| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 586-593 Original Research Beneficial effect of probiotics supplementation on quality of edible fresh table eggs during storageEsmat I. Awad*, Salah F. Abdlaal, Mohamed A. Bayoumi and Sameh M. HalawaFood Hygiene, Safety, and Technology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Esmat I Awad. Food Hygiene, Safety, and Technology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: esmatawad2 [at] yahoo.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
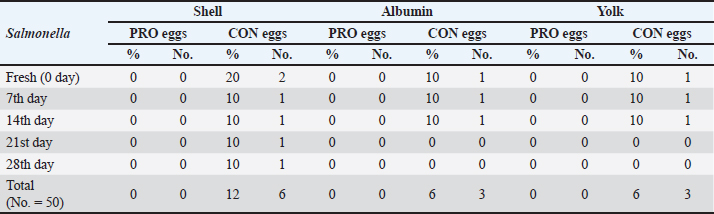
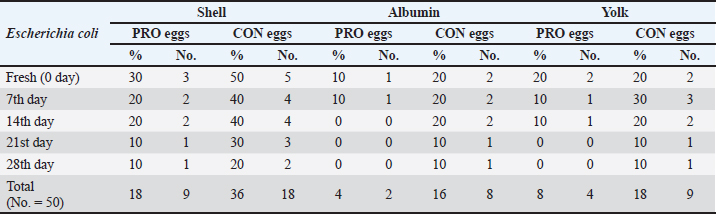
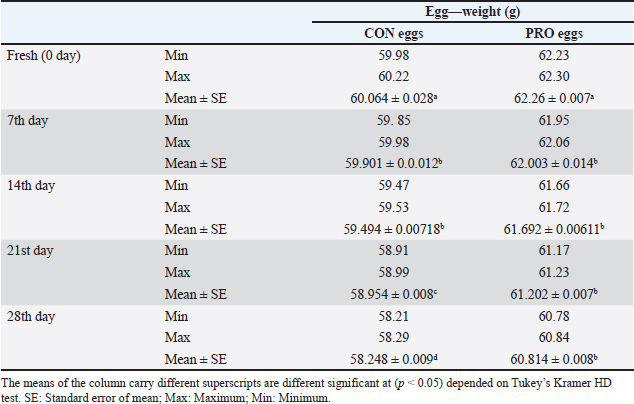
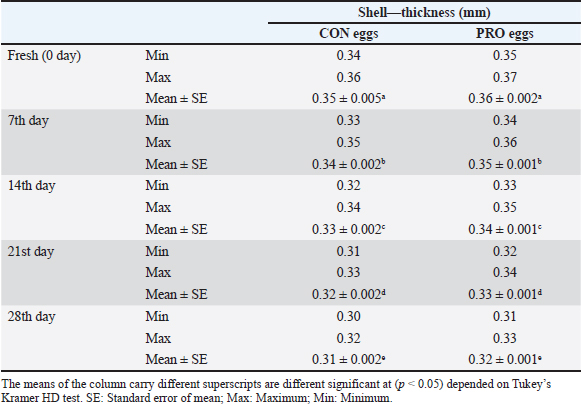
AbstractBackground: This study discussed the effect of probiotic supplementation on laying hens’ diets and the enhancement of egg quality during the cold storage period. Aim: To study the efficacy of the addition of probiotics to hen diets in terms of improving the egg’s quality during the cold storage period and protection against enteric pathogens. Methods: 100 table eggs were collected from farms of laying hens on a battery system, 46 weeks old HylineW36 white in Sharkia Government. The collected eggs were separated into 2 groups (50 each); the control group from hens fed on diets without probiotics, and the probiotic group from hens fed on diets with (100 g/ton) of supplemented probiotics preparation. All groups were separated into 5 sub-groups for the examinations; on the fresh day, 7th, 14th, 21st, and 28th days on cold storage at 4°C. Chemical, physical, and microbiological examinations were done for internal egg contents and eggshells. Results: Our results showed that probiotics supplements have advantageous effects on the quality of eggs during cold storage periods. Also, microbiological examination proved that eggshells from hens fed on diets with probiotics supplemented (100 g/ton) have decreased the level of bacterial contamination with Salmonella and Escherichia coli than hens fed on a regular diet. Conclusion: It could be shown that the probiotics supplementation may decrease and reduce the effect of the storage period on the quality of shell, albumen, and yolk. Keywords: Table eggs quality, Laying hens’ diet, Storage, Probiotic supplementation, Enteric pathogens. IntroductionEg g quality begins to decrease immediately after oviposition and continues during the storage periods, especially in a non-refrigerating environment. This drop has a great effect on egg quality as yolk and albumen pH and weight. Egg loss is considered a global problem for the economic poultry industry and for human food security (Feddern et al., 2017). Thus, enhancement of food quality and food safety for humans and extending the shelf-life of eggs are considered very important. In addition, pathogens that may contaminate laying hens are also decreasing the quality of eggs (Nyholm, 2020). Practices of hens feeding are considered effective ways to modify egg characteristics. Those treatments changed the freshness and flavor of eggs (Oliveira and Oliveira, 2013). Food additives may enhance the quality of eggs. Recently, probiotics have been considered effective approaches. Probiotics are considered as live microorganisms that promote hens’ growth that may be limited in many countries due to improper uses of antibiotics