| Research Article | ||
Open Vet. J.. 2023; 13(5): 599-603 Open Veterinary Journal, (2023), Vol. 13(5): 599–603 Original Research Comparative performance evaluation of blood film microscopy for the diagnosis of bovine trypanosomosis by some laboratories in North-central NigeriaJones Soladoye Akinbobola1*, Solomon Oluwole Okaiyeto2, Anthony Kojo Sackey3, Lushaikyaa Allam2, Bisalla Mohammed4, Jerome Nyhalah Dinga5,6, Prosper C. Chukwuemeka1 and Lucas Cunningham71Department of Veterinary Medicine, Faculty of Veterinary Medicine, University of Abuja, Abuja, Nigeria 2Veterinary Teaching Hospital, Ahmadu Bello University, Zaria, Nigeria 3Department of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria 4Department of Veterinary Pathology, Ahmadu Bello University, Zaria, Nigeria 5Michael Gahnyam Gbeugvat Foundation, Buea, Cameroon 6Biotechnology Unit, Faculty of Science, University of Buea, Buea, Cameroon 7Department of Tropical Disease Biology, Biological Sciences, Liverpool School of Tropical Medicine, Liverpool, UK *Corresponding Author: Jones Soladoye Akinbobola. Department of Veterinary Medicine, Faculty of Veterinary Medicine, University of Abuja, Abuja, Nigeria. Email: jones.akinbobola [at] uniabuja.edu.ng. Submitted: 13/10/2022 Accepted: 12/04/2023 Published: 13/05/2023 © 2023 Open Veterinary Journal
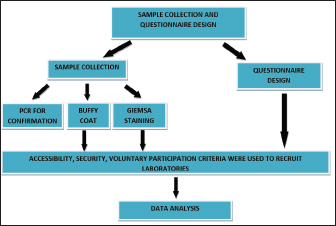
AbstractBackground: Due to its affordability in disease-affected communities and suitability for field application, microscopy has historically been considered the gold standard for field diagnosis of trypanosomosis in rural settings. Aim: This works aims to compare the performance of microscopists on bovine trypanosome microscopy by organizing the first comparative assessment on a correct reading of slides by laboratory professionals using the read slide results and a structured interviewer-administered questionnaire in North-central Nigeria. Methods: Ten participants were addressed, as they were sent a panel of two slides (Slide 1: No Trypanosome present; Slide 2: Trypanosome present) and a questionnaire. Results: All participants greater than 41 years old reported correctly the presence and absence of parasites on slides. Only 3/8 of microscopists from routine diagnostic laboratories reported correctly the presence of the parasite. Conclusion: Our study confirmed errors in reading slides. Therefore, training of microscopists besides a nationwide quality assessment is recommended. Keywords: Blood film microscopy, Bovine trypanosomosis, North-central Nigeria, Performance, Evaluation. IntroductionTrypanosomosis is considered the most important constraint to the health and improved productivity of cattle in sub-Saharan Africa (Taylor, 1998). It is ranked among the most important genera of hemoparasites of veterinary importance affecting cattle (Bakre et al., 2020). The disease is characterized by fever, anemia, reduced productivity, and frequently high mortality, which among the other factors limit the development of rural areas in tropical Africa (Abenga et al., 2002; Fajinmi et al., 2007). As of 2011, the annual financial burden of animal trypanosomosis in Nigeria was estimated to be above 22 million dollars (Fadiga et al., 2013). In the southern states, African animal trypanosomosis (AAT) prevalence values ranged between 2.7% and 35.45% (Opasina and Ekwuruke, 1987; Unigwe et al., 2022). In northern states, trypanosome prevalence using microscopy is 0.8% in Bagudo local government of Kebbi state (Yusuf et al., 2015), 26.67% in Song local government of Adamawa state (Zubairu et al., 2013), 2.2% in Kaduna central (Samdi et al., 2011), and 1.8% in Sokoto main abattoir (Fajinmi et al., 2011). Similarly, prevalence rates of 8.4% (Enwezor et al., 2009) and 3.98% (Machina et al., 2017) have all been reported in Kaduna state. Trypanosoma vivax is reported to be the dominant species in ruminants in most parts of northern Nigeria (Omotainse et al., 2004). AAT is being controlled by chemotherapy or targeting the tsetse fly (Steverding, 2008). However, the effect of PATTEC Nigeria, which was officially inaugurated at the 26th ISCTRC conference in 2001 on trypanosomosis and tsetse elimination, is not yet felt among local farmers considering the financial limitations (Odeniran, 2022). Diagnosis is an essential element in disease’s management among animal populations. Microscopy-based techniques using direct observation of wet blood