| Original Article | ||
Open Vet. J.. 2022; 12(1): 129-137 Open Veterinary Journal, (2022), Vol. 12(1): 129–137 Original Research Dietary aflatoxin B1-related risk factors for the presence of aflatoxin M1 in raw milk of cows from EcuadorByron Puga-Torres1,2*, Lenin Ron3, and Carlos Gómez21Laboratorio de Control de Calidad de Leche, Facultad de Medicina Veterinaria y Zootecnia, Universidad Central del Ecuador, Quito, Ecuador 2Doctorado en Ciencia Animal, Facultad de Zootecnia y Escuela de Postgrado, Universidad Nacional Agraria La Molina, Lima, Perú 3Instituto de Investigación en Salud Pública y Zoonosis-CIZ, Facultad de Medicina Veterinaria y Zootecnia, Universidad Central del Ecuador, Quito, Ecuador *Corresponding Author: Byron Puga-Torres. Laboratorio de Control de Calidad de Leche, Facultad de Medicina Veterinaria y Zootecnia, Universidad Central del Ecuador, Quito, Ecuador. Email: bpuga [at] uce.edu.ec Submitted: 13/09/2021 Accepted: 08/02/2022 Published: 22/02/2022 © 2022 Open Veterinary Journal
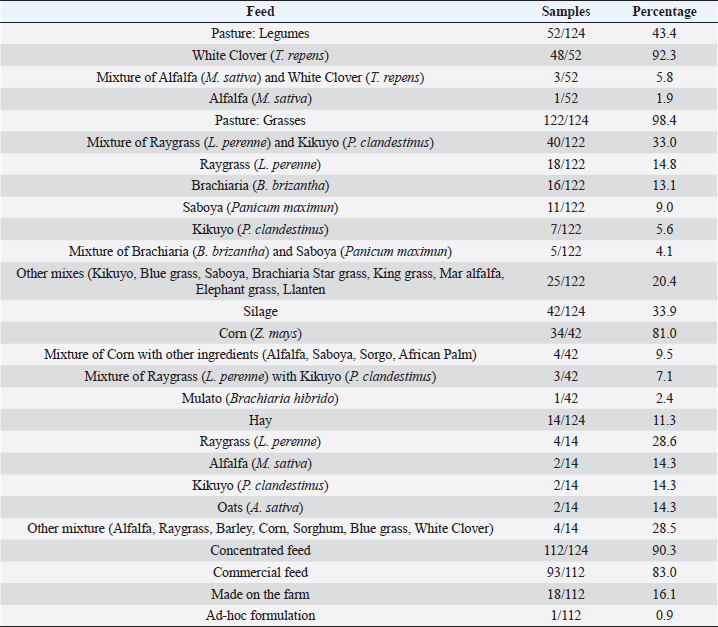
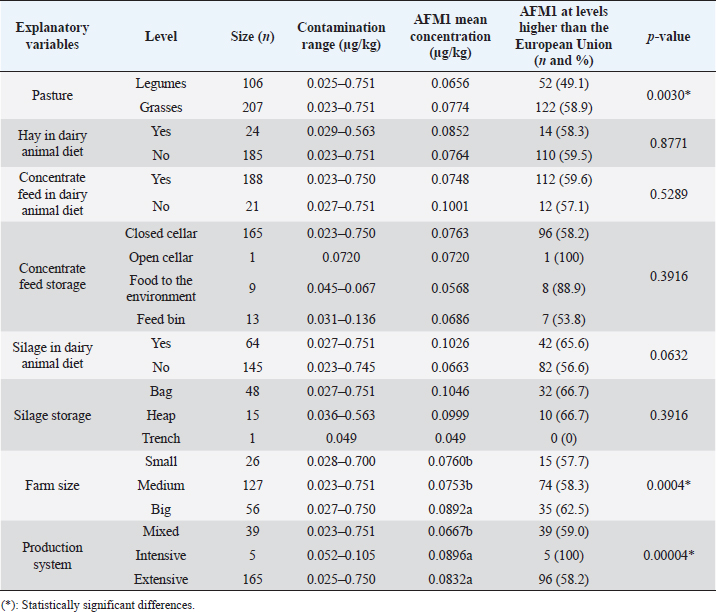
AbstractBackground: Aflatoxin M1 (AFM1) is hepatotoxic and carcinogenic, and it may be present in milk due to dairy cow’s ingestion when feed is contaminated with Aflatoxin B1 (AFB1). Aim: The objective of this research was to determine the potential risk factors of dietary AFB1 contamination in dairy cows, which causes AFM1 contamination of the raw milk, through an epidemiological survey and statistical analysis. Methods: 209 raw milk samples were collected, and AFM1 concentrations were detected by lateral flow immunochromatographic assay. Results: It was determined that 100% of the samples contained concerning levels of this mycotoxin with a mean of 0.077 μg/kg, which exceeds the maximum allowed by the European Union legislations (0.05 μg/kg) but not Ecuadorian Regulations (0.5 μg/kg). An adjustment to a linear model by weighted least squares was used to correct the presented heteroscedasticity. Potential risk factors for dietary AFB1 contamination were analyzed in relation to the appearance of AFM1 in milk from Ecuador. Among factors including legumes consumption, the use of silage, type of production system (intensive, extensive, and mixed), and farm size (small, medium, and big), the intensive production system and the big producers presented higher levels of AFM1. Conclusion: Considering that all the milk samples presented AFM1 and since there is no specific feed determined to be a risk factor, it was concluded that any of the feed offered to dairy cows may be contaminated with AFB1. It is necessary to reduce AFB1 levels in feed by implementing good agricultural practices and improving feed storage to decrease milk AFM1 levels. This study identified that intensive systems have a lot of animals, and silage is incorporated into the diet, which can significantly increase AFM1 levels. Keywords: Aspergillus, AFB1, AFM1, Feed, Milk. IntroductionMycotoxins are secondary metabolites of fungi (Toso et al., 2018), and among them, aflatoxins are the most toxic (Serrano and Cardona, 2015; Udomkun et al., 2017). The most common are produced mainly by Aspergillus flavus and Aspergillus parasiticus and, to a lesser extent, by Aspergillus nomius, Aspergillus ochraceoroseus, Aspergillus bombycis, and Aspergillus pseudota—mari (Campagnollo et al., 2016; Xiong et al., 2020). Aflatoxin B1 (AFB1) contaminates various feeds offered to animals, including those of dairy cows. After the dairy cow consumes feed contaminated with AFB1, it is partially metabolized in the rumen and later in the liver. Through this process, it is transformed mainly to the hydroxylated metabolite called Aflatoxin M1 (AFM1), which is eliminated in the urine, bile, and milk (Kuilman et al., 1998; Xiong et al., 2018; Hamzeh Pour et al., 2020; Xu et al., 2021). Between 12 and 24 hours after ingestion of AFB1, when the intake of AFB1 ceases, the concentration of AFM1 decreases rapidly and is not detected until three days after consuming the contaminated feed (Soriano del Castillo, 2007; Battacone et al., 2009). Recent research maintains that the transfer rate of AFB1 that cattle ingest and transform into AFM1 is between 1% and 6% (Intanoo et al., 2020). AFB1 and AFM1 can cause grave danger to public health, as they can cause kidney and liver damage, and liver, lung, and colon cancer (Marchese et al., 2018; Li et al., 2019; Saha Turna and Wu, 2019). These contaminants and their effects also alter dairy cows’ health, reproduction, and production (Kosicki et al., 2016; Jiang et al., 2019, 2020; Bani Ismail et al., 2020). Because the presence of AFM1 in milk is due to the consumption of feeds contaminated with AFB1 by lactating cows (Van Eijkeren et al., 2006; Abyaneh et al., 2019), it is necessary to identify the risk factors within milk production systems