| Short Communication | ||
Open Vet. J.. 2023; 13(3): 358-364 Open Veterinary Journal, (2023), Vol. 13(3): 358–364 Short Communication Expression of a recombinant ASFV P30 protein and production of monoclonal antibodiesRosario Liberti1, Claudia Colabella1, Lucia Anzalone1, Giulio Severi1, Antonella Di Paolo1, Cristina Casciari1, Anna Beatrice Casano2, Monica Giammarioli1, Monica Cagiola1, Francesco Feliziani1 and Antonio De Giuseppe1*1Istituto Zooprofilattico Sperimentale dell’Umbria e delle Marche “Togo Rosati”, Perugia, Italy 2Dipartimento di Medicina Veterinaria, Università di Perugia, Perugia, Italy *Corresponding Author: Antonio De Giuseppe. Istituto Zooprofilattico Sperimentale dell’Umbria e delle Marche “Togo Rosati”, Perugia, Italy. Email: a.degiuseppe [at] izsum.it. Submitted: 12/10/2022 Accepted: 22/02/2023 Published: 19/03/2023 © 2023 Open Veterinary Journal
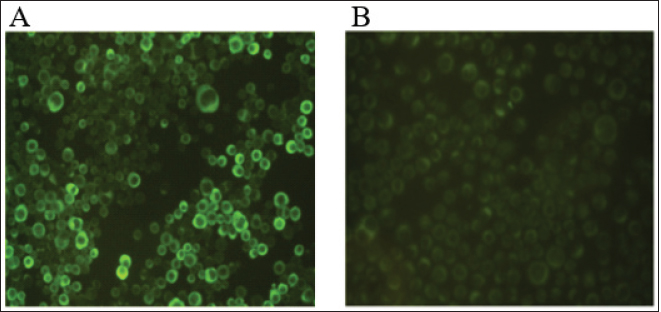
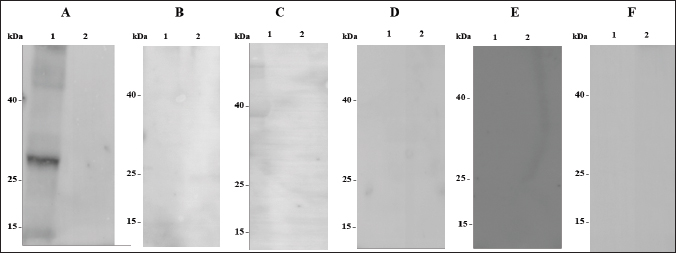
AbstractBackground: African Swine Fever (ASF) is an infectious disease that affects domestic pig and wild boar populations. The ASF Virus (ASFV) has a genome characterized by a very complex DNA (170–193 kb) that encodes for more than 200 different proteins. Among these, the highly immunogenic phosphoprotein p30 plays a fundamental role in the induction of specific antibodies. To date, the lack of a vaccine against the disease requires continuous studies to improve knowledge about the virus and the development of new tests in addition to virological ones. Aim: The aim of this work was to produce specific monoclonal antibodies (mAbs) against the p30 protein of ASFV, which could find useful applications in routine diagnostics and the implementation of new diagnostic tools. Methods: ASFV p30 encoding gene was amplified and used for the generation of the recombinant baculovirus by transfection of the Sf21 insect cells. The recombinant protein was analyzed by immunofluorescence assay, purified, and used for mice Balb-c immunization. The hybridomas obtained were cultured and screened, using an indirect Enzyme-linked Immunosorbent Assay (iELISA), in order to select clones that secrete the mAbs of interest. Results: The expression of recombinant p30 protein was assessed using direct immunofluorescence. The purified p30 protein fractions were analyzed by Coomassie gels staining confirming the presence of bands with a molecular weight of 30 kDa and used for the immunization of Balb-c mice. Six clones of pure hybridomas secreting the specific mAbs against recombinant p30 were obtained and tested in iELISA. The mAbs were also characterized by Western blot and immunofluorescence assay. The best results were obtained with the anti-p30 mAb 2B8E10 clone which showed high reactivity with both recombinant and viral p30 protein, respectively. Conclusion: In this work, a recombinant p30 protein produced in an insect cell system was purified and used to immunize Balb-c mice. Six anti-p30 mAbs-secreting hybridomas clone cells were obtained. These mAbs displayed high reactivity against the recombinant protein, but only 2B8E10 mAb showed excellent functionality against the p30 protein produced by ASFV. These results open the possibility to develop different diagnostic assays. Keywords: ASFV, ASFV p30 protein, Baculovirus, Monoclonal antibody. IntroductionAfrican Swine Fever (ASF) is a highly contagious viral disease that affects domestic pigs and wild boar populations and is transmitted through contact with contaminated fomites or infected animals (Giménez-Lirola et al., 2016; Petrovan et al., 2019; Li et al., 2022). The African Swine Fever Virus is a unique member of the Asfarviridae family (Dixon et al., 2008). This infection is one of the most feared diseases for pig farming due to the high rate of mortality (Sánchez-Cordón et al., 2018). Until the late 1990s, ASF infection has been underestimated, due to eradication