| Original Article | ||
Open Vet. J.. 2023; 13(2): 193-201 Open Veterinary Journal, (2023), Vol. 13(2): 193–201 Original Research Development of unconventional treatments for mastitis in dairy cattleAigerim A. Kukeeva1*, Talgat Zh. Abdrakhmanov1, Aibar N. Akhmetov2, Askar A. Terklibaev1 and Kanat M. Kamsaev11Department of Veterinary Medicine, Faculty of Veterinary Sciences and Animal Husbandry, Saken Seifullin Kazakh Agro Technical University, Astana, Republic of Kazakhstan 2Department of Veterinary Sanitation, Faculty of Veterinary Sciences and Animal Husbandry, Saken Seifullin Kazakh Agro Technical University, Astana, Republic of Kazakhstan *Corresponding Author: Aigerim A. Kukeeva. Department of Veterinary Medicine, Faculty of Veterinary Sciences and Animal Husbandry, Saken Seifullin Kazakh Agro Technical University, Astana, Republic of Kazakhstan. Email: ai.kukeeva [at] gmail.com Submitted: 13/10/2022 Accepted: 16/01/2023 Published: 13/02/2023 AbstractBackground: The increase in the intensity of livestock industries, by improving the technology of animal product manufacturing, largely depends on the correct organization of herd reproduction and the intensity of using the biological capabilities of the animal organism. Various diseases, including such common diseases as mastitis, complicate the successful reproduction and growth of enterprise productivity. The widespread use of antibiotic-containing drugs for the treatment of mastitis creates a number of inevitable consequences for the body. The relevance of the study is due to the fact that the residual content of antibiotics in the collected milk after the course of treatment poses a serious danger to human health and reduces the quality of dairy products obtained from such milk. Aim: The authors set the task of developing a new, antibiotic-free method of treating bovine mastitis. This paper is devoted to the problem of improving methods of treating the subclinical mastitis in dairy cattle by alternative methods during the interlactation period. Methods: The leading method for the study of this problem is an experimental method that allows to develop and test a veterinary homeopathic substance for the treatment of subclinical mastitis in cows during the interlactation period. Results: This paper presents materials on the typification of microflora in the milk of cows with subclinical mastitis and the effectiveness of a veterinary homeopathic substance developed by the authors. The use of the veterinary homeopathic substance provided a high therapeutic effect, and did not cause side effects and complications in cows. Conclusion: The resulting veterinary substance was tested and introduced into the “Izhevskiy” natural complex of Akmola region as a new alternative method of treating subclinical mastitis in cows. Based on this substance, a drug for the treatment of mastitis will be developed and proposed for production. Keywords: Dairy cattle, Mastitis, Unconventional therapies, Homeopathic substance, Pharmacotherapeutic effect. IntroductionBovine mastitis is the most common disease affecting dairy farms around the world. The economic losses associated with mastitis derive mainly from reduced milk production and, to a lesser extent, culling of lactating animals, veterinary costs, and milk quality penalties (Mylostyvyi et al., 2021a,b; Huilca-Ibarra et al., 2022). Mastitis is caused by a wide range of pathogenic agents that penetrate the lacteous glands and multiply in the udder. Often the cause of mastitis is a small group of bacteria, including Staphylococci, Streptococcus uberis, mycoplasmas, and Escherichia coli Carrillo-Casas and Miranda-Morales, 2012). Bovine mastitis is recognized as the most common and costly disease affecting dairy herds. The disease results in huge financial losses for the dairy industry as a result of lower yields and milk quality, death and culling of affected cows, and associated treatment costs. The disease occurs as a result of the invasion of the lacteous glands by pathogenic bacteria, followed by their reproduction in the mammary tissues (Mushtaq et al., 2017). The clinical symptoms of the cows with mastitis (Libisch et al., 2022):
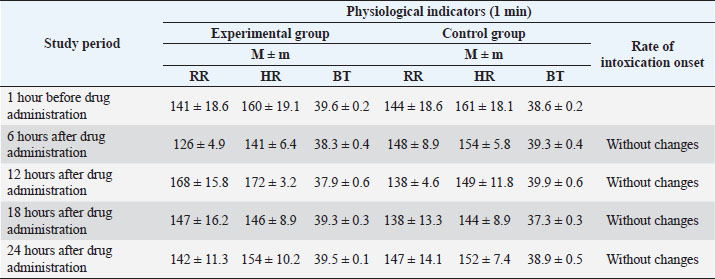
The most common treatment for bovine mastitis is intrauterine antibiotic infusion (Ferreira et al., 2022). However, its use is associated with the problem of resistance of microorganisms to antimicrobial drugs, which contributed to the search for alternative approaches to treatment. Medicinal plants, with their long history, are an excellent natural resource used as alternative therapy (Boonstra et al., 2020). Plant antibacterial agents can act as important sources of new antibiotics, and compounds that target bacterial virulence or can be used in combination with existing drugs. Plants are an integral component of ethno-veterinary medicine used in the treatment of various diseases, including bovine mastitis (Schroeder, 2012). It is known that the medicinal effect of plants is due to the biologically active substances contained in them (Cheng et al., 2020). However, it should be borne in mind that the content of active substances in plants depends on many factors, their