| Original Article | ||
Open Vet. J.. 2022; 12(4): 578-583 Open Veterinary Journal, (2022), Vol. 12(4): 578–583 Original Research Alterations of selected serum biochemical and urinary parameters in dogs with chronic enteropathyEleonora Gori1, Ilaria Lippi1, Giulia Ansaldo1, Paola Gianella2, Francesca Perondi1, Alessio Pierini1* and Veronica Marchetti11Department of Veterinary Sciences, Veterinary Teaching Hospital “Mario Modenato”, University of Pisa, Pisa, Italy 2Department of Veterinary Sciences, Veterinary Teaching Hospital, University of Turin, Torino, Italy *Corresponding Author: Alessio Pierini. Department of Veterinary Sciences, Veterinary Teaching Hospital “Mario Modenato”, University of Pisa, Pisa, Italy. Email: pierini.alessio2004 [at] gmail.com Submitted: 09/04/2022 Accepted: 22/07/2022 Published: 23/08/2022 © 2022 Open Veterinary Journal
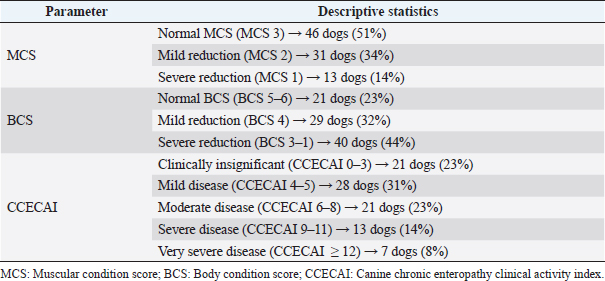
AbstractBackground: No specific study on concurrent nephropathy has been conducted in dogs with chronic enteropathy (CE), except for soft-coated Wheaten Terriers. Moreover, limited information exists regarding the urinary profile in dogs with CE. Aim: To describe, compare, and discuss the alterations in selected serum biochemical and urinary parameters in dogs with CE. Methods: Multicentric retrospective study on dogs with CE diagnosed after exclusion of extra-gastrointestinal diseases. In addition, dogs with azotemia and lower urinary tract diseases were excluded. Information on canine chronic enteropathy clinical activity index (CCECAI) score, muscular condition score (MCS), presence of glycosuria, proteinuria [urine protein-to-creatinine (UPC) ratio > 0.5], and/or cylindruria (>1–2 casts/hpf) at diagnosis were gleaned from the medical records. Dogs were retrospectively classified as food-responsive enteropathy, immunosuppressant-responsive enteropathy, or nonresponsive enteropathy based on the presence of gastrointestinal histological inflammation and the treatment response. In addition, based on the serum albumin concentration (ALB), dogs were classified as having protein-losing enteropathy (PLE). Results: Ninety CE dogs were included. Fifty-two dogs had mild-to-severely decreased MCS and 38 dogs showed altered urinary parameters. No significant associations were found between CCECAI and altered urinary parameters. No significant association was found between PLE dogs and altered urinary parameters. PLE dogs showed higher prevalence of proteinuria than non-PLE dogs (p =0.03; OR=2.8; 95% CI=1–6.8). Conclusion: Despite the presence of altered urinary profile in dogs with CE, further studies are needed to explore a possible link between gastrointestinal and renal inflammation. Keywords: Dog, Intestinal disease, Urine, Proteinuria. IntroductionThe relationship between inflammatory bowel disease (IBD) and kidney injury has been extensively studied in human medicine. Approximately the half of IBD human patients show extraintestinal manifestations, and 4%–23% of the patients may develop renal involvement (Ambruzs and Larsen, 2018; Greuter and Vavricka, 2019). These patients showed renal symptoms, such as urinary stones, urinary obstructions, glomerulonephritis, interstitial tubular nephritis, or amyloidosis with proteinuric renal insufficiency (Ambruzs and Larsen, 2018). Rothfuss et al. (2006) highlighted renal manifestations in IBD human patients, hypothesizing that a systemic involvement of intestinal diseases may be the trigger for the onset of renal dysfunction. In humans, the so called “gut–kidney axis” underlines the relationship between these two organs. This relationship is mainly based on immunological responses and metabolism-dependent mechanisms (Evenepoel et al., 2017). The “gut–kidney axis” has also been taken into consideration in veterinary medicine and two mechanisms have been hypothesized: deposition of immune