programs applied in many countries, including European and extra-European countries where the Sardinia region in Italy, remained the only endemic area outside the African continent. In 2007, the presence of the infection was notified in the Caucasian area, where the disease found the ideal conditions for spreading due to the high number of backyard pig farms with low biosafety levels and the frequent direct and indirect contact between wild boars and domestic pigs (Beltran-Alcrudo, 2008). The infection quickly spread to Russia and other European countries as well as to Asian countries (Danzetta et al., 2020; Jurado et al., 2018). Since then, the disease has spread in Europe, reaching ASF-free countries such as Belgium and Germany, in 2022, the regions of Liguria, Piedmont, and Lazio, in Italy (Iscaro et al., 2022). Currently, the presence of ASFV genotype II covers all continents worldwide. ASFV genome is a large double-stranded DNA (170-193 kbp) encoding approximately 200 structural and non-structural proteins (Alejo et al., 2018; Wang et al., 2021). Structural proteins, such as p30, p54, and p72, are involved in viral replication and infection (Jia et al., 2017). Phosphoprotein p30, encoded by the CP204L gene and located on the inner membrane of the viral envelope, is one of the most immunogenic proteins (Zhang et al., 2021; Kazakova 2016). This protein induces high-level production of viral and mostly neutralizing antibodies (Gómez-Puertas et al., 1998, Guanglei et al., 2021). To date, due to the lack of commercial vaccines against ASFV, active surveillance remains a fundamental tool to contrast the disease and requires continuous studies to improve knowledge about the virus. For these reasons, the improvement of rapid and low-cost diagnostic tests, such as ELISA, Immunoblotting test, or others are needed (Bosch-Camós et al., 2020; WOAH, 2021). In this work, we described the development of recombinant p30 protein and the production of monoclonal antibodies for potential use in serological/virological diagnosis techniques. Materials and MethodsMolecular techniques and generation of recombinant baculovirusASFV p30 encoding gene (CP204L) was amplified with Platinum™ SuperFi™ PCR Master Mix (Life Technologies) from attenuated viral strain (Genotype I) provided by Pestivirus and Asfivirus National Reference Centre. The 600 base pairs gene was amplified using the following primer sequences: p30ASFV-F (5’-TCGAGAATT CACAAAATGGATTTTATTTTAAATATATCC), p30 ASFV-R (5’-GGCCAACCGGTAAACATTA AATGTAGGTGAGATAAA) and cloned in frame with the 6xHis tag into the baculovirus transfer vector pOET2C-6xHis (Oxford Technologies Expression). The obtained construct was analyzed by restriction analysis and sequenced (BigDye Terminator v 3.1 Cycle Sequencing Applied Biosystem, Foster City, CA). Recombinant baculovirus was obtained by co-transfection of Sf21 insect cells (Thermo Fischer Scientific) with the construct, which contained the full-length p30 gene, and bacmid DNA following the manufacturer’s instructions of the FlashBac system (Oxford Technologies Expression). Immunofluorescence assayRecombinant ASFV p30 expression in Sf21 cells was evaluated by immunofluorescence assay. Sf21 cells were seeded at 15,000 cells/well in 96 well plates and infected with p30 recombinant baculovirus. 