in animal production (European Commission, 2021). Probiotics enhance protein digestibility and provide the animal with good performance (Tang et al., 2019). Also increase the activity of the immune system (Wang et al., 2019). Probiotics have activities of anti-inflammatory action (Tomosada et al., 2013), and many different mechanisms of action (Callaway et al., 2008). For instance, the chemical effects of bacteriocins, the biological effect of probiotic bacteria, preventing pathogens proliferation, and physical actions as competitive exclusion for binding sites (Vallance et al., 2008). Thus, our study was conducted to study the effect of probiotic supplementation in laying hens’ diets on the effect on the quality of eggs through different cold storage periods. Materials and MethodsExperimental designThe study was conducted in June 2023, at Sharkia Governorate, Egypt. Eggs samples were collected from white hens, HylineW36, aged 46 weeks old. These hens were fed in 2 types of diet, the 1st was control (CON), which was a basal diet, with non-supplements, and the 2nd was a probiotic-supplemented diet (PRO) which was a basal diet mixed with (100 ppm) of a probiotic additive. The probiotic supplement (Baymix, Grobig BS, Bayer) included Bacillus subtilis (QST 713) (1010 cfu/g). Basal diet feed is prepared following that prepared by Hy-Line-Product-PDFs (Hy-Line-Products, 2021). Water and feed used during these experiments were the drinker's nipple and automatic feeders. The lighting system was (8 hours of dark and 16 hours of light) per day. The hens were housed in cages from wire (long 50 cm, wide 60 cm, and high 50 cm), six hens on the cage, resting in the middle raw during time of experimental. Collection of samplesEgg samples were collected and put on plastic sterile containers and immediately transferred for examination (chemical, physical, and microbiological) at the laboratory. Eggs were stored at 4°C on the refrigerator until examination. All groups were categorized into 5 groups (fresh eggs, 7th, 14th, 21st, and 28th days). Physical analysisWeight of eggsEgg samples were weighed using a digital scale weighing (model: AX 1000). Then, egg samples were broken on a smooth surface. Albumin was separated from the yolk and then put into two glass beakers. After that, the weight of the albumen and yolk was recorded. Eggshell’s thicknessUsing a micrometer (Testex TX-GAGE, USA), eggshell thickness was recorded. Chemical analysispH determinationFollowing albumin separation from the yolk, the pH of albumin and yolk was measured by using a pH meter (Electronic Instrument Ltd). Two grams from each sample were homogenized into 20 ml of de-ionized water in a glass beaker. Initially, following pH meter standardization, electrodes were washed with distilled water and egg homogenate pH was measured. Microbiological analysisThe eggs hell was disinfected with 70% ethanol, and then the eggshell was broken with a sterilized hard spatula. Then, albumin was separated from the yolk then put into a sterilized container. Serial dilution of egg content was initially performed (Cruickshank et al., 1978). Peptone water (Oxoid Ltd; pH 6.2 ± 0.0) was used as primary enrichment media for Salmonella and Escherichia coli isolation. Four selective media were used for the isolation of those pathogens. SS