complexes (Littman et al., 2000; Vaden et al., 2013) and intestinal dysbiosis (Lippi et al., 2017). However, it is important to underline that specific studies about kidney injury in dogs with chronic enteropathy (CE) are lacking. We hypothesized that a kidney injury in dogs with CE may occur and that less severe forms of canine CE may have less severe and lower prevalence of kidney injury compared to more severe and unresponsive forms of CE (Dandrieux, 2016; Dandrieux and Mansfield, 2019). Thus, the aim of this study was to describe concurrent changes in selected serum biochemical and urinary parameters indicative of kidney injury in dogs with CE. Materials and MethodsThe electronic medical records databases of the Veterinary Teaching Hospitals of the University of Pisa and the University of Turin were searched to identify dogs with primary CE presented from February 2015 to August 2020. Medical records of each veterinary teaching hospital were reviewed to confirm the diagnosis of primary CE. According to the current literature (Dandrieux, 2016; Dandrieux and Mansfield, 2019), primary CE was defined for dogs showing signs of chronic gastrointestinal disease (including weight loss, vomiting, diarrhea, and decreased appetite) for at least 3 weeks. All dogs had to have hematobiochemical panel, including serum trypsin-like immunoreactivity, canine pancreatic lipase, serum basal cortisol, and complete urinalysis with urinary protein-to-creatinine ratio (UPC), fecal flotation, to rule out other causes of chronic gastrointestinal signs. In addition, each dog had to have a full abdominal ultrasound performed for the exclusion of extraintestinal diseases or nonprimary intestinal disease (e.g., intussusception, foreign bodies, or intestinal tumors) (Dandrieux, 2016; Dandrieux and Mansfield, 2019). Afterward, selected CE dogs, based on treatment response extrapolated from medical records, were retrospectively classified as from food-responsive enteropathy (FRE), immunosuppressant-responsive enteropathy (IRE), or nonresponsive enteropathy (NRE) (Dandrieux, 2016; Dandrieux and Mansfield, 2019). Specifically, FRE was defined as complete and sustained remission of clinical signs while on elimination or novel protein diet (hydrolyzed or restricted antigen diets) associated with a multistrain probiotic (Dandrieux, 2016; Dandrieux and Mansfield, 2019). IRE was defined as clinical improvement after the start of an immunomodulatory therapy based on prednisolone (0.5–1 mg/kg every 12 hours) or budesonide (3 mg/m2 every 24 hours) in association or alternatively with cyclosporine (5 mg/kg every 12 hours) or chlorambucil (2–4 mg/m2 every 24 hours) (Dandrieux, 2016; Dandrieux and Mansfield, 2019). Finally, NRE was defined as poor or no clinical response to diet or immunosuppressants (Dandrieux, 2016; Dandrieux and Mansfield, 2019). According to the literature, all dogs that failed the diet trial, thus classified as IRE and NRE, underwent digestive endoscopy to confirm and characterize gastrointestinal inflammation using WSAVA guidelines before the use of immunomodulatory therapy, as described elsewhere (Dandrieux, 2016; Dandrieux and Mansfield, 2019). Based on recently published information, the real clinical value of antibiotic-responsive enteropathy (ARE) is now questioned (Cerquetella et al., 2020) and dogs with ARE (if responding to an antibiotic trial with tylosin at 15 mg/kg every 12 hours for at least 3 weeks) (Dandrieux, 2016; Dandrieux and Mansfield, 2019) were then excluded from the statistical analysis. In addition, based on serum ALB, dogs were classified as having protein-losing enteropathy (PLE; if ALB < 27 g/l) (Nakashima et al., 2015) and non-PLE. Dogs lacking hematobiochemical profile and/or urinalysis and/or abdominal ultrasound were excluded from the study. In addition, dogs with a history of previous kidney or lower