that are associated with the presence of aflatoxin, which is essential for the formulation of control programs and national policies (Patyal et al., 2020). In particular, Ecuadorian dairy production systems operate in clearly defined climatic conditions, which are temperate conditions of both highlands and under tropical conditions in the coastal and Amazonian regions. There are intensive production systems with a high demand of inputs in temperate climates and where cattle breeds are adapted primarily to dairy production. On the other hand, tropical zones of Ecuador have mixed systems dedicated to dual-purpose and where extensive systems are dominant, therefore having less demand for inputs. Our parallel study found that the highest mean of AFM1 in Ecuador occurs in the dry season and the tropical climatic region (Puga-Torres et al., 2020), assuming that food under these conditions is more contaminated by AFB1. Since there are no studies on the risk factors associated with the presence of AFM1 in raw milk from Ecuador, the objective of this research; therefore, AFM1 contamination in raw milk from Ecuador, was to determine these factors associated with the bovine diet through an epidemiological survey of Ecuadorian cattle production systems. Materials and MethodsSample collectionTwo hundred and nine pooled samples (~ 100 ml) of raw milk were collected from different farms between April and November 2019. They were collected directly from the refrigeration tanks, the same day of their milking, according to NTE INEN-ISO 707 standard: “Milk and dairy products. Guidelines for taking samples “ (INEN, 2014). Farms were classified by their size according to the number of cows in dairy production: 1 to 20 (small farms); from 21 to 100 (medium); and more than 100 (large). They were also classified by the production system (extensive, intensive, or mixed). Farms were located in the central-northern region of Ecuador, and its two climatic regions include: inter-Andean region (represented by the Province of Pichincha); and the coastal region (Provinces of Manabí and Santo Domingo de los Tsáchilas). Using cold chain for transport (between 2°C and 5°C), the samples were transferred to the Milk Quality Control Laboratory of the Faculty of Veterinary Medicine and Zootechnics at the Central University of Ecuador, where they were stored at −20°C until the respective analysis, whose maximum time was 48 hours from the collection. Epidemiological survey of producersWhile sampling was carried out, a questionnaire was performed with participating farmers. It aimed to gather information on the diet that their animals received in the days prior to the sampling and the different characteristics of their production systems. The questionnaire consisted of the following topics: a) Type of feed (grass, silo, hay, concentrated feed); b) Type of grass (legumes, grasses, and variety of each); c) Silo raw material and storage form; d) Type of haylage and its ingredients; e) Type of concentrated feed (prepared or augmented on the farm itself) and how it is stored; f) Farm size (Small, Medium, Big); and g) Production system (mixed, intensive, extensive). Regarding the concentrated feed offered to the animals, we do not know its formulation since the vast majority of it was commercial feed. Analysis of AFM1 in raw milk by Lateral Flow ImmunochromatographyMilk samples were thawed at room temperature and then subjected to centrifugation for 10 minutes at 4,000 × g. After centrifugation, the top layer of fat was removed, and the skim milk was analyzed for specific monoclonal antibodies against AFM1, following the procedure recommended by the AFLA M1-V VICAM® Assay Tests (Vicam, 34 Maple Street, Milford, MA 01757). The lower limit of detection was 0.025 μg/kg (25 ppt), and the limit of quantification was 0.01 μg/kg (10 ppt). The recovery rate was approximately 95% (10 milk samples were spiked with standard AFM1 working solutions at concentrations of 0, 0.01, 0.05, 0.1, 0.3, 1.0, and 10 μg/kg). The coefficient of variation was approximately 15%. 200 µl of each milk sample was added to the Afla M1-V strip test vial, which contains the conjugate. It was mixed 3 times for 5 seconds each and incubated at 40°C for 10 minutes with the test strip. After the indicated time, the strip was inserted into the reader, and the reading proceeded. The incubation was carried out in a Delvotest® Incubator DSM-MiniS-11548 (Hangzhou Allsheng Instrument Co., Ltd, Building 1 & 2, Zheheng Science Park, Zhuantang Town, Xihu District, Hangzhou, Zhejiang 310024, China) and reading with a Vertu 1648 Lateral Flow Reader medium (Vicam, 34 Maple Street, Milford, MA 01757). The result obtained was reflected directly on the screen interface. Statistical analysisThe results and surveys were tabulated in a Microsoft Excel sheet. A frequency table was used