films, buffy coat technique (BCT) (Murray et al., 1977), or hematocrit centrifugation technique (Woo, 1970) are the most common methods of parasite detection. Although its main drawbacks reflect in its analytical sensitivity (low analytical sensitivity in chronic infection and nil in healthy carriers), microscopy is the ideal diagnostic technique for bovine trypanosomosis because it is suitable for field application and has test cost within the means of the communities affected by the disease (Hailu, 2019; Desquesnes et al., 2022). For this reason, microscopy has historically been considered the gold standard for field diagnosis in rural settings. In rural areas, the quality of microscopy in trypanosomosis endemic areas is often inadequate because of poor quality equipment, insufficient training, and lack of quality assurance (Rosenblatt et al., 2009; Moura et al., 2014). External quality assessment (EQA) is a quality assurance method for objectively checking the laboratory’s performance through panel testing, blinded rechecking of slides, and on-site supervision. Studies by Mukadi et al. (2013) showed that EQA methods were feasible and acceptable to improve the quality of blood film microscopy. In 2002, the World Health Organization (WHO) launched an EQA program to improve the proficiency of microbiological testing for diseases prone to cause epidemics in the WHO Africa Region (Frean et al., 2012). The first and second national large-scale EQAs on trypanosomosis were done in humans in The Democratic Republic of the Congo (Mukadi et al., 2011, 2016). However, such assessments have not focused on the microscopy-based diagnosis of diseases of economic importance in animals. Hence, this study aims to assess the ability of microscopists to detect and identify Trypanosoma brucei in infected cattle and point out quality improvement areas in microscopic diagnosis for effective case management and control of the disease. Materials and MethodsStudy designBetween July and October 2021, 10 clinical laboratories in the country’s North-central part of Nigeria were contacted. Laboratories were recruited from both public and private sectors, and this was based on accessibility, security, and voluntary participation. Collected samples were analyzed in the laboratory using two different methods [microscopy and polymerase chain reaction (PCR) for confirmation]. Slides were read by two microscopists using Olympus Microscope CX23 (Japan), questionnaires responses were recorded, and all data was analyzed (Fig. 1). Sample collectionUsing the ear and jugular vein of each of the five cattle used for the study, the demonstration of the presence of T. brucei was done using a buffy coat (Woo, 1970) and the Giemsa staining method (Wilson, 1969). To maintain quality, slides were prepared, viewed, and double-checked by two professional microscopists before it was sent out to the participating laboratories for viewing. Stained slides were examined using oil immersion under × 100 microscopic magnification (Fig. 2). For all slides, the presence or absence of T. brucei was confirmed using internal transcribed spacer PCR at the National Veterinary Research Institute, Jos, Nigeria. The PCR reactions that used 5× firepol master mix were carried out at a final volume of 25 μl each containing the following reagents: 2.5 μl of 10× PCR buffer (Bioline), 200 μM of each of the deoxynucleotide triphosphates (ThermoFisher), 1.2 mM of MgCl2 (Bioline), 0.4 μM of both the forward 5′-CCG-GAA-GTTCAC-CGA-TAT-TG-3′ and reverse 5′-TTG-CTG-CGT-TCT-TCA-ACG-AA-3′ primers (Njiru et al., 2005), and 10 μl of BIOTAQ Red DNA polymerase (Bioline). The PCR reactions were carried out on a BioRad T100TM thermal cycler. The thermal reactions were an initial denaturation step at 95°C for 5 minutes, 35 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds followed by a final extension step of 72°C for 3 minutes. Signals on gel were judged and T. brucei was identified based on a band size of 480 bp. Upon slide viewing, participants were to report the presence or absence of T. brucei. Participants were also asked to complete a questionnaire on: (i) their use of microscopy and other diagnostic tests for trypanosome diagnosis; (ii) the number of tests performed each month; and (iii) and their staff training. The results of the microscopic analysis were categorized as correct and incorrect.
Fig. 1. Flow chart demonstrating the study design.
