| Original Article | ||
Open Vet. J.. 2021; 11(4): 764-770 Open Veterinary Journal, (2021), Vol. 11(4): 764–770 Original Research Analysis of normal electrocardiographic patterns in mandarin ducks (Aix galericulata)Rocky La Maestra, Michela Pugliese*, Annamaria Passantino and Filippo SpadolaDepartment of Veterinary Sciences, University of Messina Via Umberto Palatucci, Messina, Italy *Corresponding Author: Michela Pugliese. Department of Veterinary Sciences, University of Messina Via Umberto Palatucci, Messina, Italy. Email: mpugliese [at] unime.it Submitted: 08/09/2021 Accepted: 01/12/2021 Published: 25/12/2021 © 2021 Open Veterinary Journal
AbstractBackground: Electrocardiographic (ECG) examination in birds is considered an inexpensive and noninvasive diagnostic aid for the recognition of infectious and metabolic diseases. Contrary to other bird species, studies describing normal ECG patterns for mandarin ducks (Aix galericulata) are lacking. Aim: The aim of this study was to describe and evaluate normal ECG patterns in A. galericulata. Methods: Ten clinically healthy mandarin ducks of both sexes, aged between 1 and 2 years and with body weight between 0.8 and 1.2 kg were included. Electrocardiograms were performed in a quiet room with animals conscious and manually restrained. All electrocardiographic parameters were recorded using standard (I, II, and III) and augmented (aVR, aVL, and aVF) limb leads. The paper speed was set at 50 mm/second. The amplitude was 1 mV=20 mm. Morphological patterns of P, QRS, and T deflections were evaluated in all limb leads. The amplitude and the duration of waves and, their intervals were determined in lead II. The mean electrical axis (MEA) in the frontal plane was calculated using the Bailey hexaxial system. Results: In all birds examined, a regular sinus rhythm was observed. P waves were mainly positive in I, II, III, and aVF. A negative P wave was identified in aVR, while in aVL a great variability was present. The QRS complex was mainly isoelectric in lead I, while it was negative with rS or QS morphology in leads II, III, and aVF. A positive polarity of QRS was detected in aVL with R pattern, while it was negative or positive with QS or R morphology, respectively, in aVF. T waves were mostly positive in leads II, III, and aVF and, isoelectric in lead I. ST slurring and Ta wave were observed in 2/10 and 8/10, respectively. The mean heart rate was 246 ± 90 beats per minute and the MEA was −88.8° ± 9.57°. Conclusion: The ECG tracings of mandarin ducks show similarities and some differences with other avian species. The electrocardiographic values provided here can be used to assist in the interpretation of ECG in A. galericulata. Keywords: ECG, Electrocardiogram, Avian, Mandarin ducks, Aix galericulata. IntroductionECG examination is a non-invasive method used to evaluate the electrical activity of the heart both in human (Amsterdam et al., 2015) and veterinary medicine (Tilley, 1994; Smith et al., 2015). The first ECG examination in birds was performed by Buchanan in 1909 (Buchanan, 1909). In the following decades, ECG traces of turkeys (McKenzie et al., 1971; Boulianne et al., 1992), ducks (Kisch, 1951; Hassanpour and Khadem, 2013), chickens (Sturkie, 1949; Kisch, 1951), geese (Kaya and Çenesiz, 2018), and other species have been recorded (Kisch, 1949; Rodríguez et al., 2004; Uzun et al., 2004; Lopez Murcia et al., 2005; Talavera et al., 2008; Hassanpour et al., 2010, 2011a, 2011b; Kaya and Soylu, 2013; Hassanpour et al., 2014, 2016). There are important differences between the circulatory system of mammals and birds: the hearts of birds is proportionally larger, the heart rate is faster, and blood pressure levels are higher. In addition, the cardiomyocytes are smaller and they lack the M line and T tubules (Sturkie, 1998; Whittow, 2014). Birds also have some unique characteristics regarding their anatomy of the conduction system (e.g., subepicardial Purkinje network, truncobulbar node, the ring of