to obtain the percentage of each feed in relation to the levels of AFM1 that exceeded the Maximum Allowed Limit of the Ecuadorian and/or European Regulations. Then, a 95% confidence interval was generated for the proportion of samples that did not meet these regulations. The level of AFM1 in raw milk from dairy farms in Ecuador was used as the dependent variable, and other qualitative factors were used as explanatory variables. An adjustment to a linear model by weighted least squares was used to take into account the over-dispersion (heteroscedasticity) existing in the different combinations of factor levels. Weights were obtained in such a way that the observations with lower variance are given more weight. Thus, the inverse of the square root of the fitted values were estimates of the weights. For the results, the analysis of variance is presented for the factors under study with the sums of squares type III. If necessary, multiple tests of comparisons were done to establish the differences between the levels of the different factors. The free statistical software Rstudio version 1.2.5019 (Rstudio Inc. Boston, MA) was used, and the level of statistical significance was set at 0.05. Ethical approvalEthical approval does not apply to this research, since no imples was performed on animals. Milk imples were taken directly from the farms’ refrigeration tanks. ResultsMilk contamination by AFM1In a previously published study (Puga-Torres et al., 2020), it was determined that 100% (209/209) of the samples presented AFM1 levels, reaching a mean of 0.0774 μg/kg. Only 1.9% of the samples (4/209) fail to comply with the Ecuadorian Regulations’ Maximum Allowed Limit of 0.5 μg/kg. Regarding the European Regulation, 59.3% of the samples (124/209) are above their maximum allowed concentration of 0.05 μg/kg. Significant differences were found between provinces (being higher in Manabí), climatic regions (higher levels in the coastal region), and in the climatic season (with a greater presence of AFM1 in the dry season). Risk factors associated with the presence of AFM1Table 1 details the type of pasture, hay, silage, and concentrated feed consumed by the animals of the sampled farms, based on the 124 livestock farms whose milk contains levels higher than the maximum allowed by European legislation. The most consumed legume was White Clover (Trifolium repens), while for grasses, the most used are the mixture of Raygrass (Lolium perenne) with Kikuyo (Pennisetum clandestinus), Raygrass (L. perenne), and Brachiaria (Brachiaria brizantha). Among producers who offer silage to their animals, the majority manage Corn silos (Zea mays), and they store it in bag silos to a greater extent. Regarding hay feeding, the main ingredient was Raygrass (L. perenne), followed by Alfalfa (Medicago sativa), Kikuyo (P. clandestinus), and Oats (Avena sativa). Finally, the vast majority of concentrated feed was a commercial source, and it was predominantly stored in a closed cellar. Regarding the risk factors associated with the presence of AFM1 (Table 2), it was found that there is no statistically significant relationship (p > 0.05) with respect to the consumption of legumes, hay, and concentrated feed by the animals. It was only significantly associated (p < 0.05) with the cattle fed with grasses; however, there are no studies that demonstrate the presence of Aspergillus in fresh pastures (Merlassino, 2014; Gallo et al., 2015). In the case of silage used for dairy cow diets, this was the only feed that presented very close association levels for the contaminants. For this reason and because all the milk samples presented levels of AFM1, any of the feeds can constitute a source of AFB1. In the case of production systems and farm size, there is a statistically significant relationship (p < 0.05) between the intensive system and large producers. They both presented higher levels of AFM1 when correcting the heteroscedasticity, probably due to the greater use of silage and concentrated feed in the bovine diet. DiscussionThis study revealed significant differences concerning large producers and the intensive system. This corroborates reports from Kenya, where feed tends to be contaminated by aflatoxin in intensive systems (Kagera et al., 2019). However, these data do not agree with those obtained in Iran and Serbia, where the highest levels of AFM1 were found in small producers (Hashemi, 2016; Horvatović et al., 2016). Similarly, it does not concur with a study in India, where there was no significant difference between the different producers and types of farms (Patyal et al., 2020). In our research, the animals’ high consumption of grass was observed. This is because Ecuador has favorable environmental conditions, in most territories, for the continuous production of forages throughout the year (León et al., 2018). For