24 hours post-infection, infected and uninfected Sf21cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde solution Overnight (ON) at + 4°C. Sf21 monolayers were permeabilized for 10 minutes at room temperature (RT) with 0.5% TritonX-100, and then incubated with monoclonal antibody anti-p30 FITC diluted 1:200 (provided by Pestivirus and Asfivirus National Reference Centre) in PBS for 45 minutes at RT. Plates were monitored using a fluorescence microscope. Immunofluorescence assay was also carried out with Vero cell line, to evaluate the reactivity of monoclonal antibodies against the viral antigen. Vero cells (ATCC CCL-81™) were plated at 32,000 cells per well in 96 well plates and infected with 0.03 MOI of ASFV attenuated strain. Eighteen hours post infection, infected and uninfected Vero cells were fixed with a fixing solution (30% acetone and 70% methanol) for about 10 minutes at RT and incubated with anti-p30 monoclonal antibodies produced for 45 minutes at 37°C. The immunocomplex was evaluated with a fluorescence microscope after 45 minutes at 37°C incubation with an anti-mouse FITC conjugated antibody (Thermo Fischer Scientific). Production and purification of p30 recombinant proteinInsect cell line Sf21 (2 × 106 cells/ml at 27°C) was infected with recombinant baculovirus at one MOI for cells. Seventy-two hours post-infection, Sf21 cells were harvested and centrifuged at 400× g for 10 minutes. The cell pellet was resuspended in Lysis Buffer (0.1 M NaH2PO4/Na2HPO4, 8 M Urea, and 4 mM DTT) and stored at −20°C. After centrifugation (15,000 × g for 30 minutes at +4°C), recombinant protein was purified by affinity chromatography (His-select® Nickel affinity gel, SIGMA-ALDRICH) following a modified manufacturer’s protocol. Briefly, denaturing condition protocol was performed until the first chromatographic column wash, while a native condition protocol was applied until recombinant protein elution. Purified protein samples were dialyzed against PBS, filtered with 0.2 µ filter (Sartorius Minisart® Syringe Filter), mixed with NuPAGE™ LDS Sample Buffer (4×) and 10 mM DTT, denatured at 99°C for 7 minutes and resolved by SDS-PAGE in 10% polyacrylamide pre-cast NuPAGE gels (Thermo Fischer Scientific). After electrophoresis, the gel was washed with deionized water, treated with a fixing solution (50% methanol and 7% acetic acid), and then stained with coomassie blue (GelCode™ Blue Stain Reagent, ThermoFisher Scientific) for 1 hour at room temperature (RT). Monoclonal Antibodies productionSix-eight weeks female Balb-c were immunized intraperitoneally. The first inoculum was carried out with 80 µg of purified p30 recombinant protein with Freund’s Adjuvant Complete (Sigma-Aldrich) in 1:1 ratio. Second inoculum was carried out with 60 µg of recombinant protein with Freund’s Adjuvant Incomplete (Sigma-Aldrich) as reported above. The last inoculum and booster were performed with 60 µg of recombinant protein diluted in a sterile physiological solution. Three days after the booster, immunized mice sera were collected and tested in iELISA to evaluate seroconversion. Splenocytes were collected and fused with Sp2/O-Ag-14 myeloma cells (ATCC CRL1581™). After fusion, cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 20% Fetal Bovine Serum (Corning®) and 1× HAT Media (HybriMax ®, SIGMA-ALDRICH) at 37°C with 5% CO2. All the hybridomas culture supernatants were screened in iELISA for the research of anti-p30 antibodies. Positive hybridomas were subcultured following the limiting dilution method and tested again using the same immunoenzymatic assay. MAbs were purified with pre-packed rProtein A GravitrapTM, (GE Healthcare) following the manufacturer’s protocol. Eluted mAbs were neutralized with 100 µl of Tris-HCl 1 M, pH 9.0, dialyzed against PBS, and concentrated by centrifugation in Vivaspin® Turbo 50K MWCO (SARTORIUS). Finally, the purified mAbs were conjugated with Lightning-Link® HRP Antibody Labeling Kit (Novusbio) and tested in ELISA and Western Blot to evaluate their functionality. iELISA96 microwell plates (f96-NUNC-MAXISORP-IMMUNO-PLATE, Thermofisher Scientific) were coated with 100 ng of recombinant p30 protein in carbonate/bicarbonate coating buffer (pH 9.6) at +4°C overnight. Plates were blocked with PBS containing 0.05% Tween20 and 2% yeast extract (PBSTY). Pre and post-immunization mice sera were diluted (two-fold dilution in PBSTY), added to the plate, and incubated at 37°C for 1 hour. Plates were washed three