| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 553-563 Original Research Isolation and identification of multidrug resistance bacterial agents implicated in duck enteritis with first record of Salmonella enterica subspecies arizonae in EgyptAshraf Hussein1*, Amal A.M. Eid1, Mohamed Hassaan2, Eman Mohamed3, Ibrahim Elsohaby4,5 and Mohamed Shawky21Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Faculty of Veterinary Medicine, Veterinary Hospital, Zagazig, Egypt 3Department of Microbiology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 4Department of Infectious Diseases and Public Health, Jockey Club of Veterinary Medicine and Life Sciences, City University of Hong Kong, Hong Kong SAR, China 5Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Ashraf Hussein. Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: ashrafhamed1 [at] yahoo.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
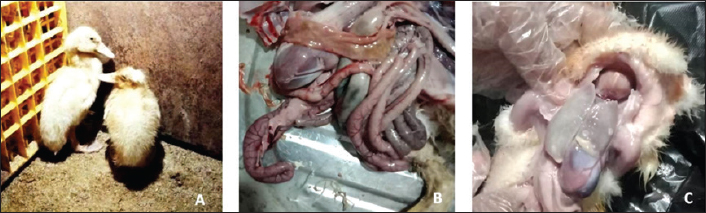
AbstractBackground: Bacterial infections causing digestive problems are among the most serious threats to Egypt's duck industry, owing to their effects on feed utilization and body weight gain. Aim: As a result, the goal of this study was to identify bacterial pathogens causing enteritis in ducks as well as testing their antimicrobials resistance capabilities. Methods: Forty-two duck flocks from different localities at four Egyptian Governorates (El-Sharkia, El-Gharbia, El-Dakahlia, and El-Qaliobia) have been subjected to clinical and postmortem examination as well as bacterial isolation and identification. The liver samples have been collected aseptically from freshly euthanized ducks for bacterial isolation followed by identification using conventional biochemical tests, VITEK 2 system, and confirmatory polymerase chain reaction (PCR) for detection of the uid A gene (beta-glucuronidase enzyme) of Escherichia coli. In addition, antimicrobial sensitivity testing for the isolates against different antimicrobials by the VITEK 2 system was used. Results: Forty-six positive bacterial isolates were identified using conventional methods and the VITEK 2 system including Staphylococcus spp. (52.17%), E. coli (41.30%), and 2.17% for each of Enterococcus casseli lavus, Salmonella enterica subspecies arizonae, and Enterobacter cloacae. PCR was positive for E. coli uid A gene at 556 bp. The antibiogram patterns of isolated pathogens from naturally infected ducks in our work demonstrated 87% multidrug resistance with varying results against different antimicrobial drugs tested. Such findings supported the fact of the upgrading multidrug resistance of Staphylococci and Enterobacteriacae. Conclusion: The most prevalent bacterial pathogens associated with duck enteritis were Staphylococcus spp. and E. coli with the first report of S. enterica subspecies arizonae causing duck enteritis in Egypt. Keywords: Duck enteritis, Staphylococci, Salmonella, E. coli, Antimicrobials. IntroductionBacterial infections in ducks have higher incidence rates compared with viral diseases. Mortality rates of bacterial infections have increased globally (Enany et al., 2018). The studies have concentrated on determining the intestinal load of pathogenic bacteria such as Staphylococci spp., Escherichia coli, and Salmonellae spp. (Cao et al., 2008). Ducks with bacterial infection experience diarrhea, lack of coordination, depression, dehydration, and a high mortality rate. In the poultry business, these illnesses result in great financial losses for various regions of the world (Brans and Gross, 1997). Staphylococci inhabit the skin and mucosal surface of the most critical organs of mammals and birds (El-Jakee et al., 2008). In poultry, it produces considerable economic losses in a variety of ways, including septicemia, lower body weight, decreased egg production, and osteomyelitis, which result in lameness and carcass condemnation at slaughter (McNamee and Smyth, 2000 and Andreasen, 2008). Escherichia coli infects ducks of all ages, causing septicemia with a death rate of 10%–50%. Young ducklings are more commonly affected, and mortality rates in birds aged 4–9 weeks can approach 20%. Colibacillosis is manifested either as a systemic or localized form. Pericarditis is commonly seen in colisepticemia. The pericardial sac becomes cloudy and the epicardium becomes edematous and covered with a light-colored exudate (Saif et al., 2010). Also, congested spleen, perihepatitis, airsacculitis, and enteritis