the vast majority of grazing dairy cattle, this is cheaper than other types of feed (Valverde, 2013). However, no studies demonstrate the preference of Aspergillus in the aforementioned pastures. On the other hand, it is known is that certain endophytic fungi perform a type of symbiosis with perennial Raygrass and are capable of storing toxic secondary metabolites within the plant (Merlassino, 2014). Therefore, pastures are not considered prone to contamination by AF (Tsiplakou et al., 2014), but this can occur in concentrated feed, silo, or hay (Fink-Gremmels, 2008). Likewise, a review of several previous publications revealed that there are few studies regarding the presence of mycotoxins in fresh pastures. It is very limited compared to the presence in cereals. However, it is that filamentous fungi, only the genera Fusarium and Alternaria, can grow in forages, while Aspergillus has a preference for grains and cereals. For this reason, none of the studies carried out worldwide report the presence of this type of fungi and AF in fresh forages (Gallo et al., 2015). Although most fungi are indeed eliminated in the production of silage feed, those of the genus Aspergillus are capable of supporting high concentrations of organic acids, carbon dioxide, and low oxygen availability (Gallo et al., 2015). Therefore, it constitutes an excellent substrate for the growth and development of Aspergillus and, consequently, AF (Del Palacio et al., 2016). This occurrence depends on the time of harvest, irrigation, pest control, humidity, and mechanical damage to the grain at the time of ensiling (Prandini et al., 2009). The present study determined that the use of silage in animal feeding was the closest to the level of significance in association with the presence of AFM1 in milk. Corn (Z. mays), being the most used ingredient in its preparation, is the grain most prone to contamination by AF (Alonso et al., 2011; Ogunade et al., 2018; Kagera et al., 2019). Maize silage is one of the main risk factors for AF in several countries. For example, a study in Kenya reported 99% of the samples (83/84) contained levels of AFM1 with a relatively high mean of 0.84 µg/kg, where 56.3% of the animals received silage (Kagera et al., 2019). In Mexico, 48 samples of organic milk presented the highest concentrations of AFM1 during the dry season due to non-compliance with good agricultural practices and silage, likely contaminating the animal feed (Gutiérrez et al., 2013). For these reasons, the use of corn silage in Ecuador could be an important source of AFB1 in the diet of dairy cows. Table 1. Types of pasture, silo, hay, and concentrated feed received by animals (124/209) whose milk does not comply with European Regulation in AFM1.
Regarding silage storage, it is known that the fungi develop better when there is poor storage (Kemboi et al., 2020). This favors their growth, particularly when there is an inadequate sealing of the silo, holes in the packaging, or inadequate storage facilities (Variane et al., 2018). The warmer and more humid environmental conditions inside the silo are also more favorable for the development of fungi (Pereyra et al., 2008). Our survey determined that the largest number of farmers store it in bags, and this corroborates the reports of several authors who found the highest percentage of contamination with AF occurring in products ensiled in bags, mainly after long and inadequate storage periods (Fink-Gremmels, 2008; Prandini et al., 2009; Baliukonien et al., 2012). These periods are notably influenced by harvest time of corn, fertilization, irrigation, pest control, and silage moisture (Prandini et al., 2009). In relation to the use of hay, various studies have determined that it is a feed where Aspergillus can grow, and therefore, the presence of AF can be found (Tsiplakou et al., 2014; Dhakal and Sbar, 2020). In several studies, alfalfa hay has presented AFB1 at levels higher than the maximum limit allowed by the European Union (Sugiyama et al., 2008; Bani Ismail et al., 2020; Rodríguez-Blanco et al., 2020). In the case of Ecuador, the most used ingredients were Alfalfa and Raygrass. Table 2. Explanatory variables included in the analysis of risk factors for occurrence of AFM1 in raw Ecuadorian milk (n=209).
