| Case Report | ||
Open Vet. J.. 2022; 12(4): 511-518 Open Veterinary Journal, (2022), Vol. 12(4): 511–518 Case Report Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitisEleanor M. Kellon* and Kathleen M. GustafsonEquine Cushing’s and Insulin Resistance Group, Inc. 2307 Rural Road, Tempe, AZ 85282 USA Submitted: 02/04/2022 Accepted: 16/07/2022 Published: 07/08/2022 *Corresponding Author: Eleanor M. Kellon. Equine Cushing’s and Insulin Resistance Group, Inc, Tempe, AZ. Email: drkellon [at] gmail.com © 2022 Open Veterinary Journal
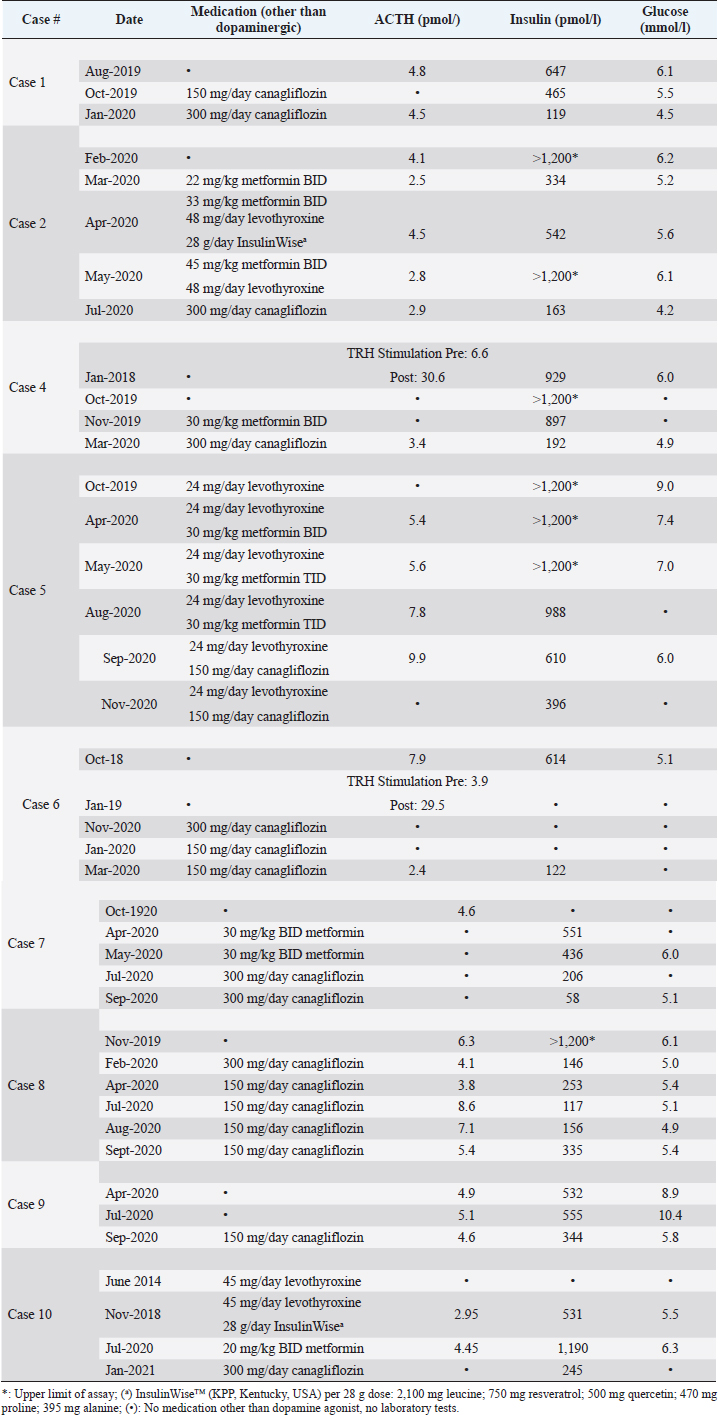
AbstractBackground: Hyperinsulinemia associated with pituitary pars intermedia dysfunction (PPID) and/or equine metabolic syndrome is well documented to put horses at high risk of laminitis. While dietary control of simple sugars and starch is the most effective therapy to control hyperinsulinemia, some horses fail to respond. Case Descriptions: Ten horses with hyperinsulinemia refractory to diet control, metformin, levothyroxine, and pergolide (if diagnosed with PPID) were treated with sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana®). Nine horses were hyperglycemic (>5.5 mmol/l) or had a history of hyperglycemia. Before instituting therapy, renal function was assessed by determining serum creatinine and blood urea nitrogen concentrations. Canagliflozin was administered orally once a day, with food. Dipstick urinalysis was performed every 2 weeks to confirm glucosuria and screen for proteinuria. Owners were also instructed regarding clinical signs consistent with urinary tract infection. All horses responded with a substantial decrease in serum insulin concentrations to normal or near normal values. Laminitis pain resolved in all cases, with regression of fat deposits. Owner satisfaction with outcomes was 100%. Conclusion: Once daily administration of the SGLT2 inhibitor canagliflozin corrected hyperglycemia, reduced insulin to normal or near normal levels, and was 100% effective in reversing or reducing abnormal fat pads and eliminating laminitis pain in horses with refractory hyperinsulinemia and laminitis. The core aspects of therapy–diet control, exercise when possible, and adequate treatment of PPID–must also be maintained if using canagliflozin. Canagliflozin should be reserved for refractory cases. Further controlled trials to investigate canagliflozin pharmacokinetics, pharmacodynamics, efficacy, and safety are needed. Keywords: Horses, Hyperinsulinemia, Laminitis, Metformin, Canagliflozin. IntroductionHyperinsulinemia associated with pituitary pars intermedia dysfunction (PPID) and/or equine metabolic syndrome (EMS) is well documented to put horses at high risk