| Case Report | ||
Open Vet. J.. 2022; 12(1): 148-155 Open Veterinary Journal, (2022), Vol. 12(1): 148–155 Case Report Use of a customized 3D-printed external splint for the correction of a severe pectus excavatum in a 3-month-old kittenGiovanni Mattioli*, Matteo Zanfabro, Mattia Bonazzi and Marina MartanoDepartment of Veterinary Medical Science, University of Parma, Parma, Italy *Corresponding Author: Giovanni Mattioli. Department of Veterinary Medical Science, University of Parma, Parma, Italy. Email: giovanni.mattioli [at] unipr.it Submitted: 02/09/2021 Accepted: 25/01/2022 Published: 27/02/2022 © 2022 Open Veterinary Journal
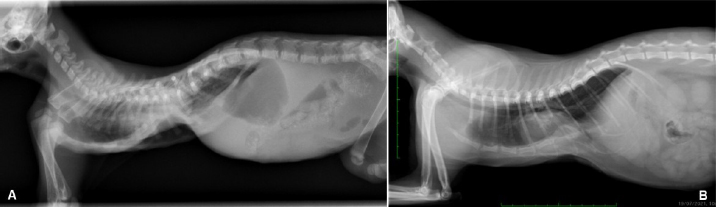
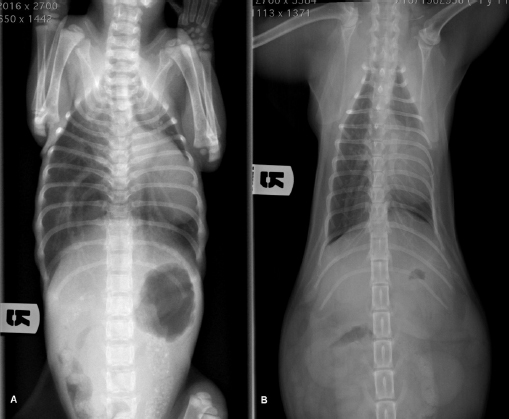
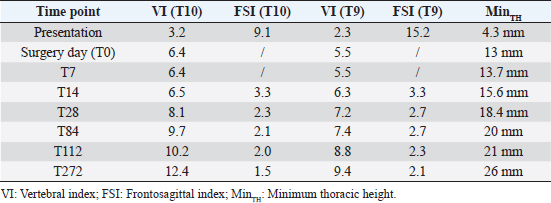
AbstractBackground: Pectus excavatum is a rare congenital or developmental deformity of the chest wall that has been reported in both dogs and cats. The clinical symptoms vary in severity and are correlated with the grade of the deformity. The most severe cases are at a very high risk of intra- and post-operative complications that could be fatal. Case Description: A 3-month-old kitten was presented for severe dyspnea and exercise intolerance. After the radiographic diagnosis of severe pectus excavatum, a splint was designed based on the computed tomography scan of the kitten. A locking mechanism was applied on a dedicated pocket on the splint and secured with commercial glue. Five sutures were placed, the most caudal three with a surgical approach to the corresponding sternebra. The three caudal traction sutures were progressively tensioned during the first 2 weeks, and then tied. The splint was removed 1 month postoperatively; neither surgical nor splint-associated complications were observed. The kitten well tolerated the splint and the owner reported no further episodes of dyspnea or exercise intolerance. Vertebral and frontosagittal indexes were 3.2 and 9.1 at presentation, respectively, and 10.2 and 2.0 at 4 months postoperatively. Conclusion: The use of a patient-specific 3D-printed external splint associated with a progressive correction of the pectus excavatum can be a better solution for the treatment of severe cases of pectus excavatum, where a sudden distension of the rib cage could cause re-expansion injuries. Keywords: Cat, Pectus excavatum, Splint, Thoracic surgery, 3D printing. IntroductionPectus excavatum is a congenital or developmental deformity of the chest wall characterized by a concave deformity of the caudal sternum in association with the deformity of the costal cartilage (Mestrinho et al., 2011). The result of this anatomic anomaly is a narrowing of the entire thorax and compressive cardiopulmonary dysfunction, leading to exercise intolerance, dyspnea, cyanosis, cardiac murmur, and arrhythmias or respiratory distress (Yoon et al., 2008; Charlesworth et al., 2016). The condition varies in severity, with some animals being asymptomatic and others showing life-threatening cardiopulmonary dysfunction (Hunt, 2012). The condition is rare and has been reported in both dogs and cats (Fossum et al., 1989a,b; Sturgess et al., 1997; Charlesworth and Sturgess, 2012). Burmese cats seem to be predisposed (Sturgess et al., 1997; Hunt, 2012). The diagnosis is based on clinical and radiographic findings, allowing the identification