agar (Merck, pH 6.9 ± 0.2) and xylose lysine deoxycholate (XLD) agar (Oxo id Ltd, pH 7.4 ± 0.2) were used for Salmonella sp. The eosin-methylene blue (EMB) agar (Merck, pH 7.1 ± 0.2) and MacConkey agar (Oxoid Ltd, pH7.4 ± 0.2) were used for E. coli. 1 ml of egg content was inoculated in a screw cap test tube containing 10 ml of nutrient broth and incubated for 24 hours at 37°C. For Salmonella sp., after inoculation of the sample on nutrient broth, one loop-full of incubated broth was streaked on the XLD agar plate and incubated for 24 hours at 37°C. Then, positive samples were further inoculated on SS agar and incubated overnight for 24 hours at 37°C. Colonies with a black center in XLD and blackish growth in SS agar were considered as presumptive Salmonella positive. While for E. coli, samples were streaked on MacConkey agar and incubated overnight. Large, pink colonies were further subcultured on EMB agar at 37°C for 24 hours. The characteristic metallic sheen colonies were suggestive of E. coli positive (Jotan et al., 2017). Isolate identification was performed by culturing, incubation, and the morphology of organisms and biochemical identification (Jotan et al., 2017). Statistical analysisThe presented values are presented as mean ± standard error (SE). Presented data were driven from the statistical package and social sciences for one-way analysis of variance at a 95% level of confidence and (SPSS-16.; Chicago, IL) software. Significant differences between the means were calculated by Tukey’s Kramer HD test, considering p < 0.05 as significant (Lee and Lee, 2018). ResultsIn our study, the results presented in Table 1 reported Salmonella occurrence in CON eggs (albumin, yolk, and eggshell) at refrigerated storage intervals also reported decreased with increased storage refrigerated period. The incidence of Salmonella (shell, albumin, and yolk) was not reported in the PRO eggs. The results presented in Table 2 reported E. coli occurrence in CON eggs and PRO eggs in (albumin, yolk, and eggshell) at refrigerated storage intervals. The incidence of E. coli in (shell, albumin, and yolk) of CON eggs was higher than in PRO eggs and it decreased with an increased refrigerated storage period. The results presented in Table 3 reported significant differences were found between egg weights. The mean weight of CON eggs and PRO eggs on the lying day was significantly the highest when compared with other examination periods. The egg weight in CON eggs and the PRO eggs group decreased with an increase in the storage period during the refrigerated storage period. The results presented in Table 4 reported the mean thickness of examined eggshells in CON and PRO eggs has a significant difference during different refrigerated storage intervals. The shell thickness in the CON eggs and PRO eggs groups decreased with an increase in the storage period during the refrigerated storage period. Table 1. Incidence of isolated Salmonella spp. from the examined control group and probiotics-supplemented group during the refrigerated storage period.
Table 2. Incidence of isolated E. coli spp. from examined control group and probiotics-supplemented group during refrigerated storage.
Table 3. Effect of probiotics supplementation on weight of eggs during refrigerated storage.