of laminitis (Patterson-Kane et al., 2018; de Laat et al., 2019). Diet control, restricting intake of simple sugar and starch, is the cornerstone of therapy for hyperinsulinemia (Johnson et al., 2004; Morgan et al., 2016; Kellon, 2017). Horses with concurrent PPID also require daily administration of a dopamine agonist (pergolide). High-dose levothyroxine may accelerate weight loss but has no effect on insulin sensitivity (Chameroy, 2010). Except for one mention of glyburide (Durham et al., 2009), the only published commercially available pharmacological option for hyperinsulinemia in horses is metformin (Rendle et al., 2013; Durham, 2017). We describe 10 chronically laminitic horses with refractory insulin that failed to respond to these standard diet and treatment modalities and were treated off-label with canagliflozin, an SGLT-2 inhibitor that blocks renal reabsorption of glucose and is approved for the treatment of type 2 human diabetes. Horses were privately owned by members of the Equine Cushing’s and Insulin Resistance group; treated and followed by owners’ private veterinarians. Owners provided their horses’ histories and laboratory data for this report. There were four Arabians, two Warmblood, one each Tennessee Walker, Quarterhorse pony cross, Spanish Mustang, Andalusian; two mares and eight geldings. Ages ranged from 8 to 30 years old. All had a history of refractory laminitis pain. Seven horses were positive for PPID. Horses were maintained on diets of predominantly grass hay with a combined simple sugar (ethanol soluble carbohydrate; ESC) and starch level of ˂10%. All PPID horses had ACTH controlled with pergolide or cabergoline. Prior treatments included levothyroxine and metformin in addition to the diet. Laboratory tests were collected under non-fasting conditions and performed by commercial laboratories. Insulin concentrations are reported as pmol/l, with a conversion factor of 6 used for laboratories reporting in uIU/ml (Knopp et al., 2019). Based on the response to a similar drug in this class (velagliflozin), canagliflozin was dosed at 0.3 to 0.6 mg/kg. The drug is available in 100 and 300 mg tablets. Renal function was assessed with serum BUN and creatinine before starting treatment. Table 1 shows details of medication regimens and laboratory results. Individual case values for insulin at baseline and post-canagliflozin therapy are shown in Figure 1. Mean grouped values for insulin and glucose (baseline, post-metformin, and post-canagliflozin) are provided in Figure 2. Further monitoring and safety considerations are described in the Discussion. Table 1. Complete medication history, ACTH, Insulin, and Glucose values for each case.