of the sternal deformity (Mestrinho et al., 2011); however, computed tomography (CT) scans allow a more accurate evaluation of the disease and of the displacement of the intrathoracic structures, and a better surgical planning (Charlesworth and Sturgess, 2012; Charlesworth et al., 2016). As reported in human and veterinary medicine, this condition may have different degrees of severity and is graded using several indexes. The pectus severity index is the most commonly employed and is based on two indexes, the vertebral (VI) and the frontosagittal (FSI) (Boudrieau et al., 1990; Nuss et al., 1998; Risselada et al., 2006; Charlesworth et al., 2016; Hassan et al., 2018). The VI is the ratio between the depth of the thorax measured at the center of the dorsal surface of the 10th thoracic vertebral body (T10) and the dorsoventral diameter of T10 at the same level; the FSI is the ratio of the thoracic width at T10 on a ventro-dorsal radiograph and the distance from the ventral surface of T10 to the nearest point on the sternum. An increase in the FSI (reference range 0.7–1.3) and a decrease in the VI (reference range 12.6–18.8) are usually reported in pectus excavatum (Fossum, 2013; Charlesworth et al., 2016; Hassan et al., 2018). The treatment of choice in very young animals with compliant sternum is surgical repair with external splint techniques (Hunt, 2012; Fossum, 2013). It has been demonstrated that these techniques are contraindicated when the animal is older and the sternum is noncompliant (Shires et al., 1988; Crigel and Moissonnier, 2005; Fossum, 2013). Case DetailsA 3-month-old domestic shorthair intact female cat weighting 330 g was referred to the Veterinary Teaching Hospital (VTH) of the University of Parma for respiratory distress, exercise intolerance, anorexia, and lethargy. The symptoms started immediately after weaning, and had been mild and intermittent until the day of presentation to the VTH. At consultation, the kitten showed signs of severe dyspnea and tachypnea, lethargy, delayed growth, and needed oxygen supplementation and hospitalization. On clinical examination, a severe dorsal displacement of the most caudal part of the sternum and a transversal flattening of the entire thorax were observed. Thoracic auscultation showed increased vesicular murmur and respiratory stridor. Routine blood work and blood gas analysis revealed no significant alterations, apart from hypoglycemia. Radiographic examination revealed severe dorsal displacement from the fifth sternebra to the xiphoid cartilage and a dorsal displacement of the cardiac silhouette on the lateral view (Fig. 1A), and a left mediastinal shift and compression of the left pulmonary lobes on the ventrodorsal view (Fig. 2A). VI and FSI were measured on the radiographs, being 3.2 and 9.1, respectively. The authors decided to measure these two indices also at the level of T9 because this vertebra corresponded to the point of minimum height of the thorax. VI and FSI at the level of T9 were 2.3 and 15.2, respectively. The authors measured also the minimum thoracic height (MinTH) that resulted 4.3 mm, at the level of the last sternebra.
Fig. 1. Left lateral radiographic view of the thorax of the cat with pectus excavatum. Comparison of patient’s thorax before (A) and after (B) surgical treatment at 9 months postoperatively.