urinary tract diseases (previous clinicopathological finding and/or imaging alterations), prerenal or post-renal proteinuria, and severe renal proteinuria (UPC > 2) or active sediment were excluded. In addition, since ultrasonographic changes in renal parenchyma may be due to previous subclinical kidney disease, dogs with ultrasonographic signs of renal impairment or renal ultrasonographic abnormalities at presentation to the two facilities were excluded. Specifically, dogs with at least one of the following ultrasound signs were excluded: increased cortical echogenicity, renal infarcts, loss of corticomedullary differentiation, decreased renal volume, and irregular renal margins (Bragato et al., 2017). Of the dogs included in the study, the following information were recorded: signalment, body condition score (BCS; 9-point scale; Laflamme, 1997), muscular condition score (MCS; 3-point scale; Freeman et al., 2011), and CCECAI (Allenspach et al., 2007). Regarding BCS, a dog was considered with normal BCS (5), mildly decreased (BCS 4), and moderately-to-severely decreased (BCS 3–1) (Laflamme, 1997). The CCECAI scoring system assessed nine categories of disease severity, including the evaluation of attitude and activity [from 0 (normal) to 3 (severely decreased)], appetite [from 0 (normal) to 3 (severely decreased)], vomiting [from 0 (<1 event/week) to 3 (>3 events/week)], fecal consistency [from 0 (normal) to 3 (watery diarrhea)], fecal frequency [from 0 (normal; <2 events/day) to 3 (severely increased; >5 events/day)], weight loss [from 0 (none) to 3 (>10%)], serum ALB [from 0 (>20 g/l) to 3 (<12 g/l)], peripheral edema and ascites [from 0 (none) to 3(severe)], and pruritus [from 0 (none) to 3 (pruritis regularly wakes dog)] (Allenspach et al., 2007). Based on the CCECAI value, the severity of CE was assessed as insignificant (0–3), mild disease (4–5), moderate disease (6–8), severe (9–11), and very severe disease (>12) (Allenspach et al., 2007). In this study, the five categories mentioned above were grouped into two categories: clinically insignificant–mild disease (CCECAI 0–5; LoCCECAI) and clinically moderate-to-very severe disease (CCECAI ≥ 6; HiCCECAI) for statistical purposes. Subsequently, from the hematobiochemical profile performed at diagnosis/presentation to the hospitals and before therapies, serum total proteins, ALB, urea, and creatinine were recorded. From the urinalysis, performed at diagnosis/presentation to the hospitals and before therapies, the presence of glycosuria, hematuria, proteinuria, cylindruria, and UPC value were recorded. Dogs with glycosuria, renal proteinuria (UPC > 0.5), and/or cylindruria were classified as having urinary kidney injury. For statistical analysis, dogs were divided into different groups: LoCCECAI and HiCCECAI, FRE and IRE-NRE, and PLE and non-PLE dogs. Continuous variables (urea, creatinine, and UPC) were non-normally distributed and were presented as the median and range. CCECAI, MCS, proteinuria, cylindruria, glycosuria, and hematuria were evaluated as categorical variables. The association between kidney injury and CCECAI groups, FRE versus IRE-NRE and PLE versus not-PLE dogs was tested using the chi-squared test. Differences in urea, creatinine, and UPC values between LoCCECAI and HiCCECAI, FRE and IRE-NRE, and PLE and not-PLE dogs were tested using Mann–Whitney U-test. Statistical analysis was carried out using IBM SPSS Statistics v.25 (IBM Corporation, New York, NY), and a p-value < 0.05 was considered statistically significant for the other tests. ResultsFrom the initial screening of medical records of Veterinary Teaching Hospitals of the University of Pisa and Turin between January 2017 and 2020, 304 dogs with primary CE were found. Of these 304 dogs, 107 were excluded because urinalysis was not available for review, 27 dogs had no UPC performed, 21 dogs had no abdominal ultrasound for review, an additional 35 dogs showed ultrasonographic kidney abnormalities, and 2 dogs were azotemic. Furthermore, eight dogs were excluded due to the lack of available biochemistry report and one dog because leishmaniosis was suspected and not ruled out based on medical records review. 