have been recorded by Aggad et al. (2010). The localized form may be in the form of coliform omphalitis/yolk sac infection (Montagomery et al., 1999), coliform cellulitis (Gomis et al., 2000), swollen head syndrome (Van de Zande et al., 2001), diarrheal disease (Saif et al., 2010), or venereal colibacillosis (acute vaginitis) (Gerardian et al., 2000). Salmonellosis is a dangerous duck disease with clinical symptoms more common in very young ducklings and more prevalent in the immediate post-hatched period (Henry, 2000). The clinically affected ducklings showed depression, inappetence, huddling together, loss of weight, closed eyes, and a staggering gait appeared 72 hours post infection, which was followed by tremors, droopy wings, diarrhea, and feather pasting around the vent (Mondal et al., 2008). Ducklings with salmonellosis have postmortem findings that range from no grossly evident lesions to a septicemic picture with congestion of the internal organs, including the liver, spleen, lungs, and kidneys. Typhlitis, pericarditis, and perihepatitis are also frequently seen (Lister and Barrow, 2008). Discrete necrotic lesions in the lungs, liver, and heart may be observed. Birds that survive the acute septicemic phase of the infection may have peritonitis and hemorrhagic enteritis (Pattison et al., 2008). The VITEK 2 system is considered an efficient and dependable method for carrying out bacterial identification and testing for antibiotic susceptibility. The polymerase chain reaction (PCR) is a very sensitive technique for identifying different pathogens in clinical samples. Numerous PCR assays have been created to detect and identify bacterial pathogens in ducks (Gomis et al., 2003). Since most bacterial illnesses in duck flocks cannot be totally prevented by vaccination, antibiotics are often recommended as a control measure. In the poultry industry, improper usage of antibiotics results in higher rates of resistance, leading to the growth of multidrug-resistant bacteria and subsequently raises concern (Ammar et al., 2021). This study aims to identify the bacterial pathogens incriminated in the duck enteritis problem using conventional biochemical tests, VITEK 2 system as well as PCR. Furthermore, the study assesses the antibiotic sensitivity of the identified pathogens. Material and MethodsExamined birdsForty-two duck flocks from four Egyptian governorates (EL-Sharkia, El-Gharbia, El-Dakahlia, and El-Qaliobia) suffering from enteric disease were subjected to clinical and postmortem examination. The examined flocks were of different breeds (Pekin, Mallard, and Muscovy) including 32 flocks of young ages 5–45 days and 10 older flocks ages 8–57 weeks. The birds were subsequently transported to the Avian and Rabbit Medicine Department at Zagazig University's Faculty of Veterinary Medicine in Egypt. For bacterial isolation and identification, 42 liver samples were collected aseptically from freshly dead ducks. Bacterial isolation and identificationA loopful of liver samples were inoculated onto five different media (nutrient agar, mannitol salt agar, MacConkey’s agar, xylose lysine desoxycholate agar, and sheep blood agar) and then incubated for 24 hours at 37°C. Separate pure colonies were identified morphologically via using Gram’s stain as well as biochemically using methods described by Quinn et al. (2002). VITEK 2 compact analysisVITEK 2 compact analysis was applied for phenotypical confirmation of bacterial isolates. Following the introduction of a uniform suspension of the unidentified organism into every single self-contained card, the device's inbuilt optics reads the cards after incubation. By comparing the results of the samples to the known species-specific reactions found in the VITEK 2 database, sophisticated colorimetry technology allowed for the identification of organisms and the testing of antibiotic sensitivity (Wallet et al., 2005). Conventional PCR for E. coli identificationBacterial DNA extractionDNA extraction by boiling was performed by mixing bacterial culture with 200 μl of distilled water then boiled for 10 minutes at 95°C in a heat block or dry water bath. The resultant solution was centrifuged using a cooling centrifuge at 4°C for 10 minutes then the supernatant was used as a template of DNA which was stored at −20°C until used. Preparation of PCR master mixThe DreamTaq Green PCR Master Mix (2×) kit, code number K1082 (Thermo ScientificTM), was utilized. 