Purkinje, etc.), which varies between species (Sturkie, 1998; Whittow, 2014). In Anatid birds, the electrical impulse is generated by the sinoatrial node and transmitted to the atrioventricular node along muscle fibers existing in the interatrial septum. The atrioventricular node is located between the interatrial and interventricular septum; it conducts the impulse to the atrioventricular bundle (Purkinje fibers) (Sturkie, 1998; Whittow, 2014). In ECG recordings, atrial depolarization is represented by the P wave, and the QRS complex represents the electrical impulse as it spreads through the ventricles and indicates ventricular depolarization (Sturkie, 1998; Whittow, 2014). The ECG in avian species is utilized to diagnose and monitor cardiac rhythm disorders in response to drugs administration (Hassanpour et al., 2008, 2009), metabolic disorders (Sturkie et al., 1954; Odom et al., 1991; Martinez et al., 1997), and infectious diseases (McKenzie and Will, 1972; Dubey et al., 2007; Cox et al., 2015). Currently, studies on the ECG patterns of Aix galericulata are insufficient and the ECG examination is, therefore, more difficult to evaluate. The aim of the study was to analyze the ECG records of healthy A. galericulata, establishing specific ECG patterns and normal value parameters. Materials and MethodsAnimalsA total of 10 A. galericulata (4 male, 6 female) were included, aged between 1 and 2 years and with a body weight ranging from 0.8 to 1.2 kg belonging to farms located in Sicily (South Italy). All ducks were considered healthy based on complete physical examination. They were housed in floor pens lined with sawdust litter and fed with corn, soybean meal, and barley. Di-calcium phosphate and a premix containing water-soluble vitamins (vitamins B group, folic acid, and biotin), lipid-soluble vitamins (A, D3, E, and K3), traces of minerals (such as iron, copper, zinc, manganese, cobalt, iodine, and selenium), and antioxidants were added at the diet. Water and food were available ad libitum. ProceduresECG traces were recorded with a direct writing electrocardiograph (Delta Tre Plus, Cardioline, Italy). Standard bipolar (I, II, and III) and augmented unipolar limb (aVR, aVL, and aVF) leads were recorded using 50 mm/seconds paper speed and 20 mm=1 mV for at least 30 seconds (Tilley, 1994; Smith et al., 2015). All procedures were carried out during the morning (10:00–12:00) in their native environment. All the animals were placed in right lateral recumbency on a plastic table in order to minimize stress and to record good quality ECG traces without artifacts. Atraumatic alligator clip electrodes were attached to the propatagium of the left and right wings and to the inguinal skin fold of the left and right legs (Talavera et al., 2008; Hassanpour and Khadem, 2013). The application of water-soluble ECG gel facilitated the parting of feathers, improved good clip-to-skin contact, and electrical conductivity (Tilley, 1994; Hassanpour and Khadem, 2013; Smith et al., 2015). No sedative drugs were used. To calm down the animals, ECG recordings started 5 minutes after the electrode placement. When birds were completely quiet and relaxed, limb leads were recorded (Fig. 1). Analysis of ECG tracesThe mean heart rate was calculated by the average of 10 consecutive RR intervals. The morphologic patterns of P, QRS, and T waves were evaluated for each lead and the QRS were labeled according to the standard nomenclature. The major deflection was indicated by a capital letter and the minor deflections by lower case letters (Tilley, 1994; Smith et al., 2015). The lead II was selected to measure amplitude and duration of P, T waves, and QRS complex, as well as the duration of PQ/PR, QT intervals, and ST segments. The mean electrical axis (MEA) in the frontal plane was determined in leads II and III using the Bailey hexaxial method (Tilley, 1994; Smith et al., 2015).
Fig. 1. Example of restrain and electrodes placement for ECG recording in female mandarin duck (A. galericulata).