accumulation in individual organs in different phases of plant development varies within very wide range. On the other hand, in order to obtain plant raw materials with high pharmacological qualities, it is necessary to know which part of the plant needs to be harvested, in which phase of its development the greatest number of chemical compounds is accumulated, which determine its healing effect (Dalanezi et al., 2020; Wollowski et al., 2021). To increase the pharmacological effectiveness of drugs from plant materials, it is necessary to carefully study its chemical composition, as well as the rationality and activity of the resulting dosage formulations (Mamonov and Muzychkina, 2008). Extensive research is necessary to explore the medicinal potential of various plant species from biodiversity-rich areas of the world. Medicinal plants used as an alternative therapeutic agent in the treatment of bovine mastitis may exhibit antibacterial, anti-inflammatory, or immunomodulatory properties. Since, with mastitis, bacterial infection of tissues leads to disruption of oxidative processes in the lacteous gland, that is, the possibility for pharmacological intervention in order to block the proteolytic or oxidative cascade. Plants are excellent sources of antioxidant substances, the use of which is justified to prevent oxidative processes in mastitis and to combat this chronic disease. The purpose of the study is to develop a veterinary substance on a plant basis and to study its pharmacotherapeutic strength in the treatment of subclinical mastitis in dairy cattle. The authors were tasked with developing an antibiotic-free drug, mainly using local plant materials, with a lower cost and an extended spectrum of action on the causative agents of bovine mastitis. Materials and MethodsSampleScientific and experimental studies were carried out at the Department of Veterinary Medicine of the Saken Seifullin Kazakh Agro Technical University, the State Institution “National Center for Monitoring, Reference, Laboratory Diagnostics and Methodology in Veterinary Medicine” of the Committee of State Inspection in the Agro-Industrial Complex of the Ministry of Agriculture of the Republic of Kazakhstan, at the L.N. Gumilyov National University, production experiments were carried out on the basis of the natural complex “Izhevskiy” in Akmola region. The object of the study was cows of the black-and-white breed of the dairy-commercial complex “Izhevsky”, Akmola region. For the experiment, 38 cows with a clear positive test reaction were selected, and also 12 rabbits were studied in the experiment. Materials collectionTo create dosage forms of the drug, the authors used raw materials from local plants obtained from wormwood, chamomile, and willow bark. In the summer–autumn period of 2016, the following medicinal plants were collected: chamomile flowers, willow bark, and wormwood herb. The collection of medicinal plant materials was carried out taking into account the phases of the vegetation of plants, in ecologically clean regions of Kazakhstan: zone of the city of Kokshetau, Talman village of Akzhar region. Materials preparationA tincture was obtained from the dried plant material. An alcohol–water mixture of 20% and 40% concentration was used as an extragent when obtaining the tincture. Obtaining substances consisted of the following technological stages: preparation of raw materials and extragent, obtaining an extract, purification from accompanying substances, and packaging. The preparation of the plant material consists of crushing and removing dust. To grind plant material, grass and root cutters (size smaller than 0.25 mm) were used. The extractor was prepared by diluting rectified alcohol with water to the desired concentration. To obtain extracts, the method of maceration and all its varieties were used with the intensification of the process (vortex) re-maceration, percolation, and dissolution of extracts. When calculating the extragent, its volume, which is absorbed by the raw material, was taken into account. The absorption coefficient of raw materials was determined by the following equation: K=P1/P2,(1) Where P1 and P2 are the mass of raw materials before and after swelling, respectively. During the period of settling, many high-molecular compounds coagulate and precipitate. The settled extract was decanted and filtered through a drook-filter or plate-press under pressure. Tinctures are stored in well-sealed bottles, protected from light. After obtaining a compound tincture from plant materials, chicken manure was added to it, 1% of the total volume. The chicken manure was preliminarily moistened with 9% acetous acid and, to weather the odor of acetous acid, kept in air (1 hour). Also, the sea-buckthorn oil was added to the resulting composition, 10% of the total volume. To stabilize the resulting composition, aminoglutaric acid powder was used. Sterilization of the resulting dosage form was carried out by autoclaving. The resulting liquid pharmaceutical formulation for veterinary purposes was analyzed to determine biological and pharmacological properties. Analyzing the microbiological purity of a substanceThe studies were carried