Case DetailsCase 116-year-old Warmblood gelding; weight 530 kg. The horse had a history of PPID which had been well controlled with pergolide. There were yearly laminitis events since 2014 with periods of intermittent discomfort between acute episodes. Insulin was 368 and 858 pmol/l in 2017 and 2018, respectively. Glucose ranged from <5.5 to as high as 7.3 mmol/l. Despite good diet management and therapeutic control of ACTH, there was ongoing hoof discomfort and persistently high insulin. In August 2019, insulin was 647 pmol/l and glucose was 6.1 mmol/l. Canagliflozin was instituted at 150 mg once daily. In October 2019, insulin was still over twice the upper reference range cutoff of 200 pmol/l; glucose 5.5 mmol/l. Canagliflozin was increased to 300 mg/day. In January 2020, insulin decreased to 119 pmol/l. The owner reports the horse has done well with no further issues with active laminitis or foot discomfort with 2019 being the first laminitis-free winter since 2014. Case 220-year-old Tennessee Walker mare, weight 443 kg. The horse has a diagnosis of PPID, controlled with pergolide. Hyperinsulinemia was first diagnosed in 2015 and was not adequately controlled with diet and pergolide. In February 2020, the mare had ongoing laminitis pain, prominent fat deposits on the neck and rump, and a depressed attitude. Insulin was >1,200 pmol/l (upper limit of the assay) and glucose was elevated at 6.2 mmol/l. In March 2020, metformin therapy was initiated at 22 mg/kg twice daily. Initially, insulin decreased to 334 pmol/l but 1 month later increased to 542 pmol/l. Metformin was increased to 33 mg/kg BW and titrated up to 45 mg/kg twice daily. Levothyroxine was added at 48 mg/day. A resveratrol-based supplement (Insulin Wise™j, Kentucky Performance Products, USA) was also added for 1 month, during which time the horse’s clinical condition worsened and the supplement was discontinued in May 2020. Insulin subsequently increased to ˃1200 pmol/l. Canagliflozin was started on July 1, 2020. On July 28, insulin had decreased from ˃1200 to 163.5 pmol/l; glucose normalized from 6.1 to 4.2 mmol/l. Clinically, the mare had decreased size of fat deposits, increased hoof comfort and spontaneous mobility, and an alert and interactive demeanor. She continues to do well.
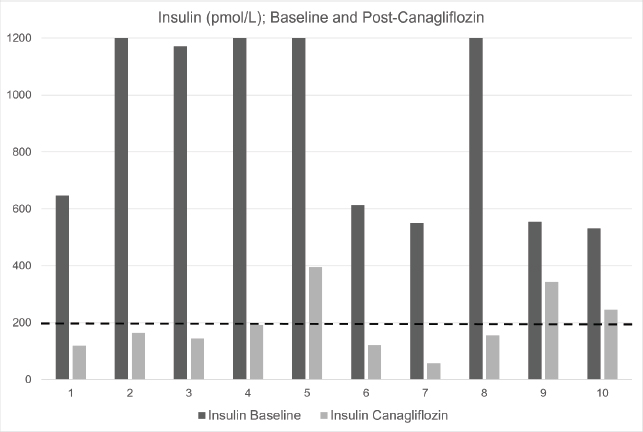
Fig. 1. Baseline and post-canagliflozin therapy insulin (pmol/l) in 10 cases of refractory hyperinsulinemia. Black bars represent baseline insulin; grey bars represent post-canagliflozin. Dashed line at 200 pmol/l represents the upper reference range. The laboratory upper limit for insulin assay is 1200 pmol/l.