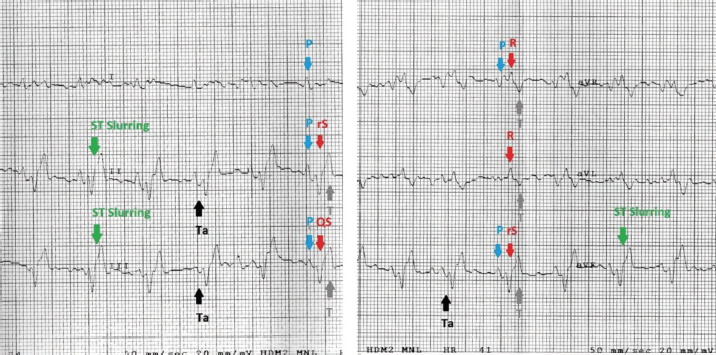
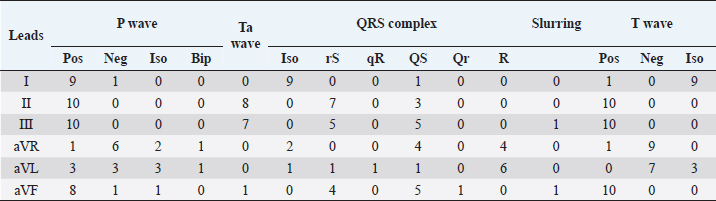
Fig. 2. Examples of mandarin duck ECG. Sinus rhythm, heart rate of 200 beats per minute with regular RR intervals. Lead I appears mostly isoelectric except for positive P waves, which are oriented downward and to the left. The P wave is often followed by the Ta, particularly clearly visible in II, III, and aVF. The QRS complex appears negative with rS or QS morphology in the lower leads; the vector is oriented mostly upward and slightly to the left. ST slurring is visible only in some beats, although the ST section always remained very short. Statistical analysisStatistical analyses were carried out using the Statistical Package for the Social Sciences Version 17.0, Inc., Chicago, IL. The values of duration and amplitude were expressed as mean ± standard deviation (SD). The 95% confidence interval for each parameter was calculated. Ethical approvalThe study was conducted in accordance with the Italian and European Regulations on Animal Welfare and with the approval of the Ethical Committee of the Veterinary Sciences Department of Messina University (no. 2017-16). ResultsTable 1 summarizes the recorded morphological parameters. P wave was mainly positive in leads II, III, and aVF, while they were negative in aVR presenting a great variability in lead I. The QRS complex was mainly isoelectric in lead I and negative with rS or QS morphology in leads II, III, and aVF. It was positive with R pattern in aVL, while it was negative or positive with QS or R morphology, respectively, in aVF. T waves were mostly positive in leads II, III, and aVF. They were negative in aVL and aVF and isoelectric in lead I. The Ta wave was detected in 8/10 ducks, while only 2/10 ST slurring was observed. The vast majority of ECG traces were obtained without major artifacts. However, in a few points of the recorded ECG traces, movement (large swing in the baseline) and tremors (narrow and rapid spikes in the baseline) artifacts were visible. A regular sinus rhythm was observed in all birds. Table 1. Morphological ECG patterns in different leads (II, III, aVR, aVL, and aVF) recorded in A. galericulata under manual restraint.
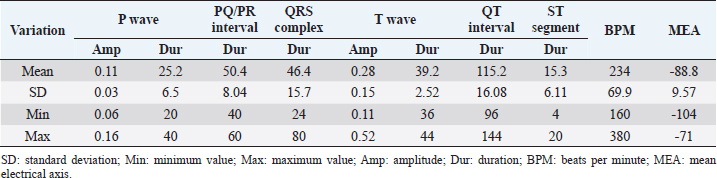
Table 2. The amplitude and duration of P and T waves, QRS complex, and their intervals in A. galericulata recorded in lead II. The amplitude is expressed in millivolts, while the duration is expressed in milliseconds.
