| Original Article | ||
Open Vet. J.. 2023; 13(2): 179-187 Open Veterinary Journal, (2023), Vol. 13(2): 179–187 Original Research A meta-analysis of the mastitis vaccination efficacy in dairy cattleFernando Mata1*, Meirielly S. Jesus1, Ricardo P. Pinto1,2 and Andreia Mata31CISAS—Centre for Research and Development in Agri-food Systems and Sustainability, Instituto Politécnico de Viana do Castelo, Viana do Castelo, Portugal 2ESTG—Escola Superior de Tecnologia e Gestão, Instituto Politécnico de Viana do Castelo, Viana do Castelo, Portugal 3Department of Biosciences, Faculty of Science, Durham University, Durham, UK *Corresponding Author: Fernando Mata. CISAS–Centre for Research and Development in Agri-Food Systems and Sustainability, Instituto Politécnico de Viana do Castelo, Viana do Castelo, Portugal. Email: fernandomata [at] ipvc.pt Submitted: 05/09/2022 Accepted: 13/01/2023 Published: 10/02/2023 © 2023 Open Veterinary Journal
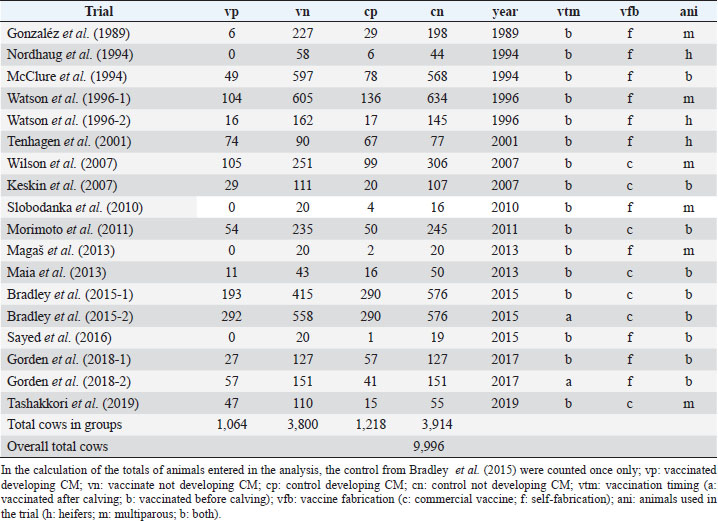
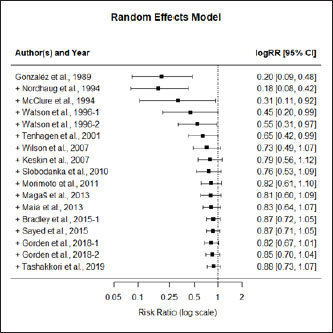
AbstractBackground: Mastitis is the most important disease in dairy cattle with impact in welfare of animals and the economy of the dairy farming activity. Attempts have been made to produce vaccines to prevent the disease, however, results have been dubious. Aim: The aim of this study was to evaluate the efficacy of the mastitis vaccination in dairy cattle by merging several trials to allow evidence synthesis. Methods: A list of publications with common methodological grounds was selected to allow a quantitative comparison in a meta-analysis with moderators. Results: A mixed methods model (p < 0.001), with four significant moderators was successfully fitted: “year of publication” (p < 0.001), “vaccination timing” (p < 0.01), “type of animal” (p < 0.001), and “vaccine fabrication” (p < 0.001). The model is homogeneous (p > 0.05), with the moderators explaining the variability. Efficacy decreases over time. Vaccines applied after calving show inefficacy [log risk ratio (RR) 1.72 (1.34, 2.21)], and applied before calving show reduced efficacy [log RR 0.86 (0.72, 1.03)]. Commercial vaccines don’t show efficacy [log RR 1.07 (0.94, 1.22)]. Self-fabricated vaccines show efficacy [log RR (0.51, 0.94)]. Conclusion: Full clarification of efficacy is not shown; however, if vaccination is used, must follow a pre-calving protocol. While not showing efficacy, the vaccination has demonstrated a reduction of the severity in clinical cases, rate of culling, and has increased the production of milk and milk solids. Vaccination may improve health and welfare but does not prevent the disease effectively; it must be seen as an additional tool to the traditional preventive measures. Keywords: Dairy cow welfare, Mastitis, Meta-analysis, Vaccination. IntroductionMastitis has impacts on the behavior and welfare of the dairy cow. There are behavioral responses to the pain and discomfort caused by the disease, including changes in activity, gait, feeding, and grooming (Petersson-Wolfe et al., 2018). Mastitis has a negative emotional impact on the cow. As a result of the disease, they can become less active, vigorous, happy, and bright (des Roches et al., 2018). It is the most relevant cause of concern in dairy cattle welfare (Silva et al., 2021) and it is the first cause of economic losses due to disease in dairy farming (Rainard et al., 2021). The costs include losses in milk production and quality, replacement heifers due to early culling, diagnostic, and treatment (Hogeveen et al., 2019). Mastitis develops due to the bacteria orchestrating an infection in one or more quarters of the udder. Afterwards, the immune system of the cow reacts, and the somatic cells count (SCC) within the milk increases. When this count reaches 200,000 ml−1, it is considered, that the animal has developed clinical mastitis (CM) (International Dairy Federation, 1997). Below these values, the cow has sub-clinical mastitis (SCM), which means that the disease is developing but does not show clinical signs (Geary et al., 2012). Distinct types of bacteria are related with the etiology of the disease presented, and these can be classified into two groups, the contagious and the environmental. The contagious are, between others, the Staphylococcus aureus, and the Streptococcus agalactiae; and the environmental are the coliforms Escherichia coli and Klebsiella spp., between others (Ruegg, 2017). More commonly the cow infects with contagious agents, with the prevalence of S. aureus ranging between 7% and 40%. The infection with coliforms has lower prevalence (Tashakkori et al., 2019). In the 1980s, research on the vaccination against mastitis was conducted to combat environmental pathogens. It was based on the J5 E. coli mutant, but the RE-17 mutant was also used (Wilson and González, 2003). Examples of these commercial vaccines include Pfizer Animal Health’s Upjohn J-5 Bacterin®, Bayer’s Mastiguard™, Biogenesis-Bago’s Rotatec® - J5, Merial’s Imocolibov® and J-Vac®, IMMVAC’s Endovac-Dairy® and Novartis’ Animal Health J-5 Shield™. Furthermore, the vaccination against S. aureus was developed in the early 1990s (Michie, 2002). Denis et al. (2009) tested experimental DNA recombinant protein and recombinant protein-only vaccines. More recently, Boehringer Ingelheim’s Lysigin® Somato-Staph® also became available in the market. HIPRA’s Startvac® and Mastivac®, are commercially available vaccines. They fight both E. coli and