Table 4. Effect of probiotics supplementation on eggshell thickness during refrigerated storage.
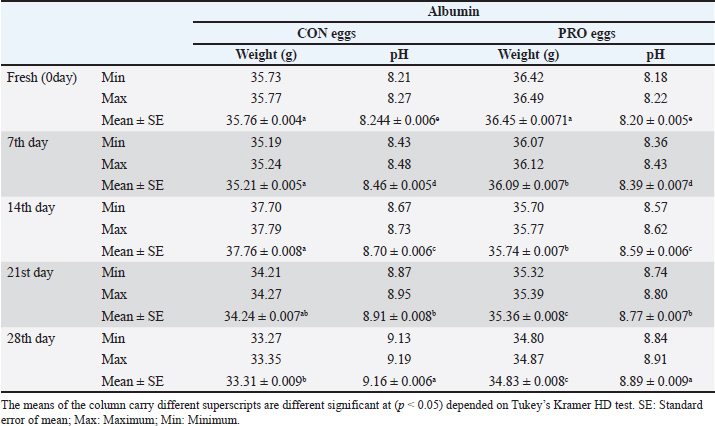
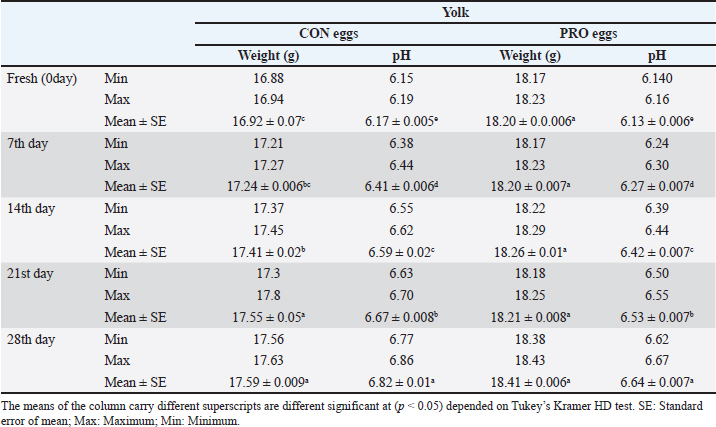
Our study showed in Table 5 a significant variation in the weight of albumin in CON eggs and PRO eggs. During the refrigerated storage period, the albumin weight in CON eggs and PRO eggs decreased in albumen weight with an increased storage period. Also, our results revealed that the statistical analysis of the pH of albumin has more significant difference variations among CON and PRO eggs at different cold storage periods. On the 28th day, it was higher significantly in pH than the other storage periods in CON and PRO eggs. Also, the albumin pH of the 2 groups increased with an increase in storage period. The significant difference in Table 6 is seen in the weight of the yolk of CON and PRO eggs at the different cold storage periods. Also, there were significant variations seen in the pH of the yolk of CON and PRO eggs at different cold storage periods. During the refrigerated storage period, the yolk weight in CON and PRO eggs increased in yolk weight with the increased storage period. Also, the yolk pH of the two groups increased with an increased storage period. DiscussionThe highest prevalence of Salmonella was found in eggshells (12% in CON eggs). On the contrary, the contents of the egg had the lowest incidence of Salmonella in albumin (6% in CON eggs) and in the yolk of eggs (6% in CON eggs). On the other hand, we failed to detect Salmonella in any part of PRO eggs samples. The obtained results of our study were similar to the results obtained by Mansour et al. (2015) who recorded the occurrence of Salmonella in the egg contents (1.2%) and it is lower than in eggshells (4.7%). In another study, Salmonella was isolated from fresh eggshells (6.5%) and egg contents (5.6%) and from stored eggshells (2.8%) and egg contents (7.5%) (Adesiyun et al., 2005). The failure of detection Salmonella from probiotic-supplemented egg contents and eggshells, as the growth promoter, may inhibit the isolation of Salmonella (Sadek et al., 2016). Salmonella coul d not be isolated from the egg contents at fresh and stored eggs until 4 weeks of storage. Isolation from the shell of the fresh egg (0.22%) decreased at storage eggs at 4 weeks of storage to (0.22%) (Stepień-Pyśniak, 2010). Regarding E. coli, it was isolated from eggshells by 36% in CON eggs, and 18% in PRO eggs, which were higher than egg contents which had the incidence of E. coli in albumin (16% in CON eggs, and 4% in PRO) and in the yolk of eggs (18% in CON eggs, and 8% in PRO eggs). Khan et al. (2016) recorded that table eggs were contaminated with E. coli and the highest incidence of E. coli was found in eggshells when compared with eggs’ components (in egg shell 15%, in albumin 12%, and in yolk 10%). Our results were in agreement with the results recorded by Adesiyun et al. (2005) who found that E. coli on eggshells was (58.7%) and (4.3%) in egg contents. Table 5. Chemical and physical characteristics of albumen (weight and pH) of the examined control group and probioticssupplemented group during refrigerated storage.
Table 6. Chemical and physical characteristics of yolk (weight and pH) of the examined control group and probiotics-supplemented group during refrigerated storage.