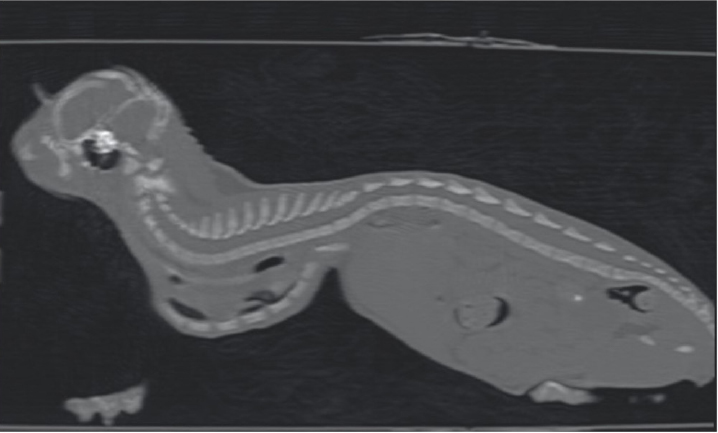
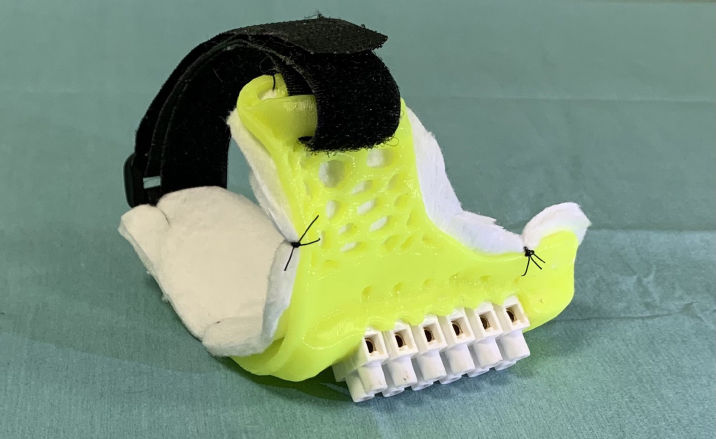
Fig. 2. Ventrodorsal radiographic view of the thorax of the cat with pectus excavatum. Comparison of patient’s thorax before (A) and after (B) surgical treatment at 9 months postoperatively. The kitten was hospitalized for 4 days until the respiratory rate and the clinical condition improved. During the hospitalization, the cat was treated with oxygen supplementation, intravenous fluid therapy with ringer lactate solution, and high-energy nutrition. Due to the very young age and the clinical condition of the patient, the surgical treatment was delayed for 1 month. The cat was discharged from hospital advising the owners to feed the kitten every 2 hours and to administer oxygen if the respiratory rate increased. One month later, the kitten weighted 550 g and presented again for intermittent tachypnea, needing oxygen administration, and exercise intolerance. A preoperative CT was performed to evaluate the exact position of the intrathoracic structures and to assess safe corridors for the needle passage. CT was performed using a 20 slice TC scanner (Siemens® Somatom Sensation Open, Munich, Germany) with a 140 Kw and 150 mAs technique, in the awake patient, using a Plexiglas “mouse trap” (VetMouse trapTM) to restrain the animal. Only basal scans were obtained with 2 mm slice thickness, using 0.5 seconds rotation time. CT confirmed radiographic findings, adding more details (Fig. 3). Tomographic images revealed a complete dislocation of the heart into the left hemithorax, with complete atelectasis of the caudal portion of the cranial left lung lobe and partial atelectasis of the caudal left lobe near the hilum. The heart and the tracheal bifurcation were dislodged cranially compared to the normal anatomical position. The aortic arch was completely left-placed, together with the heart, but properly positioned at the level of the aortic hiatus. The caudal vena cava was dislocated to the left, resulting in a more central position, exactly interposed between the 12th vertebral body and the xiphoidal cartilage apex. The caudal vena cava and the aorta completely occupied the narrowed diaphragmatic portion of the thorax at the level of the xiphoid cartilage. In order to model a customized 3D-printed splint, a segmentation of CT DICOM was performed (3DSlicer, www.slicer.org). A digital model of the skin and rib cage was generated. The sternebrae were identified and digitally projected on the skin surface reconstruction in order to find the proper position for the terminal blocks (to be used to gradually tie the sutures) and wire-passing holes. For this purpose, a commercial electrician terminal block with six holes was used. Following surgeon’s indications, a provisional digital model of the splint was designed (Meshmixer, www.meshmixer.com), adjusted according to the surgeon’s further instructions, and the final digital model was requested to PlayCast s.r.l. (Padova, Italy). The final digital model of the splint (Fig. 4), with custom wire-passing holes, dorsal velcro strap passer-by, and a tailored socket for terminal blocks was designed by PlayCast s.r.l. using a CAD commercial software (Rhyno6, Mcneel, Washignton, DC). The final version of the splint was designed foreseeing some degree of growth of the animal. After the surgeon’s approval, the definitive 3D-printed model was produced (Fig. 5) (3DBNZ Atlas 4070 Technik, ItalyMaker, Milan, Italy) with PETg material (FormFutura, Nijmegen, The Netherlands). Lastly, the terminal block was glued to the 3D-printed splint and stabilized (Superattack, Loctite, Henkel, Germany). Locking mechanism clips were tested with surgical wires in order to test locks strength. Before being applied to the animal, the splint was padded with soft cast padding to make it more comfortable.