12.5 µl of DreamTaq Green PCR Master Mix, 7.5 µl of PCR-grade water, 1 µl of each forward and reverse 20 pmol primer, and 3 µl of template DNA make up the total amount of 25 µl/reaction. Thermal cycle conditions for the PCR assay used to identify E. coliThe temperature and timing conditions of the PCR for detecting the uid A gene were as follows: Denaturation at 95°C for 5 minutes, followed by 35 cycles of denaturation at 95°C for 30 seconds, annealing at 52°C for 30 seconds, and extension at 72°C for 60 seconds, followed by final extension at 72°C for 7 minutes (Anbazhagan et al., 2011). Screening of PCR productsAbout 10 µl of the amplified PCR product was analyzed by electrophoresis on a 2% agarose gel stained with 0.5 µg of ethidium bromide/ml. Electrophoresis was carried out in 1 × TAE buffer at 80 volts for 1 hour. Gels were visualized under an ultraviolet transilluminator (UVP, UK) and photographed (Lee et al., 2012). Data analysisData analysis and visualization were performed using R software (R Core Team, 2022; version 4.2.0). Using the "Complex heatmap" program, a heatmap of the isolates' antimicrobial resistance patterns was produced (Gu et al., 2016). Ethical approvalThe study was approved by the Ethical Committee of the Faculty of Veterinary Medicine, Zagazig University. ResultsClinical and postmortem findingsThe examined ducks of different ages and breeds were suffering from severe diarrhea and general signs of illness expressed by off food, depression, ruffling feathers, and weakness. All flocks under investigation had diarrhea (watery, whitish, bloody, and greenish). In addition, 59.5% of the flocks had respiratory symptoms, 16.7% had stunted growth, and 16.7% had a swollen tongue and shorter beak. The postmortem findings include enteritis (100% of examined flocks of different ages), increased pericardial fluid, pericarditis, and perihepatitis (64% of examined flocks) (Fig. 1).
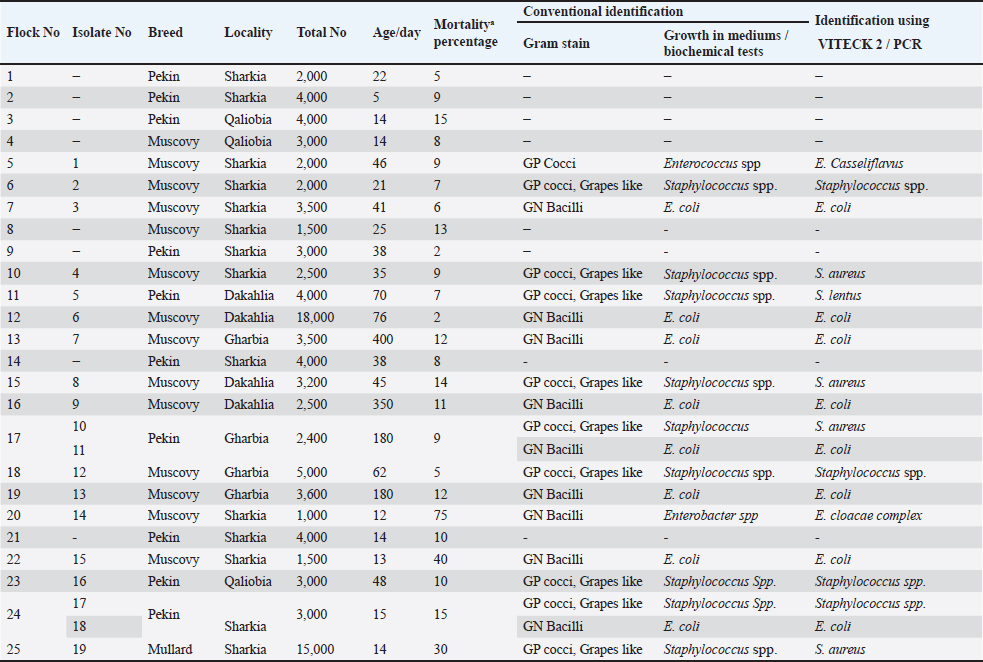
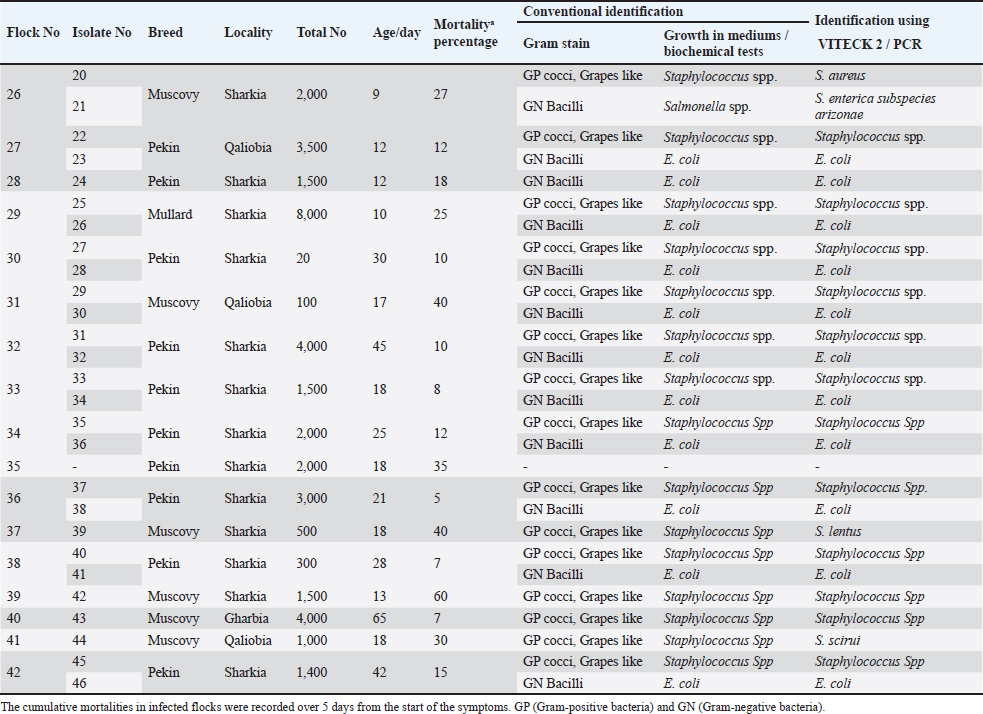
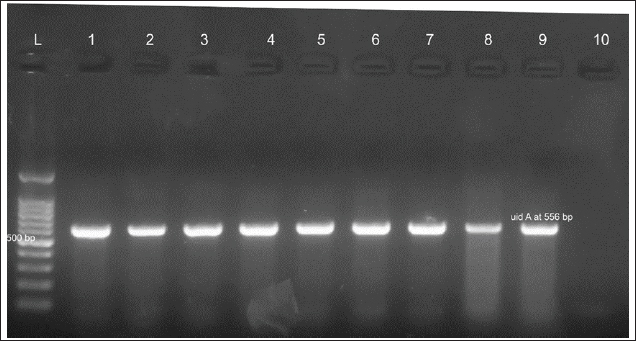
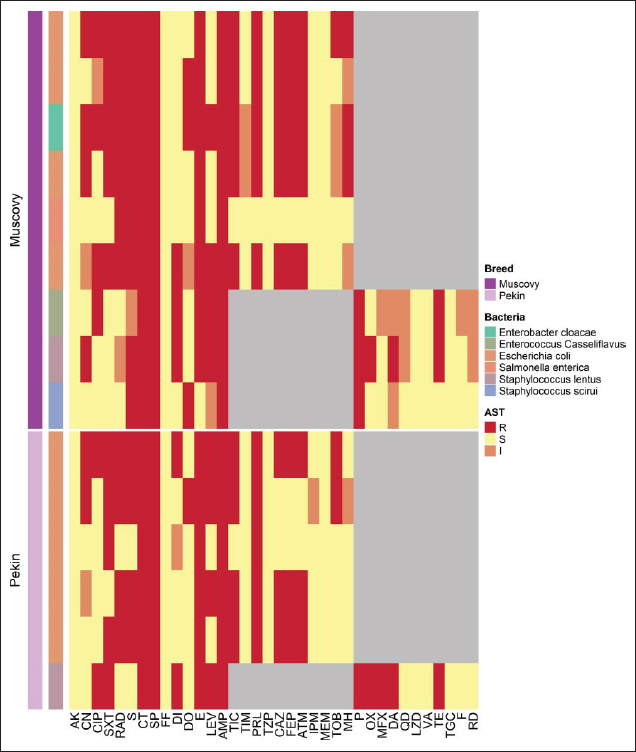
Figure 1. Clinical and postmortem lesions of ducklings, naturally infected with E. coli: a) Ruffled feathers and weakness; b) Closed and opened intestine displaying enteritis; c) Perihepatitis and pericarditis. Bacterial isolation and identification using conventional methodsOut of 42 liver samples taken from ducks suffering enteritis revealed 46 isolates either in single or mixed occurrence. They were identified as Staphylococcus spp, Enterococcus spp, E. coli, Salmonella spp, and Enterobacter spp (Table 1). Findings of bacterial identification using VITEK 2 analysis and conventional PCRPositive isolates identification was based on morphology, biochemical characters (VITEK 2), and confirmatory PCR for E. coli revealing the presence of 24 isolates of Staphylococcus spp., 19 isolates of E. coli, one isolate of each E. Casseliflavus, Salmonella spp, and Enterobacter cloacae complex. The E. coli isolates were successfully identified by the detection of the uidA gene (beta-glucuronidase enzyme) at the band size 556 bp (Table 1 and Fig. 2). Mixed infection was proved in 13 out of 42 flocks studied (30.95%) with two bacterial agents; only one flock was infected with Staphylococcus spp. and Salmonella enterica subspecies arizona, while 12 flocks got mixed infections with Staphylococcus spp. and E. coli. Antimicrobial sensitivityA representative 15 out of the 46 isolates exhibited resistance to multiple tested antimicrobial agents (Fig. 3). Antibiotic susceptibility of the Staphylococcus spp. isolates displayed high sensitivity to amikacin, cephradine, fosfomycin, vancomycin, tigecycline, linezolid, nitrofurantion, rifampicin, and quinupristin/dalfopristin, (100%); gentamycin, ciprofloxacin, moxifloxacin, trimethoprim/sulfamethoxazole, and doxycycline (66.7%) while isolates were highly resistant to penzylpenicillin, ampicillin, colistin, and spiramycin (100%); levofloxacin, erythromycin, oxacillin, clindamycin, tetracycline, streptomycin, and difloxacin (66.7%). While E. coli isolates showed high sensitivity to amikacin, fosfomycin, ticarcillin/clavulanic acid, piperacillin/tazobactam, meropenem, and imipenem (100%); minocycline, doxycycline, and difloxacin (78%); tobramycin and ciprofloxacin (66.7%), while isolates were resistant to colistin, erythromycin, spiramycin, ampicillin, piperacillin, and ticarcillin (100%); cephradine, trimethoprim/sulfamethoxazole, and streptomycin (88.9%); ceftazidime, cefepime, and aztreonam (78%). The antimicrobial sensitivity pattern of E. Casseliflavus isolate showed high sensitivity to amikacin, cephradine, fosfomycin, gentamycin, vancomycin, tigecycline, doxycycline, linezolid, oxacillin, and trimethoprim/sulfamethoxazole; moderately sensitive to streptomycin, clindamycin, moxifloxacin, nitrofurantion, rifampicin, and quinupristin/dalfopristin, and resistant to penzylpenicillin, ampicillin, colistin, and spiramycin, erythromycin, tetracycline, ciprofloxacin, levofloxacin, and difloxacin. Table 1. Results of bacterial isolation and identification from duck flocks suffering from enteritis.