14 dogs were classified as ARE and were excluded from the final cohort. 90 dogs with primary CE were retrospectively included. Most dogs were mixed breed (n =17), and the most represented breeds were German shepherd (n =11), Rottweiler and Poodle (n =5 each), Bolognese and Jack Russell terrier (4 dogs each breed), Beagle, Boxer, Border collie, English setter, Kurzhaar (3 dogs each breed), Bernese Mountain dog, Corso, Maltese, Weimaraner, Yorkshire Terrier, and Golden retriever (2 dogs each breed). The other 17 dogs belonged to other breeds (1 dog each one). 45 dogs were female, 28 of them were spayed, and 47 dogs were males, only one castrated. The median age was 3.6 years (IQR=5.1, range=0.3–14.9 years). Descriptive statistics of MCS, BCS, and CCECAI are reported in Table 1. Dividing the study population in LoCCECAI and HiCCECAI, 50 (55.5%) dogs belonged to the former group and 40 (44.4%) dogs to the latter one. Based on the treatment response, 50, 25, and 15 dogs were classified as FRE, IRE, and NRE, respectively. Based on serum ALB, 38 dogs (42%) were classified as inflammatory PLE (10 FRE, 25 IRE, and 12 NRE). Overall median creatinine was 0.96 mg/dl (84.8 µmol/l) [IQR=0.38; range=0.48–1.6 mg/dl (42.4–140.8 µmol/l)]. Overall median urea was 31 mg/dl (11.1 mmol/l) [IQR=17; range=12–110 mg/dl (4.3–39.3 mmol/l)]. Dogs with normal MCS had a significantly higher serum creatinine [median=1 mg/dl (88.4 µmol/l); IQR=0.39, range=0.65–1.6 mg/dl [57.5–140.8 µmol/l)] than those with a reduction in MCS [median=0.8 mg/dl (70.7 µmol/l); IQR=0.3, range=0.48–1.6 mg/dl (42.4–140.8 µmol/l); p =0.008]. Based on urinary parameters, 34 out of 90 dogs (38%) showed kidney injury. In particular, dog showed glycosuria, 21 dogs had proteinuria, and 21 dogs had cylindruria. No differences in prevalence of proteinuria, cylindruria, glycosuria, and hematuria and UPC values between FRE and IRE–NRE dogs were found (all p > 0.05). Dogs with LoCCECAI and HiCCECAI did not differ in urinary kidney injury prevalence (p =0.9). The prevalence of urinary kidney injury was not different between PLE and non-PLE (p =0.2) dogs, whereas PLE dogs showed a higher frequency (62%) of proteinuria than non-PLE dogs (p =0.04 OR 2.8 95%CI 1–7.6). DiscussionThis retrospective study described concurrent changes in selected serum and urinary parameters indicative of kidney injury in a population of dogs with CE. Even if no dogs had azotemia, proteinuria was present in approximately 40% of the dogs and was more frequent in PLE dogs, compared to non-PLE dogs. The use of urinary markers might be more indicative that serum urea and creatinine for a concurrent kidney injury, since approximately the half of CE dogs of our study showed a moderate-to-severe reduction in BCS and MCS, might affect serum creatinine levels. In addition, CE dogs may display various degrees of dehydration and intestinal, often occult, blood losses and, thus, an increase in serum urea may not be specific for kidney impairment (Syme, 2016). There are no studies evaluating MCS in dogs with CE. The presence of a reduction in muscle mass could be attributed to a deficit in protein synthesis, due to intestinal malabsorption (Ward, 2013). In fact, in IBD human patients, a reduction in muscle mass is attributable to reduced intestinal absorption, increased catabolism, malnutrition, drug side effects, and chronic inflammation (Greuter and Vavricka, 2019). Therefore, a similar condition may be hypothesized also in canine patients with CE. In our population, dogs with azotemia were