Our research also found that most farmers feed their animals concentrated feed, which is of different commercial brands or has been made on the farm itself. This agrees with the study carried out in Punjab, India, where feeding with ready-made concentrated feed resulted in the presence of AFM1 in milk (Patyal et al., 2020). Similarly, in Mexico and Kenya, the greater presence of AFM1 was due to higher consumption of concentrate during milking, attributed to the prepared feed (Gutiérrez et al., 2013; Kagera et al., 2019). This can be explained because Aspergillus prefers cereals or grains (Alpízar Solís, 2016; Giovanni et al., 2019), affirmed by the correlation between AFM1 in milk and the presence of AFB1 in the concentrated feed received by dairy cows (Sugiyama et al., 2008). It is also necessary to control the storage of concentrated feed since the temperature and humidity contribute to the production of AFB1 (Ismail et al., 2015; Yunus et al., 2019). This is because poor storage results in it becoming contaminated with AFB1 (Milićević et al., 2019). In Ecuador, there is possibly inadequate storage of the concentrate, which causes contamination of the feed with fungi. In several studies in China, statistical variation was found only between regions for the presence of AFM1 in raw cow milk (Xiong et al., 2015, 2018; Xu et al., 2021). On the other hand, the study carried out in three regions of Algeria (north-east, north-central, and north-west) on the risk factors associated with milk contamination presented significant differences between both regions and seasons (higher levels between spring and autumn), as well as in small farms (Mohammedi-Ameur et al., 2020). We found something different in Kenya, where urban and peri-urban farmers in Nairobi county and Kasarani sub-county. The authors determined no correlation between farmer knowledge and gender concerning the prevalence of AFM1, but on the other hand, this relationship existed for intensive production operations where they administered silage (Kagera et al., 2019). In our previous study, statistical analysis was carried out with non-parametric tests and reveal significant differences between regions (greater in the coastal area compared to the Inter-Andean region), in climatic season (greater in the dry season), months of study (differences found in June) (Puga-Torres et al., 2020). Evidence has been found that current climate change, which causes droughts and floods, can increase the production of mycotoxigenic fungi and, therefore, explain their increase in feed (Milićević et al., 2019). Likewise, the prevalence of mycotoxins in feed can be high, with AFB1 being found in peanut flour, cottonseeds, and corn, which are ingredients of dairy cow feed (Marin et al., 2013). Therefore, producers must be aware of the detrimental effects of AF on their animals and public health, prompting them to consider strategies to reduce their presence in the feed offered to dairy cattle (Mohammedi-Ameur et al., 2020). This can be achieved through good agricultural practices in pre and post-harvest, correct storage of forage crops, and physical or chemical decontamination of feed (Giovati et al., 2015). It is interesting to note that the studies that report 100% occurrence of AFM1 in milk come from those experiments using screening techniques, such as ELISA or LFIA. These are reported mainly in developing countries where it is costly to use measurement techniques such as HPLC. Thus, investigators should aim to use such techniques for improving their results and studies. ConclusionAFM1 contamination in raw milk from Ecuador was widespread, and AFM1 levels in a small number of milk samples exceeded the legal limit set by Ecuador. By analyzing dietary AFB1-related risk factors for the presence of AFM1, it was found that no feed was determined as a specific risk factor for the presence of AFM1 in the raw milk of Ecuador. In the case of using silage as part of the diet of dairy cows, there was only one group that had a level very close to that of association. Caution should be exercised with all the feed offered to animals because any of them can constitute a source of AFB1. Therefore, it is important to reduce the levels of AFB1 in raw materials and food through good farming practices and to educate farmers on this problem through training. AcknowledgmentsWe extend our gratitude to the General Research Directorate of the Central University of Ecuador for funding this research (Grant: DOCT-DI-2018-21). The authors would also like to thank Gabriela Cisneros, Mayra Cachiguango, Denisse Alarcón, David Salazar, Miguel Cáceres and Ms. Rosario Tigse for their valuable collaboration. We would also like to thank Michael James Stablein of the University of Illinois Urbana-Champaign for his translation services and review of this work. Conflict of interestThe authors declare that there are no conflicts of interest. Authors contributionConceptualization, B.P.T. and C.G.B; data curation, B.P.T, L.R.; formal analysis, B.P.T, L.R; funding acquisition, B.P.T; investigation, B.P.T and C.G.B; methodology, B.P.T; supervision, C.G.B; validation, L.R., C.G.B; writing—original draft, B.P.T; writing—review and editing, C.G.B. ReferencesAbyaneh, H.K., Bahonar, A., Noori, N., Yazdanpanah, H. and Aliabadi, M.H.S. 2019. Exposure to aflatoxin M1 through milk consumption in Tehran population, Iran. Iran. J. Pharm. Res. 18, 1332–1340; doi:10.22037/ijpr.2019.1100764 Alonso, V., Gonzalez-Pereyra, M., Armando, M., Dogi, C., Dalcero, A., Rosa, C., Chiacchiera, S. and Cavaglieri, L. 2011. Silage contribution to aflatoxin b1 contamination of dairy cattle feed. In (Ed.), Aflatoxins - Detection, Measurement and Control. IntechOpen. https://doi.org/10.5772/22136. Alpízar Solís, C. 2016. Presencia de hongos y contaminación con micotoxinas en ensilajes para alimentación de rumiantes. Artículo de Revisión. Rev. Ciencias Vet. 33, 7; doi:10.15359/rcv.33-1.1 Baliukonien, V., Bakutis, B., Vaivadait, T., Bartkiené, E. and Jovaišien, J. 2012. Prevalence of fungi and mycotoxins in silage and milk in Lithuania. Vet. ir Zootech. 