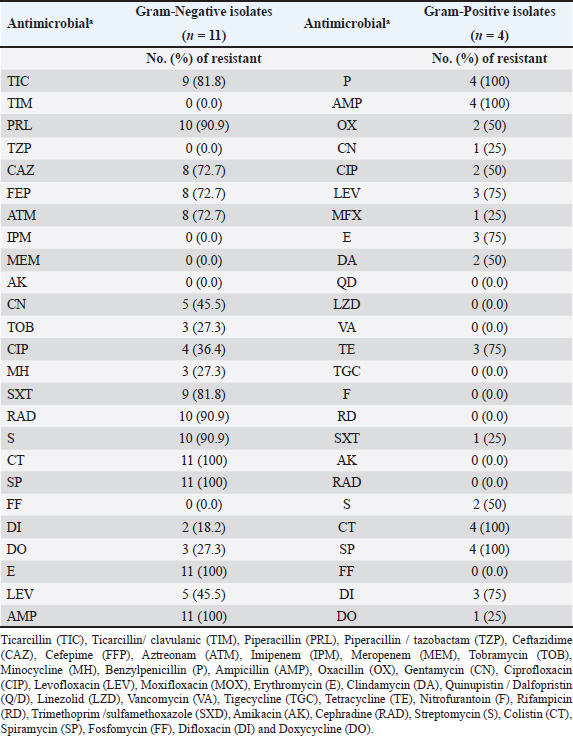
Figure 2. Agarose gel electrophoresis of the PCR products of E. coli :Lanes L: 100 bp ladder, 1: 13, 2: 18, 3: 23, 4: 28, 5: 30, 6: 32, 7: 34, 8: 36, and 9: positive control uid A at 556 bp. 10: negative control. The E. cloacae complex isolate showed high sensitivity to amikacin, fosfomycin, difloxacin, piperacillin/tazobactam, meropenem, and imipenem; moderately sensitive to ticarcillin/clavulanic acid and tobramycin, and resistant to ampicillin, piperacillin, ticarcillin, cephradine, ceftazidime, cefepime, aztreonam, gentamycin, streptomycin, erythromycin, spiramycin, ciprofloxacin, levofloxacin, minocycline, doxycycline trimethoprim/sulfamethoxazole. Interestingly S. enterica subspecies arizonae isolate showed high sensitivity to amikacin, gentamycin, ciprofloxacin, difloxacin, levofloxacin, minocycline, doxycycline, fosfomycin, piperacillin, piperacillin/tazobactam, meropenem, imipenem, ticarcillin, ticarcillin/clavulanic acid, tobramycin, ceftazidime, cefepime, aztreonam, and trimethoprim/sulfamethoxazole, and resistant to cephradine, colistin, ampicillin, streptomycin, erythromycin and spiramycin. Results of antimicrobial resistance testing show an increase in the number of isolates causing duck enteritis that is multidrug resistant, with 87% of the tested isolates having resistance to five or more antimicrobials (Table 2). DiscussionEnteritis is a devastating disease for the poultry performance particularly in ducklings which subtracts from the economic outcome of this poultry sector. Consequently, the bacterial agents causing duck enteritis among 42 flocks from four Egyptian governorates (Sharkia, Gharbia, Dakahlia, and Qliobia) were investigated. The birds were subjected to clinical examination and bacterial and molecular identification as well as antimicrobial testing using VITEK 2. The frequently observed clinical signs among the examined flocks were watery, whitish, bloody, and greenish diarrhea. The obtained results were analogs to that previously recorded by Ibrahim, (2003) who infected 14-day-old ducklings intranasal with 7.5 × 106 cfu E. coli O86:K61 experimentally and noticed whitish and greenish diarrhea 48 hours post infection. The clinically affected ducklings showed diarrhea with pasting of feathers around the vent which is comparable with Barrow et al. (1999) findings that isolated and identified Salmonella typhimurium from ducklings during the first 2 weeks of life in the United Kingdom. Most of the flocks under examination had respiratory distress, labored breathing, and gasping. The obtained results were parallel to those previously stated by Abd El-Samie et al. (2019) who observed respiratory symptoms in 40 diseased ducks of different ages (1–8 weeks old) in Sharkia governorate, Egypt, between June and September 2018 affected by colibacillosis. The necropsy revealed enteritis in the ducks under investigation. Similarly, Abd El Tawab et al. (2015) observed enteritis in ducks infected with S. Inganda, S. Infantis, and S. Larochelle in the Egyptian governorates of Dakahlia and Damietta. In addition, in this study, increased pericardial fluid, pericarditis, and serofibrinous perihepatitis were noted as necropsy findings and these results were agreed with Aggad et al. (2010) who isolated E. coli from 1 to 8-week-old chickens in western Algeria. The results were also consistent with those reported previously by Abd El-Samie et al. (2019), who noted air sacculitis, pericarditis, perihepatitis, and peritonitis in young diseased ducks suffering from colibacillosis in Sharkia governorate, Egypt, between June and September 2018. Bacterial isolation, bacterial identification utilizing (VITEK 2), and confirmatory PCR were carried out to evaluate the bacterial etiology of enteritis in the examined ducks.