Table 2 summarizes the amplitude and duration parameters of the recorded ECG parameters in II limb lead. The heart rate ranged between 160 and 380 beats/minute (mean=246; SD ± 90; 95% CI=190.22–301.78). The duration of the P wave ranged between 20 and 40 milliseconds (mean=25.2; SD ± 6.5; 95% CI=21.17–29.22), and the amplitude was between 0.06 and 0.16 mV (mean=0.11; SD ± 0.03; 95% CI=0.09–0.12). The QRS duration ranged between 24 and 80 milliseconds (mean=46.4; SD ± 15.7; 95% CI=36.66–56.13). The duration of the T wave was between 36 and 44 milliseconds (mean=39.2; SD ± 2.52; 95% CI=37.63–40.76), while the amplitude ranged between 0.11 and 0.52 mV (mean=0.28; SD ± 0.15; 95% CI=0.18–0.37). The duration of PQ/PR interval ranged between 40 and 60 milliseconds (mean=50.4; SD ± 8.04; 95% CI=45.41–55.38). The ST segment was very short with a duration between 4 and 20 milliseconds (mean=15.3; SD ± 6.11; 95% CI=11.51–19.08). The duration of the QT segment was between 96 and 144 milliseconds (mean=115.2; SD ± 16; 95% CI=105.28–125.11). In all ducks examined, the MEA on the frontal plane was between −104° and −71° (mean=−88.84; SD ± 9.57; 95% CI=−90.73 to –78.86). Figure 2 show examples of a mandarin duck ECG trace. DiscussionMandarin ducks are native to the Asian continent, but have spread as farm animals around the world. Furthermore, large groups of wild mandarin ducks are present in various European countries (Davies, 1988). The role of this species in the transmission of viral infectious diseases is under study (Kang et al., 2017). The ECG examination is considered a useful, cheap, and noninvasive diagnostic tool in the evaluation of the cardiovascular system. The electrocardiographic examination is, therefore, easy to perform even in farms or rural environments. It is also helpful to assess the clinical evaluation during severe trauma and monitor arrhythmias associated with conduction disorders (Sturkie et al., 1954; Martinez et al., 1997). Normal ECG patterns have been described in different species of birds, and it is widely used to evaluate cardiac rhythm (Kisch, 1949; McKenzie et al., 1971; Boulianne et al., 1992; Lopez Murcia et al., 2005; Talavera et al., 2008; Hassanpour et al., 2010; Hassanpour and Khadem, 2013; Hassanpour et al., 2016; Kaya and Çenesiz, 2018). Several infectious diseases such as toxoplasmosis (Dubey et al., 2007), West Nile virus (Cox et al., 2015), influenza (McKenzie and Will, 1972), adenovirus (Ivanics et al., 2010), and parvovirus infection (Shehata et al., 2016) may affect Anatids, compromising heart function. The aim of this study was to provide physiological ECG patterns of healthy adult ducks (A. galericulata), in order to improve the health monitoring of mandarin ducks with noninvasive methods. No sedative drugs were used during ECG recording. The manual restraint in a quiet place allowed to record good quality ECG traces without artifacts. Indeed, anesthetic drugs may result in ECG change such as the slowing of heart rate, prolongation of PR and QT intervals, and atrial premature contractions (Rashmi, 2002; Oji et al., 2013; Shintaku et al., 2014; Biernawska et al., 2016). Data reported here are partially in accordance with other studies carried out on birds and, in particular, on Anatids (Hassanpour and Khadem, 2013; Kaya and Çenesiz, 2018). The morphology of P wave was