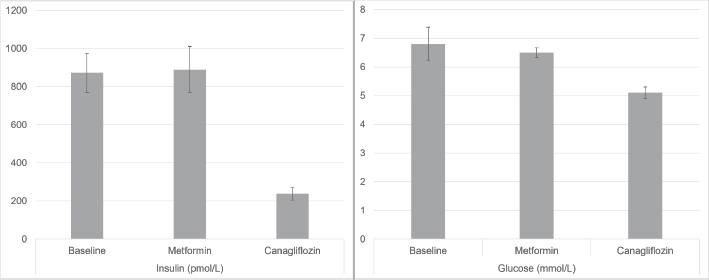
Fig. 2. Mean insulin (pmol/l) and glucose (mmol/l) at baseline, post-metformin and post-canagliflozin therapy. Error bars represent the standard error of the mean. Case 330-year-old Warmblood gelding, weight 500 kg. He was diagnosed with PPID in 2012 and confirmed hyperinsulinemic in 2015. The first episode of laminitis occurred in 2016 with ongoing mild to moderate hoof pain between episodes. PPID was initially treated with pergolide, switching to compounded injectable cabergoline (BET Pharmacy, Lexington Kentucky, USA) in April 2018, due to incomplete ACTH control with pergolide. Between 2016 and 2018, hyperglycemia up to 11.1 mmol/l was noted while insulin concentrations ranged from 900 pmol/l to over 1200 pmol/l. Metformin therapy was initiated in December of 2016 and continued to February 2017, but insulin remained very high (620 pmol/l) and lameness did not improve. In November 2018, insulin was again >1200 pmol/l. A trial of InsulinWise™ began in March 2019 through mid-April 2019 with no change in insulin. In July 2019, insulin was 928 pmol/l and glucose had increased to 10.8 mmol/l. In August 2019, canagliflozin therapy was started at 300 mg/day. In September 2019, both insulin and glucose decreased to normal (144 pmol/l and 4.4 mmol/l, respectively) for the first time in 7 years and the horse remains on that dose. Nuchal fat deposits resolved with a return to soundness and increased spontaneous movement. The horse continued to do well clinically in 2019 and 2020 with no laboratory work repeated until August 2020 at which time insulin had risen again (504 pmol/l). ACTH was elevated indicating poor control of PPID. Cabergoline dose was adjusted and 30 days later ACTH had decreased to values within seasonal reference ranges, and insulin decreased to 221 pmol/l. The horse was asymptomatic throughout this period. Case 424-year-old Arabian gelding, weight 410 kg. PPID is treated with pergolide. He had his first acute laminitis in January of 2018, second in October 2019. Following the second bout, the horse was confined to his stall and reluctant to move. Insulin at this time was ˃1200 pmol/l. Metformin was started at 30 mg/kg twice daily with minimal improvement in insulin after 30 days and no change in clinical status. Canagliflozin was started in February 2020 at 300 mg/day. Within 5 days, the horse was more alert and interactive, willing to leave the stall. Insulin decreased to 192 pmol/l in March 2020. Comfort has steadily improved. He was freely trotting and cantering at liberty and nuchal fat deposits decreasing. Case 515-year-old Quarter Horse/Shetland Pony cross gelding, weight 225 kg. The first episode of laminitis was March 2019 with recurrence in May 2020. Levothyroxine therapy was initiated in May 2019. Following a diagnosis of PPID, pergolide therapy began in October 2019. Metformin therapy began in April 2020, given twice daily, increased to three times daily in August 2020. Despite these measures and a tightly controlled diet since 2019, he became laminitic again in May 2020 with refractory pain, isolating himself, very reluctant to move and constantly needing hoof boots and pads through August 2020. From October 2019 through August 2020, glucose was elevated and insulin ˃1,200 pmol/l on three occasions. Metformin was stopped and canagliflozin started early September 2020 at 150 mg/day. Within 24 hours, he was more alert, socializing with herd mates, and showed more spontaneous movement. After 8 days of treatment, he was out of boots and galloping freely in the pasture. At 28 days after starting canagliflozin glucose had normalized, insulin reduced but still abnormal at 610 pmol/l. Two months later, insulin was further reduced to 397 pmol/l. The horse continues to be energetic and sound. Case 616-year-old Spanish Mustang gelding, weight 340 kg. History of easy weight gain earlier in life. First known episode of laminitis in October of 2017, with repeat severe episode in September 2018 and ongoing pain thereafter. In October 2018, insulin was 614 pmol/l and glucose 5.1 mmol/l. He was started on pergolide after a positive TRH Stimulation Test in January 2019 but