Figure 3. Heatmap representation of isolates source and antimicrobial resistance patterns. In this study, the examined livers yielded 46 bacterial isolates identified as follows Staphylococcus spp (52.17%), E. coli (41.30%), and the least percentage of 2.17% for E. casseliflavus (S. enterica subspecies arizonae and E. cloacae complex). These results were obtained by Bisgaard (1981) who isolated a higher percentage of Staphylococcus species than E. coli from ducks (Mallard, Muscovy, and white Pekin) at the age of 55 days. Also, partially agreed with Safwat et al. (1986) who isolated Salmonella, E. coli, Enterobacter species, and Pasteurella multocida from the internal organs of 150 balady ducks (4 weeks old) at a percentage of 19%, 15%, 13.3%, and 5%, respectively. The inability to isolate Pasteurella in our study may be due to medicated or vaccinated flocks. The second highest percentage of isolated bacteria in this study was E. coli (41.30%). This result was agreed with Asway and Abd-El latif (2010) who isolated E. coli from the internal organs of ducks with a higher percentage in the liver (42.5%). Table 2. Resistant proportions of 15 isolates from ducks with enteritis.
Infections with two bacterial pathogens were detected in 30.95%. Whereas 12 flocks had mixed infections with Staphylococcus spp. and E. coli, just one flock had Staphylococcus spp. and S. enterica subspecies arizonae infection. These findings are similar to those obtained by Asway and Abd-El latif (2010), who studied bacterial enteritis in 120 freshly dead ducks and 40 fecal samples from clinically diseased ducks of various ages (1–30 days) obtained from private farms in the Dakahlia Governorate and recorded mixed infections of E. coli, Staphylococcus aureus, and Salmonella. However, negative results from bacterial isolation testing, enteritis was found in four of the duck flocks (1–4) under investigation. This could be related to various factors such as enteric viruses, as explored by Abd El-Ghany (2021) who investigated the common emerging viral infections affecting ducks in Egypt, parasites, or poor hygiene and sanitary conditions. In our study, different species of Staphylococcus were isolated including S. aureus, Staphylococcus lentus, and Staphylococcus scirui. The identification of both later new species of Staphylococci from cases of enteritis in our study may be due to using one of the most sensitive techniques for bacterial identification (VITEK 2 automated system). These results are similar to that obtained by Marek et al. (2016) who isolated Staphylococcus species including S. lentus and S. scirui from heart, liver, tarsal joints, and bone marrow from poultry flocks (broilers, laying hens, breeding hens, turkeys, ducks, and geese). The bird in the aforementioned study had increased mortality, inflammation of the skin and s/c tissue (dermatitis and cellulitis), lameness, arthritis, decreased weight gain, omphalitis, and yolk sac infections. This finding emphasizes the elevated significance of both staphylococcus species as disease-causing agents in poultry flocks. Salmonella enterica subspecies arizonae was isolated from 9 days duckling in this study. To the best of our knowledge, this is considered the first record of S. arizonae isolation from ducks suffering enteritis in Egypt. Previously Abd El Tawab et al. (2015) isolated S. Inganda, S. Infantis, and S. Larochelle from 104 ducks from flocks scattered in two Egyptian governorates (Dakahlia and Damietta). Conventional techniques that we used in this study for isolation and identification recognized the genus of the pathogenic bacteria, whereas VITEK 2 identified the bacterium to the species level. Furthermore, the VITEK 2 system is a simpler approach to implement than the traditional method (Carroll and Weinstein, 2007). VITEK 2 is quickly becoming a common approach for laboratory microbiology (Wallet et al., 2005), and we used it in this study for bacterial identification and antibiotic susceptibility testing. PCR was able to confirm VITEK 2 automated system results in the case of E. coli isolates by the detection of the uidA gene (beta-glucuronidase enzyme) at the band size 556 bp which agreed with Anbazhagan et al. (2011) who employed the uidA E. coli O177-specific gene sequence as the target of a single-plex PCR experiment. The antibiogram patterns of isolated pathogens from naturally infected ducks in our work demonstrated varying results against different antimicrobial drugs tested, indicating the rise of drug- and multidrug-resistance of Staphylococci, E. coli, Enterococcus, and Enterobacter. Staphylococci isolates were extremely resistant penzylpenicillin, ampicillin, colistin, spiramycin (100%), and levofloxacin, erythromycin, oxacillin, clindamycin, tetracycline, streptomycin and difloxacin (66.7%). This result partially matched with Awad et al. (2023) who examined the prevalence and antimicrobial resistance of S. aureus isolated from 500 broilers and ducks in the Egyptian governorates of El-Dakahlia and El-Sharkia. The authors found that chicken S. aureus isolates had higher resistance rates to trimethoprim/ sulfamethoxazole (84.2%) and oxytetracycline (73.7%) while duck S. aureus isolates were resistant to oxacillin (87.5%), followed by trimethoprim/ sulfamethoxazole (50%). Conversely, enterobateriacae isolates were extremely resistant to