different in the six leads examined, probably due to physiologic variations in the species (Hassanpour and Khadem, 2013). The amplitude of the P wave ranged between 0.06 and 0.16 mV, which is similar to the values described in partridge (Uzun et al., 2004), helmeted guinea fowl (Hassanpour et al., 2011b), green peafowl (Hassanpour et al., 2011a), rooks (Hassanpour et al., 2016), and Muscovy ducks (Hassanpour and Khadem, 2013). An atrial repolarization wave (Ta) was detected in 8/10 ducks. In dogs, the Ta wave is typically hidden by the QRS complex and may be normal findings, although it is more frequently visualized when there is a delay in atrial repolarization due to a conduction disturbance or with atrial chamber enlargement (Santilli et al., 2018). The presence of Ta is considered evidence of right atrial hypertrophy in some animals such as dogs. The Ta wave is reported as a normal finding in the ECG traces of birds (Yogeshpriya et al., 2018). Similar to previous studies published in other avian species, the QRS complex was mainly isoelectric in lead I and negative in leads II, III, and aVF (Kisch, 1949; Lopez Murcia et al., 2005; Talavera et al., 2008; Hassanpour et al., 2010; Hassanpour and Khadem, 2013; Kaya and Soylu, 2013; Hassanpour et al., 2016; Kaya and Çenesiz, 2018). ST slurring, or the fusion of the QRS complex with the T wave and, therefore, absence of the ST segment, has been recorded several times in the past in various bird species with highly variable percentages (Lopez Murcia et al., 2005; Hassanpour and Khadem, 2013; Kaya and Soylu, 2013; Kaya and Çenesiz, 2018). Also, in the mandarin ducks, the ST tract is often very short, and in 2/10 cases there was a real ST slurring. T waves were always positive in leads II, III, and aVF, while they were mainly isoelectric in lead I (9/10) and negative in leads aVL (7/10) and aVR (9/10). These findings of T wave morphology were similar to data reported previously in other avian species (Kaya and Soylu, 2013; Kaya and Çenesiz, 2018). The negative MEA as recorded in this study (−88.8) is characteristic of the ECG of birds which present a negative polarity of the QRS complex in leads II, III, and aVF. Conversely to mammals, in avian species, the QRS complex is always negative in II limb lead for an opposite spread of the depolarization front from the epicardium to the endocardium (Sturkie, 1998; Whittow, 2014). Finally, this study has three main limitations: a low number of subjects enrolled and a recording speed of the ECG tracks at 50 mm/second instead of 100 mm/second more suitable for such high heart rates. Another limitation, common to other previous studies, is the lack of a guideline on the best patient positioning for which to record the ECG trace in avian species (Sturkie, 1998; Whittow, 2014; Yogeshpriya et al., 2018). Different positions such lateral decubitus, upright position, dorsal, and ventral decubitus have been described (Lopez Murcia et al., 2005; Hassanpour and Khadem, 2013; Kaya and Soylu, 2013; Omóbòwálé et al., 2017; Kaya and Çenesiz, 2018; Yogeshpriya et al., 2018). ConclusionIn the ECG of A. galericulata, there are some characteristics common to other bird species, such as the presence of atrial T wave and negative QRS complex in lead II, while ST slurring is found more rarely, probably linked to an increase in heart