Fig. 3. Longitudinal reconstruction from CT scan. Notice the close relationship between the xiphoid process and the ventral aspect of the vertebral column.
Fig. 4. CAD model of the splint. A 3D-printed model (Ultimaker S3, Ultimaker, The Netherlands) of the rib cage in PolyLactic Acid (3DiTALY, Rome, Italy) was used for surgical planning. The kitten was premedicated with butorphanol 0.2 mg/kg intramuscularly, and anesthesia was induced with 2% isoflurane in 100% oxygen, via a face mask. After endotracheal intubation, inhaling anesthesia was maintained with 2% isoflurane in 100% oxygen via a nonrebreathing circuit. Twenty minutes before skin incision, 20 mg/kg of cefazolin was administered intravenously for antibiotic prophylaxis. The kitten was placed in dorsal recumbency and prepared for aseptic surgery. A 1.5 cm skin incision was made over the xiphoid process, just caudal to the point of maximum dorsal displacement of the sternum. The seventh sternebra was grasped with Adson Brown forceps and pulled ventrally to allow the passage of the needle, avoiding lesion to the caudal vena cava and aorta. The needle was inserted in reverse manner to avoid damage to vascular structures. The second and the third sutures were placed under direct vision at the level of the sixth and fifth sternebra, respectively, applying gentle traction on the first suture to lift the sternum and facilitate the passage of the needle. The incision was routinely sutured with 4–0 PDS. The fourth and fifth sutures were blindly placed percutaneously around the first and third sternebra, respectively. Each suture was kept long and tagged with a mosquito hemostat. When all the sutures were placed, each end of the suture was inserted in the corresponding hole of the cast using an 18-gauge syringe needle. At this point, the two ends of each suture were passed in the locking mechanism clip and, by applying a gentle traction, the bolt of the locking mechanism was screwed while an operator held the suture tight. The caudalmost three sutures were tightened and held in place by the bolt, starting from the most caudal; conversely, the cranialmost two sutures were ligated. A velcro strap, encompassing the cast, was placed around the caudal part of the thorax to ensure proper splint positioning. Postoperative radiographs were obtained in lateral and ventrodorsal projections.
Fig. 5. Definitive 3D-printed splint with soft cast padding. The kitten recovered well from anesthesia, and she was discharged from hospital 24 hours postoperatively, with a normal respiratory and heart rate. During hospitalization, the cat was strictly monitored and buthorphanol 0.2 mg/kg every 4 hours was administered intravenously for pain control. The splint was well tolerated and the kitten returned to a normal activity immediately. The splint was first protected with a bandage made of cotton and vetrap and, after 2 days, it was replaced by a custom-made bodysuit. No activity restriction was required in the postoperative period. At follow-up examination 7 days postoperatively, the kitten was in good clinical condition and no episodes of dyspnea were reported by the owner. Increased appetite, exercise tolerance, and a more active attitude were also reported. Therefore, the cat was premedicated with butorphanol 0.2 mg/kg intramuscularly and anesthesia was induced as previously described (Fig. 6). The three most caudal sutures were unlocked screwing out the bolt and, applying a gentle traction, and tightened more firmly. This procedure was repeated at the next follow-up examination after further 7 days, but this time the sutures were tied definitively. No splint-associated complications were encountered during follow-up examinations. The splint was removed without sedation 1 month from the first surgery, after a radiographic control of the chest; by that time the surgical incision was healed completely and no skin dermatitis or suture reaction was found under the splint and in the cutaneous contact point. Radiographs were obtained as previously described at each follow-up examination (Figs. 1B and 2B). IV, FSI, and MinTH were calculated and the values are reported in Table 1. Four months postoperatively the kitten increased her body weight from 550 to 2,436 g and the skeletal deformity was dramatically improved at physical examination. No episodes of dyspnea or exercise intolerance were noticed by the owner. The VI and FSI measured on the radiographs obtained 4 months after surgery were 10.2 and 2.0, respectively. The same indexes measured at the level of T9 were 8.8 and 2.3, respectively, and MinTH was 21 mm. Nine months postoperatively the cat underwent an ovariohysterectomy with no anesthetic-related complications. The cat was discharged from hospital 6 hours postoperatively and recovered uneventfully. Radiographs were obtained as previously described before the surgery. VI and FSI resulted in 12.4 and 1.5, respectively; the same indexes measured at the level of T9 were 9.4 and 2.1, respectively, and MinTH was 26 mm.