colistin, erythromycin, spiramycin, ampicillin, piperacillin, and ticarcillin (100%); cephradine, trimethoprim/sulfamethoxazole, and streptomycin (88.9%); ceftazidime, cefepime, aztreonam (78%), and less resistant to gentamicin and levofloxacin (45%). This finding was consistent with that of Bushen et al. (2021), who reported that the enterobateriacae isolates found in 140 fresh chicken dropping samples collected in Southwest Ethiopia from April to June of 2018 were highly resistant to ampicillin (91.7%) and trimethoprim-sulfamethoxazole (70.8%) and less resistant to gentamicin (41.7%). In addition, during 7 weeks of duck breeding in Slovakia, Hleba et al. (2011) noted resistance of Enterobacteriaceae to tetracycline (32.43%), streptomycin, and ampicillin on the same level of 8.10%, and chloramphenicol (5.40%). Antibiotic susceptibility of the Staphylococcus spp. isolates revealed high sensitivity to amikacin, cephradine, fosfomycin, vancomycin, tigecycline, linezolid, nitrofurantion, rifampicin, and quinupristin/dalfopristin, (100%); gentamycin, ciprofloxacin, moxifloxacin, trimethoprim/sulfamethoxazole, and doxycycline (66.7%) and levofloxacin, difloxacin, streptomycin, oxacillin, and tetracycline (33.3%). These results were similar to Nabil (2010) who stated that Staphylococcus isolates were sensitive to ciprofloxacin, norofloxacin, streptomycin, and gentamycin. The E. coli isolates were highly sensitive to amikacin, fosfomycin, ticarcillin/clavulanic acid, piperacillin/tazobactam, meropenem, and imipenem (100%); minocycline, doxycycline, and difloxacin (78%); tobramycin and ciprofloxacin (66.7%). These findings partially matched to those of Cambrea (2014), who noted that E. coli isolates were highly sensitive to amikacin (85.7%), cephalosporins (80%), ceftazidime (79.5%), ceftriaxone (75%), trimethoprim/sulfamethoxazole (38.4%), and tetracycline (29.5%), as well as Adam et al. (2022) who noted that all E. coli isolates were highly sensitive to imipenem (100%). Salmonella enterica subspecies arizonae isolate was highly sensitive to amikacin, gentamycin, ciprofloxacin, difloxacin, levofloxacin, minocycline, doxycycline, fosfomycin, piperacillin, piperacillin/tazobactam, meropenem, imipenem, ticarcillin, ticarcillin/clavulanic acid, tobramycin, ceftazidime, cefepime, aztreonam, and trimethoprim/sulfamethoxazole. These findings agreed with Lebdah et al. (2017), who found that Salmonella isolates were susceptible to amikacin (80.9%); ciprofloxacin (76.2%); norofloxacin (71.2%); and cefotaxime (52.4%). In addition, Ćwiek et al. (2020) reported that Salmonella isolates were susceptible to gentamicin, tazobactam, cefotaxime, meropenem, ciprofloxacin, azithromycin, tigecycline, and trimethoprim. ConclusionBecause enteric pathogens are recognized as one of the most serious concerns confronting the duck business, various studies to understand such sickness situations are required. In Egypt, the most common bacterial pathogens causing duck enteritis were Staphylococcus spp. and E. coli. To the best of our knowledge, the isolation of S. lentus, S. scirui, E. casseliflavus, S. enterica subspecies arizonae, and E. cloacae complex was first recorded in Egypt from duck enteritis. Eighty percent of the duck enteritis isolates were multidrug resistant to five or more antimicrobials. This underlines the ways in which duck pathogens may serve as a source of antimicrobial resistance traits and may spread to humans and other birds via the food chain and environment. AcknowledgmentThe authors are grateful to the Faculty of Veterinary Medicine, Zagazig University. Conflict of interestThe authors declared that there is no conflict of interest. FundingThis research received no specific grant or funding. Authors' contributionsAH, MH, and EM designed this study, collected samples, and epidemiological data, performed the bacterial identification and molecular diagnosis, and wrote the manuscript. IE performed statistical analysis. AE and MS revised and commented on the manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. Any extra data needed are available from the corresponding authors upon reasonable request. ReferencesAbd El-Ghany, W.A. 2021. A comprehensive review on the common emerging viral diseases affecting ducks with special emphasis on Egyptian situation. Solv. Vet. Res. 58, 259–270. Abd El-Samie, L.K., Abd-Elmoaty, A.M., Ibrahim, N.A. and Darwish, W.S. 2019. Prevalence and molecular characterization of Avian pathogenic E. coli in ducks. Adv. Anim. Vet. Sci. 7, 163–168. Abd El Tawab, A.A., Ammar, A.M., Nasef, S.A. and Reda, R.M. 2015. Antibacterial resistance and resistance gene detriments of E. coli isolated from chicken. Benha. Vet. Med. J. 28, 231–240. Adam, A.F., Ismaael, N.A., Atiyahullah, T.M., Bentaher, E.T., Gaidan, O.K. and Meriz, O.M. 2022. Antibiotic susceptibility, serotyping and pathogenicity determination of avian Escherichia coli isolated from colibacillosis cases in broiler chicken in Aljabel Alakhar Region, Libya. Assiut Vet. Med. J. 172, 1–8. Aggad, H., Ammar, Y.A., Hammoudi, A., and Kihal, M. 2010. Antimicrobial resistance of Escherichia coli isolated from chicken with colibacillosis. Glob. Vet. 4, 303–306. Ammar, A.M., El-Naenaeey, E.Y., El-Malt, R.M.S., El-Gedawy, A.A., Khalifa, E., Elnahriry, S.S. and Abd El-Hamid, M.I. 2021. Prevalence, antimicrobial susceptibility, virulence and genotyping of Campylobacter jejuni with a special reference to the anti-virulence potential of eugenol and beta-resorcylic acid on some multi-drug resistant isolates in Egypt. Animals 11, 3. Anbazhagan, D., Mui, W.S., Mansor, M., Yan, G.O., Yusof, M.Y. and Sekaran S.D. 2011. Development of conventional and real time multiplex PCR assays for the detection of nosocomial pathogens. Braz. J. Microbiol. 