rate. In conclusion, morphology, duration, and amplitude of P, QRS and T waves, heart rates, and MEA of mandarin ducks show similarities and differences with the other avian species studied previously. These variations in avian species explain the need for the specific ECG patterns and reference values for all wild bird species. This study provides a description of the normal ECG in healthy ducks that can be used as a reference in clinical settings. Further studies with more enrolled animals are needed to establish normal ECG parameters for A. galericulata. ReferencesAmsterdam, E.A., Wenger, N.K., American College of Cardiology and American Heart Association. 2015. The 2014 American College of Cardiology ACC/American Heart Association guideline for the management of patients with non-ST-elevation acute coronary syndromes: ten contemporary recommendations to aid clinicians in optimizing patient outcomes. Clin. Cardiol. 38(2), 121–123. Biernawska, J., Kaźmierczak, J. and Kotfis, K. 2016. The influence of regional anaesthesia and local anaesthetics on cardiac repolarization. Anaesthesiol. Intensive Ther. 48(2), 135–141. Boulianne, M., Hunter, D.B. and Julian, R.J. 1992. Cardiac muscle mass distribution in the domestic Turkey and relationship to electrocardiogram. Avian Dis. 36(3), 582–589. Buchanan, F. 1909. The frequency of the heartbeat and the form of the electrocardiogram in birds. J. Physiol. 38, 62–66. Cox, S.L., Campbell, G.D. and Nemeth, N.M. 2015. Outbreaks of West Nile virus in captive waterfowl in Ontario, Canada. Avian Pathol. 44(2), 135–141. Davies A.K., 1988. The distribution and status of the mandarin duck Aix galericulata in Britain. Bird Study 35(3), 203–207. Dubey, J.P., Webb, D.M. and Sundar N., 2007. Endemic avian toxoplasmosis on a farm in Illinois: clinical disease, diagnosis, biologic and genetic characteristics of Toxoplasma gondii isolates from chickens (Gallus domesticus), and a goose (Anser anser). Vet. Parasitol. 148, 207–212. Hassanpour, H. and Khadem, P. 2013. Normal electrocardiogram patterns and values in Muscovy ducks (Cairina moschata). J. Avian Med. Surg. 27(4), 280–284. Hassanpour, H., Dehkordi, H.A. and Khosravi, M. 2016. Analysis of the normal electrocardiogram in wild Rooks (Corvus frugilegus). J. Avian Med. Surg. 30(4), 329–334. Hassanpour, H., Hojjati, P. and Zarei, H. 2011a. Electrocardiogram analysis of the normal unanesthetized green peafowl (Pavo muticus). Zoo Biol. 30(5), 542–549. Hassanpour, H., Shamsabadi, M.G. and Dehkordi, I.N. 2014. Normal electrocardiogram of the laughing dove (Spilopelia senegalensis). J. Zoo Wild Med. 45(1), 41–46. Hassanpour, H., Zamami Moghadam, A.K. and Zarei, H. 2009. Effect of citric acid on the electrocardiographic parameters of broiler chickens with pulmonary hypertension. Acta Vet. Hung. 57(2), 229–238. Hassanpour, H., Zamami Moghadam, A.K., Teshfam, M. and Zarei, H. 2008. Effect of ascorbic acid on the electrocardiogram of broiler chickens raised at high altitude. Niger. Vet. J. 29(2), 8–14. Hassanpour, H., Zamani Moghaddam, A.K. and Cheraghchi Bashi, M. 2010. The normal electrocardiogram of conscious golden eagles (Aquila chrysaetos). J. Zoo Wild Med. 41(3), 426–431. Hassanpour, H., Zarei, H. and Hojjati, P. 2011b. Analysis of electrocardiographic parameters in helmeted guinea fowl (Numida meleagris). J. Avian Med. Surg. 25(1), 8–13 Ivanics, E., Palya, V. and Markos, B. 2010. Hepatitis and hydropericardium syndrome associated with adenovirus infection in goslings. Acta Vet. Hung. 58(1), 47–58. Kang, Y.M., Lee, E., Song, B., Heo, G., Jung, J. and Jang, I. 2017. Experimental infection of mandarin duck with highly pathogenic avian influenza A (H5N8 and H5N1) viruses. Vet. Microbiol. 