out at the State Institution “National Centre for Monitoring, Reference, Laboratory Diagnostics and Methodology in Veterinary Medicine” of the Committee for State Inspection in the Agro-Industrial Complex of the Ministry of Agriculture of the Republic of Kazakhstan. Testing for microbiological purity includes the quantitation of revivable bacteria and fungi, as well as the identification of certain types of microorganisms, the presence of which is unacceptable in non-sterile medicines. Medicines (solutions, extracts, syrups, ointments, suppositories, aerosols, etc.) that are not sterilized during the production process can be contaminated with microorganisms and, therefore, must be tested for microbiological purity. The test is carried out under aseptic conditions, using the methods and culture media given in the State Pharmacopoeia of the Republic of Kazakhstan (2008) for the control of all types of non-sterile medicines, as well as raw materials used in their production. Determining the antiradical substance activityTo study the antiradical activity (ARA), 20% and 40% solutions of the veterinary substance were used. To determine the inhibition of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) to 0.1 ml of an alcohol solution of the test solutions in the concentration range of 0.1, 0.25, 0.5, 0.75, and 1.0 mg/ml were added 3 ml of 6*10-5 M radical solution. The beakers were in a rack wrapped in black polyethylene. e. After vigorous stirring, the solutions remained in the dark for 30 minutes, then the optical densities were measured at 520 nm. The values of the ARA of the studied objects were determined by the following equation: where A0—optical density of the control sample; At—optical density of the working sample. Measurement of optical densityDepending on the concentration, the optical density of the investigated solutions was measured on a spectrophotometer at a wavelength of 520 nm. Next, the authors conducted an analysisof the cytotoxic activity of the substance. The separating tube was filled with artificial seawater (55 ml), with Artemia salina eggs (200 mg), and supplied with air for 3 days until the larvae emerged. One side of the tube was covered with aluminum foil. After 5 minutes, the larvae, that had gathered on the bright side of the tube, were extracted with a Pasteur pipette. Approximately 20–40 larvae were housed in 990 μl of seawater in each of the 24 micro-wells. Dead larvae were counted under a microscope. Dimethyl sulfoxide solution (10 μl) was added to the sample 10 mg/ml. The reference drug was actinomycin D or staurosporin. Only dimethyl sulfoxide (10 μl) was treated as a negative control. After incubation for 24 hours and further storage of the micro-wells for 24 hours (to ensure immobility), dead larvae were counted under a microscope. Mortality (p) was determined using the following equation: where A—number of dead larvae after 24 hours; N—the number of dead larvae before the test; B—average number of dead larvae in negative control; and Z—total number of larvae. Ethical approvalThe study protocol was approved by the Animal Welfare and Ethics Committee of Saken Seifullin Kazakh Agro Technical University, Astana, Republic of Kazakhstan. Verbal permission from the owners of the dairy-commercial complex “Izhevsky” was also obtained before the experiment. ResultsTo determine the safety of the obtained veterinary substance, studies were carried out to study acute and chronic toxicity on rabbits. To study acute toxicity, the substance was administered in a dose of 0.2 ml intramuscularly, during the day, with an interval of 6 hours. The experimental animals were clinically observed. The results are presented in Table 1. As can be seen from the data in Table 1, the respiratory rate , heart rate , and body temperature (BT) indicators of the animals from the experimental group was significantly reduced after 6 hours. Then the dynamics of increase and alignment with the indicators of control group increases. Fluctuations in the dynamics of clinical indicators, apparently, are due to the body's response to the drug administration. However, no significant deviations from physiological parameters were observed, the general condition of the animals was satisfactory (Chepky and Zhalko-Titarenko, 1983; The State Pharmacopoeia of the Republic of Kazakhstan: Vol. 1., 2008; The State Pharmacopoeia of the Republic of Kazakhstan, 2009). To study chronic toxicity, the veterinary substance was administered to rabbits at a dose of 1 ml, intramuscularly, once a day, for 21 days (Table 2). Throughout the entire period of the study, the clinical indicators of health of the experimental group of animals were at the level of the control group, no intoxication or death of animals was observed. The sterility test of the obtained substance was carried out by direct inoculation. After incubation in a nutrient medium for 5 days, no visible growth of microorganisms was observed in the plating containing the drug. The presence of ARA of the obtained veterinary substance was studied in its solutions of 20% and 40% concentration (Table 3). Table 1. Study of the acute toxicity of the obtained substance on rabbits (n=5).