Fig. 6. Cat in dorsal recumbency after sedation at first postoperative control. Note the 3D-printed cast (yellow), terminal block with screws (white), and velcro strap (black). Table 1. Radiographic indices and measurements before and after surgery.
DiscussionPectus excavatum is the dorsal elevation of the caudal part of the sternum that can be associated with a dorsoventral flattening of the entire thorax (Mestrinho et al., 2011; Hunt, 2012; Fossum, 2013). Surgery is considered the treatment of choice in dogs and cats in case of mild to severe deformity of the sternum with functional impairment (Shires et al., 1988). Different surgical techniques have been described in the literature to treat this disease, both with internal or external fixation (Crigel and Moissonnier, 2005; Mestrinho et al., 2011; Hunt, 2012; Fossum, 2013). External splinting techniques are indicated in case of very young animals, where the costal cartilages and sternum are pliable and the thorax can be reshaped (Fossum et al., 1989a,b; Crigel and Moissonnier, 2005; Fossum, 2013). The main goal of the surgery is to correct the sternal concavity and flattening of the thorax so that the intrathoracic structures could develop normally (Risselada et al., 2006; Mestrinho et al., 2011). In those animals, due to compliant sternum and high growth potential, this is achieved by applying a permanent traction in a ventral direction to the malformed sternebrae and keeping them in that position with an external device until bone ossification occurs. Successful treatment with internal splinting using a bone plate (Risselada et al., 2006) and a combination of a longitudinal sternebral pin with external splint (Crigel and Moissonnier, 2005) was described in older cats with noncompliant sternum.
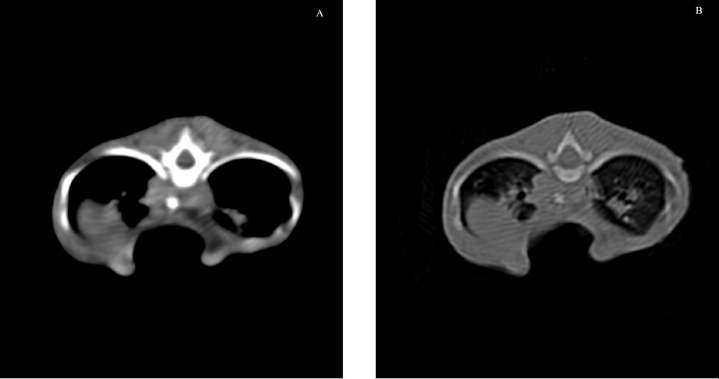
Fig. 7. Transverse CT scan of the cat’s chest at the level of the xiphoid process, soft tissue window view (A), and bone window view (B). The cat in this report was 3 months old and, based on the pectus severity index, had a severe pectus excavatum; therefore, an external splinting technique with a 3D-printed cast, customized on the base of CT images was chosen. CT scan was useful to better assess skeletal deformities, anomaly of the intrathoracic structures, and safe corridors for the needle passage. Moreover, CT was fundamental to design the splint. Five sutures were applied, the most caudal three being gradually pulled outward the concave part of the sternum and the other two directly ligated to the splint. The decision to surgically approach the seventh sternebra and xiphoid process to place the corresponding suture under direct vision was taken based on the result of the CT scan. CT images showed a close relationship between the seventh sternebra and the xiphoid process (Fig. 7), with the aorta and caudal vena cava occupying most of the thoracic space, making blind passage of the needle too risky. With this approach, the skeletal segment could be grasped and pulled ventrally, increasing the space for a secure passage of the needle. There were no surgical complications such as organ laceration or puncture, nor postoperative complications such as re-expansion pulmonary edema. The concern of the development of the re-expansion pulmonary edema, based on the sudden increase of the intrathoracic pressure due to the correction of the sternal concave deformity, lead the authors to the decision to gradually correct the sternal deformity. In this way, the increase in intrathoracic pressure and, therefore, lung re-expansion occurred little by little, reducing the risk for this complication. For these reasons, the sutures were gradually tightened during the first 2 weeks in response to the skeletal development of the kitten, and then kept in place for further 2 weeks to