continued to have bouts of laminitis. Metformin was tried but the horse refused to eat it, resisted attempts at administration by dose syringe and the owner had difficulty providing twice daily dosing. Canagliflozin therapy began November 2020 at 300 mg/day, decreased to 150 mg/day in January 2020 due to the horse’s lightweight and remains on that dose. Insulin decreased from 614 to 122 pmol/l. Within 1 week of starting canagliflozin, his attitude brightened, he become sound and spontaneous movement increased. He has remained sound. His ectopic fat deposits resolved. Case 78-year-old Andalusian mare, weight 445 kg. Diagnosed with laminitis and elevated insulin at age 5. Relatively stable on a low sugar/starch diet except for exaggerated pain with estrus cycles and intermittent lameness, until winter of 2019 when she developed constant hoof pain and dramatic increase in neck crest and fat deposits. Metformin was started in mid-March 2020 but failed to control clinical signs or insulin (April 551 pmol/l, May 436 pmol/l). Metformin was stopped mid-May and canagliflozin started at the end of May 2020 at 300 mg/day. Soundness and attitude rapidly improved to pre-2017 levels over the first 3 weeks. In July 2020, insulin had decreased to 206 and to 58 pmol/l in September. As of December 2020, she was sound at all gaits and ready to resume work under saddle. Glucose decreased from 6.0 to 5.1 mmol/l. Neck crest and other fat deposits resolved. Case 816-year-old Arabian gelding, weight 400 kg. He was diagnosed with PPID and EMS following a laminitis episode in July of 2017 at age 13 years. Glucose was 7.2 pmol/l at time of diagnosis. ACTH has been well controlled with pergolide since diagnosis, but from 2017 through 2019 insulin concentrations remained elevated between 600 and ˃1200 pmol/l, with lethargy, cresty neck, and other abnormal fat deposits. There were two recurrent bouts of laminitis in 2019 with residual lameness and difficulty holding feet up for hoof trims. The owner declined a trial of metformin and opted for canagliflozin which was started in January of 2020 at 300 mg/day. Insulin decreased to within reference range (146 pmol/l) on retest in February 2020. Canagliflozin was decreased to 150 mg/day for financial reasons. There has been no recurrent laminitis although mobility remained restricted secondary to hoof deformity. Insulin concentrations have remained normal on 150 mg canagliflozin except for one episode in September 2020 when the owner attempted to liberalize the diet. Case 919-year-old Arabian gelding, weight 340 kg. PPID was diagnosed in 2017 and controlled with pergolide. He developed acute laminitis in March 2020. In April, he had normal ACTH concentration but insulin was 532 pmol/l and glucose 8.9 mmol/l. Strict diet control was instituted; however, in late July 2020, there was essentially no change in insulin (555 pmol/l) with an increase in glucose (10.4 mmol/l). Lameness continued. Canagliflozin was started at 300 mg/day on September 1, reduced to 150 mg/day by the owner on September 12 for financial reasons. On September 23, he had normal glucose at 5.8 mmol/l, and insulin was reduced to 344 pmol/l. The horse was alert, active, and sound. Case 1021-year-old Arabian gelding, weight 500 kg. Diagnosed EMS at age 14 with elevated leptin and insulin 265 pmol/l. Levothyroxine was started in June 2014 and discontinued in June 2020 when T4 was elevated. First laminitis episode was in November 2018 with insulin of 531 pmol/l and glucose 5.5 mmol/l. He made a full recovery. The second laminitis episode was in July 2019 with repeated bouts of left front abscessing until November 2019. The horse was on three courses of metformin and InsulinWise™ from November 2018 to July 2020 with no change in clinical signs: insulin 1,190 pmol/l and glucose 6.3 mmol/l in December 2020. He had hoof soreness alternating with repeated severe bouts of hoof pain. He was started on canagliflozin in early January 2021 at 300 mg/day. After 6 days of canagliflozin, the owner reported hooves were no longer warm. On day 7, the neck crest was greatly reduced and lameness improved. On day 8, the horse was walking normally, bucking on a lunge line