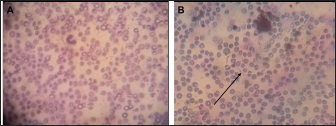
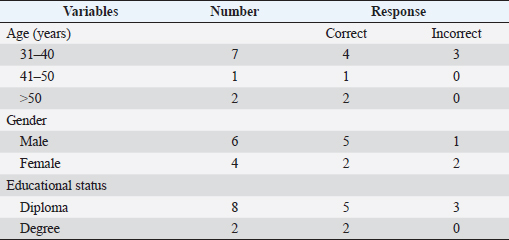
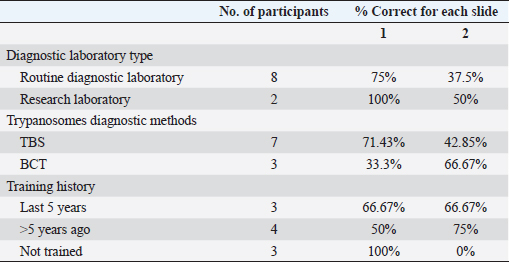
Fig. 2. (A) Uninfected sample. (B) Trypanosoma brucei (arrowed) infected sample. Statistical analysisData were entered into Excel spreadsheets (Microsoft, Redmond, USA) and analyzed using Stata version 10.0 (StataCorp. LP, College Station, TX, USA). Ethical approvalEthical clearance for the present study was obtained from the Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC) of Ahmadu Bello University; Ref: ABUCAUC/2019/26. The purpose of the study was explained to the participants and consent was obtained before responding to the questions on the survey. ResultsA total of 10 laboratory professionals took part in the study. All microscopists responded to the microscopy request, and the questionnaire was submitted. More males (6/10) than females took part in the study. The educational qualifications of the microscopists were a diploma (8/10) and a degree (2/10). All respondents greater than 41 years old reported correctly the presence and absence of parasites on slides. Similarly, microscopists who had university degrees reported correctly the presence and absence of parasites (Table 1). The type of diagnostic laboratories that took part in the study were routine diagnostic laboratories (8/10) and research laboratories (2/10). For trypanosome diagnosis, microscopists reported using only thin blood film (7/10) and buffy coat (3/10). For slide 2, only 3/8 of microscopists from a routine diagnostic laboratory reported correctly the presence of trypanosome. Among the 3/10 that reported using BCT for trypanosome diagnosis, 2/3 reported correctly the presence of the parasite. A total of 7/10 reported having received training on microscopy, among which only 3/10 were trained in the last 5 years (Table 2). DiscussionThe present study assesses the ability of some microscopists in North-central Nigeria to detect and diagnose bovine trypanosomosis. Table 1. Demography and performance of laboratory professionals on trypanosome microscopy in the selected diagnostic laboratories in Gwagwalada Area Council, Federal Capital Territory, Abuja.
Table 2. Questionnaire results according to diagnostic laboratory type, Trypanosoma diagnostic method, and participation in recent training on microscopy.
The study revealed that there are more male microscopists than females. Although data on gender participation in various disciplines is lacking in Nigeria, this could be because science is robustly male gender-typed. This finding is like the report of Madume (2021) who reported that women were concentrated in what is perceived as a traditional female social science and education disciplines. Although new diagnostics techniques have been developed for the laboratory diagnosis of bovine trypanosomosis, nevertheless, the microscopic examination of stained blood smears remains the acceptable gold standard method for bovine trypanosomosis. This could be because it is simple, cheap and can also simultaneously detect other hemoparasites (Babesia and Anaplasma) simultaneously. This result is in concordance with that report by Mulenga et al. (2021) that microscopy is regarded as the gold standard in detecting trypanosomes. The present comparative assessment did not show an excellent performance of the microscopists in the diagnosis of bovine trypanosomosis in North-central Nigeria. This could be because of the competency of the microscopist and the quality of the microscope, which makes it difficult to identify and morphologically differentiate subgenus of trypanosomes in positive blood film using flagellum, the kinetoplast, undulating membrane, and nucleus. This result is like the report of Mukadi et al. (2016), who reported poor performance of blood parasite microscopy among diagnostic laboratories in the Democratic Republic of