excluded, therefore, we decided to evaluate the relationship between serum creatinine and BCS and MCS. Although the first relationship was not statistically significant, serum creatinine resulted to be higher in dogs with normal MCS compared to those with MCS reduction. This agrees with the information reported in the literature. Given its muscle origin, if muscle mass tends to decrease, serum creatinine will be decreased (Syme, 2016). This finding suggests that creatinine may have some limitations to detect kidney injury in dogs with CE. For this reason, we decided to consider urinary markers of kidney injury (proteinuria, cylindruria, and hematuria), which were highlighted in approximately 40% of the CE dogs. In humans, renal manifestations during IBD occur in 4%–23% of the patients (Ambruzs and Larsen, 2018; Greuter and Vavricka, 2019) and a relationship between IBD and renal damage was demonstrated (Rothfuss et al., 2006; Ambruzs and Larsen, 2018; Jang et al., 2018; Greuter and Vavricka, 2019). Veterinary medicine lacks specific studies on kidney damage in dogs with CE, except for two studies on soft-coated Wheaten Terrier with concomitant PLE and protein-losing nephropathy (Littman et al., 2000; Vaden et al., 2013). In addition, only a few studies have been conducted to underline the importance of controlling intestinal dysbiosis using probiotics in renal insufficiency (Ranganathan et al., 2004, 2006; Lippi et al., 2017). Another possible explanation is linked to an interstitial tubule damage associated with an increase in circulating uremic toxins (Inoue and Ishibe, 2015). However, this hypothesis in our case might be excluded since our patients were mainly non-azotemic, and only a few had an increase in urea and creatinine. Another hypothesis of renal damage in CE may take into consideration an immune-mediated origin. In CE dogs, dysbiosis and accumulation of pathogenic microorganisms are highlighted as triggers to the activation of an uncontrolled immune response (Luckschander et al., 2006; Baumgart and Carding, 2007; Dandrieux, 2016; Dandrieux and Mansfield, 2019). This inflammatory status may contribute to renal injury (Yang et al., 2018). Table 1. Descriptive statistics of MCS, BCS, and CCECAI.
We also hypothesized that dogs with more severe forms of CE might have a higher prevalence of kidney damage, especially dogs with a diagnosis of PLE. We failed to find an association between CCECAI and altered renal parameters. Our hypothesis was based on various possible pathogenic mechanisms. First, a positive correlation between CCECAI and dysbiosis index has been recently highlighted (AlShawaqfeh et al., 2017). More severe enteropathies may be characterized by increased dysbiosis index, which may be responsible for kidney damage (Sirich et al., 2014; Minamoto et al., 2019). Other possible pathogenesis may be due to immune complex deposition (Littman et al., 2000; Vaden et al., 2013), dehydration (McGrotty et al., 2016), and endotoxemia (Anders et al., 2013). In CE dogs, all these mechanisms might be possible in various degrees. The lack of significance of this association might be perhaps due to CCECAI assessment, which was performed by different clinicians throughout the study period; however, dehydration, endotoxemia, and immune complex deposition might have played a role. In addition, also the prevalence of kidney injury in dogs with or without PLE was not significantly different, although approximatily 60% of PLE dogs had proteinuria and the prevalence of proteinuria was significantly higher in PLE dogs. No differences in serum urea, creatinine, presence of kidney injury and proteinuria, cylindruria, glycosuria, and hematuria, evaluated individually, between FRE and, IRE/NRE were found. The lack of significance of the association between kidney injury in PLE dogs may be explained by our choice to include not only