59, 3–9. Bani Ismail, Z., Al-Nabulsi, F., Abu-Basha, E. and Hananeh, W. 2020. Occurrence of on-farm risk factors and health effects of mycotoxins in dairy farms in Jordan. Trop. Anim. Health Prod. 52, 2371-2377. Battacone, G., Nudda, A., Palomba, M., Mazzette, A. and Pulina, G. 2009. The transfer of aflatoxin M1 in milk of ewes fed diet naturally contaminated by aflatoxins and effect of inclusion of dried yeast culture in the diet. J. Dairy Sci. 92, 4997–5004; doi:10.3168/jds.2008-1684 Campagnollo, F.B., Ganev, K.C., Khaneghah, A.M., Portela, J.B., Cruz, A.G., Granato, D., Corassin, C.H., Oliveira, C.A.F. and Sant’Ana, A.S. 2016. The occurrence and effect of unit operations for dairy products processing on the fate of aflatoxin M1: a review. Food Control 68, 310–329; doi:10.1016/j.foodcont.2016.04.007 Del Palacio, A., Bettucci, L. and Pan, D. 2016. Fusarium and Aspergillus mycotoxins contaminating wheat silage for dairy cattle feeding in Uruguay. Brazilian J. Microbiol. 47, 1000–1005; doi:10.1016/j.bjm.2016.06.004 Dhakal, A. and Sbar, E. 2020. Aflatoxin toxicity. StatPearls Publishing. StatPearls Publishing. 2022 Jan–. PMID: 32491713. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557781/. Fink-Gremmels, J. 2008. Mycotoxins in cattle feeds and carry-over to dairy milk: a review. Food Addit. Contam. Part A 25, 172–180; doi:10.1080/02652030701823142 Gallo, A., Giuberti, G., Frisvad, J., Bertuzzi, T. and Nielsen, K. 2015. Review on mycotoxin issues in ruminants: occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins (Basel) 7, 3057–3111; doi:10.3390/toxins7083057 Giovanni, S. Di, Zambrini, V., Varriale, A. and Auria, S.D. 2019. Sweet sensor for the detection of aflatoxin M1 in whole milk. ACS Omega 4, 12803–12807; doi:10.1021/acsomega.9b01300 Giovati, L., Magliani, W., Ciociola, T., Santinoli, C., Conti, S. and Polonelli, L. 2015. AFM1 in milk: physical, biological, and prophylactic methods to mitigate contamination. Toxins (Basel) 7, 4330–4349; doi:10.3390/toxins7104330 Gutiérrez, R., Vega, S., Ruiz, J.L., Yamazaki, A., Rivera, J.G. and Escobar, A. 2013. Evaluación de aflatoxina M1 en leche orgánica producida en Tecpatán, Chiapas, México. Rev. Salud Anim. 35, 33–37. Hamzeh Pour, S., Mahmoudi, S., Masoumi, S., Rezaie, S., Barac, A., Ranjbaran, M., Oliya, S., Mehravar, F., Sasani, E., Noorbakhsh, F. and Khodavaisy, S. 2020. Aflatoxin M1 contamination level in Iranian milk and dairy products: a systematic review and meta-analysis. World Mycotoxin J. 13, 67–82; doi:10.3920/WMJ2019.2485 Hashemi, M. 2016. A survey of aflatoxin M1 in cow milk in Southern Iran. J. Food Drug Anal. 24, 888–893; doi:10.1016/j.jfda.2016.05.002 Horvatović, M.P., Glamočić, D., Jajić, I., Krstović, S., Guljaš, D. and Gjorgjievski, S. 2016. Aflatoxin M 1 in raw milk in the region of Vojvodina. M. Polovinski Horvatović 66, 239–245; doi1:10.15567/mljekarstvo.2016.0309 INEN. 2014. Leche y productos lácteos. Directrices para la toma de muestras (ISO 707:2008, IDT). Available from: https://www.normalizacion.gob.ec/buzon/normas/nte-inen-iso-707compl.pdf. Intanoo, M., Kongkeitkajorn, M.B., Suriyasathaporn, W., Phasuk, Y., Bernard, J.K. and Pattarajinda, V. 2020. Effect of Supplemental Kluyveromyces marxianus and Pichia kudriavzevii on Aflatoxin M1 Excretion in Milk of Lactating Dairy Cows. Animals (Basel). 10(4), 709. doi: 10.3390/ani10040709. Ismail, A., Akhtar, S., Levin, R.E., Ismail, T., Riaz, M. and Amir, M. 2015. Aflatoxin M1: Prevalence and decontamination strategies in milk and milk products. Crit. Rev. Microbiol. 42(3), 418–427. Jiang, Y., Hansen, P.J., Xiao, Y., Amaral, T.F., Vyas, D. and Adesogan, A.T. 2019. Aflatoxin compromises development of the preimplantation bovine embryo through mechanisms independent of reactive oxygen production. J. Dairy Sci. 102, 10506–10513; doi:10.3168/jds.2019-16839 Jiang, Y., Ogunade, I.M., Arriola, K.G., Pech-Cervantes, A.A., Kim, D.H., Li, X., Xue, Y.L., Vyas, D. and Adesogan, A.T. 2020. Short communication: effects of a physiologically relevant concentration of aflatoxin B1 with or without sequestering agents on in vitro rumen fermentation of a dairy cow diet. J. Dairy Sci. 103, 1559–1565; doi:10.3168/jds.2019-17318 Kagera, I., Kahenya, P., Mutua, F., Anyango, G., Kyallo, F., Grace, D. and Lindahl, J. 2019. Status of aflatoxin contamination in cow milk produced in smallholder dairy farms in urban and peri-urban areas of Nairobi County: a case study of Kasarani sub county, Kenya. Infect. Ecol. Epidemiol. 