Congo. Despite reports of increased sensitivity to the buffy coat method, it appeared that only thin blood film microscopy was used by most diagnostic laboratories for the parasitological diagnosis of bovine trypanosomosis. This could be because of the unavailability of microscopists that are experts in another method of microscopic diagnosis of trypanosomosis and the availability of more sensitive diagnostic techniques within the geographical zone. Using Giemsa-stained thin blood smear (TBS) could lead to misdiagnosis, and affect the reliability of the result. ConclusionThe overall performance of microscopists on the diagnosis of bovine trypanosomosis is poor. This study, to the best of our knowledge, is the first to document the quality of blood film microscopy among laboratories in Nigeria and shows that the performance. Therefore, repeated training, national-level assessment, and availability of rapid diagnostic tests are advocated to improve trypanosome diagnosis for better disease management. AcknowledgmentsThe authors express their gratitude to the laboratory professionals who took part in the study. We wish to also thank the staff of the Parasitology Unit (National Veterinary Research Institute). Conflict of interestThe authors declare that there is no conflict of interest regarding the publication of this paper. Authors contributionSOO, AKBS, LA, MB supervised the study. AJS and PCC collected the blood samples and distributed the questionnaire. AJS also wrote the first manuscript while LC and JND reviewed the manuscript before submission. ReferencesAbenga, J.N., Ewenzor, F.N.C., Lawani, F.A.G., Ezebuiro, C., Sule, J. and David, K.M. 2002. Prevalence of trypanosomiasis in trade cattle at slaughter in Kaduna State, Nigeria. J. Parasitol. 23, 107–110. Bakre, A., Omotosho, O., Adelakun, O. and Alaba, B. 2020. Prevalence of haemoparasites in cattle slaughtered at Central Abattoir in Igboora, Oyo State, Nigeria. Int. J. Livest. Res. 10(10), 74–79. Desquesnes, M., Gonzatti, M., Sazmand, A., Thévenon, S., Bossard, G., Boulangé, A., Gimonneau, G., Truc, P., Herder, S., Ravel, S., Sereno, D., Jamonneau, V., Jittapalapong, S., Jacquiet, P., Solano, P. and Berthier, D. 2022. A review on the diagnosis of animal trypanosomoses. Parasit. Vectors 15(1), 1–24. Enwezor, F.N., Umoh, J.U., Esievo, K.A., Halid, I., Zaria, L.T. and Anere, J.I. 2009. Survey of bovine trypanosomosis in the Kachia Grazing Reserve, Kaduna State, Nigeria. Vet. Parasitol. 159(2), 121–125. Fadiga, M.L., Jost, C. and Ihedioha, J. 2013. Financial costs of disease burden, morbidity and mortality from priority livestock diseases in Nigeria: disease burden and cost-benefit analysis of targeted interventions. ILRI Research Report. Fajinmi, A.O., Kalgo, A.M., Wyorkson, M.A., Yohanna, J.A. and Faleke, O.O. 2007. Impact of trypanosomiasis on food security in Nigeria: a review. Anim. Prod. Res. Adv. 3, 191–194. Fajinmi, A.O., Faleke, O.O., Magaji, A.A., Daneji, A.I. and Gweba, M. 2011. Presence of trypanosome species and determination of anaemia in trade cattle at Sokoto Abattoir, Nigeria. Res. J. Parasitol. 6, 31–42. Frean, J., Perovic, O., Fensham, V., McCarthy, K., Gottberg, A.V., Gouveia, L.D., Poonsamy, B., Dini, L., Rossouw, J., Keddy, K. and Alemu, W. 2012. External quality assessment of national public health laboratories in Africa, 2002-2009. Bull. WHO. 90(3), 191–199. Hailu, M. 2019. Review on diagnostic methods of trypanasomosis. J. Dairy Vet. Sci. 11(3), 1–5. Machina, I.B., Suleiman, A., Ladan, H.I., Hassan, A., Abubakar, A.T., Baba, U.M. and Aliyu, Z. 2017. The prevalence of trypanosomes infection in cattle in five local government areas of Kaduna State North-Western Nigeria. J. Agric. Vet. Sci. 10(11), 77–81. Madume, I.O. 2021. Influence of students’ factors on the enrolment of female students in science-oriented courses in Nigeria higher