allow consolidation. The use of an electrician terminal block applied to the ventral part of the splint between the two rows of holes for the passage of the sutures perfectly fit this purpose. These locking clips allowed holding the sutures in position without the need to untie them each week. The 3D-printed splint adapted perfectly to the thorax of the cat and no adjustments were necessary. The T form of the splint was comfortable for the kitten because all the area around the forelimb was left free and the thorax had the space to develop without compression. The arms of the T stabilized the splint and, with the velcro strap positioned around the thorax, prevented its displacement. The velcro strap around the thorax was adjusted at the end of the third week postoperatively to adapt to the increased body weight of the kitten, while the splint was tailored to take into account the increase in body volume and was filled with cotton in the inner part to be more comfortable and better tolerated. There were no splint-associated complications such as dermatitis, cutaneous lesion, or compressive wounds in the area of splint contact. The successful management of this case, complicated by the poor physical development of the kitten and the severity of the symptoms and malformation, suggests that the use of patient-specific 3D-printed splints associated with progressive correction of the malformation can be a better solution for these severe cases. The authors consider CT scan very important the correct evaluation of the malformation and the surgical planning, especially when such a narrow passage is present for suture placement. The placement of the traction sutures under direct vision, with or without manipulation of the corresponding sternebra, avoided intraoperative complications. AcknowledgmentThe authors thank student Noemi Mercati for her commitment in assisting with her kitten. Authors’ contributionsMattioli G. and Martano M. contributed to the conception and design of the work, acquisition of data, data analysis, interpretation, and surgical management of the case. Zanfabro M. contributed to the conception of the study, splint design, and 3D printing. Bonazzi M. contributed to the acquisition of CT data and data analysis. Conflict of interestZanfabro M. was a research fellow at University of Parma (Research Fellowship sponsored by PlayCast s.r.l.) at the time of the study. All other authors report no conflict of interest. ReferencesBoudrieau, R.J., Fossum, T.W. and Harstfield, S.M. 1990. Pectus excavatum in dogs and cats. Compend. Contin. Educ. Vet. 12, 341–335. Charlesworth, T.M. and Sturgess, C.P. 2012. Increased incidence of thoracic wall deformities in related Bengal kittens. J. Feline Med. Surg. 14, 365–368. Charlesworth, T.M. Schwarz, T. and Sturgess, C.P. 2016. Pectus excavatum: computed tomography and medium-term surgical outcome in a prospective cohort of 10 kittens. J. Feline Med. Surg. 18, 613–619. Crigel, M.H. and Moissonnier, P. 2005. Pectus excavatum surgically repaired using sternum realignment and splint techniques in a young cat. J. Small Anim. Pract. 46, 352–356. Fossum, T.W. 2013. Surgery of the lower respiratory system. In Small animal surgery. Eds., Fossum, TW. St. Louis, MO: Mosby, pp: 983–988. Fossum, T.W., Boudrieau, R.J. and Hobson, H.P. 1989a. Pectus excavatum in eight dogs and six cats. J. Am. Vet. Med. Assoc. 25, 595–605. Fossum, T.W., Boudrieau, R.J. and Hobson, H.P. 1989b. Surgical correction of pectus excavatum using external splintage in two dogs and a cat. J. Am. Vet. Med. Assoc. 