198, 59–63. Kaya, M. and Çenesiz, M. 2018. The electrocardiogram of the conscious Chinese goose (Anser cygnoides). Thai J. Vet. Med. 48(3), 487–492. Kaya, M. and Soylu, S.M. 2013. Analysis of electrocardiographic parameters in the conscious common pheasants (Phasianus colchicus). Kafkas Univ. Vet. Fak. Derg. 19(6), 1039–1044. Kisch, B. 1949. Electrocardiographic studies in seagulls. Exp. Med. Surg. 7(4), 345–357. Kisch, B. 1951. The electrocardiogram of birds (chicken, duck, pigeon). Exp. Med. Surg. 9(1), 103–124. Lopez Murcia, M.M., Bernai, L.J., Montes, A.M., Garcia Martinez, J.D. and Ayala, I. 2005. The normal electrocardiogram of the unanaesthetized competition “Spanish Pouler” pigeon (Columba livia gutturosa). J. Vet. Med. A Physiol. Pathol. Clin. Med. 52(7), 347–349. Martinez, L.A., Jeffrey, J.S. and Odom, T.W. 1997. Electrocardiographic diagnosis of cardiomyopathies in aves. Poult. Avian Biol. Rev. 8(1), 9–20. McKenzie, B.E. and Will, A.J. 1972. Electrocardiographic changes following influenza infection in turkeys. Avian Dis. 16(2), 308–318. McKenzie, B.E., Will, A.J. and Hardien, A. 1971. The electrocardiogram of turkey. Avian Dis. 15(4), 737–744. Odom, T.W., Hargis, B.M., Lopez, C.C., Arce, M.J., Ono, Y. and Avila, G.E. 1991. Use of electrocardiographic analysis for investigation of ascites syndrome in broiler chickens. Avian Dis. 35(4), 738–744. Oji, M., Terao, Y., Toyoda, T. and Kuriyama, T. 2013. Differential effects of propofol and sevoflurane on QT interval during anesthetic induction. J. Clin. Monit. Comput. 27(3), 243–248. Omóbòwálé, T.O., Esan, O.O., Adejumobi, O.A. and Oladele, O.A. 2017. Six-lead electrocardiographic studies of the pigeon (Columba livia) in Nigeria. Afr. J. Biomed. Res. 20, 273–276. Rashmi, R.S. 2002. The significance of QT interval in drug development. Br. J. Clin. Pharmacol. 54(2), 188–202. Rodríguez, R., Prieto-Montaña, F., Montes, A.M., Bernal, L.J., Gutierrez-Panizo, C. and Ayala, I. 2004. The normal electrocardiogram of the unanesthetized peregrine falcon (Falco peregrinus rookei). Avian Dis. 48, 405–409. Santilli, R., Sidney Moïse, N., Pariaut, R. and Perego M. 2018. Electrocardiography of the dog and cat. Diagnosis of arrhythmias, 2nd ed. Palm Beach Gardens, FL: Edra. Shehata, A.A., Gerry, D.M. and Heenemann, K. 2016. Goose parvovirus and circovirus coinfections in ornamental ducks. Avian Dis. 60(2), 516–522. Shintaku, T., Ohba, T. and Niwa, H. 2014. Effects of propofol on electrocardiogram measures in mice. J. Pharmacol. Sci. 126(4), 351–358. Smith, F., Tilley and L. and Oyama, M. 2015. Manual of canine and feline cardiology, 5th ed. Philadelphia, PA: Saunders. Sturkie, P.D. 1949. The electrocardiogram of the chicken. Am. J. Vet. Res. 10(35), 168–175. Sturkie, P.D. 1998. Heart: contraction, conduction, and electrocardiography. In Avian physiology. Ed., Sturkie, P.D. New York, NY: Springer, pp: 167–190. Sturkie, P.D., Singsen, E.P., Matterson, L.D., Kozeff, A. and Jungherr, E. L. 1954. The effects of dietary