times with PBST and incubated for 1 hour at 37°C with Goat anti-Mouse IgG Fc Secondary Antibody-HRP (Thermofisher Scientific) diluted 1:20,000 in PBSTY. Plates were further washed with PBST and incubated with 100 μl of 3, 3´, 5, 5´-tetramethylbenzidine peroxidase substrate (TMB) for 20 minutes. The reaction was stopped with 0.5 M H2SO4 and the optical density at 450 nm (OD450) was measured. The same procedure was used for the screening of hybridomas culture supernatants diluted 1:2 in PBSTY. Positive hybridomas were also tested using the ID Screen ASF Competition Kit (ID-vet) following the manufacturer’s instructions. Western blotAnti-p30 monoclonal antibodies were characterized with recombinant p30 and heat-inactivated viral antigens. After sample preparation, as previously described, the resolved proteins were transferred onto the PVDF membrane. The membrane was incubated with TBST buffer (20 mM Tris–HCl, 150 mM NaCl, 0.05% Tween 20 (v/v) at pH 7.4) containing 5% dry milk for 3 hours at RT, and incubated ON at +4°C with diluted hybridomas supernatant culture (1:3 in 5% fat dry milk-TBST). Membranes were washed four times for 15 minutes each with TBST, and incubated for 1 hour at RT with Goat anti-Mouse IgG Fc Secondary Antibody-HRP (Thermofisher Scientific). The immune reaction was visualized by chemiluminescence using the Super Signal West Pico Substrate kit (Thermo Fischer Scientific). Ethical approvalAnimal experimentation was carried out in compliance with the Italian National Law (Legislative Decree 4th March, 2014, n. 26) implementing Directive 2010/63/UE of the Council of the European Communities on the protection of animals used for experimental and other scientific purposes (Italian Ministry of Health authorization number 150/2018-PR). Results and DiscussionExpression and purification of recombinant p30 proteinThe immunofluorescence signal observed in infected Sf21 and its absence in non-infected cells confirmed the expression of recombinant p30 protein (Fig. 1). SDS-PAGE and Coomassie gel staining of purified p30 recombinant protein showed the presence of a colorimetric band with the expected molecular weight of 30 kDa (Fig. 2). As shown in Figure 2, high recovery and a good degree of purity of the protein was observed. Monoclonal antibodiesSix hybridoma cell clones secreting anti-p30 mAbs were obtained after immunization of Balb-c mice with recombinant p30 protein. The high immunogenicity of this antigen was confirmed by the iELISA used to evaluate the seroconversion after immunization (Table 1). Anti-p30 monoclonal antibodies were tested in iELISA, where three of them showed 450 higher than 2.0, while the others showed an 450 ranging from 0.4 to 1.5 (Table 2). Hybridomas with OD450 higher of 0.150 were considered positive. These results were confirmed using the commercial kit ID Screen ASF Competition (data not shown). Western Blot analysis showed that all six mAbs highlighted an immunoreactive band of 30 kDa corresponding to the recombinant protein (Fig. 3) and no cross-reactivity with uninfected Sf21 lysate. Furthermore, only the monoclonal antibody 2B8E10 reacted also with the viral antigen (Fig. 4). The same results were obtained in immunofluorescence assay, where only mAb 2B8E10 was able to react against produced p30 viral antigen (Fig. 5A). The lack of reactivity against the viral antigen evidenced by the other five monoclonal antibodies could depend on different antigenic characteristics between the recombinant and viral protein. Since the post-translational modification in insect cells lines (i.e., N-glycosylation, etc.) are similar but not identical to those in mammalian cells, the antigenic characteristics or folding of the p30 recombinant protein, could not be the same as wild-type p30. Furthermore, 2B8E10 preserved its excellent functionality even after conjugation with horseradish peroxidase (data not shown).
