, . M. A., El-Borady, . O. M., El-khaiat, . I. & Ismail, . M. M. (2024) Comparative efficacy of difloxacin and nano-emulsion difloxacin as antibacterial agents against Salmonella Enterica Serovar Enteritidis in chickens. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 449-458. doi:10.5455/OVJ.2024.v14.i1.41 Turabian Style Ashosh, Mohammed I., Moshira A. El-Abasy, Ola M. El-Borady, Ibrahim El-khaiat, and Mahmoud M. Ismail. 2024. Comparative efficacy of difloxacin and nano-emulsion difloxacin as antibacterial agents against Salmonella Enterica Serovar Enteritidis in chickens. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 449-458. doi:10.5455/OVJ.2024.v14.i1.41 Chicago Style Ashosh, Mohammed I., Moshira A. El-Abasy, Ola M. El-Borady, Ibrahim El-khaiat, and Mahmoud M. Ismail. "Comparative efficacy of difloxacin and nano-emulsion difloxacin as antibacterial agents against Salmonella Enterica Serovar Enteritidis in chickens." Open Veterinary Journal 14 (2024), 449-458. doi:10.5455/OVJ.2024.v14.i1.41 MLA (The Modern Language Association) Style Ashosh, Mohammed I., Moshira A. El-Abasy, Ola M. El-Borady, Ibrahim El-khaiat, and Mahmoud M. Ismail. "Comparative efficacy of difloxacin and nano-emulsion difloxacin as antibacterial agents against Salmonella Enterica Serovar Enteritidis in chickens." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 449-458. Print. doi:10.5455/OVJ.2024.v14.i1.41 APA (American Psychological Association) Style Ashosh, M. I., El-Abasy, . M. A., El-Borady, . O. M., El-khaiat, . I. & Ismail, . M. M. (2024) Comparative efficacy of difloxacin and nano-emulsion difloxacin as antibacterial agents against Salmonella Enterica Serovar Enteritidis in chickens. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 449-458. doi:10.5455/OVJ.2024.v14.i1.41 |