S. aureus infections through inactivated E. coli J5 and S. aureus (CP8) SP 140 strains. Difficulties with the development of a mastitis vaccination have been identified and discussed by other authors (Mellenberger, 1977; Pyörälä, 2002; Rainard et al., 2021). Among the difficulties identified, many of the issues are linked to a large number of different organisms and strains that engage in the pathology. This also includes the remaining pathogens in the duct and alveoli as subclinical infections, which are not attacked by the humoral antibodies. Several vaccine trials have been implemented but doubts regarding efficacy are an issue (Leitner et al., 2003). To date, the results presented from different trials have been contradictory, and different trials favor (Gorden et al., 2018) or not (Tashakkori et al., 2019) the vaccination. This meta-analysis aims to contribute to the shedding of light on the efficacy of mastitis vaccination by using as many trials as possible merged into a single overall study. Providing, therefore, conditions for evidence synthesis to stand out in favor of the dairy cows’ welfare. Materials and MethodsFor this meta-analysis, the methodologies recommended within the Cochrane Handbook for Systematic Reviews of Interventions, have been followed (Higgin et al., 2019). Literature searching strategyThe library search engine b-on Biblioteca do Conhecimento Online, b-on.pt, was initially used. The following Booleans and keywords were used: (vaccination trial OR vaccine trial) AND (mastitis) AND (cattle OR cow OR heifer). A total 1,669 hits were retrieved in 30 of September 2021. Then academic peer reviewed journals only, were selected, refining the search to 1,513 hits. After, relevant data bases were selected (Complementary Index, Academic Search Complete, Gale in Context-Science, Medline, Science Citation Index, Scopus, Directory of Open Access Journals, Science Direct, Supplemental Index, and Scielo) refining to 1,507 hits. Finally, by unselecting the topics sheep and antibiotic, 959 hits were refined. From this point, a manual search of relevant papers, systematic reviews, meta-analyses, and clinical trials was conducted. After searching for any relevant clinical trial in the reference lists of the systematic reviews and meta-analysis, the inclusion and exclusion criteria were used to reach the final list of studies included in this meta-analysis. Despite the search in the English language, we have included a study (Maia et al., 2013) published in Portuguese, with abstract in English. Inclusion and exclusion criteriaOnly independent studies, without conflict of interests reported, were included. Only case-control clinical trials have been used where, at least, two groups of animals are present in the trial. One treated (vaccinated) group and a control group, with or without a placebo administered. The presence of CM is defined as the outcome observation. Studies reporting SCC without a clear differentiation between CM and SCM were excluded. Results needed to include counts of cases with CM, as well as cases without CM, in each of the trial groups. Sometimes it was possible to deduce these counts (e.g., if the number of animals in the two groups of the trial together with the positive cases is reported, we can deduce the negative cases even if not reported directly). Therefore, only studies reporting data to allow the construction of a 2 × 2 contingency table to deduce the risk ratio (RR) were considered. Several studies report their results in terms of udder quarts infected with CM. When no other information was provided, which would not allow the deduction of the number of animals infected, the studies were excluded. Only trials, where the animals in the analysis groups were used under normal farming conditions, were considered. Therefore, studies including the artificial challenging of animals with pathogens were excluded. The minimum duration of the trials considered was 3 months. No maximum was considered. Therefore, different trial durations were considered in this meta-analysis. However, the duration of the trial was tested as a covariate moderator. Data extractionAfter the selection of the papers to include in this meta-analysis, the following data were extracted to construct a contingency table for each trial: Number of animals vaccinated with CM; Number of animals vaccinated without CM; Number of control animals with CM; Number of control animals without CM. Additional data were also extracted to create variables to be used as moderators. Variables used as covariates: Year of publication, not necessarily the year when the trial was conducted; Trial duration (in months); Absolute latitude of the place where the trial took place. Variables used as factors (levels within brackets): Type of trial (“randomized control trial” or “controlled clinical trial”); Control group (“unvaccinated” or “sham vaccine”); Vaccination timing (“before calving” or “after calving”), where we have considered the date of the first shot and sometimes boosts occurred after calving; Vaccine type (“environmental,” “contagious” or “both”); Vaccine fabrication (“commercial vaccine” or “self-fabrication”); Animals used in the trial (“multiparous,” “heifers” or “both together”). Some papers, while meeting the prerequisites for inclusion, required additional attention: Watson et al. (1996) reported two trials, one using heifers only and a second one using multiparous cows. As