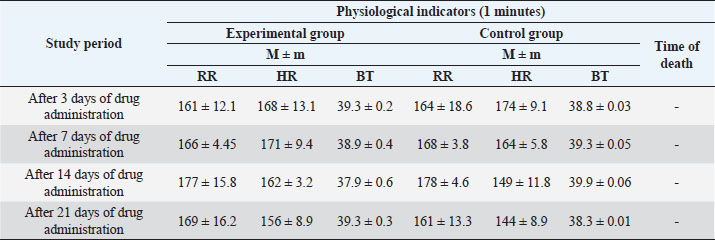
Table 2. Study of chronic toxicity of a veterinary substance on rabbits (n=5).
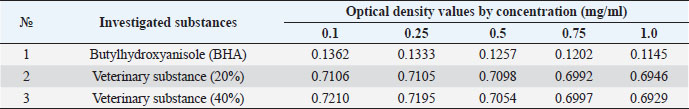
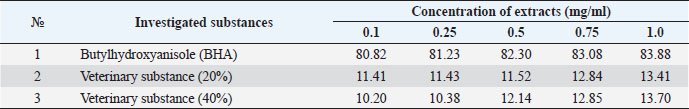
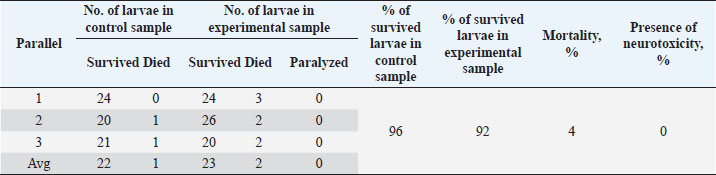
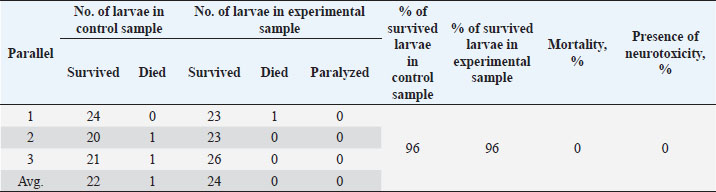
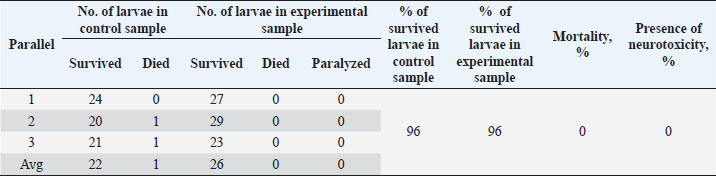
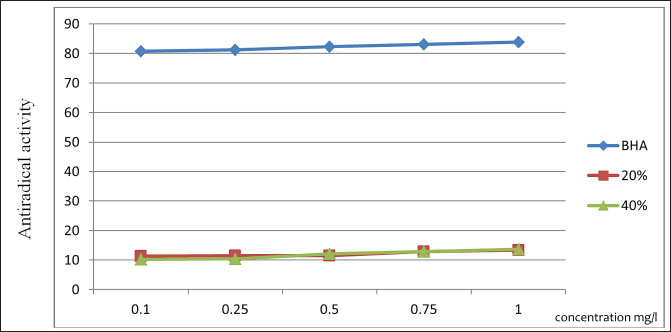
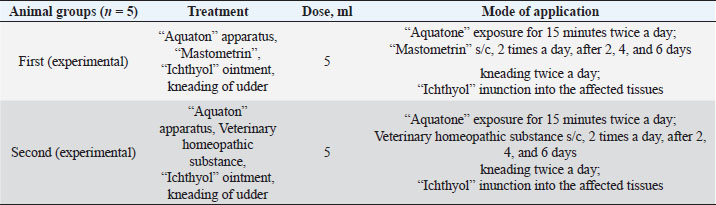
The ARA of the investigated substances was compared with the ARA of butylhydroxyanisole (BHA). The values of the antiradical effect of substances, calculated by the formula (2), are given in Table 4. Based on the analysis of the data from Tables 5–7 and Figure 1, it is evident that 20% and 40% of veterinary substances have low ARA compared to BHA. Based on the experiment conducted, it can be assumed that the resulting composition (40%) does not exhibit cytotoxicity in all tested concentrations. The larvae remained alive, but their activity was significantly inhibited. The next stage in the study of the obtained drug was the study of pharmacotherapeutic activity in the treatment of subclinical mastitis in dairy cattle. The study of milk samples for the detection of a subclinical form of bovine mastitis during the interlactation period, as well as the prophylactic and therapeutic efficacy of the drug, was carried out according to standard methods—sampling for a somatic test, sampling for a Lange test, and a sedimentation test. In order to determine the therapeutic dose of the drug, two groups of animals with a subclinical form of mastitis, five animals in each, were selected. At the same time, the animals from the first group were injected with the drug “Mastometrin” at a dose of 5 ml, the animals from the second group were injected with the obtained substance at a dose of 5 ml. The mammary glands of animals of both groups were exposed to the “Aquaton” apparatus with an exposure time of 15 minutes. Clinical observation of the animals (BT, pulse, and respiration rate) was conducted daily for 6 days. A comparative treatment regimen for bovine mastitis is presented in Table 8. As a result of the therapeutic measures, it was found that cows with subclinical mastitis showed recovery at different times (Table 9). Thus, in the animals of the second experimental group, the inflammatory processes in the lacteous gland decreased much earlier than in the animals of the first experimental group. Table 3. Change in optical density of the investigated substances with change in concentration.
Table 4. ARA (%) of investigated substances at different concentrations.
Table 5. Results of the analysis of the cytotoxic activity of the resulting substances (40%) 10 mg/ml.
Table 6. Results of the analysis of the cytotoxic activity of the resulting substances (40%) 5 mg/ml.