Fig. 1. Immunofluorescence assay of p30 recombinant protein expressed in Sf21 cells using anti-p30 FITC mAb: (A) infected Sf21 cells; (B) uninfected Sf21 cells (negative control).
Fig. 2. SDS-PAGE and Coomassie gel staining of purified recombinant p30 protein; recombinant protein elution fractions (lanes 1–7); protein standard molecular weight is reported. Table 1. Evaluation of the immunogenicity of p30 recombinant protein. iELISA of pre- and post-immunisation serum. OD450 values are shown.
Table 2. iELISA of six hybridomas culture media secreting anti-p30 monoclonal antibodies. OD450 are shown.
Fig. 3. Western Blot analysis of anti-p30 mAbs using purified recombinant p30. (A) mAb 2B8E10; (B) mAb 2B8D8B1; (C) mAb 2B8G5D2; (D) mAb 7C3E3G5; (E) mAb 4G6F4; (F) mAb 4F11C2. Recombinant p30 protein (lane 1) and uninfected Sf21 cells (lane 2). Protein standard molecular weight is reported (spectra multicolor low range protein ladder).
Fig. 4. Western Blot analysis of anti-p30 mAbs. (A) mAb 2B8E10; (B) mAb 2B8D8B1; (C) mAb 2B8G5D2; (D) mAb 7C3E3G5; (E) mAb 4G6F4; (F) mAb 4F11C2. ASFV infected vero cells (lane 1) and uninfected Vero cells (lane 2). Protein standard molecular weight is reported (spectra multicolor low range protein ladder).
Fig. 5. Immunofluorescence assay carried out using anti p30 mAbs on ASFV infected Vero cells. (A) mAb 2B8E10; (B) mAb 2B8D8B1; (C) mAb 2B8G5D3; (D) mAb 7C3E3G5; (E) mAb 4G6F4; (F) mAb 4F11C2; (G) uninfected Vero cells. ConclusionIn this work, we evaluated the expression of ASFV p30 recombinant protein that allowed the production of a reagent with excellent immunogenic characteristics. Indeed, when the purified p30 protein was used to immunize Balb-c mice for monoclonal antibody production, we obtained six hybridomas secreting anti-p30 monoclonal antibodies. These mAbs showed a great reactivity against the recombinant protein, although only 2B8E10 mAb, displayed also high reactivity against the viral antigen, as shown in both Western Blot and Immunofluorescence assay. To date, the excellent functionality demonstrated by 2B8E10 mAb opens the possibility for its potential use in the development of screening diagnostic assays, such as competitive ELISA or rapid field test for antigen detection. Furthermore, this reagent could be used in more sophisticated investigations if, for examples, conjugated with fluorescent molecules for immunofluorescence/immunochromatography assays. AcknowledgementThe research was funded by Ministero della Salute (Italia), progetto di Ricerca Corrente RC002/2018. Conflict of interestThe Authors declare that there is no conflict of interest. Authors’ contributionConceptualization, ADG, FF, MG and MC; Data curation, RL, CCo, LA, ADP; Formal analysis RL, CCo, LA, GS, ADP, CCa, ABC; Methodologies GS and ADG; Supervision, ADG; writing-original draft RL; writing-review and editing CCo, ADP, FF, MG and ADG. All authors have read and agreed to the published version of the manuscript. ReferencesAlejo, A., Matamoros, T., Guerra, M. and Andrés, G. 2018. A proteomic atlas of the African swine fever virus particle. J. Virol. 92(23), e01293–e01318. Beltran-Alcrudo, D., Lubroth, J., Depner, K., La Rocque, S. 2008. African swine fever in the Caucasus. doi: 10.13140/RG.2.1.3579.1200. Bosch-Camós, L., López, E. and Rodriguez, F. 2020. African swine fever vaccines: a promising work still in progress. Porc. Health Manag. 