parity was used as one of the moderators, this trial was entered as two different ones [Watson et al. (1996-1), with multiparous cows and Watson et al. (1996-2), with heifers only]; Bradley et al. (2015) used a commercial vaccine. A trial was designed using three separate groups of animals, the control and two vaccination regimens (label and rolling). Animals in the label regimen were vaccinated as per commercial vaccine producer recommendation before calving. The second group of animals followed a different regimen, resulting in vaccination schemes varying between animals within the group, before and after calving, ignoring the vaccine producer’s recommendation. All three groups in the trial were allocated an identical number of animals. In this meta-analysis, only balanced studies were used, and therefore it would not make sense to include a group of vaccinated animals, doubling the number of the non-vaccinated. Furthermore, one of the moderators is exactly the “vaccination timing” and the inclusion of the two vaccinated groups in the meta-analysis as one would not be correct. We have taken the decision of including, in the first model, the control and the vaccinated as per label group only (Bradley et al., 2015-1). Later, as the moderator “vaccination timing” was found significant (p < 0.01), we have also included the same control group and the rolling vaccination group (Bradley et al., 2015-2) in a marginal analysis with after calving vaccinations only. Gorden et al. (2018), using a self-fabricated vaccine, designed a protocol where approximately half of the vaccinated group was vaccinated before calving and the other half after calving. Again, as the moderator “vaccination timing” was being analyzed two study groups have been created: Gorden et al. (2018-1), vaccinated before calving, and Gorden et al. (2018-2), vaccinated after calving. Assessment of qualityFollowing the Cochrane Collaboration definitions for randomized controlled trials (RCT) and controlled clinical trials (CCT), the selected studies were divided for assessment of quality. For RCT the risk of bias 2 Cochrane Collaboration’s tool (Stern et al., 2019) was used. For CCT the Cochrane Collaboration’s tool ROBIN-I (Stern et al., 2016) was used. Only the trials passing these quality assessment protocols were included in this meta-analysis. In the end, trials entering this study were identified after passing both the quality and the inclusion/exclusion criteria. StatisticsThe outcome measure considered for this meta-analysis was the logarithm of the RR, also known as relative risk. A mixed effects model was initially tested and adjusted, using the factors and covariates already identified as moderators. A backwards stepwise procedure to eliminate non-significant moderators was implemented. The residual heterogeneity (τ2) was estimated with the maximum-likelihood estimator. The percentage of the total variability in the models due to heterogeneity was estimated with the I2 statistic. The H2 statistic was used to estimate the ratio of the total amount of variability in the observed outcomes to the amount of sampling variability. The heterogeneity of the model and the moderators were tested with Cochran’s Q-test. The parameters of the models were estimated via weighted least squares and tested via Wald-type tests and confidence intervals (CI). The fitness of the model was evaluated through the deviance and the Akaike information criterion (AIC). The publication bias was evaluated via funnel plot and tested with the regression test, with the sample size of the studies used as predictors to add for the moderators’ effects. The assumption of the residuals’ distribution normality was evaluated via normal Q-Q plot. The best linear unbiased predictors of the true outcomes, combining the fitted values and the estimated contributions of the random effects (Robinson, 1991), were also calculated and added to the forest plot of the main model. After the identification of the significant moderators, they were analyzed individually as suggested by Deeks et al. (2019). All level interactions were unsuccessfully tested (p > 0.05) and therefore did not enter into further analyses. The covariate “year of publication” was subject to a meta-regression, and a cumulative meta-analysis was also performed to observe the evolution of the RR over time. The factors were analyzed in separate groups accordingly to the levels of each factor. Random effects models and the maximum likelihood estimator were used. The statistical analysis was performed using the freeware R CRAN for Windows® version 4.0.4 platform x86_64-w64-mingw32/x64 (64-bit) (Comprehensive R Archive Network, http://cran.r-project.org/). The specific meta-analysis package “metaphor” (Viechtbauer, 2010) was used. Ethical approvalNot needed for this study. ResultsThe data set analysis is presented in Table 1, indicating the Trials selected for this meta-analysis and the moderators considered. The adjusted modelThe adjusted mixed effects model was fitted without intercept and has the parameters identified in Table 2. The forest plot in Fig. 1 illustrates the model. Due to the significance found in four of the moderators used to fit the model, it does not make sense to consider an overall RR. We instead analyzed these moderators individually to produce marginal RRs. The full model has deviance 18.21 and AIC 20.02. Table 1. Trials and data entered in the meta-analysis.