Table 7. Results of the analysis of the cytotoxic activity of the resulting substances (40%) 1 mg/ml.
Fig. 1. Dynamics of ARA with a change in the substance concentration. Table 8. Treatment regimen for bovine mastitis during the interlactation period.
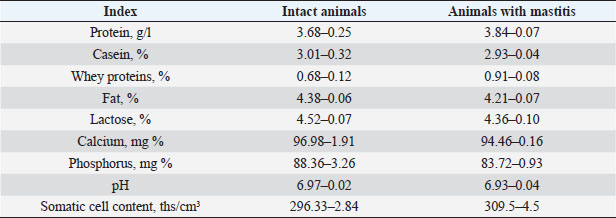
Also, studies were carried out to assess the physical and chemical parameters of milk from contaminated cattle. Organoleptic testing has shown that the milk of healthy animals was white, without any foreign smell or taste. In the results of the physical and chemical studies, the following data were obtained, which are presented in Table 10. DiscussionFrom the analysis of the above, it follows that in the group of contaminated animals, an increase in total milk protein is observed by 0.16% due to an increase in the content of whey proteins, with a simultaneous decrease in casein, respectively, by 2.65%. The increase in the content of whey proteins was caused by lactoglobulins and immunoglobulins in milk. This is apparently associated with inflammatory processes in the genitals and, as a consequence, with an increase in the concentration of immunoglobulins in the blood. In cows with mastitis, a decrease in the content of fat, lactose, calcium, and phosphorus was observed with a simultaneous increase in the content of total and whey milk proteins, catalase activity, and also changes in the technological properties of milk, a decrease in its buffer capacity. Changes in the content of proteins, and fats lead to a decrease in the energy value of milk. Belkin et al. (2015) devoted a number of studies to the research of bovine mastitis as the most important scientific and practical problem of veterinary hygiene and obstetrics. Being a polymicrobial and multifactorial disease, inflammation of the lacteous gland—mastitis, is widespread in cows on farms and complexes. Along with summarizing the most important literature data, the papers give special attention to the results of scientific developments and research by the authors in the field of creation and application in practice of new methods for the diagnosis and treatment of bovine mastitis. Mestorino and Errecalde (2011) believe that bovine mastitis is a disease characterized by significant economic losses due to a decrease in milk secretion, a potential decrease in cow productivity, and an increase in production costs due to contamination of milk with microorganisms. Intramammary infection is the most common cause of mastitis in dairy cows. Antimicrobial drugs have been used to treat mastitis for over 50 years, but there is still no consensus on the most effective, safest, and most economical treatment. Belkin et al. (2009) developed a new treatment for bovine mastitis in interlactation period using antimicrobial drug apramycin in combination with xanthan gum. Table 9. Dynamics of cow recovery.