proteinuria, but also glycosuria and cylindruria. In the study of Lippi et al. (2017), median UPC values in CKD dogs treated with multistrain probiotics had a significant reduction compared to untreated CKD dogs, proving that anti-inflammatory and microbiota-modulating properties of probiotics in kidney injury may be clinically relevant (Rossi et al., 2014; Lippi et al., 2017). An immunocomplex deposition nephropathy in CE has been hypothesized in soft-coated Wheaten Terriers with PLE (Littman et al., 2000; Vaden et al., 2013). It has been hypothesized that an increase in intestinal permeability and hypersensitivity to luminal antigens can lead to the deposition of immune complexes in kidneys, resulting in kidney damage (Littman et al., 2000; Vaden et al., 2013). It should be considered that these two latter studies take into consideration a specific breed, which is genetically predisposed to other immunomediated disorders and hypersensitivity disorders, and that is also absent in our population. In fact, other possible etiologies on the data about proteinuria in PLE dogs should be considered. This study has some limitations. First, early serum or urinary biomarkers of renal injury, such as symmetric dimethylarginine (SDMA), urinary gamma-glutamyl transpeptidase, and urinary neutrophil gelatinase-associated lipocalin were not evaluated because of the retrospective nature of the study. Despite serum creatinine, SDMA is not influenced by age, MCS, breed, sex, physical activity, and, in general, by extrarenal factors (Hall et al., 2015; Sargent et al., 2021). Another important limitation is linked to the two different veterinary facilities in which dogs were evaluated, and consequently followed by different clinicians with different clinical approaches. Finally, although we have applied strict exclusion criteria, dogs with mild and subclinical nephropathy cannot be certainly ruled out. ConclusionTo date, in the veterinary literature, there are no specific studies evaluating kidney injury in dogs with CE. Our study identified a significant presence of CE dogs with concomitant renal damage. Of these dogs, only a minority showed azotemia, thus emphasizing the importance of adding other markers to serum urea and creatinine, especially if reduced muscle mass is present. The elevated frequency of proteinuria in PLE dogs should encourage further investigations on the pathogenetic mechanisms, with particular interest toward dysbiosis. Conflict of interestThe authors declare that there is no conflict of interest. FundingNo funding was obtained for this study. Authors’ contributionsConceptualization: Ilaria Lippi, Paola Gianella, and Veronica Marchetti; Data curation: Eleonora Gori, Giulia Ansaldo, and Paola Gianella; Statistical analysis: Eleonora Gori and Alessio Pierini. All the authors revised the final version of the manuscript. Ethical approvalThis is a retrospective study. Therefore, official Ethics Committee approval is not required. ReferencesAllenspach, K., Wieland, B., Gröne, A. and Gaschen, F. 2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 21, 700–708. AlShawaqfeh, M.K., Wajid, B., Minamoto, Y., Markel, M.E., Lidbury, J.A., Steiner, J., Serpedin, E. and Suchodolski, J.S. 2017. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS. Microbiol. Ecol. 93; doi:10.1093/femsec/fix136. Ambruzs, J.M. and Larsen, C.P. 2018. Renal manifestations of inflammatory bowel disease. Rheum. Dis. Clin. North Am. 44, 699–714. Anders, H.J., Andersen, K. and Stecher, B. 2013. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 83, 1010–1016. Baumgart, D.C. and Carding, S.R. 2007. Inflammatory bowel disease: cause and immunobiology. Lancet. 369, 1627–1640. Bragato, N., Borges, N.C. and Fioravanti, M.C.S. 2017. B-mode and doppler ultrasound of chronic kidney disease in dogs and cats. Vet. Res. Commun. 