Upon storage, on egg albumin, E. coli at fresh eggs (0.67%) decreased with storage to (0.44%) at 4 weeks of storage. Similarly, E. coli at fresh eggs (0.45%) decreased with storage to (0.17%) at 4 weeks of storage. Also, on eggshells E coli at fresh eggs (2.63%) decreased with storage times to (0.47%) at 4 weeks of storage (Stepień-Pyśniak, 2010). In contrast, isolated microorganisms from eggs and contents might live at several different temperatures i.e. (4°C and 60°C). However, Salmonella could not be isolated, and this explains why all eggs were free from Salmonella (Osei-Somuah et al., 2006). Since E. coli is normally considered an inhabitant in a hen’s intestine; it is easily found on eggshells, and moreover, in egg contents. Thus, it gets its pathogenic importance. It also causes public health hazards and diarrhea (Awny et al., 2018). The presence of E. coli in eggs is a major indication of fecal contamination in eggshells, which causes hazards to human public health (Sadek et al., 2016). The Microbial Gut community plays a great role in the performance and health of the host. Therefore, the ability of probiotic supplements to promote the health effects gained a higher scientific interest in the last years (Duggan et al., 2002). For PRO eggs at fresh days were (62.26 ± 0.007 g) and at 28 days were (60.814 ± 0.008 g) with 1.4% weight loss. For CON eggs at fresh day was (60.064 ± 0.28 g) and at 28 days was (58.248 ± 0.009 g) with 1.816% weight loss. The results declared a decrease in egg weight loss by increasing the storage time in PRO eggs than CON eggs. The eggs from PRO-supplemented hens saw an accumulative loss of weight 11% lower than the eggs from control hens with a 15% lower weight loss was agreement with the result (Camila et al., 2022). Egg weight loss increased by the time of cold storage periods (7th day-21st day) from (1.91 g) to (3.60 g) these results were shown by Okoleh and Eze (2016). The decrease in the weight of the egg on the 7th day (0.36 g) and on the 14th day (0.57 g) during cold storage periods was concluded by Walsh et al. (1995). The significant decrease in the weight of eggs observed during the period of storage (4 weeks) could be caused by the increase of pores on the shells of aged eggs. The increase of the pores of the shell may cause easy escaping of gases and moisture from eggs. The carbonic acid breakdown occurred inside the albumin and then produced water and carbon dioxide. Carbon dioxide escapes from the pores of the shell and the albumin becomes watery and loses its thickness, this is the cause of weight loss of eggs (Eke et al., 2013). The highest thickness of eggshell (mm) was reported on laying day (fresh) in PRO (0.36 ± 0.002) and in CON eggs (0.35 ± 0.005); however, on the 28th day, the lowest thickness of shell egg in PRO (0.32 ± 0.001) and in CON eggs (0.31 ± 0.002). The PRO eggs reported an increase in the weight of shell eggs by 1% when compared to the control group (Camila et al., 2022). These results differed from the results obtained by Kralik et al. (2014). They concluded that the cold storage periods have no effect on the thickness of eggshells. In our study, the statistical analysis of the weight of albumin showed significant variation in PRO eggs on the fresh day (36.45 ± 0.0071) and on the 28th day (34.83 ± 0.008). Also, in CON eggs there was a significant difference on a fresh day (35.76 ± 0.004) and on the 28th day (33.31 ± 0.009). The mean albumin weight of PRO eggs on the lying day was (36.16 ± 0.623) and on the 28th day was (33.83 ± 0.793). While in CON eggs, there was a significant difference on the laying day (35.76 ± 0.613) and on the 28th day (33.31 ± 0.227) (Camila et al., 2022). Also, a decrease in the weight of albumin in fresh eggs (41.12 g) and in eggs stored for the 28th day (39.96 g) was seen (Kralik et al., 2014). Albumin produced in the magnum by the epithelial cells also contains protein and a clear colloidal solution. The quality of albumin is considered a parameter of protein quality and the freshness of an egg. Loss of albumin weight showed in the probiotic supplements, due to high deposition of protein on eggs. It happens due to intestinal microbiota modulation that provides good digestion and absorption of nutrients (Sobczak and