Table 2. Parameters of the adjusted mixed effects model.
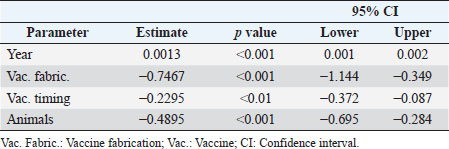
Moderators and source of heterogeneityWhile considering all the trials (excluding however and for the reasons explained), (Bradley et al., 2015-2) the best fit was reached with a mixed effects model, containing four significant moderators explaining all the variability (Q=27.99; p < 0.001). These moderators are the covariate “year of publication” (p < 0.001), and the factors “vaccine fabrication” (p < 0.001), “vaccination timing” (p < 0.001), and “animals used in the trial” (p=0.02). The model does not show heterogeneity (Q=18.21; p=0.15), explaining therefore all the variability (τ2=0.0000, SE=0.0065; I2=0.00%; H2=1.00). While conducting this meta-analysis it was difficult to find common grounds to include some studies. To be able to compare studies, the same type of procedures and outcome variables needed to be considered. Due to the different methodologies and reporting techniques used by different authors, limitations for the inclusion of several studies were identified, as previously indicated. The relatively small number of trials included in this meta-analysis is a limitation factor. No interactions were produced due to the lack of studies, replicating the combinations between the levels of the several factors. In the group analysis, each of the significant moderators were analyzed individually and, therefore, no other moderators are considered once no interactions are present.
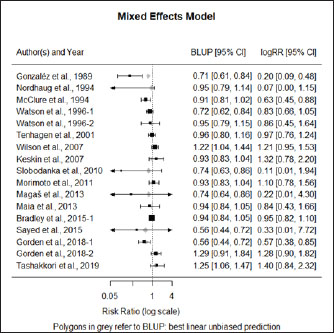
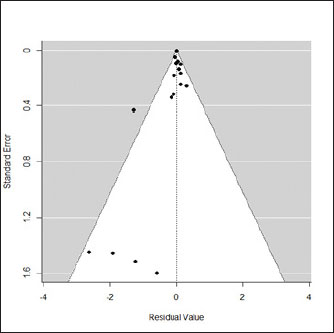
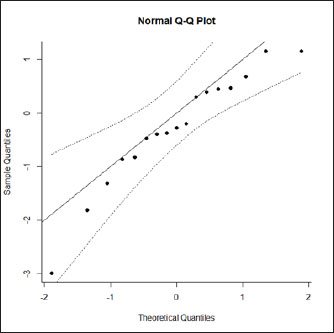
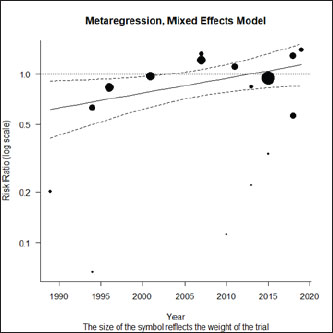
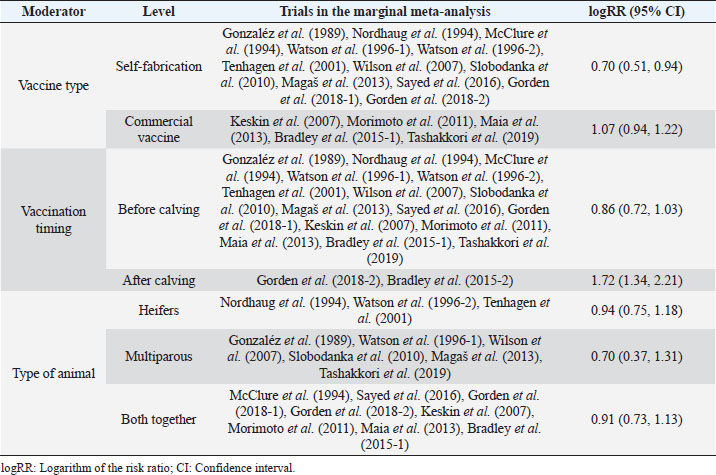
Fig. 1. Forest plot of the adjusted model. Publication bias, normal distribution of residuals, and sensitivity analysisThe regression test applied to the adjusted model shows non-significant publication bias (Z=0.316, p=0.75), which is confirmed by the graphical analysis of the funnel plot (Fig. 2). The model also shows to be smooth, with the simulated pseudo residuals falling within the pseudo confidence envelop of the normal Q-Q plot shown on Fig. 3. Analysis of the covariate “year of publication”The vaccination efficacy reported by the trials used in this meta-analysis has been decreasing over time. The meta-regression performed to the covariate “year of publication” illustrates this fact (Fig. 4). This can also be illustrated by the cumulative meta-analysis (Fig. 5) performed, using a random effects model. As we add new trials over time, the vaccine efficacy decreases. Despite overall values of the logRR below one, and therefore favoring the vaccine efficacy, we can also observe that shorter CIs, due to the increase of the sample size, still include the value “one” where the limit for lack of vaccine efficacy seats. Analysis of the factors used as moderatorsTable 3 summarizes the results of the application of random effects models to the different levels of the factor moderators used in this study. Within the factor “vaccine fabrication” the levels “self-fabrication” and “commercial vaccine” were considered. This factor was included with significance (p < 0.001) in the main model. The “self-fabrication” subgroup has shown some vaccination efficacy, which is not the case with “commercial vaccine.”