and insulin concentration had decreased to 245.4 pmol/l. DiscussionHyperinsulinemia (HI) with EMS or as a complication of PPID is the leading cause of laminitis (Patterson-Kane et al ., 2018; de Laat et al., 2010). Many horses are laminitic at the time of diagnosis. In addition to treatment of PPID and appropriate hoof care, the first line therapy for HI-associated laminitis is controlled diet with combined ESC (simple sugars) and starch content of no higher than 10%, Exercise is an effective tool (Menzies-Gow, 2010) but not an option with laminitis pain. Horses that fail to improve with these measures have limited options. High dose levothyroxine supplementation has been suggested but does not influence insulin sensitivity (Chameroy, 2010). Despite conflicting early reports, metformin has demonstrated efficacy in lowering insulin in horses, but the effect tends to decrease over time (Durham et al., 2008) and some horses fail to respond. It also requires twice daily administration and medication refusals often necessitate it be given by oral syringe. SGLT2 inhibitors are a class of drugs which block renal glucose reabsorption. Two studies have shown the SGLT2 inhibitor velagliflozin reduced glucose and insulin responses to a high simple carbohydrate diet, and prevented laminitis (Meier et al., 2018; Meier et al., 2019), with indications that the drug was safe and effective over a 16-week period (Meier, 2019). Insulin concentrations were not normalized but were decreased below the threshold for acute laminitis. Because velagliflozin is not currently available, the flagship SGLT2 inhibitor, canagliflozin, was used in these cases. Since it is less expensive and has a long history of use, a trial of metformin was suggested before trying canagliflozin but one owner declined and three were either unable to do the twice daily dosing, or their horses refused the drug or worsened significantly before the end of the metformin trial. All owners maintained strict diet control and pergolide if warranted for a PPID diagnosis. Estimated effective dosage was 0.3 to 0.6 mg/kg so 150 to 300 mg for a 500 kg horse. The drug is only available in 100 and 300 mg tablets. The cost of these tablets is very similar. We saw more rapid improvements in insulin levels and hoof comfort at the higher end of the dosage scale and it was recommended that animals which were close to but under the 500 kg mark should at least start with 300 mg/day. Canagliflozin is very expensive and because of the unknown side effect profile in horses, it was only considered in advanced cases that failed to respond to all other measures. It is known that the elevated insulin drives laminitis but in longstanding cases, there may be complicating problems with improper hoof care or issues with unresolved abscesses/collections causing pain. Relief of laminitis pain aligns best with drops in insulin levels but those hoof issues can complicate that response. However, the universal level of owner satisfaction in response to treatment indicates this is an approach worth pursuing for refractory cases. We saw no differences in response by age, sex, breed, duration of laminitis, severity of pain, or duration of hyperinsulinemia. As might be expected from the ages of the horses and duration of their problems, in addition to the severe hyperinsulinemia and refractory laminitis pain, 8/10 cases had documented PPID with an additional horse in the high-risk age group and 7/10 had at least one documented episode of hyperglycemia, attesting to the severity of their insulin resistance. Absence of side effects in the velagliflozin trials was encouraging but several possible concerns have been reported with human use of SGLT2 inhibitors. The drug induces glucosuria and a resultant degree of polyuria. The potential for volume depletion is likely an important factor in the increased risk of acute renal failure with this class of drug, particularly in individuals with pre-existing compromised renal function (Perlman et al., 2017). Because of this, and the increased risk with concurrent use of NSAIDs (Szalat, 2018) which are often heavily prescribed in laminitis cases, horses were screened for renal