41, 307–315. Cerquetella, M., Rossi, G., Suchodolski, J.S., Schmitz, S.S., Allenspach, K., Rodríguez-Franco, F., Furlanello, T., Gavazza, A., Marchegiani, A., Unterer, S., Burgener, I.A., Pengo, G. and Jergens, A.E. 2020. Proposal for rational antibacterial use in the diagnosis and treatment of dogs with chronic diarrhoea. J. Small Anim. Pract. 61, 211–215. Dandrieux, J.R.S. 2016. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J. Small Anim. Pract. 57, 589–599. Dandrieux, J.R.S. and Mansfield, C.S. 2019. Chronic enteropathy. In canines: prevalence, impact and management strategies. Vet. Med. 10, 203–214. Evenepoel, P., Poesen, R. and Meijers, B. 2017. The gut-kidney axis. Pediatr. Nephrol. 32, 2005–2014. Freeman, L., Becvarova, I., Cave, N., MacKay, C., Nguyen, P., Rama, B., Takashima, G., Tiffin, R., Beukelen, P. van, Yathiraj, S. and Force, W.N.A.G.T. 2011. WSAVA nutritional assessment guidelines. Compend. Contin. Educ. Vet. 33, 1–9. Greuter, T. and Vavricka, S.R. 2019. Extraintestinal manifestations in inflammatory bowel disease–epidemiology, genetics, and pathogenesis. Exp. Rev. Gastroenterol. Hepatol. 13, 307–317. Hall, J.A., Yerramilli, M., Obare, E., Yerramilli, M., Melendez, L.D. and Jewell, D.E. 2015. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J. Vet. Intern. Med. 29, 808–814. Inoue, K. and Ishibe, S. 2015. Podocyte endocytosis in the regulation of the glomerular filtration barrier. Am. J. Physiol. Renal Physiol. 309, 398–405. Jang, H.M., Baek, H.S., Kim, J.E., Kim, J.Y., Lee, Y.H., Cho, H.Y., Choe, Y.H., Kang, B., Choe, B.-H., Choi, B.S. and Cho, M.H. 2018. Renal involvement in children and adolescents with inflammatory bowel disease. Korean J. Pediat. 61, 327–331. Laflamme, D. 1997. Nutritional management. Vet. Clin. 27, 1561–1577. Lippi, I., Perondi, F., Ceccherini, G., Marchetti, V. and Guidi, G. 2017. Effects of probiotic VSL#3 on glomerular filtration rate in dogs affected by chronic kidney disease: a pilot study. Can. Vet. J. 58, 1301–1305. Littman, M.P., Dambach, D.M., Vaden, S.L. and Giger, U. 2000. Familial protein-losing enteropathy and protein-losing nephropathy in soft coated wheaten terriers: 222 cases (1983-1997). J. Vet. Intern. Med. 14, 68–80. Luckschander, N., Allenspach, K., Hall, J., Seibold, F., Gröne, A., Doherr, M.G. and Gaschen, F. 2006. Perinuclear antineutrophilic cytoplasmic antibody and response to treatment in diarrheic dogs with food responsive disease or inflammatory bowel disease. J. Vet. Intern. Med. 20, 221–227. McGrotty, Y., Bell, R. and McLauchlan, G. 2016. Disorder of plasma proteins. In BSAVA manual of canine and feline clinical pathology. Eds., Villiers, E. and Ristić, J. Gloucester, UK: British Small Animal Veterinary Association, pp: 123–141. Minamoto, Y., Minamoto, T., Isaiah, A., Sattasathuchana, P., Buono, A., Rangachari, V.R., McNeely, I.H., Lidbury, J., Steiner, J.M. and Suchodolski, J.S. 2019. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 33, 1608–1618. Nakashima, K., Hiyoshi, S., Ohno, K., Uchida, K., Goto-Koshino, Y., Maeda, S., Mizutani, N., Takeuchi, A. and Tsujimoto, H. 2015. Prognostic factors in dogs with protein-losing enteropathy. Vet. J. 205, 28–32. Ranganathan, N., Patel, G., Ranganathan, P., Dheer R., Pamquist, R. and Van Engelenberg, G. Effect of feeding Kibow Biotics® to cats and dogs in kidney failure. In Proceedings of the 39th Annual Meeting of the American Society of Nephrology, San Diego, CA, 2006, p 897A. Ranganathan, P., Marczely, J., Dheer J., Patel, B., Ranganathan, N., Friedman, A. and Thornill, J.A. Initial trial of probiotic bacteria as therapy for uremia in dogs. In Proceedings of the American Society of Nephrology, St. Louis, MO, 2004, p 768A. Rossi, G., Pengo, G., Caldin, M., Piccionello, A.P., Steiner, J.M., Cohen, N.D., Jergens, A.E. and Suchodolski, J.S. 2014. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS One 9, 94699; doi:10.1371/journal.pone.0094699. Rothfuss, K.S., Stange, E.F. and Herrlinger, K.R. 2006. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J. Gastroenterol. 12, 4819–4831. Sargent, H.J., Elliott, J. and Jepson, R.E. 2021. The new age of renal biomarkers: does SDMA solve all of our problems? J. Small Anim. Pract. 62, 71–81. Sirich, T.L., Plummer, N.S., Gardner, C.D., Hostetter, T.H. and Meyer, T.W. 2014. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 9, 1603–1610. Syme, H.M. 2016. Laboratory evaluation of renal disorders. In BSAVA manual of canine and feline clinical pathology. Eds., Villiers, E. and Ristić, J. Gloucester, UK: British Small Animal Veterinary Association, pp: 219–236. Vaden, S.L., Littman, M.P. and Cianciolo, R.E. 2013. Familial renal disease in soft-coated wheaten terriers. J. Vet. Emerg. Crit. Care 23, 174–183. Ward, C.R. 2013. Weight loss and cachexia. In Canine and feline gastroenterology. Eds., Washabau, R.J. and Day, M.J. St. Louis, MO: Elsevier Saunders, pp: 174–176. Yang, T., Richards, E.M., Pepine, C.J. and Raizada, M.K. 2018. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 14, 442–456. | ||
| How to Cite this Article |
| Pubmed Style Gori E, Lippi I, Ansaldo G, Gianella P, Perondi F, Pierini A, Marchetti V. Alterations of selected serum biochemical and urinary parameters in dogs with chronic enteropathy. Open Vet. J.. 2022; 12(4): 578-583. doi:10.5455/OVJ.2022.v12.i4.21 Web Style Gori E, Lippi I, Ansaldo G, Gianella P, Perondi F, Pierini A, Marchetti V. Alterations of selected serum biochemical and urinary parameters in dogs with chronic enteropathy. https://www.openveterinaryjournal.com/?mno=11459 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i4.21 AMA (American Medical Association) Style Gori E, Lippi I, Ansaldo G, Gianella P, Perondi F, Pierini A, Marchetti V. Alterations of selected serum biochemical and urinary parameters in dogs with chronic enteropathy. Open Vet. J.. 2022; 12(4): 578-583. doi:10.5455/OVJ.2022.v12.i4.21 Vancouver/ICMJE Style Gori E, Lippi I, Ansaldo G, Gianella P, Perondi F, Pierini A, Marchetti V. Alterations of selected serum biochemical and urinary parameters in dogs with chronic enteropathy. Open Vet. J.. (2022), [cited January 25, 2026]; 12(4): 578-583. doi:10.5455/OVJ.2022.v12.i4.21 Harvard Style Gori, E., Lippi, . I., Ansaldo, . G., Gianella, . P., Perondi, . F., Pierini, . A. & Marchetti, . V. (2022) Alterations of selected serum biochemical and urinary parameters in dogs with chronic enteropathy. Open Vet. J., 12 (4), 578-583. doi:10.5455/OVJ.2022.v12.i4.21 Turabian Style Gori, Eleonora, Ilaria Lippi, Giulia Ansaldo, Paola Gianella, Francesca Perondi, Alessio Pierini, and Veronica Marchetti. 2022. Alterations of selected serum biochemical and urinary parameters in dogs with chronic enteropathy. Open Veterinary Journal, 12 (4), 578-583. doi:10.5455/OVJ.2022.v12.i4.21 Chicago Style Gori, Eleonora, Ilaria Lippi, Giulia Ansaldo, Paola Gianella, Francesca Perondi, Alessio Pierini, and Veronica Marchetti. "Alterations of selected serum biochemical and urinary parameters in dogs with chronic enteropathy." Open Veterinary Journal 12 (2022), 578-583. doi:10.5455/OVJ.2022.v12.i4.21 MLA (The Modern Language Association) Style Gori, Eleonora, Ilaria Lippi, Giulia Ansaldo, Paola Gianella, Francesca Perondi, Alessio Pierini, and Veronica Marchetti. "Alterations of selected serum biochemical and urinary parameters in dogs with chronic enteropathy." Open Veterinary Journal 12.4 (2022), 578-583. Print. doi:10.5455/OVJ.2022.v12.i4.21 APA (American Psychological Association) Style Gori, E., Lippi, . I., Ansaldo, . G., Gianella, . P., Perondi, . F., Pierini, . A. & Marchetti, . V. (2022) Alterations of selected serum biochemical and urinary parameters in dogs with chronic enteropathy. Open Veterinary Journal, 12 (4), 578-583. doi:10.5455/OVJ.2022.v12.i4.21 |