Table 10. Physicochemical parameters of milk in healthy cows and cows with mastitis.
In West Bengal and India, the Gram-negative bacteria, resistant to antibiotics such as β-lactams and tetracyclines, have been found in animals with mastitis (Das et al., 2017). The prevalence of mastitis pathogens and their antimicrobial resistance have been investigated in numerous studies by scientists from Ethiopia and Estonia. Thus, in these countries, a high prevalence of penicillin-resistant S. aureus strains was revealed (Kalmus et al., 2011). India, in recent years, has been working on the development of traditionally used medicinal plants in the Kashmir Himalayas to combat bovine mastitis. During these preliminary studies, some medicinal plants and their antimicrobial potential against important mastitis pathogenic agents were researched. For example, the methanol extract obtained from the underground elements of the Aquilegia fragrans plant has caused moderate and weak antibacterial activity against mastitis pathogens (Mushtaq et al., 2016). Pasca et al. (2017) have investigated the antimicrobial efficacy of an ethanol extract from 11 plant species, 8 of which showed antimicrobial activity against 32 tested microorganisms isolated from milk samples. The results showed that three plant species, namely Evernia prunastri, Artemisia absinthium, and Lavandula angustifolia, inhibited the growth of the test microorganisms to a maximum level. Similarly, of the eight evaluated herbal products, only three samples R3, R4, and R7 showed better antimicrobial activity, comparable to standard antimicrobials such as florfenicol and enrofloxacin (Pașca et al., 2017). Natural products have proven to be an inexhaustible source of antibacterial treatment. About 66% of all medicines approved as antibacterial are natural or derived from natural products (Brown et al., 2014). The mechanism of action of phytochemicals can be very different from that of routine antibiotics, and this trait can be of vital importance in the treatment of diseases caused by resistant bacteria. The combination of phytochemical antibacterial drugs with already existing drugs provides another field for their use. Although there are no herbal antibacterial drugs available on the market based on individual chemicals, this natural source is worth exploring for several important reasons that have been sufficiently reviewed by a number of authors (Gibbons, 2008; Kuete, 2010; Saleem et al., 2010). ConclusionA veterinary homeopathic substance has been developed and tested for the treatment of subclinical bovine mastitis during interlactation period. The use of the veterinary homeopathic substance provided a high therapeutic effect, and did not cause side effects and complications in cows. To determine the safety of the obtained veterinary composition, studies were carried out to research acute and chronic toxicity in rabbits. To study acute toxicity, the drug was administered in a dose of 0.2 ml intramuscularly, during one day, with an interval of 6 hours. The experimental animals were clinically observed. When studying some technological properties of milk, it was found that milk from sick and healthy animals did not differ from each other in terms of heat resistance. The resulting veterinary substance was tested and introduced into the “Izhevskiy” natural complex of Akmola region as a new unconventional method of treating subclinical bovine mastitis. Based on this substance, a drug for the treatment of bovine mastitis will be developed and proposed for production ReferencesBelkin, B.L., Cherepakhina, L.A., Sotnikova, V.M., Popkova, T.V. and Skrebneva, E.N. 2009. Cow mastitis: etiology, diagnosis, treatment and prevention. Orel, Russia: Orel State Agrarian University. Belkin, B.L., Komarov, V.Yu. and Andreev, V.B. 2015. Mastitis of cows: Etiology, pathogenesis, diagnosis, treatment and prevention. Orel, Russia: Orel State Agrarian University named after N.V. Parahin. Boonstra, M.B., Spijkerman, D.C.M., Voor In 't Holt, A.F., Van Der Laan, R.J., Bode, L.G.M., Van Vianen, W., Klaassen, C.H.W. and Severin, J.A. 2020. An outbreak of ST307 extended-spectrum beta-lactamase (ESBL)-producing Klebsiella pneumoniae in a rehabilitation center: an unusual source and route of transmission. Infect. Contr.Hosp. Epidem. 