deficiencies of vitamin E and the B complex vitamins on the electrocardiogram of chickens. Am. J. Vet. Res. 15(56), 457–462. Talavera, J., Guzmán, M.J., Fernández del Palacio, M.J., Albert, A.P. and Bayon, A. 2008. The normal electrocardiogram of four species of conscious raptor. Res. Vet. Sci. 84, 119–125. Tilley, L.P. 1994. The approach to the electrocardiogram. In Essentials of canine and feline electrocardiography, 3rd ed. Ed., Tilley, L.P. Philadelphia, PA: Lea & Febiger, pp: 22–33. Uzun, M., Yildiz, S. and Onder, F. 2004. Electrocardiography of rock partridges (Alectoris graeca) and cuckar partridges (Alectoris chuckar). J. Zoo Wild Med. 35(4), 510–514. Whittow, G.C. 2014. Cardiovascular system. In Sturkie’s avian physiology, 6th ed. Eds., Scanes, C. and Dridi, S. San Diego, CA: Academic Press, pp: 230–244. Yogeshpriya, S., Selvaraj, P., Ramkumar, P.K., Veeraselvam, M., Saravanan, M., Venkatesan, M. and Jayalakshm, K. 2018. Review on avian electrocardiogram. Int. J. Curr. Microbiol. Appl. Sci. 7(8), 1389–1395. | ||
| How to Cite this Article |
| Pubmed Style Maestra RL, Pugliese M, Passantino A, Spadola F. Analysis of normal electrocardiographic patterns in Mandarin ducks (Aix galericulata). Open Vet. J.. 2021; 11(4): 764-770. doi:10.5455/OVJ.2021.v11.i4.29 Web Style Maestra RL, Pugliese M, Passantino A, Spadola F. Analysis of normal electrocardiographic patterns in Mandarin ducks (Aix galericulata). https://www.openveterinaryjournal.com/?mno=121184 [Access: January 25, 2026]. doi:10.5455/OVJ.2021.v11.i4.29 AMA (American Medical Association) Style Maestra RL, Pugliese M, Passantino A, Spadola F. Analysis of normal electrocardiographic patterns in Mandarin ducks (Aix galericulata). Open Vet. J.. 2021; 11(4): 764-770. doi:10.5455/OVJ.2021.v11.i4.29 Vancouver/ICMJE Style Maestra RL, Pugliese M, Passantino A, Spadola F. Analysis of normal electrocardiographic patterns in Mandarin ducks (Aix galericulata). Open Vet. J.. (2021), [cited January 25, 2026]; 11(4): 764-770. doi:10.5455/OVJ.2021.v11.i4.29 Harvard Style Maestra, R. L., Pugliese, . M., Passantino, . A. & Spadola, . F. (2021) Analysis of normal electrocardiographic patterns in Mandarin ducks (Aix galericulata). Open Vet. J., 11 (4), 764-770. doi:10.5455/OVJ.2021.v11.i4.29 Turabian Style Maestra, Rocky La, Michela Pugliese, Annamaria Passantino, and Filippo Spadola. 2021. Analysis of normal electrocardiographic patterns in Mandarin ducks (Aix galericulata). Open Veterinary Journal, 11 (4), 764-770. doi:10.5455/OVJ.2021.v11.i4.29 Chicago Style Maestra, Rocky La, Michela Pugliese, Annamaria Passantino, and Filippo Spadola. "Analysis of normal electrocardiographic patterns in Mandarin ducks (Aix galericulata)." Open Veterinary Journal 11 (2021), 764-770. doi:10.5455/OVJ.2021.v11.i4.29 MLA (The Modern Language Association) Style Maestra, Rocky La, Michela Pugliese, Annamaria Passantino, and Filippo Spadola. "Analysis of normal electrocardiographic patterns in Mandarin ducks (Aix galericulata)." Open Veterinary Journal 11.4 (2021), 764-770. Print. doi:10.5455/OVJ.2021.v11.i4.29 APA (American Psychological Association) Style Maestra, R. L., Pugliese, . M., Passantino, . A. & Spadola, . F. (2021) Analysis of normal electrocardiographic patterns in Mandarin ducks (Aix galericulata). Open Veterinary Journal, 11 (4), 764-770. doi:10.5455/OVJ.2021.v11.i4.29 |