Kozlowski, 2021). The weight of yolk gradually increases from the fresh day (lying day) in PRO eggs (18.20 ± 0.006) and (18.41 ± 0.006) on the 28th day of storage, also fresh day (laying day) in CON eggs (16.92 ± 0.07) and (17.59 ± 0.009) at the 28th day of storage. Other scientists reached nearly the same results that probiotics gradually increased the yolk weight from fresh day in PRO eggs (21.34 ± 0.005) and (23.05 ± 0.003) until the 28th day of storage, also from fresh day in CON eggs (21.07 ± 0.04) and (22.36 ± 0.002) until the 28th day of storage (Gunhan and Kirikçi, 2017, Camila et al., 2022). The yolk weight increase is due to the functionality of hepatocytes to synthesize the Vitellogenin (Kasiyati et al., 2016). Vitellogenin is considered a protein that is responsible for the transportation of lipids to the yolk from the liver. Regarding the pH of albumin, it determines the quality of albumin. So that it measured the egg freshness (Scott and Silversides, 2000). Our results revealed that the statistical analyses of the pH of albumin on a fresh day (lying day) PRO egg pH mean was (8.20 ± 0.005) and (8.89 ± 0.009) on the 28th day of storage. Fresh day (laying day) in CON egg pH mean was (8.244 ± 0.006) and (9.16 ± 0.006) on the 28th day of storage. Camila et al. (2022) also discussed that and found that Albumin pH increased gradually from the fresh day in PRO eggs (9.15 ± 0.003) and (9.42 ± 0.008) until the 28th day of storage and from the fresh day in CON eggs (9.16 ± 0.02) and (9.51 ± 0.002) until the 28th day of storage. Therefore, the significant increase in pH of albumin with the increase of the storage time. Our results were similar to the findings of (Okoleh and Eze, 2016), who concluded that the pH of albumin was higher in the eggs stored on the 7th day (0.44) and (0.80%) on the 21st day than in the fresh eggs. Other scientists stated that the pH of albumin increased from (7.6 to 9.7) due to the storage time (Gavril and Usturoi, 2012). The increase in the alkalinity of the albumin in the egg occurred during the storage of the egg, due to the water losses by the evaporation then carbon dioxide of albumin escaped from the pores of the eggshell (Vlaicu et al., 2021). Our results revealed that the 28th day saw more significance of pH in PRO and CON eggs, on the fresh day PRO eggs pH was (6.13 ± 0.006) and (6.64 ± 0.007) on the 28th day of storage. On a fresh day, CON eggs' pH was (6.17 ± 0.005) and (6.82 ± 0.01) on the 28th day of storage. Camila et al. (2022) also found that probiotics showed a gradual increase in the pH of yolk from fresh day PRO eggs (5.85 ± 0.005) and (6.52 ± 0.002) until the 28th day of storage and from fresh day CON eggs (5.89± 0.003) and (6.52 ± 0.004) until the 28th day of storage. Also, there was a significant increase in the pH of the yolk with increasing storage periods; these results were concluded by Akyurek and Okur (2009) and Kralik et al. (2014). Moreover, the Probiotic-supplemented eggs had the lowest pH value in the yolk; it may be caused by the concentration of antioxidants in the yolk that lead to peroxidation inhibition (Eke et al., 2013). ConclusionThe probiotics supplementation may decrease and reduce the effect of the storage period on the quality of shell, albumen, and yolk. Probiotic supplementations enhanced the ecosystem of the gut in laying hens and enforced a balance to many of the microbial genera and, also promoted the health of the intestine of the hen. This study showed that storage has significant effects on egg quality produced by the PRO and CON eggs. Also, eggshells showed higher microbial contamination than egg contents. Conflict of interestThe authors declare that there is no conflict of interest. FundingSelf-funding as no external funding has been provided for this study. Author contributionEsmat I. Awad: Main idea, field work, sampling laboratory analyses, statistical analyses, and manuscript writing. Salah F. Abdelaal: Field work and sampling laboratory analyses, and manuscript writing. Mohamed A. Bayoumi: Field work and sampling, laboratory analyses, and manuscript writing. Sameh M. Halawa: Field work and sampling, laboratory analyses, and manuscript writing. Data availabilityData are available from the authors upon reasonable request. ReferencesAdesiyun, A., Offiah, N., Seepersadsingh, N., Rodrigo, S. , Lashley, V., Musai, L. and Georges, K. 2005. Microbial health risk posed by table eggs in Trinidad. Epidemiol. Infect. 