9, 1547095; doi:10.1080/20008686.2018.1547095 Kemboi, D.C., Antonissen, G., Ochieng, P.E., Croubels, S., Okoth, S., Kangethe, E.K., Faas, J., Lindahl, J.F. and Gathumbi, J.K. 2020. A review of the impact of mycotoxins on dairy cattle health: challenges for food safety and dairy production in Sub-Saharan Africa. Toxins (Basel) 12, 222; doi:10.3390/toxins12040222 Kosicki, R., Błajet-Kosicka, A., Grajewski, J. and Twarużek, M. 2016. Multiannual mycotoxin survey in feed materials and feedingstuffs. Anim. Feed Sci. Technol. 215, 165–180; doi:10.1016/j.anifeedsci.2016.03.012 Kuilman, M., Maas, R.F.M., Judah, D.J. and Fink-Gremmels, J. 1998. Bovine hepatic metabolism of aflatoxin B 1. J. Agric. Food Chem. 46, 2707–2713; doi:10.1021/jf980062x León, R., Bonifaz, N. and Gutiérrez, F. 2018. Pastos y forrajes del Ecuador: siembra y producción de pasturas, Quito, Ecuador: Primera Ed. Li, H., Li, S., Yang, H., Wang, Y., Wang, J. and Zheng, N. 2019. L-proline alleviates kidney injury caused by AFB1 and AFM1 through regulating excessive apoptosis of kidney cells. Toxins (Basel) 11, 1–12; doi:10.3390/toxins11040226 Marchese, S., Polo, A., Ariano, A., Velotto, S., Costantini, S. and Severino, L. 2018. Aflatoxin B1 and M1: biological properties and their involvement in cancer development. Toxins (Basel) 10, 1–19; doi:10.3390/toxins10060214 Marin, S., Ramos, A.J., Cano-Sancho, G. and Sanchis, V. 2013. Mycotoxins: iccurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 60, 218–237; doi:10.1016/j.fct.2013.07.047 Merlassino, J.L. 2014. Micotoxinas. Universidad Nacional del Mar del Plata-Argentina. Estación Experimental Agropecuaria Balcarce, Instituto Nacional de Tecnología Agropecuaria. Available from: http://www.biblioteca.unlpam.edu.ar/rdata/tespo/0_mermic162.pdf. Milićević, D., Petronijević, R., Petrović, Z., Đjinović-Stojanović, J., Jovanović, J., Baltić, T. and Janković, S. 2019. Impact of climate change on aflatoxin M1 contamination of raw milk with special focus on climate conditions in Serbia. J. Sci. Food Agric. 99, 5202–5210; doi:10.1002/jsfa.9768 Mohammedi-Ameur, S., Dahmane, M., Brera, C., Kardjadj, M. and Ben-Mahdi, M.H. 2020. Occurrence and seasonal variation of aflatoxin M1 in raw cow milk collected from different regions of Algeria. Vet. World 13, 433–439; doi:10.14202/vetworld.2020.433-439 Ogunade, I.M., Martinez-Tuppia, C., Queiroz, O.C.M., Jiang, Y., Drouin, P., Wu, F., Vyas, D. and Adesogan, A.T. 2018. Silage review: mycotoxins in silage: occurrence, effects, prevention, and mitigation. J. Dairy Sci. 101, 4034–4059; doi:10.3168/jds.2017-13788 Patyal, A., Gill, J.P.S., Bedi, J.S. and Aulakh, R.S. 2020. Potential risk factors associated with the occurrence of aflatoxin M1 in raw milk produced under different farm conditions. J. Environ. Sci. Heal.—Part B Pestic. Food Contam. Agric. Wastes 55, 827–834; doi:10.1080/03601234.2020.1787019 Pereyra, M.L.G., Alonso, V.A., Sager, R., Morlaco, M.B., Magnoli, C.E., Astoreca, A.L., Rosa, C.A.R., Chiacchiera, S.M., Dalcero, A.M. and Cavaglieri, L.R. 2008. Fungi and selected mycotoxins from pre- and postfermented corn silage. J. Appl. Microbiol. 104, 1034–1041; doi:10.1111/j.1365-2672.2007.03634.x Prandini, A., Tansini, G., Sigolo, S., Filippi, L., Laporta, M. and Piva, G. 2009. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem. Toxicol. 47, 984–991; doi:10.1016/j.fct.2007.10.005 Puga-Torres, B., Salazar, D., Cachiguango, M., Cisneros, G. and Gómez-Bravo, C. 2020. Determination of aflatoxin M1 in raw milk from different provinces of Ecuador. Toxins (Basel). 12, 498; doi:0.3390/toxins12080498 Rodríguez-Blanco, M., Ramos, A.J., Prim, M., Sanchis, V. and Marín, S. 2020. Usefulness of the analytical control of aflatoxins in feedstuffs for dairy cows for the prevention of aflatoxin M1 in milk. Mycotoxin Res. 36, 11–22; doi:10.1007/s12550-019-00362-y Saha Turna, N. and Wu, F. 2019. Risk assessment of aflatoxin-related liver cancer in Bangladesh. Food Addit. Contam. Part A 36, 320–326; doi:10.1080/19440049.2019.1567941 Serrano, H. and Cardona, N. 2015. Micotoxicosis y micotoxinas: generalidades y aspectos básicos. Rev. CES Med. 29, 143–152. Soriano del Castillo, J.M. 2007. Micotoxinas en alimentos. Valencia, España: Primera ed. Sugiyama, K., Hiraoka, H. and Sugita-Konishi, Y. 2008. Aflatoxin M1 contamination in raw bulk milk and the presence of aflatoxin B1 in corn supplied to Dairy Cattle in Japan. J. Food Hyg. Soc. Japan (Shokuhin Eiseigaku Zasshi) 49, 352–355; doi:10.3358/shokueishi.49.352 Toso, R.E., Toribio, M.S., Diesser, M., Borello, A.B. and