6, 17. Danzetta, M.L., Marenzoni, M.L., Iannetti, S., Tizzani, P., Calistri, P. and Feliziani, F. 2020. African Swine Fever: lessons to learn from past eradication experiences. A systematic review. Front. Vet. Sci. 7, 296. Directive 2010/63/EC of the Parliament and of the Council of 22 September 2010, on the protection of the animals used for scientific purposes. J. Eur. Union 2010, 276, 1–47. Dixon, L.K. and Chapman, D. 2008. African swine fever virus. In Encyclopedia of virology, 3rd ed. Eds., Brian, W.J., Mahy, Marc, H.V., and Van Regenmortel. New York, NY: Academic Press, pp: 43–51. Giménez-Lirola, L.G., Mur, L., Rivera, B., Mogler, M., Sun, Y., Lizano, S., Goodell, C., Harris, D.L., Rowland, R.R., Gallardo, C., Sánchez-Vizcaíno, J.M. and Zimmerman, J. 2016. Detection of African swine fever virus antibodies in serum and oral fluid specimens using a recombinant protein 30 (p30) dual matrix indirect ELISA. PLoS ONE 11(9), e0161230. Gómez-Puertas, P., Rodríguez, F., Oviedo, J.M., Brun, A., Alonso, C. and Escribano, J.M. 1998. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology 243(2), 461–471. Iscaro, C., Dondo, A., Ruocco, L., Masoero, L., Giammarioli, M., Zoppi, S., Guberti, V., Feliziani, F. 2022. January 2022: Index case of new African Swine Fever incursion in mainland Italy. Transbound. Emerg. Dis. 69(4), 1707–1711. Jia, N., Ou, Y., Pejsak, Z., Zhang, Y. and Zhang, J. 2017. Roles of African swine fever virus structural proteins in viral infection. J. Vet. Res. 61(2), 135–143. Jurado, C., Fernández-Carrión, E., Mur, L., Rolesu, S., Laddomada, A. and Sánchez-Vizcaíno, J.M. 2018. Why is African swine fever still present in Sardinia? Transbound. Emerg. Dis. 65(2), 557–566. Kazakova, A.S., Imatdinov, I.R., Dubrovskaya, O.A., Imatdinov, A.R., Sidlik, M.V., Balyshev, V.M., Krasochko, P.A. and Sereda, A.D. 2017. Recombinant Protein p30 for serological diagnosis of african swine fever by immunoblotting assay. Transbound. Emerg. Dis. 64(5), 1479–1492. Li, Z., Chen, W., Qiu, Z., Li, Y., Fan, J., Wu, K., Li, X., Zhao, M., Ding, H., Fan, S. and Chen, J. 2022. African swine fever virus: a review. Life (Basel). 12(8), 1255. OIE Terrestrial Manual, chapter 3.9.1, WOAH. 2021. Available via https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/ Petrovan, V., Yuan, F., Li, Y., Shang, P., Murgia, M.V., Misra, S., Rowland, R.R.R. and Fang, Y. 2019. Development and characterization of monoclonal antibodies against p30 protein of African swine fever virus. Virus Res. 269, 197632. Sánchez-Cordón, P.J., Montoya, M., Reis, A.L. and Dixon, L.K. 2018. African swine fever: a re-emerging viral disease threatening the global pig industry. Vet. J. 233, 41–48. Wang, Y., Kang, W., Yang, W., Zhang, J., Li, D., Zheng, H. 2021. Structure of African swine fever virus and associated molecular mechanisms underlying infection and immunosuppression: a review. Front Immunol. 12, 715582. Zhang, G., Liu, W., Gao, Z., Yang, S., Zhou, G., Chang, Y., Ma, Y., Liang, X., Shao, J. and Chang, H. 2021. Antigenicity and immunogenicity of recombinant proteins comprising African swine fever virus proteins p30 and p54 fused to a cell-penetrating peptide. Int Immunopharmacol. 101(Pt A), 108251. Zhang, Y., Wang, A., Zhou, J., Liu, H., Chen, Y., Ding, P., Zhu, X., Liang, C., Qi, Y. and Zhang, G. 2021. Epitope mapping of African swine fever virus (ASFV) structural protein p30. Authorea. doi: 10.22541/au.163412179.91835999/v1. | ||