Fig. 2. Funnel plot of the adjusted model.
Fig. 3. Normal Q-Q plot of the adjusted model. Within the factor “vaccination timing” the levels “before calving” and “after calving” were considered. This factor was included with significance (p < 0.001) in the main model. The vaccination before calving shows some vaccine efficacy, with the CI slightly intercepting logRR=1, while the opposite is observed with vaccination after calving, where clearly there is lack of vaccine efficacy. Please note that for the subgroup “after calving” we have included Bradley et al. (2015-2), not included in the main model for the reasons explained before. Anyway, while not considering this study the result is similar. The difference being the 95% CI interception of logRR=1.
Fig. 4. Meta-regression using the covariate “year of publication.”
Fig. 5. Cumulative meta-analysis. Within the factor “animals used in the trial” we have considered the levels “heifers,” “multiparous” and “both together.” This factor was included with significance (p=0.002) in the main model. The vaccination efficacy cannot be clearly differentiated within these three groups. This fact is a good illustration of the limitation identified before, regarding lack of interactions, which could eventually explain the results obtained in the main model. DiscussionTo produce this meta-analysis, a list of high-quality publications with common methodological grounds was selected to allow a quantitative comparison. While capturing the data, we have identified a set of variables to be used as moderators to explain any eventual heterogeneity found in the chosen model. We have adjusted a mixed methods model with four significant moderators that will now be discussed: the covariate “year of publication,” and the factors (with levels) “vaccination timing” (“before calving” and “after calving”), “type of animal” (“heifers,” “multiparous” and “both together”) and “vaccine fabrication” (“commercial vaccine” or “self-fabrication”). The covariate “Year”The vaccine efficacy shows a decreasing efficacy over time. As stated by Bradley et al. (2015), the pathogens causing mastitis evolve with the disease. The SCM shows a high prevalence in dairy herds and the constant use of antibiotics may result in the development of resistant strains, for which the vaccines may also show decreased efficacy. The factor “Vaccination timing”The protocols used in mastitis vaccinations have consistently used vaccination before calving. The commercial vaccines clearly recommend pre-calving vaccination. Eventually, a boost is given after calving, especially if the cow calves before the expected delivery date. Highly productive dairy cows are subject to important metabolic changes, entering in physiological stress during their periparturient period and, therefore, may be immunocompromised and susceptible to mastitis (Burvenich et al., 2000). Also, after the first shoot, cows will take on average about 4 weeks to develop antibodies (Pyörälä, 2002). Menichetti et al. (2021) have also recently concluded while comparing IgG levels in animals vaccinated with booster 21- and 28-days pre-calving, that the earlier the vaccination and booster, the higher the concentration in the colostrum at calving. Therefore, early vaccination in the pre-calving period is advised and it is not a surprise to verify the inefficacy of the vaccine in post-calving vaccination. The factor “type of animal”The relatively small number of studies included in these meta-analysis results in limitations made evident while considering “type of animal” as a moderator. While in the main model (mixed methods) we find this to be a significant moderator, that is not made evident while using a random effects model. A higher number of trials would have resulted in replications in the combinations of the levels of the significant factors within the main model. This would have allowed the analysis of interactions between levels which could, eventually, explain the differences in significance between models. We could expect differences between heifers and multiparous cows, as the former was not previously exposed, while the latter are also associated with a lower probability of cure and higher SCC and duration of mammary infections as age progresses (Rainard et al., 2018). Table 3. Results of the application of random effects models to the different levels of the factor moderators used in this metaanalysis.