Ardoino, S.M. 2018. Affections in animals and humans due to ingestion or exposure to aflatoxins. Preventive measures to avoid toxic effects. Cienc. Vet. 20, 51–67; doi:10.19137/cienvet-20182013 Tsiplakou, E., Anagnostopoulos, C., Liapis, K., Haroutounian, S.A. and Zervas, G. 2014. Determination of mycotoxins in feedstuffs and ruminant׳s milk using an easy and simple LC–MS/MS multiresidue method. Talanta 130, 8–19; doi:10.1016/j.talanta.2014.06.018 Udomkun, P., Nimo, A., Nagle, M., Müller, J., Vanlauwe, B. and Bandyopadhyay, R. 2017. Innovative technologies to manage a fl atoxins in foods and feeds and the pro fi tability of application e a review. Food Control 76, 127–138; doi:10.1016/j.foodcont.2017.01.008 Valverde, M. 2013. Análisis estadístico de la influencia de la Fiebre Aftosa en el desarrollo del sector ganadero al 2009. Quito, Ecuador: Universidad Central del Ecuador. Van Eijkeren, J.C.H., Bakker, M.I. and Zeilmaker, M.J. 2006. A simple steady-state model for carry-over of aflatoxins from feed to cow’s milk. Food Addit. Contam. 23, 833–838; doi:10.1080/02652030600779890 Variane, A.C.F., Santos, F.C. dos, Castro, F.F. de, Barbosa-Tessmann, I.P., Santos, G.T. dos and Pozza, M.S. dos S. 2018. The occurrence of aflatoxigenic Aspergillus spp. in dairy cattle feed in Southern Brazil. Brazilian J. Microbiol. 49, 919–928; doi:10.1016/j.bjm.2018.05.005 Xiong, J., Peng, L., Zhou, H., Lin, B., Yan, P., Wu, W., Liu, Y., Wu, L. and Qiu, Y. 2020. Prevalence of aflatoxin M1 in raw milk and three types of liquid milk products in central-south China. Food Control 108, 106840; doi:10.1016/j.foodcont.2019.106840 Xiong, J., Wang, Y., Nennich, T., Li, Y. and Liu, J. 2015. Transfer of dietary aflatoxin B 1 to milk aflatoxin M 1 and effect of inclusion of adsorbent in the diet of dairy cows. Am. Dairy Sci. Assoc. 98, 2545–2554; doi:10.3168/jds.2013-7842 Xiong, J., Xiong, L., Zhou, H., Liu, Y. and Wu, L. 2018. Occurrence of aflatoxin B1 in dairy cow feedstuff and aflatoxin M1 in UHT and pasteurized milk in central China. Food Control 92, 386–390; doi:10.1016/j.foodcont.2018.05.022 Xu, N., Xiao, Y., Xie, Q., Li, Y., Ye, J. and Ren, D. 2021. Occurrence of aflatoxin B1 in total mixed rations and aflatoxin M1 in raw and commercial dairy milk in northern China during winter season. Food Control 124, 107916; doi:10.1016/j.foodcont.2021.107916 Yunus, A., Imtiaz, N., Khan, H., Ibrahim, M. and Zafar, Y. 2019. Aflatoxin contamination of milk marketed in Pakistan: a longitudinal study. Toxins (Basel). 11, 110; doi:10.3390/toxins11020110 | ||
| How to Cite this Article |
| Pubmed Style Puga-torres B, Ron L, Gomez C. Dietary Aflatoxin B1-related risk factors for the presence of Aflatoxin M1 in raw milk of cows from Ecuador. Open Vet. J.. 2022; 12(1): 129-137. doi:10.5455/OVJ.2022.v12.i1.16 Web Style Puga-torres B, Ron L, Gomez C. Dietary Aflatoxin B1-related risk factors for the presence of Aflatoxin M1 in raw milk of cows from Ecuador. https://www.openveterinaryjournal.com/?mno=118014 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i1.16 AMA (American Medical Association) Style Puga-torres B, Ron L, Gomez C. Dietary Aflatoxin B1-related risk factors for the presence of Aflatoxin M1 in raw milk of cows from Ecuador. Open Vet. J.. 2022; 12(1): 129-137. doi:10.5455/OVJ.2022.v12.i1.16 Vancouver/ICMJE Style Puga-torres B, Ron L, Gomez C. Dietary Aflatoxin B1-related risk factors for the presence of Aflatoxin M1 in raw milk of cows from Ecuador. Open Vet. J.. (2022), [cited January 25, 2026]; 12(1): 129-137. doi:10.5455/OVJ.2022.v12.i1.16 Harvard Style Puga-torres, B., Ron, . L. & Gomez, . C. (2022) Dietary Aflatoxin B1-related risk factors for the presence of Aflatoxin M1 in raw milk of cows from Ecuador. Open Vet. J., 12 (1), 129-137. doi:10.5455/OVJ.2022.v12.i1.16 Turabian Style Puga-torres, Byron, Lenin Ron, and Carlos Gomez. 2022. Dietary Aflatoxin B1-related risk factors for the presence of Aflatoxin M1 in raw milk of cows from Ecuador. Open Veterinary Journal, 12 (1), 129-137. doi:10.5455/OVJ.2022.v12.i1.16 Chicago Style Puga-torres, Byron, Lenin Ron, and Carlos Gomez. "Dietary Aflatoxin B1-related risk factors for the presence of Aflatoxin M1 in raw milk of cows from Ecuador." Open Veterinary Journal 12 (2022), 129-137. doi:10.5455/OVJ.2022.v12.i1.16 MLA (The Modern Language Association) Style Puga-torres, Byron, Lenin Ron, and Carlos Gomez. "Dietary Aflatoxin B1-related risk factors for the presence of Aflatoxin M1 in raw milk of cows from Ecuador." Open Veterinary Journal 12.1 (2022), 129-137. Print. doi:10.5455/OVJ.2022.v12.i1.16 APA (American Psychological Association) Style Puga-torres, B., Ron, . L. & Gomez, . C. (2022) Dietary Aflatoxin B1-related risk factors for the presence of Aflatoxin M1 in raw milk of cows from Ecuador. Open Veterinary Journal, 12 (1), 129-137. doi:10.5455/OVJ.2022.v12.i1.16 |