41(1), 31–36. Brown, D.G., Lister, T. and May-Dracka, T.L. 2014. New natural products as new leads for antibacterial drug discovery. Bioorg. Med. Chem. Lett. 24, 413–418. Carrillo-Casas, E.M. and Miranda-Morales, R.E. 2012. Bovine mastitis pathogens: prevalence and effects on Somatic Cell Count. Paris, France: IntechOpen. Cheng, J., Zhang, J., Han, B., Barkema, H.W., Cobo, E.R., Kastelic, J.P., Zhou, M. and Gao, J. 2020. Klebsiella pneumoniae isolated from bovine mastitis is cytopathogenic for bovine mammary epithelial cells. J. Dairy Sci. 103(4), 3493–3504. Chepky, L.P. and Zhalko-Titarenko, V.F. 1983. Anesthesiology and resuscitation. Vyshcha. shkola. Dalanezi, F.M., Schmidt, E.M.S., Joaquim, S.F., Guimarães, F.F., Guerra, S.T., Lopes, B.C., Cerri, R.L.A. and Langoni, H. 2020. Concentrations of acute-phase proteins in milk from cows with clinical mastitis caused by different pathogens. Pathogens 9(9), 1–12. Das, A., Guha, C., Biswas, U., Jana, P.S., Chatterjee, A. and Samanta, I. 2017. Detection of emerging antibiotic resistance in bacteria isolated from subclinical mastitis in cattle in West Bengal. Vet. World. 10(5), 517–520. Ferreira, E.M., Romero, L.C., Cunha, M.R., Junior, W.M., Camargo, C.H. and Zafalon, L.F. 2022. Persistence of staphylococcus spp. in milk from cows undergoing homeopathy to control subclinical mastitis. BMC. Vet. Res. 18, 273. Gibbons, S. 2008. Phytochemicals for bacterial resistance—strengths, weaknesses and opportunities. Plan. Med. 74, 594–602. Huilca-Ibarra, M.P., Vasco-Julio, D., Ledesma, Y., Guerrero-Freire, S., Zurita, J., Castillejo, P., Barceló Blasco, F. and Waard, J.H.D. 2022. High prevalence of prototheca Bovis infection in dairy cattle with chronic mastitis in Ecuador. Vet. Sci. 9(12), 659. Kalmus, P., Aasmäe, B., Kärssin, A., Orro, T. and Kask, K. 2011. Udder pathogens and their resistance to antimicrobial agents in dairy cows in Estonia. Acta Vet. Scand. 53, 4. Kuete, V. 2010. Potential of cameroonian plants and derived products against microbial infections: a review. Plan. Med. 76, 1479–1491. Libisch, B., Picot, C., Ceballos-Garzon, A., Moravkova, M., Klimesová, M., Telkes, G., Chuang, S.-T. and Le Pape, P. 2022. Prototheca infections and ecology from a one health perspective. Microorganisms 10(5), 938. Mamonov, L.K. and Muzychkina, R.A. 2008. Introduction to phytochemical researches and detection of biological activity of plant substances. XXI Century School. Mestorino, N. and Errecalde, J.O. 2011. Pharmacokinetic-pharmacodynamic considerations for bovine mastitis treatment. Paris, France: IntechOpen. Mushtaq, S., Aga, M.A., Qazi, P.H., Ali, M.N., Shah, A.M., Lone, S.A., Shah, A., Hussain, A., Rasool, F., Dar, H., Shah, Z.H. and Lone, Sh. H. 2016. Isolation, characterization and HPLC quantification of compounds from Aquilegia fragrans Benth: their in vitro antibacterial activities against bovine mastitis pathogens. J. Ethnopharm. 178, 9–12. Mushtaq, S., Shah, A.M., Shah, A., Lone, S.A., Hussain, A., Hassan, Q.P. and Ali, M.N. 2017. Bovine mastitis: an appraisal of its alternative herbal cure. Microb. Pathogen. 114, 357–361. Mylostyvyi, R., Sejian, V., Izhboldina, O., Kalinichenko, O., Karlova, L., Lesnovskay, O., Begma, N., Marenkov, O., Lykhach, V., Midyk, S., Cherniy, N., Gutyj, B. and Hoffmann, G. 2021a. Changes in the spectrum of free fatty acids in blood serum of dairy cows during a prolonged summer heat wave. Animals 11(12), 3391. Mylostyvyi, R., Lesnovskay, O., Karlova, L., Khmeleva, O., Kalinichenko, O., Orishchuk, O., Tsap, S., Begma, N., Cherniy, N., Gutyj, B. and Izhboldina, O. 2021b. Brown Swiss cows are more heat resistant than Holstein cows under hot summer conditions of the continental climate of Ukraine. J. Anim. Behav. Biometeorol. 