function with serum BUN and creatinine concentrations before beginning therapy and NSAID use during therapy was discouraged. Acetaminophen should probably also be avoided since both drugs are metabolized by the hepatic UGT enzyme system. Full serum chemistry at 4 to 6 weeks after the institution of canagliflozin was recommended. In addition, the provision of 30 to 60 g/day of sodium chloride with constant access to water was suggested to encourage good fluid intake. A significant risk of mycotic genital infections and strong trend for increased risk of urinary tract infections has been demonstrated in humans (Lega et al., 2019). Owners were instructed in signs of these disorders and free catch urine samples for dipstick analysis at 2-week interval were encouraged for monitoring of proteinuria and degree of glucosuria. All tests remained normal with the exception of glucosuria readings as high as the upper detection limit (>2,000 mg/dl). Hypoglycemia is a potential concern but was not identified in the velagliflozin studies or in these horses, even in animals that were normoglycemia prior to therapy. Rarely reported adverse events in humans included Fournier gangrene or need for extremity amputation but these occur in people with a pre-existing diabetic microvascular disease which does not occur in horses and a recent human meta-analysis showed there is no increased risk with SGLT2 inhibitors (Ryan et al., 2018). In conclusion, once daily administration of the SGLT2 inhibitor canagliflozin corrected hyperglycemia, reduced insulin to normal or near normal levels, and was 100% effective in reversing or reducing abnormal fat pads and eliminating laminitis pain in horses with refractory hyperinsulinemia and laminitis. There were no drug refusals. The expected polyuria was noted, which was mild. Despite its effectiveness, increases in insulin were observed when concomitant PPID was not controlled or diet was liberalized. It is recommended that the core aspects of therapy–diet control, exercise when possible and adequate treatment of PPID– must also be maintained if using canagliflozin. Furthermore, since these modalities are often successful themselves, canagliflozin should be reserved for refractory cases. Further controlled trials to investigate canagliflozin pharmacokinetics, pharmacodynamics, efficacy, safety, and long-term use in horses are needed. AcknowledgmentThe authors recognize the effort and commitment of the horse owners and attending veterinarians who provided the case histories for this report. The authors also acknowledge the all-volunteer members of the nonprofit corporation, Equine Cushing’s and Insulin Resistance Group, Inc. whose mission is to improve the welfare of equines with metabolic disorders in order to prevent laminitis. Conflict of interestThe authors declare that there is no conflict of interest. Author’s contributionsDr. Kellon supervised the data collection, communication with horse owners and their veterinarians and wrote the manuscript. Dr. Gustafson managed the database, generated the graphs and figures, and contributed to the writing of the manuscript. ReferencesChameroy, K.A. Diagnosis and management of horses with equine metabolic syndrome (EMS). In Ph.D. thesis, 2010 University of Tennessee, Knoxville, TN. de Laat, M.A., McGowan, C.M., Sillence, M.N. and Pollitt, C.C. 2010. Hyperinsulinemic laminitis. Vet. Clin. North. Am. Equine Pract. 26(2), 257–264. Durham, A.E. 2017. Therapeutics for equine endocrine disorders. Vet. Clin. North. Am. Equine Pract. 33(1), 127–139. Durham, A.E., Hughes, K.J., Cottle, H.J., Rendle, D.I. and Boston, R.C. 2009. Type 2 diabetes mellitus with pancreatic beta cell dysfunction in 3 horses confirmed with minimal model analysis. Equine. Vet. J. 41(9), 924–929. Durham, A.E., Rendle, D.I. and Newton, J.E. 2008. The effect of metformin on measurements of insulin sensitivity and beta cell response in 18 horses and ponies with insulin resistance. Equine. Vet. J. 40(5), 493–500. Johnson, P.J., Messer, N.T. and Kellon, E. 2004. Treatment of equine metabolic syndrome. Compend. Cont. Educ. Pract. Vet. 26(2), 122–130. Kellon, E.M. Tiered management approach to EMS and PPID. In Proceedings of the 2017 NO Laminitis Conference, 2017, Tucson, AZ. Knopp, J.L., Holder-Pearson, L. and Chase, J.G. 2019. Insulin units and conversion factors: a story of truth, boots, and faster half-truths. J. Diabetes. Sci. Technol. 