42, 448–458. Andreasen, C.B. 2008. Diseases of poultry. In Editorial Board for the American Association of Avian Pathologist. Chapter 23 Other bacterial diseases (Staphylococcosis), 12th ed. Eds., Saif, Y.M., Fadly, A.M., Glisson, J.R., McDougald, L.R., Nolan, L.K. and Swayne, D.E. Blackwell Publishing, pp: 892–900. Asway, A.M.E. and Abd-El latif, M.M. 2010. Some bacteriological and serological studies on enteritis in ducks. Assiut Vet. Med. J. 56, 125. Awad, N.F., Abd El-Hamid, M.I., Nabil, N.M., Tawakol, M.M., Eid, S., Al-Zaban, M.I., Farouk, H., Zakai, S.A., Elkelish, A., Ibrahim, M.S., Mahmoud, H.A., Salem, S.M., Ismail, H.M., Rehab, I. and Hamed, R.I. 2023. Multidrug resistant and multivirulent bacterial pathogens in chickens and ducks: tackling experimental leg disorders using phytobiotics and antibiotics alone or in combination. Poult. Sci. 102,102889. Barrow, P.A., Lovell, M.A., Murphy, C.K. and Page, K. 1999. Salmonella infection in a commercial line of ducks. Experimental studies on virulence intestinal colonization and immune protection. Epidemiol. Infect. 123, 121–132. Bisgaard, M. 1981. Arthritis in ducks. Aetiology and public health aspects. Avian. Pathol. 10, 11–21. Brans, L.H. and Gross, W.B. 1997. Colibacillosis. In Disease of poultry. Eds., Calnek, B.W., Branes, J.H., Beard, C.W., Mac Dougold, R.L. and Saif, Y.M. Ames, IA: Iowa State University Press, pp: 131–141. Bushen, A., Tekalign, E. and Abayne. M. 2021. Drug- and multidrug-resistance pattern of Enterobacteriaceae isolated from droppings of healthy chickens on a poultry farm in Southwest Ethiopia. Infect. Drug. Resist. 14, 2051–2058. Cambrea, S.C. 2014. Antibiotic susceptibility of Escherichia coli strains isolated in a pediatric population from South Eastern Romania. J. Pediat. Infect. Dis. 9, 157–162 Cao, S.Y., Wang, M.S., Chang, A.C., Oi, X.F., Yang, X.Y., Deng, S.X., Yin, N.C., Zhang, Z.H., Zhou, D.C., Zhu, D.K. and Luo, Q.H. 2008. Comparative analysis of intestinal microbial community diversity between healthy and orally infected duckling with Salmonella enteritidis by ERIC–PCR. World. Gatroenterol. 14, 1120–1125. Carroll, K.C. and Weinstein, M.P. 2007. Manual and automated systems for detection and identification of microorganisms. In Manual of clinical. Microbiology, 9th ed. Eds., Murry, P.R., Baron, E.J., Jorgensen, J.H. et al. Washington, DC: American Society for Microbiology Press, pp: 192–217. Ćwiek, K., Korzekwa, K., Tabiś, A., Bania, J., Bugla-Płoskońska, G. and Wieliczko, A. 2020. Antimicrobial resistance and biofilm formation capacity of Salmonella enterica Serovar enteritidis strains isolated from poultry and humans in Poland. Pathogens 9, 643. Enany, M.E., Algammal, A.M., Shagar, G.I., Hanora, A.M., Elfeil, W.K. and Elshaffy, N.M. 2018. Molecular typing and evaluation of sidr honey inhibitory effect on virulence genes of MRSA strains isolated from catfish in Egypt. Pak. J. Pharm. Sci. 31, 1865–1870. Eid, H.I., Algammal, A.M., Nasef, S.A., Elfeil, W.K. and Mansour, G.H. 2016. Genetic variation among avian pathogenic E. coli strains isolated from broiler chickens. Asian J. Anim. Vet. Adv. 11(6), 350–356. El-Jakee, J., Nagwa, A.S., Bakry, M., Zouelfakar, S.A., Elgabry, E. and Gad El-Said, W.A. 2008. Characteristics of Staphylococcus aureus strains isolated from human and animal Sources. American-Eurasian J. Agric. Environ. Sci. 4, 221–229. Gerardian, J., Lalioui, L., Jacquemin, E., Mainel, J.G. and Bouguenec, C. 2000. The age-related gene cluster in necrotoxigenic and other Escherichia coli from animal. Vet. Microbial. 76, 175–184. Gomis, S., Babiuk, L., Godson, D.L., Allan, B., Thrush, T., Townsend, H., Willson, P., Waters, E., Hecker, R. and Potter, A. 2003. Protection of chickens against Escherichia coli infections by DNA containing CpG motifs. Infect. Immun. 71, 857–863. Gomis, S.M., Gomis, A.I., Horadagonda, N.U., Wijewardene, T.G., Allan, B.J. and Potter, A.A. 2000. Studies on cellulites and other disease syndrome caused by E. coli in broiler in Srilanka. Trop. Anim. Health. Prod. 32, 341–351. Gu, Z., Eils, R. and Schlesner, M. 2016. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849. Henry, R.R. 2000. Salmonella infection in ducks. Chapter 9. Lincolin, UK. Hleba, L., Kačániová, M., Lejková, J. and Pochop, J. 2011. Antibiotic resistance of Enterobacteriaceae species associated with faecal bacterial cenosis of ducks. Anim. Sci. Biotechnol. 44, 408–14. Ibrahim, I.A. 2003. Diarrhea in duckling. M.V.Sc. Thesis, Faculty of Veterinary Medicine Zagazig University, Zagazig, Egypt. Lebdah, M.A., Eid, Amal, A.M., Nasef, Soad, A. and Hamad, Enas, M. 2017. Phenotypic and genotypic characterization of paratyphoid Salmonellae isolated from poultry in delta area- Egypt. Zagazig. Vet. J. 45, 262–272. Lee, P.Y., Costumbrado, J., Hsu, C.Y. and Kim, Y.H. 2012. Agarose gel electrophoresis for the separation of DNA fragments. J. Vis. Exp. 20, 3923. Lister, S.A. and Barrow, P., 2008. Enterobacteriaceae. In Poultry diseases, 6th ed. Eds., Pattison, M., McMullin, P., Bradbury, J. and Alexander, D. Elsevier Ltd. Marek, A., Pysniak, D.S., Pyzik, E., Adaszek, L., Wilczynski, J. and Winiarczyk, S. 2016. Occurrence and characterization of Staphylococcus bacteria isolated from poultry in Western Poland. Berl. Munch. TierarZtl. Wochenschr. 