The factor “vaccine fabrication”We have found that commercial vaccines are not effective in preventing mastitis while self-fabricated vaccines show some level of protection. This is a result that apparently does not make sense once the commercial vaccines must have been approved after trials validated by the accreditation authorities. One explanation for this result may again be related to the limitations already identified in our study. Another reason may be the existence of a shadow factor. A plausible reason may be associated with the use of local bacteria in the production of self-fabricated vaccines, determining herd-specific immunity. Ismail (2017) has made a review of studies using commercial vaccines and herd-specific autovaccines and concluded that both types of vaccines produce equivalent results. We have only found one trial (Freick et al., 2016) comparing a commercial vaccine with a herd-specific autovaccine, but no significant differences were found between the two vaccines. ConclusionThis analysis does not provide full clarification of vaccine efficacy. However, if vaccination is to be used, this must follow the pre-calving protocol clearly prescribed by the commercial vaccines. It must also be noted that while not showing efficacy in some trials, the vaccination has demonstrated a reduction in the severity of the clinical disease, rate of culling, and increased the production of milk and milk solids. Organic farming is assuming increasing importance worldwide under the sustainability agenda and, due to the limitations in the use of traditional medication, any additional tool available for the prevention of disease. Therefore, improving welfare should be in the equation. The common preventive measures to control mastitis should not be discarded, and vaccination must be seen as an additional preventive tool in the provision of health and welfare. AcknowledgmentsTo the Foundation for Science and Technology (FCT, Portugal) for financial support to the CISAS UIDB/05937/2020 and UIDP/05937/2020, including the contract of the first author, a postdoc grant of the second author and a PhD grant to third. Conflict of interestThe authors declare that they have no conflict of interest or financial conflicts to disclose. Author contributionsFernando Mata: conceptualization, methodology, data acquisition, quality analysis, statistical analysis, results, writing—original draft. Meirielly S. Jesus: data acquisition, quality analysis, writing—review and editing. Ricardo Pinto: data acquisition, quality analysis, writing—review and editing. Andreia Mata: data acquisition, quality analysis, writing—review and editing. Data availabilityThe dataset used in this meta-analysis is provided within this paper. ReferencesBradley, A.J., Breen, J.E., Payne, B., White, V. and Green, M.J. 2015. An investigation of the efficacy of a polyvalent mastitis vaccine using different vaccination regimens under field conditions in the United Kingdom. J. Dairy Sci. 98, 1706–1720. Burvenich, C., Detilleux, J., Paape, M. and Massart-Leen, A. 2000. Physiological and genetic factors that influence the cows resistance to mastitis, especially during early lactation. In Proceedings of the IDF Symposium on Immune Ruminant Mammary Gland. Ed., Zecconi, A. Stresa, Italy, pp: 9–20. Deeks, J.J., Higgins, J.P.T. and Altman, D.G. (Eds.). 2019. Analysing data and undertaking meta-analyses. In Cochrane handbook for systematic reviews of interventions, 2nd ed. Eds., Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J. and Welch, V.A. JJohn Wiley & Sons, Chichester, UK.. Denis, M., Wedlock, D.N., Lacy-Hulbert, S.J., Hillerton, J.E. and Buddle, B.M. 2009. Vaccines against bovine mastitis in the New Zealand context: what is the best way forward? N. Z. Vet. J. 57, 132–140. des Roches, A.D.B., Lussert, A., Faure, M., Herry, V., Rainard, P., Durand, D., Françoise, W. and Foucras, G. 2018. Dairy cows under experimentally-induced Escherichia coli mastitis show negative emotional states assessed through Qualitative Behaviour Assessment. Appl. Anim. Behav. Sci. 206, 1–11. Freick, M., Frank, Y., Steinert, K., Hamedy, A., Passarge, O. and Sobiraj, A. 2016. Mastitis vaccination using a commercial polyvalent vaccine or a herd-specific Staphylococcus aureus vaccine. Results of a controlled field trial on a dairy farm. Tierarztl. Prax. 4, 219–229. Geary, U., Lopez-Villalobos, N., Begley, N., McCoy, F., O’Brien, B., O’Grady, L. and Shalloo, L. 2012. Estimating the effect of mastitis on the profitability of Irish dairy farms. J. Dairy Sci. 95, 3662–3673. Gonzaléz R.B., Cullor, J.S., Jasper, D.E., Farver, T.B., Bushnell, R.B. and Oliver, M.N. 1989. Prevention of clinical coliform mastitis in dairy cows by a mutant Escherichia coli vaccine. Can. J. Vet. Res. 53, 301–305. Gorden, P.J., Kleinhenz, M.D., Ydstie, J.A., Brick, T.A., Slinden, L.M., Peterson, M.P., Straub, D.E. and Burkhardt, D.T. 2018. Efficacy of vaccination with a Klebsiella pneumoniae siderophore receptor protein vaccine for reduction of Klebsiella mastitis in lactating cattle. J. Dairy Sci. 101, 10398–10408. Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J. and Welch, V.A. (Eds.). 2019. Cochrane handbook for systematic reviews of interventions, 2nd ed. Chichester, UK: John Wiley & Sons. Hogeveen, H., Steeneveld, W. and Wolf, C.A. 2019. Production diseases reduce the efficiency of dairy production: a review of the results, methods, and approaches regarding the economics of mastitis. Annu. Rev. Resour. Econ. 11, 289–312. International Dairy Federation. 1997. Recommendations for presentation of mastitis-related data. Bulletin No 321/1997, Brussels, Belgium. Ismail, Z.B. 2017. Mastitis vaccines in dairy cows: recent developments and recommendations of application. Vet. World. 10, 1057–1062. Keskin, A., Seyrek-İntas, K., Tek, H.B., Tuna, B., Yilmazbas, G., Ozakin, C. and Ertas, S. 2007. Efficiency of polyvalent mastitis vaccine in lactating dairy cows. J. Biol. Environ. Sci. 1, 87–92. Leitner, G., Yadlin, N., Lubashevsy, E., Ezra, E., Glickman, A., Chaffer, M., Winkler, M., Saran, A. and Trainin, Z. 2003. Development of a Staphylococcus aureus vaccine against mastitis in dairy cows. II. Field trial. Vet. Immunol. Immunopathol. 93, 153–158. Magaš V., Vakanjac, S., Pavlović, V., Velebit, B., Mirilović, M., Maletić, M., Đurić, M. and Nedić, S., 2013. Efficiency evaluation of a bivalent vaccine in the prophylaxis of mastitis in cows. Acta Vet-Beograd. 63, 525–536. Maia, P.V., Molina, L.R., Filho, E.J.F., Gonçalves, R.L., Moreira, L.P.V. and Carvalho, A.Ú. 2013. Vacinação com Escherichia coli J5 no pré-parto e ocorrência de mastite e produção de leite de vacas mestiças leiteiras. Arq. Bras. Med. Vet. Zootec. 65, 1367–1375. McClure, A., Christopher, E., Wolff, W., Fales, W., Gary, Z., Krause, F. and Miramonti, J. 1994. Effect of Re-1 7 mutant Salmonella typhimurium bacterin toxoid on clinical coliform mastitis. J. Dairy Sci. 77, 2272–2280. Mellenberger, R.W. 1977. Vaccination against mastitis. J. Dairy Sci. 60, 1016–1021. Menichetti, B.T., Garcia-Guerra, A., Lakritz, J., Weiss, W.P., Velez, J.S., Bothe, H., Merchan, D. and Schuenemann, G.M. 2021. Effects of prepartum vaccination timing relative to pen change with an acidogenic diet on serum and colostrum immunoglobulins in Holstein dairy cows. J. Dairy Sci. 104, 11072–11081. Michie, C.A. 2002. Staphilococcal vaccines. Trends Immunol. 23,461–463. Morimoto, K., Shimizu, M., Kurose, T., Nakatami, K., Akita, S., Shinozuka, Y. and Isobe, N. 2011. Efficacy of enterotoxigenic Escherichia coli vaccine for bovine clinical mastitis. J. Dairy Res. 78, 149–153. Nordhaug, M.L., Nesse, L.L., Norcross, N.L. and Gudding, R. 1994. A field trial with an experimental vaccine against Staphylococcus aureus mastitis in cattle. 1 clinical parameters. J. Dairy Sci. 77, 1267–1275. Petersson-Wolfe, C.S., Leslie, K.E. and Swartz, T.H. 2018. An update on the effect of clinical mastitis on the welfare of dairy cows and potential therapies. Vet. Clin. North Am. Food Anim. 34, 525–535. Pyörälä, S. 2002. New strategies to prevent mastitis. Reprod. Domest. Anim. 37, 211–216. Rainard, P., Foucras, G., Fitzgerald, J.R., Watts, J.L., Koop, G. and Middleton, J.R. 2018. Knowledge gaps and research priorities in Staphylococcus aureus mastitis control. Transbound. Emerg. Dis. 65, 149–165. Rainard, P., Gilbert, F.B., Germon, P. and Foucras, G. 2021. Invited review: a critical appraisal of mastitis vaccines for dairy cows. J. Dairy Sci. 104, 10427–10448. Robinson, G.K. 1991. “That BLUP is a good thing: the estimation of random effects.” Stat. Sci. 6, 15–32. Ruegg, P.L. 2017. A 100-year review: mastitis detection, management, and prevention. J. Dairy Sci. 100, 10381–10397. Sayed, M.L., Shell, W.S., Hanan, A.A., Ibrahim, H.M., Al, A.M. and Nasr E.A. 2016. Efficacy of a locally prepared bovine mastitis vaccine. Benha Vet. Med. J. 30, 302–311. Silva, S.R., Araujo, J.P., Guedes, C., Silva, F., Almeida, M. and Cerqueira J. 2021. Precision technologies to address dairy cattle welfare: focus on lameness, mastitis and body condition. Animals 11, 2253. Slobodanka, V., Pavlović, M. and Pavlović, V. 2010. Testing the efficiency of different treatments of subclinical Staphylococcus aureus mastitis in cows during the dry period. Acta Vet Beograd. 