9(4), 2134. Pașca, C., Mărghitaș, L., Dezmirean, D., Bobiș, O., Bonta, V., Chirilă, F., Matei, I. and Fiț, N. 2017. Medicinal plants-based products tested on pathogens isolated from mastitis milk. Molecules 22, 1–16. Saleem, M., Nazir, M., Ali, M.S., Hussain, H., Lee, Y.S., Riaz, N. and Jabbar, A. 2010. Antimicrobial natural products: an update on future antibiotic drug candidates. J. Nat. Prod. 27, 238–254. Schroeder, J.W. 2012. Mastitis control programs: Bovine mastitis and milking management. NDSU Exten. Serv. 1129, 1–16. State Pharmacopoeia of the Republic of Kazakhstan: Vol. 1. 2008. Zhibek Zholy. Available via http://pharmacopoeia.ru/wp-content/uploads/2017/08/Gosudarstvennaya-farmakopeya-respubliki-Kazahstan.pdf State Pharmacopoeia of the Republic of Kazakhstan: Vol. 2. 2009. Zhibek Zholy. Available via https://www.studmed.ru/gosudarstvennaya-farmakopeya-respubliki-kazahstan-tom-ii_3ae8a7677a9.html Wollowski, L., Heuwieser, W., Kossatz, A., Addis, M.F., Puggioni, G.M.G., Meriaux, L. and Bertulat, S. 2021. The value of the biomarkers cathelicidin, milk amyloid A, and haptoglobin to diagnose and classify clinical and subclinical mastitis. J. Dairy Sci. 104(2), 2106–2122. | ||
| How to Cite this Article |
| Pubmed Style Kukeeva AA, Abdrakhmanov TZ, Akhmetov AN, Terklibaev AA, Kamsaev KM. Development of unconventional treatments for mastitis in dairy cattle. Open Vet. J.. 2023; 13(2): 193-201. doi:10.5455/OVJ.2023.v13.i2.7 Web Style Kukeeva AA, Abdrakhmanov TZ, Akhmetov AN, Terklibaev AA, Kamsaev KM. Development of unconventional treatments for mastitis in dairy cattle. https://www.openveterinaryjournal.com/?mno=118262 [Access: January 24, 2026]. doi:10.5455/OVJ.2023.v13.i2.7 AMA (American Medical Association) Style Kukeeva AA, Abdrakhmanov TZ, Akhmetov AN, Terklibaev AA, Kamsaev KM. Development of unconventional treatments for mastitis in dairy cattle. Open Vet. J.. 2023; 13(2): 193-201. doi:10.5455/OVJ.2023.v13.i2.7 Vancouver/ICMJE Style Kukeeva AA, Abdrakhmanov TZ, Akhmetov AN, Terklibaev AA, Kamsaev KM. Development of unconventional treatments for mastitis in dairy cattle. Open Vet. J.. (2023), [cited January 24, 2026]; 13(2): 193-201. doi:10.5455/OVJ.2023.v13.i2.7 Harvard Style Kukeeva, A. A., Abdrakhmanov, . T. Z., Akhmetov, . A. N., Terklibaev, . A. A. & Kamsaev, . K. M. (2023) Development of unconventional treatments for mastitis in dairy cattle. Open Vet. J., 13 (2), 193-201. doi:10.5455/OVJ.2023.v13.i2.7 Turabian Style Kukeeva, Aigerim A., Talgat Zh. Abdrakhmanov, Aibar N. Akhmetov, Askar A. Terklibaev, and Kanat M. Kamsaev. 2023. Development of unconventional treatments for mastitis in dairy cattle. Open Veterinary Journal, 13 (2), 193-201. doi:10.5455/OVJ.2023.v13.i2.7 Chicago Style Kukeeva, Aigerim A., Talgat Zh. Abdrakhmanov, Aibar N. Akhmetov, Askar A. Terklibaev, and Kanat M. Kamsaev. "Development of unconventional treatments for mastitis in dairy cattle." Open Veterinary Journal 13 (2023), 193-201. doi:10.5455/OVJ.2023.v13.i2.7 MLA (The Modern Language Association) Style Kukeeva, Aigerim A., Talgat Zh. Abdrakhmanov, Aibar N. Akhmetov, Askar A. Terklibaev, and Kanat M. Kamsaev. "Development of unconventional treatments for mastitis in dairy cattle." Open Veterinary Journal 13.2 (2023), 193-201. Print. doi:10.5455/OVJ.2023.v13.i2.7 APA (American Psychological Association) Style Kukeeva, A. A., Abdrakhmanov, . T. Z., Akhmetov, . A. N., Terklibaev, . A. A. & Kamsaev, . K. M. (2023) Development of unconventional treatments for mastitis in dairy cattle. Open Veterinary Journal, 13 (2), 193-201. doi:10.5455/OVJ.2023.v13.i2.7 |