13(3), 597–600. Lega, I.C., Bronskill, S.E., Campitelli, M.A., Guan, J., Stall, N.M., Lam, K., McCarthy, L.M., Gruneir, A. and Rochon, P.A. 2019. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: A population-based study of older women and men with diabetes. Diabetes. Obes. Metab. 21(11), 2394–2404. Meier, A., Reiche, D., de Laat, M., Pollitt, C., Walsh, D. and Sillence, M. 2018. The sodium-glucose co-transporter 2 inhibitor velagliflozin reduces hyperinsulinemia and prevents laminitis in insulin-dysregulated ponies. PLoS One 13(9), e0203655. Meier, A., de Laat, M., Reiche, D., Fitzgerald, D. and Sillence, M. 2019. The efficacy and safety of velagliflozin over 16 weeks as a treatment for insulin dysregulation in ponies. BMC. Vet. Res. 15(1), 65. Menzies-Gow, N.J. 2010. Endocrinopathic laminitis: reducing the risk through diet and exercise. Vet. Clin. North. Am. Equine Pract. 26(2), 371–378. Morgan, R.A., Keen, J.A. and McGowan, C.M. 2016. Treatment of equine metabolic syndrome: a clinical case series. Equine. Vet. J. 48(4), 422–426. Patterson-Kane, J.C., Karikoski, N.P. and McGowan, C.M. 2018. Paradigm shifts in understanding equine laminitis. Vet. J. 231, 33–40. Perlman, A., Heyman, S.N., Matok, I., Stokar, J., Muszkat, M. and Szalat, A. 2017. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: analysis of the FDA adverse event report system database. Nutr. Metab. Cardiovasc. Dis. 27(12), 1108–1113. Rendle, D.I., Rutledge, F., Hughes, K.J., Heller, J. and Durham, A.E. 2013. Effects of metformin hydrochloride on blood glucose and insulin responses to oral dextrose in horses. Equine. Vet. J. 45(6), 751–754. Ryan, P.B., Buse, J.B., Schuemie, M.J., DeFalco, F., Yuan, Z., Stang, P.E., Berlin, J.A. and Rosenthal, N. 2018. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: A real-world meta-analysis of 4 observational databases (OBSERVE-4D). Diabetes. Obes. Metab. 20(11), 2585–2597. Szalat, A., Perlman, A., Muszkat, M., Khamaisi, M., Abassi, Z. and Heyman, S.N. 2018. Can SGLT2 inhibitors cause acute renal failure? plausible role for altered glomerular hemodynamics and medullary hypoxia. Drug. Saf. 41(3), 239–252. | ||
| How to Cite this Article |
| Pubmed Style Kellon EM, Gustafson KM. Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis. Open Vet. J.. 2022; 12(4): 511-518. doi:10.5455/OVJ.2022.v12.i4.14 Web Style Kellon EM, Gustafson KM. Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis. https://www.openveterinaryjournal.com/?mno=103770 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i4.14 AMA (American Medical Association) Style Kellon EM, Gustafson KM. Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis. Open Vet. J.. 2022; 12(4): 511-518. doi:10.5455/OVJ.2022.v12.i4.14 Vancouver/ICMJE Style Kellon EM, Gustafson KM. Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis. Open Vet. J.. (2022), [cited January 25, 2026]; 12(4): 511-518. doi:10.5455/OVJ.2022.v12.i4.14 Harvard Style Kellon, E. M. & Gustafson, . K. M. (2022) Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis. Open Vet. J., 12 (4), 511-518. doi:10.5455/OVJ.2022.v12.i4.14 Turabian Style Kellon, Eleanor Marie, and Kathleen M Gustafson. 2022. Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis. Open Veterinary Journal, 12 (4), 511-518. doi:10.5455/OVJ.2022.v12.i4.14 Chicago Style Kellon, Eleanor Marie, and Kathleen M Gustafson. "Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis." Open Veterinary Journal 12 (2022), 511-518. doi:10.5455/OVJ.2022.v12.i4.14 MLA (The Modern Language Association) Style Kellon, Eleanor Marie, and Kathleen M Gustafson. "Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis." Open Veterinary Journal 12.4 (2022), 511-518. Print. doi:10.5455/OVJ.2022.v12.i4.14 APA (American Psychological Association) Style Kellon, E. M. & Gustafson, . K. M. (2022) Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis. Open Veterinary Journal, 12 (4), 511-518. doi:10.5455/OVJ.2022.v12.i4.14 |