institutions. Int. J. Innov. Soc. Sci. Educ. Res. 9(2), 80–87. Moura, S., Fançony, C., Mirante, C., Neves, M., Bernardino, L., Fortes, F., do Rosário Sambo, M. and Brito, M. 2014. Impact of a training course on the quality of malaria diagnosis by microscopy in Angola. Malar. J. 13(1), 1–7. Mukadi, P., Gillet, P., Lukuka, A., Atua, B., Kahodi, S., Lokombe, J., Muyembe, J.J. and Jacobs, J. 2011. External quality assessment of malaria microscopy in the Democratic Republic of the Congo. Malar. J. 10(1), 1–9. Mukadi, P., Gillet, P., Lukuka, A., Mbatshi, J., Otshudiema, J., Muyembe, J.J., Buyze, J., Jacobs, J. and Lejon, V. 2013. External quality assessment of reading and interpretation of malaria rapid diagnostic tests among 1849 end-users in the Democratic Republic of the Congo through short message service (SMS). PLoS One. 8(8), e71442. Mukadi, P., Lejon, V., Barbé, B., Gillet, P., Nyembo, C., Lukuka, A., Likwela, J., Lumbala, C., Mbaruku, J., Vander Veken, W. and Mumba, D. 2016. Performance of microscopy for the diagnosis of malaria and human African trypanosomiasis by diagnostic laboratories in the Democratic Republic of the Congo: results of a nation-wide external quality assessment. PLoS One. 11(1), e0146450. Mulenga, G.M., Namangala, B., Chilongo, K., Mubamba, C., Hayashida, K., Henning, L. and Gummow, B. 2021. Challenges in the diagnostic performance of parasitological and molecular tests in the surveillance of African trypanosomiasis in eastern Zambia. Trop. Med. Infect. Dis. 6(2), 68. Murray, M., Murray, P.K. and McIntyre, W.I. 1977. An improved parasitological technique for the diagnosis of African trypanosomiasis. Trans. R. Soc. Trop. Med. Hyg. 71(4), 325–326. Njiru, Z.K., Constantine, C.C., Guya, S., Crowther, J., Kiragu, J.M., Thompson, R.C. and Dávila, A.M. 2005. The use of ITS1 rDNA PCR in detecting pathogenic African trypanosomes. Parasitol. Res. 95(3), 186–192. Odeniran, P.O. 2022. Transmission patterns of trypanosomes in fly vector populations and bovine host in Southwestern Nigeria. Doctoral dissertation, University of Ibadan. Available via http://repository.pgcollegeui.com:8080/xmlui/handle/123456789/1404?show=full Omotainse, S.O., Kalejaiye, J.O., Dede, P.M. and Dadah, A.J. 2004. The current status of tsetse and animal trypanosomiasis in Nigeria. Vom. J. Vet. Sci. 1, 1–7. Opasina, B.A. and Ekwuruke, J.O. 1987. Trypanosomiasis in Nigerian trade cattle. Trop. Anim. Health Prod. 19(4), 251–252. Rosenblatt, J.E., Reller, L.B. and Weinstein, M.P. 2009. Laboratory diagnosis of infections due to blood and tissue parasites. Clin. Infect. Dis. 49(7), 1103–1108. Samdi, S.M., Fajinmi, A.O., Kalejaye, J.O., Wayo, B., Haruna, M.K., Yarnap, J.E., Mshelia, W. P., Usman, A. O., Hamra, A., Jijitar, A. Ogunwole, R., Ovbagbedia, R. P. and Bizi, R. 2011. Prevalence of trypanosomosis in cattle at slaughter in Kaduna central abattoir. Asian J. Anim. Sci. 5(2), 162–165. Steverding, D. 2008. The history of African trypanosomiasis. Parasites Vectors 1(1), 3. Taylor, K.A. 1998. Immune responses of cattle to African trypanosomes: protective or pathogenic? Int. J. Parasitol. 28(2), 219–240. Unigwe, C.R., Egwu, L.U., Enibe, F., Njoku, C.P., Kenneth-Chukwu, O., Okey, S.N. and Madubuike, A.J. 2022. Haematological implications of haemoparasitism among slaughtered white fulani cattle at Bodija Abattoir, Ibadan, Oyo State, Nigeria. Nigeria Agric. J. 53(1), 47–54. Wilson, A.J. 1969. Value of the indirect fluorescent antibody test as a serological aid to diagnosis of glossina-transmitted bovine trypanosomiasis. Trop. Anim. Health Prod. 1(2), 89–95. Woo, P.T. 1970. The haematocrit centrifuge technique for the diagnosis of African trypanosomiasis. Acta Trop. 27(4), 384–386. Yusuf, A.B., Abubakar, A., Musa, U.B., Haruna, M.K., Garba, H.A., Maigari, A.K., Zubair, A. I., Machina, I. B., Shehu, A. A., Galadima, U. S., Adamu, A., Chechet, G. D. and Diggi, S. 2015. Surveillance for tsetse and trypanosomosis in Bagudo local government area north-western Nigeria. IOSR J. Agric. Vet. Sci. 8, 43–48. Zubairu, A.I., Midau, A., Dazala, I.U., Yahya, M.M. and Buba, Z.M. 2013. The prevalence of bovine trypanosomiasis in Song local government area of Adamawa state, Nigeria. Global Vet. 11(3), 310–313. | ||