129, 147–152. McNamee, P.T. and Smyth, J.A. 2000. Bacterial chondronecrosis with osteomyelitis (‘femoral head necrosis’) of broiler chickens: a review. Avian. Pathol. 29, 253–270. Mondal, T., Khan, M.S.R., Alam, M., Purakayastha, M., Das, M. and Siddique, M.P. 2008. Isolation, identification and characterization of salmonella from duck. Bangl. J. Vet. Med. 6, 7–12. Montagomery, R.D., Boyle, C.R., Lenarduzzi, T.A. and Jones, L.S. 1999. Consequence of chicks hatched from Escherichia coli-inoculated embryo. Avian. Dis. 43, 553–563. Nabil-Hala, M. 2010. Studies on bacterial causes of arthritis in chickens. M.V.Sc. Thesis, (Avian and Rabbit Med.), Faculty of Veterinary Medicine Zagazig University, Zagazig, Egypt. Pattison, M., McMullin, P. and Bradbury, J.M., 2008. Poultry diseases, 6th ed. Elsevier Ltd. Quinn, P.J., Markey, B.K., Carter, M.E., Donnelly, W.J. and Leonard, F.C. 2002. Veterinary microbiology and microbial diseases. Oxford, UK: Block Well Science Ltd.,. Saif, Y.M., Barnes, H.J., Glisson, J.R., Fadly, A.M., McDouglad, L.R. and Swayne, D.E. 2010. Diseases of poultry. Ames, IA: Iowa State Press. Safwat, E.E.A., Wahba, S., Metwally, N. and Refai, M. 1986. Incidence of Salmonella and other gram negative organisms in balady ducks and infertile eggs. Egypt. J. Vet. Med. 46, 199–206. Van de Zande, S., Nauwyhock, H. and Pensaert, M. 2001. The clinical, pathological and microbiological outcome of Escherichia coli O2:KI infection in avian pneumovirus infected in turkeys. Vet. Microbial. 81, 353–365. Wallet, F., Loiez, C., Renaux, E., Lemaitre, N. and Courcol, R.J. 2005. Performance of VITEK2 colorimetric cards for identification of Gram positive and Gram negative bacteria. J. Clin. Microbiol. 43, 4402–4406. | ||
| How to Cite this Article |
| Pubmed Style Hussein A, Eid AA, Hassaan M, Mohamed E, Elsohaby I, Shawky M, . Isolation and identification of multidrug resistance bacterial agents implicated in duck enteritis with first record of Salmonella enterica subspecies arizonae in Egypt. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 553-563. doi:10.5455/OVJ.2024.v14.i1.50 Web Style Hussein A, Eid AA, Hassaan M, Mohamed E, Elsohaby I, Shawky M, . Isolation and identification of multidrug resistance bacterial agents implicated in duck enteritis with first record of Salmonella enterica subspecies arizonae in Egypt. https://www.openveterinaryjournal.com/?mno=182431 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.50 AMA (American Medical Association) Style Hussein A, Eid AA, Hassaan M, Mohamed E, Elsohaby I, Shawky M, . Isolation and identification of multidrug resistance bacterial agents implicated in duck enteritis with first record of Salmonella enterica subspecies arizonae in Egypt. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 553-563. doi:10.5455/OVJ.2024.v14.i1.50 Vancouver/ICMJE Style Hussein A, Eid AA, Hassaan M, Mohamed E, Elsohaby I, Shawky M, . Isolation and identification of multidrug resistance bacterial agents implicated in duck enteritis with first record of Salmonella enterica subspecies arizonae in Egypt. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 553-563. doi:10.5455/OVJ.2024.v14.i1.50 Harvard Style Hussein, A., Eid, A. A., Hassaan, M., Mohamed, E., Elsohaby, I., Shawky, M. & (2024) Isolation and identification of multidrug resistance bacterial agents implicated in duck enteritis with first record of Salmonella enterica subspecies arizonae in Egypt. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 553-563. doi:10.5455/OVJ.2024.v14.i1.50 Turabian Style Hussein, Ashraf, Amal A.M. Eid, Mohamed Hassaan, Eman Mohamed, Ibrahim Elsohaby, Mohamed Shawky, and . 2024. Isolation and identification of multidrug resistance bacterial agents implicated in duck enteritis with first record of Salmonella enterica subspecies arizonae in Egypt. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 553-563. doi:10.5455/OVJ.2024.v14.i1.50 Chicago Style Hussein, Ashraf, Amal A.M. Eid, Mohamed Hassaan, Eman Mohamed, Ibrahim Elsohaby, Mohamed Shawky, and . "Isolation and identification of multidrug resistance bacterial agents implicated in duck enteritis with first record of Salmonella enterica subspecies arizonae in Egypt." Open Veterinary Journal 14 (2024), 553-563. doi:10.5455/OVJ.2024.v14.i1.50 MLA (The Modern Language Association) Style Hussein, Ashraf, Amal A.M. Eid, Mohamed Hassaan, Eman Mohamed, Ibrahim Elsohaby, Mohamed Shawky, and . "Isolation and identification of multidrug resistance bacterial agents implicated in duck enteritis with first record of Salmonella enterica subspecies arizonae in Egypt." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 553-563. Print. doi:10.5455/OVJ.2024.v14.i1.50 APA (American Psychological Association) Style Hussein, A., Eid, A. A., Hassaan, M., Mohamed, E., Elsohaby, I., Shawky, M. & (2024) Isolation and identification of multidrug resistance bacterial agents implicated in duck enteritis with first record of Salmonella enterica subspecies arizonae in Egypt. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 553-563. doi:10.5455/OVJ.2024.v14.i1.50 |