133, 1049–1056. Akyurek, H. and Okur, A.A. 2009. Effect of storage time, temperature and hen age on egg quality in free-range layer hens. J. Anim. Vet. Adv. 8, 1953–1958. Awny, C., Amer, A.A. and Abo El-Makarem, H.S. 2018. Microbial hazards associated with consumption of table eggs. AJVS 58, 139–146. Callaway, T.R., Edrington, T.S., Anderson, R.C., Harvey, R.B., Genovese, K.J., Kennedy, C.N., Venn, D.W. and Nisbet, D.J. 2008. Probiotics prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim. Health. Res. Rev. 9, 217–225. Camila, L.C., Ines, A., Gabriela, M.G. and Thais, B.S. 2022. Effects of dietary probiotic supplementation on egg quality during storage. Poultry 1, 180–192. Cruickshank, R., Dugunde, J.P., Marmionb, P. and Swain, S. 1978. Medical microbiology. A book of practice of medical microbiology, 12th ed. Edinburgh, London, UK: Churchill Livingston. Duggan, C., Gannon, J. and Walker, W.A. 2002. Protective nutrients and functional foods for the gastrointestinal tract. AJCN 75, 789–808. Eke, M., Olaitan, N. and Ochefu, J.H. 2013. Effect of storage conditions on the quality attributes of shell (Table) eggs. NIFOJ 31, 18–24. European Commission. 2021. Available via http://europa.eu/rapid/press-release_IP-05-1687-en.htm Feddern, V., Pra, M., Mores, R., Nicoloso, R., Coldebella, A. and Abreu, P. 2017. Egg quality assessment at different storage conditions, seasons and laying hen strains. Vet. Anim. Sci. 41, 322–333. Gavril, R. and Usturoi, M.G. 2012. Effect of storage time and temperature on hen egg quality. Lucrări Ştiinţifice - Seria Zootehnie. 57, 221–229. Gunhan, S. and Kirikçi, K. 2017. Effects of different storage time on hatching results and some egg quality characteristics of rock partridge (A. graeca) (management and production). Poul. Sci. 96, 1628–1634. Hy-Line-Products. 2021. Available via https://www.hyline.com/filesimages/Hy-Line-Products/Hy-Line-Product-PDFs/W-36/36%20COM%20ENG.pdf Jotan, T., Roy Barman, A., Sen, S. and Kanti, N. 2017. Isolation and identification of Escherichia coli and Salmonella sp from apparently healthy Turkey. Int. J. Adv. Res. Biol. Sci. 4(6), 72–78. Kasiyati, S., Sumiati, S., Ekastuti, D.R. and Manalu, W. 2016. Roles of curcumin and monochromatic light in optimizing liver function to support egg yolk biosynthesis in magelang ducks. Int. J. Poul. Sci. 15, 414–424. Khan, A., Rind, R., Shoaib, M., Kamboh, A.A., Mughal, G.A., Lakho, S.A., Malhi, K.K., Nizamani, A.R. and Yousaf, A. 2016. Isolation, identification and antibiogram of Escherichia coli from table eggs. J. Anim. Health. Prod. 4, 1–5. Kralik, Z., Kralik, G., Grčević, M. and Galović, D. 2014. Effect of storage period on the quality of table eggs acta Agraria Kaposváriensis. 18, 200–206. Lee, S. and Lee, D.K. 2018. What is the proper way to apply the multiple comparison tests? Korean. J. Anaesthesiol. 71, 353–356. Mansour, F.A., Zayed, A.F. and Basha, O.A.A. 2015. Contamination of the shell and internal content of table eggs with some pathogens during different storage periods. Assiut. Vet. Med. J. 61, 146–149. Nyholm, S.V. 2020. In the beginning: egg–microbe interactions and consequences for animal hosts. Philos. Trans. R. Soc. B Biol. Sci. 375, 90–93. Okoleh, V. and Eze, J. 2016. Effect of storage period and method on internal egg quality traits of the Nigerian native chicken. Livest. Res. Rural. Develop. 28, 6–9. Oliveira, B.L. and Oliveira, D.D. 2013. Qualidade e Tecnologia de Ovos. Lavras, Brazil, UFLA: pp: 223. Osei-Somuah, A., Otsyina, H.R., Arthur, C.T., Nortey, P.W.K. and Hammond, M. 2006. Microbial qual ity of table eggs sold on markets in Accra. Animal Research Institute, Vol. 6, pp: 18–26. Sadek, O.A., Hussein, M.F. and EL Berbawy, S. 2016. Microbiological status of farms and Baladi hens’ eggs. Assiut. Vet. Med. J. 62, 58–68. Scott, T.A. and Silversides, F.G. 2000. The effect of storage and strain of hen on egg quality. Poult. Sci. 79, 1725–1731. Sobczak, A. and Kozlowski, K. 2021. The effect of a probiotic preparation containing Bacillus subtilis ATCC PTA-6737 on egg production and physiological parameters of laying hens. Sci. Rep. 