| How to Cite this Article |
| Pubmed Style Akinbobola JS, Okaiyeto SO, Sackey AK, Allam L, Bisalla M, Dinga JN, Chukwuemeka PC, Cunningham L. Comparative performance evaluation of blood film microscopy for the diagnosis of bovine trypanosomosis by some laboratories in North-central Nigeria. Open Vet. J.. 2023; 13(5): 599-603. doi:10.5455/OVJ.2023.v13.i5.12 Web Style Akinbobola JS, Okaiyeto SO, Sackey AK, Allam L, Bisalla M, Dinga JN, Chukwuemeka PC, Cunningham L. Comparative performance evaluation of blood film microscopy for the diagnosis of bovine trypanosomosis by some laboratories in North-central Nigeria. https://www.openveterinaryjournal.com/?mno=120710 [Access: October 31, 2025]. doi:10.5455/OVJ.2023.v13.i5.12 AMA (American Medical Association) Style Akinbobola JS, Okaiyeto SO, Sackey AK, Allam L, Bisalla M, Dinga JN, Chukwuemeka PC, Cunningham L. Comparative performance evaluation of blood film microscopy for the diagnosis of bovine trypanosomosis by some laboratories in North-central Nigeria. Open Vet. J.. 2023; 13(5): 599-603. doi:10.5455/OVJ.2023.v13.i5.12 Vancouver/ICMJE Style Akinbobola JS, Okaiyeto SO, Sackey AK, Allam L, Bisalla M, Dinga JN, Chukwuemeka PC, Cunningham L. Comparative performance evaluation of blood film microscopy for the diagnosis of bovine trypanosomosis by some laboratories in North-central Nigeria. Open Vet. J.. (2023), [cited October 31, 2025]; 13(5): 599-603. doi:10.5455/OVJ.2023.v13.i5.12 Harvard Style Akinbobola, J. S., Okaiyeto, . S. O., Sackey, . A. K., Allam, . L., Bisalla, . M., Dinga, . J. N., Chukwuemeka, . P. C. & Cunningham, . L. (2023) Comparative performance evaluation of blood film microscopy for the diagnosis of bovine trypanosomosis by some laboratories in North-central Nigeria. Open Vet. J., 13 (5), 599-603. doi:10.5455/OVJ.2023.v13.i5.12 Turabian Style Akinbobola, Jones Soladoye, Solomon Oluwole Okaiyeto, Anthony Kojo Sackey, Lushaikyaa Allam, Mohammed Bisalla, Jerome Nyhalah Dinga, Prosper C. Chukwuemeka, and Lucas Cunningham. 2023. Comparative performance evaluation of blood film microscopy for the diagnosis of bovine trypanosomosis by some laboratories in North-central Nigeria. Open Veterinary Journal, 13 (5), 599-603. doi:10.5455/OVJ.2023.v13.i5.12 Chicago Style Akinbobola, Jones Soladoye, Solomon Oluwole Okaiyeto, Anthony Kojo Sackey, Lushaikyaa Allam, Mohammed Bisalla, Jerome Nyhalah Dinga, Prosper C. Chukwuemeka, and Lucas Cunningham. "Comparative performance evaluation of blood film microscopy for the diagnosis of bovine trypanosomosis by some laboratories in North-central Nigeria." Open Veterinary Journal 13 (2023), 599-603. doi:10.5455/OVJ.2023.v13.i5.12 MLA (The Modern Language Association) Style Akinbobola, Jones Soladoye, Solomon Oluwole Okaiyeto, Anthony Kojo Sackey, Lushaikyaa Allam, Mohammed Bisalla, Jerome Nyhalah Dinga, Prosper C. Chukwuemeka, and Lucas Cunningham. "Comparative performance evaluation of blood film microscopy for the diagnosis of bovine trypanosomosis by some laboratories in North-central Nigeria." Open Veterinary Journal 13.5 (2023), 599-603. Print. doi:10.5455/OVJ.2023.v13.i5.12 APA (American Psychological Association) Style Akinbobola, J. S., Okaiyeto, . S. O., Sackey, . A. K., Allam, . L., Bisalla, . M., Dinga, . J. N., Chukwuemeka, . P. C. & Cunningham, . L. (2023) Comparative performance evaluation of blood film microscopy for the diagnosis of bovine trypanosomosis by some laboratories in North-central Nigeria. Open Veterinary Journal, 13 (5), 599-603. doi:10.5455/OVJ.2023.v13.i5.12 |