| How to Cite this Article |
| Pubmed Style Liberti R, Colabella C, Anzalone L, Severi G, Paolo AD, Casciari C, Casano AB, Giammarioli M, Cagiola M, Feliziani F, Giuseppe AD. Expression of a recombinant ASFV P30 protein and production of monoclonal antibodies. Open Vet. J.. 2023; 13(3): 358-364. doi:10.5455/OVJ.2023.v13.i3.13 Web Style Liberti R, Colabella C, Anzalone L, Severi G, Paolo AD, Casciari C, Casano AB, Giammarioli M, Cagiola M, Feliziani F, Giuseppe AD. Expression of a recombinant ASFV P30 protein and production of monoclonal antibodies. https://www.openveterinaryjournal.com/?mno=120515 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i3.13 AMA (American Medical Association) Style Liberti R, Colabella C, Anzalone L, Severi G, Paolo AD, Casciari C, Casano AB, Giammarioli M, Cagiola M, Feliziani F, Giuseppe AD. Expression of a recombinant ASFV P30 protein and production of monoclonal antibodies. Open Vet. J.. 2023; 13(3): 358-364. doi:10.5455/OVJ.2023.v13.i3.13 Vancouver/ICMJE Style Liberti R, Colabella C, Anzalone L, Severi G, Paolo AD, Casciari C, Casano AB, Giammarioli M, Cagiola M, Feliziani F, Giuseppe AD. Expression of a recombinant ASFV P30 protein and production of monoclonal antibodies. Open Vet. J.. (2023), [cited January 25, 2026]; 13(3): 358-364. doi:10.5455/OVJ.2023.v13.i3.13 Harvard Style Liberti, R., Colabella, . C., Anzalone, . L., Severi, . G., Paolo, . A. D., Casciari, . C., Casano, . A. B., Giammarioli, . M., Cagiola, . M., Feliziani, . F. & Giuseppe, . A. D. (2023) Expression of a recombinant ASFV P30 protein and production of monoclonal antibodies. Open Vet. J., 13 (3), 358-364. doi:10.5455/OVJ.2023.v13.i3.13 Turabian Style Liberti, Rosrio, Claudia Colabella, Lucia Anzalone, Giulio Severi, Antonella Di Paolo, Cristina Casciari, Anna Beatrice Casano, Monica Giammarioli, Monica Cagiola, Francesco Feliziani, and Antonio De Giuseppe. 2023. Expression of a recombinant ASFV P30 protein and production of monoclonal antibodies. Open Veterinary Journal, 13 (3), 358-364. doi:10.5455/OVJ.2023.v13.i3.13 Chicago Style Liberti, Rosrio, Claudia Colabella, Lucia Anzalone, Giulio Severi, Antonella Di Paolo, Cristina Casciari, Anna Beatrice Casano, Monica Giammarioli, Monica Cagiola, Francesco Feliziani, and Antonio De Giuseppe. "Expression of a recombinant ASFV P30 protein and production of monoclonal antibodies." Open Veterinary Journal 13 (2023), 358-364. doi:10.5455/OVJ.2023.v13.i3.13 MLA (The Modern Language Association) Style Liberti, Rosrio, Claudia Colabella, Lucia Anzalone, Giulio Severi, Antonella Di Paolo, Cristina Casciari, Anna Beatrice Casano, Monica Giammarioli, Monica Cagiola, Francesco Feliziani, and Antonio De Giuseppe. "Expression of a recombinant ASFV P30 protein and production of monoclonal antibodies." Open Veterinary Journal 13.3 (2023), 358-364. Print. doi:10.5455/OVJ.2023.v13.i3.13 APA (American Psychological Association) Style Liberti, R., Colabella, . C., Anzalone, . L., Severi, . G., Paolo, . A. D., Casciari, . C., Casano, . A. B., Giammarioli, . M., Cagiola, . M., Feliziani, . F. & Giuseppe, . A. D. (2023) Expression of a recombinant ASFV P30 protein and production of monoclonal antibodies. Open Veterinary Journal, 13 (3), 358-364. doi:10.5455/OVJ.2023.v13.i3.13 |