11, 207–212. Stepień-Pyśniak, D. 2010. Occurrence of Gram-negative bacteria in hens’ eggs depending on their source and storage conditions. Polish. Vet. Sci.Vol. J. 13(3), 507–513. Tang,W., Qian, Y., Yu, B., Zhang, T., Gao, J., He, J., Huang, Z., Zheng, P., Mao, X. and Luo, J. 2019. Effects of Bacillus subtilis DSM32315 supplementation and dietary crude protein level on performance, gut barrier function and microbiota profile in weaned piglets. J. Anim. Sci. 29, 2125–2133. Tomosada, Y., Villena, J., Murata, K., Chiba, E., Shimazu, T., Aso, H., Iwabuchi, N., Xiao, J., Saito, T. and Kitazawa, H. 2013. Immunoregula tory effect of bifidobacterial strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression. PLoS One 8, e59259. Vallance, B.A., Wu, X., Boyer, L., Bergstrom, K.S.B., Walker, J., Madsen, K., Kusky, J.R.O., Buchan, A.M. and Jacobson, K. 2008. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am. J. Physiol.-Gastrointest. Liver. 294, 295–306. Vlaicu, P.A., Panaite, T.D. and Turcu, R.P. 2021. Enriching laying hens eggs by feeding diets with different fatty acid composition and antioxidants. Sci. Rep. 11, 207–213. Walsh, T.J., Rizk, R.E. and Brake, J. 1995. Effects of temperature and carbon dioxide on albumen characteristics, weight loss, and early embryonic mortality of long stored hatching eggs. Poult. Sci. 74(9), 1403–1410. Wang, Y., Yan, X., Zhang, W., Liu, Y., Han, D., Teng, K. and Ma, Y. 2019. L actobacillus casei prevents jejunal epithelial damage to early-weaned piglets induced by Escherichia coli K88 via regulation of intestinal mucosal integrity, tight junction proteins and immune factor expression. J. Microbiol. Biotechnol. 28, 863–876. | ||
| How to Cite this Article |
| Pubmed Style Awad EI, Abdlaal SF, Bayoumi MA, Halawa SM. Beneficial Effect of probiotics supplementation on quality of edible fresh table eggs during storage. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 586-593. doi:10.5455/OVJ.2024.v14.i1.54 Web Style Awad EI, Abdlaal SF, Bayoumi MA, Halawa SM. Beneficial Effect of probiotics supplementation on quality of edible fresh table eggs during storage. https://www.openveterinaryjournal.com/?mno=190013 [Access: May 08, 2024]. doi:10.5455/OVJ.2024.v14.i1.54 AMA (American Medical Association) Style Awad EI, Abdlaal SF, Bayoumi MA, Halawa SM. Beneficial Effect of probiotics supplementation on quality of edible fresh table eggs during storage. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 586-593. doi:10.5455/OVJ.2024.v14.i1.54 Vancouver/ICMJE Style Awad EI, Abdlaal SF, Bayoumi MA, Halawa SM. Beneficial Effect of probiotics supplementation on quality of edible fresh table eggs during storage. Open Vet J. (2024), [cited May 08, 2024]; 14((1) (Zagazig Veterinary Conference)): 586-593. doi:10.5455/OVJ.2024.v14.i1.54 Harvard Style Awad, E. I., Abdlaal, . S. F., Bayoumi, . M. A. & Halawa, . S. M. (2024) Beneficial Effect of probiotics supplementation on quality of edible fresh table eggs during storage. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 586-593. doi:10.5455/OVJ.2024.v14.i1.54 Turabian Style Awad, Esmat I., Salah F. Abdlaal, Mohamed A. Bayoumi, and Sameh M. Halawa. 2024. Beneficial Effect of probiotics supplementation on quality of edible fresh table eggs during storage. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 586-593. doi:10.5455/OVJ.2024.v14.i1.54 Chicago Style Awad, Esmat I., Salah F. Abdlaal, Mohamed A. Bayoumi, and Sameh M. Halawa. "Beneficial Effect of probiotics supplementation on quality of edible fresh table eggs during storage." Open Veterinary Journal 14 (2024), 586-593. doi:10.5455/OVJ.2024.v14.i1.54 MLA (The Modern Language Association) Style Awad, Esmat I., Salah F. Abdlaal, Mohamed A. Bayoumi, and Sameh M. Halawa. "Beneficial Effect of probiotics supplementation on quality of edible fresh table eggs during storage." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 586-593. Print. doi:10.5455/OVJ.2024.v14.i1.54 APA (American Psychological Association) Style Awad, E. I., Abdlaal, . S. F., Bayoumi, . M. A. & Halawa, . S. M. (2024) Beneficial Effect of probiotics supplementation on quality of edible fresh table eggs during storage. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 586-593. doi:10.5455/OVJ.2024.v14.i1.54 |