195, 91–97. Hassan, E.A., Hassan, M.H. and Torad, F.A. 2018. Correlation between clinical severity and type and degree of pectus excavatum in twelve brachycephalic dogs. J. Vet. Med. Sci. 80, 766–771. Hunt, G.B. 2012. Thoracic wall. In Veterinary surgery small animal. Eds., Tobias, KM. and Johnston, SA. St. Louis, MO: Saunders, pp: 1779–1782. Mestrinho, L.A., Ferreira, C.A., Lopes, A.M., Niza, M. and Hamaide, A.J. 2011. Open surgical correction combined with an external splint for correction of a non-compliant pectus excavatum in a cat. J. Feline Med. Surg. 14, 151–154. Nuss, D., Kelly, R.E. Jr., Croitoru, D.P. and Katz, M.E. 1998. A 10 years review of a minimally invasive technique for the correction of pectus excavatum. J. Pediatr. Surg. 33, 545–552. Risselada, M., De Rooster, H., Liuti, T., Polis, H. and Van Bree, H. 2006. Use of internal splinting to realign a noncompliant sternum in a cat with pectus excavatum. J. Am. Vet. Med. Assoc. 228, 1047–1052. Shires, P.K., Waldon, D.R. and Payne, J. 1988. Pectus excavatum in three kittens. J. Am. Anim. Hosp. Assoc. 24, 203–208. Sturgess, C.P., Waters, L., Gruffydd-Jones, T.J., Nott, H.M. and Earle, K.E. 1997. Investigation of the association between whole blood and tissue taurine levels and the development of thoracic deformities in neonatal Burmese kittens. Vet. Rec. 141, 566–570. Yoon, H.Y., Mann, F.A. and Jeong, S.W. 2008. Surgical correction of pectus excavatum in two cats. J. Vet. Sci. 9, 335–337. | ||
| How to Cite this Article |
| Pubmed Style Mattioli G, Zanfabro M, Bonazzi M, Martano M. Use of a customised 3D-printed external splint for the correction of a severe pectus excavatum in a 3-month-old kitten. Open Vet. J.. 2022; 12(1): 148-155. doi:10.5455/OVJ.2022.v12.i1.18 Web Style Mattioli G, Zanfabro M, Bonazzi M, Martano M. Use of a customised 3D-printed external splint for the correction of a severe pectus excavatum in a 3-month-old kitten. https://www.openveterinaryjournal.com/?mno=117883 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i1.18 AMA (American Medical Association) Style Mattioli G, Zanfabro M, Bonazzi M, Martano M. Use of a customised 3D-printed external splint for the correction of a severe pectus excavatum in a 3-month-old kitten. Open Vet. J.. 2022; 12(1): 148-155. doi:10.5455/OVJ.2022.v12.i1.18 Vancouver/ICMJE Style Mattioli G, Zanfabro M, Bonazzi M, Martano M. Use of a customised 3D-printed external splint for the correction of a severe pectus excavatum in a 3-month-old kitten. Open Vet. J.. (2022), [cited January 25, 2026]; 12(1): 148-155. doi:10.5455/OVJ.2022.v12.i1.18 Harvard Style Mattioli, G., Zanfabro, . M., Bonazzi, . M. & Martano, . M. (2022) Use of a customised 3D-printed external splint for the correction of a severe pectus excavatum in a 3-month-old kitten. Open Vet. J., 12 (1), 148-155. doi:10.5455/OVJ.2022.v12.i1.18 Turabian Style Mattioli, Giovanni, Matteo Zanfabro, Mattia Bonazzi, and Marina Martano. 2022. Use of a customised 3D-printed external splint for the correction of a severe pectus excavatum in a 3-month-old kitten. Open Veterinary Journal, 12 (1), 148-155. doi:10.5455/OVJ.2022.v12.i1.18 Chicago Style Mattioli, Giovanni, Matteo Zanfabro, Mattia Bonazzi, and Marina Martano. "Use of a customised 3D-printed external splint for the correction of a severe pectus excavatum in a 3-month-old kitten." Open Veterinary Journal 12 (2022), 148-155. doi:10.5455/OVJ.2022.v12.i1.18 MLA (The Modern Language Association) Style Mattioli, Giovanni, Matteo Zanfabro, Mattia Bonazzi, and Marina Martano. "Use of a customised 3D-printed external splint for the correction of a severe pectus excavatum in a 3-month-old kitten." Open Veterinary Journal 12.1 (2022), 148-155. Print. doi:10.5455/OVJ.2022.v12.i1.18 APA (American Psychological Association) Style Mattioli, G., Zanfabro, . M., Bonazzi, . M. & Martano, . M. (2022) Use of a customised 3D-printed external splint for the correction of a severe pectus excavatum in a 3-month-old kitten. Open Veterinary Journal, 12 (1), 148-155. doi:10.5455/OVJ.2022.v12.i1.18 |