60, 227–239. Sterne, J.A.C., Savović, J., Page, M.J., Elbers, R.G., Blencowe, N.S., Boutron, I., Cates, C.J., Cheng, H. I., Corbett, M.S., Eldridge, S.M., Hernán, M.A., Hopewell, S., Hróbjartsson, A., Junqueira, D.R., Jüni, P., Kirkham, J.J., Lasserson, T., Li, T., McAleenan, A., Reeves, B.C., Shepperd, S., Shrier, I., Stewart, L.A., Tilling, K., White, I.R., Whiting, P.F. and Higgins, J.P.T. 2019. RoB 2: a revised tool for assessing risk of bias in randomised trials. B. M. J. 366, 14898. Sterne, J.A.C., Hernán, M.A., Reeves, B.C., Savović, J., Berkman, N.D., Viswanathan, M., Henry, D., Altman, D.G., Ansari, M.T., Boutron, I., Carpenter, J.R., Chan, A.W., Churchill, R., Deeks, J.J., Hróbjartsson, A., Kirkham, J., Jüni, P., Loke, Y.K., Pigott, T.D., Ramsay, C.R., Regidor, D., Rothstein, H.R., Sandhu, L., Santaguida, P.L., Schünemann, H.J., Shea, B., Shrier, I., Tugwell, P., Turner, L., Valentine, J.C., Waddington, H., Waters, E., Wells, G.A., Whiting, P.F. and Higgins, J.P.T. 2016. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ 355, i4919. Tashakkori, N., Khoramian, B., Farhoodi, M.M., Moghadam, M., Heidarpour, M., Mashayekhi, K. and Farzaneh, N. 2019. Evaluating the effectiveness of two bovine mastitis vaccines and their influences on oxidant and antioxidant capacities of milk. Trop. Anim. Health Prod. 52, 1493–1501. Tenhagen, B.A., Edinger, D., Baumgärtner, B., Kalbe, P., Klünder, G. and Heuwieser, W. 2001. Efficacy of a heard-specific vaccine against Staphylococcus aureus to prevent postpartum mastitis in dairy heifers. J. Vet. Med. A. 48, 601–607. Viechtbauer, W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. Watson, D.L., McColl, M.L. and Davies, H.I. 1996. Field trial of a staphylococcal mastitis vaccine in dairy herds: clinical, subclinical and microbiological assessments. Aust. Vet. J. 74, 447–450. Wilson, D.J. and González, R.N. 2003. Vaccination strategies for reducing clinical severity of coliform mastitis. Vet. Clin. North Am. Food Anim. Pract. 19, 187–197. Wilson, D.J., Grohn, Y.T., Bennett, G.J., González, R.N., Schukken, Y.H. and Spatz, J. 2007. Comparison of J5 vaccinates and controls for incidence, etiologic agent, clinical severity, and survival in the herd following naturally occurring cases of clinical mastitis. J. Dairy Sci. 90, 4282–4288. | ||
| How to Cite this Article |
| Pubmed Style Mata F, Jesus M, Pinto R, Mata A. A meta-analysis of the mastitis vaccination efficacy in dairy cattle. Open Vet. J.. 2023; 13(2): 179-187. doi:10.5455/OVJ.2023.v13.i2.5 Web Style Mata F, Jesus M, Pinto R, Mata A. A meta-analysis of the mastitis vaccination efficacy in dairy cattle. https://www.openveterinaryjournal.com/?mno=111708 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i2.5 AMA (American Medical Association) Style Mata F, Jesus M, Pinto R, Mata A. A meta-analysis of the mastitis vaccination efficacy in dairy cattle. Open Vet. J.. 2023; 13(2): 179-187. doi:10.5455/OVJ.2023.v13.i2.5 Vancouver/ICMJE Style Mata F, Jesus M, Pinto R, Mata A. A meta-analysis of the mastitis vaccination efficacy in dairy cattle. Open Vet. J.. (2023), [cited January 25, 2026]; 13(2): 179-187. doi:10.5455/OVJ.2023.v13.i2.5 Harvard Style Mata, F., Jesus, . M., Pinto, . R. & Mata, . A. (2023) A meta-analysis of the mastitis vaccination efficacy in dairy cattle. Open Vet. J., 13 (2), 179-187. doi:10.5455/OVJ.2023.v13.i2.5 Turabian Style Mata, Fernando, Meirielly Jesus, Ricardo Pinto, and Andreia Mata. 2023. A meta-analysis of the mastitis vaccination efficacy in dairy cattle. Open Veterinary Journal, 13 (2), 179-187. doi:10.5455/OVJ.2023.v13.i2.5 Chicago Style Mata, Fernando, Meirielly Jesus, Ricardo Pinto, and Andreia Mata. "A meta-analysis of the mastitis vaccination efficacy in dairy cattle." Open Veterinary Journal 13 (2023), 179-187. doi:10.5455/OVJ.2023.v13.i2.5 MLA (The Modern Language Association) Style Mata, Fernando, Meirielly Jesus, Ricardo Pinto, and Andreia Mata. "A meta-analysis of the mastitis vaccination efficacy in dairy cattle." Open Veterinary Journal 13.2 (2023), 179-187. Print. doi:10.5455/OVJ.2023.v13.i2.5 APA (American Psychological Association) Style Mata, F., Jesus, . M., Pinto, . R. & Mata, . A. (2023) A meta-analysis of the mastitis vaccination efficacy in dairy cattle. Open Veterinary Journal, 13 (2), 179-187. doi:10.5455/OVJ.2023.v13.i2.5 |