| Review Article | ||
Open Vet. J.. 2023; 13(2): 143-149 Open Veterinary Journal, (2023), Vol. 13(2): 143–149 Review Article Analysis of mice (Mus musculus L.) and hamster embryo development using culture and vitrification medium: Systematic reviewMaslichah Mafruchati1*, and Jonathan Makuwia21Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Malawi University of Science and Technology, Limbe, Malawi *Corresponding Author: Maslichah Mafruchati. Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: maslichahmafruchati01 [at] gmail.com Submitted: 01/11/2022 Accepted: 17/01/2023 Published: 04/02/2023 © 2023 Open Veterinary Journal
AbstractBackground: The success of embryo production is largely determined by the accuracy of making medium formulations that are adapted to the age of embryo growth. It is known that the cryopreservation method is widely used for the vitrification of embryos frozen at –196°C. Aims: This study aimed to analyze the embryonic development of mice (Mus musculus L.) and hamsters using culture and vitrification media. Method: This method uses the preferred guide to reporting items for systematic review and meta-analysis. Results: Based on the search results, a total of 700 articles were obtained which then entered the elimination stage, resulting in 37 articles on the development of mice embryos (M. musculus L.) and hamsters using culture and vitrification media. Conclusion: Thus, it can be concluded that the identification of the embryonic development of mice (M. musculus L.) and hamsters can be used with the use of culture media and the development of vitrification methods. Keywords: Embryo, Mus musculus, Hamster, Culture, Vitrification media. IntroductionIn the practice of embryo production to obtain embryos with superior genetic potential, the aggregation method is often used, because this method is considered the cheapest and easiest method only by utilizing the potential of the embryo. Embryos to be aggregated can come from two sources; first are non-clonal embryos, namely embryos which are growths from zygotes without any treatment, while the second is clonal embryos obtained from splitting results. Several problems are often encountered in vitro embryo production, both related to internal and external factors. One example of a problem that is often encountered is the low level of viability of aggregated embryos. This low viability value is often caused by the absence of a match in the selection of embryonic stages that will be used as a source for the aggregation process with the medium to be used. The biggest obstacle to in vitro embryo production through the aggregation method is the occurrence of the “cell block” phenomenon in embryo growth. The success of embryo production is again largely determined by the accuracy of making a medium formulation that is adapted to the growth age of the embryo to be used. There are several types of medium as a source of nutrients to support the growth and development of embryos, ranging from simple compound media, such as M16 (Verschoor et al., 2003; Kurniawati, 2006) used for the growth of mouse embryos, CR1AA medium (Rosenkrans and First, 1994) for embryonic growth of sheep and goats (Verschoor et al., 2003) to more complex ones such as tissue culture medium 199 which is widely used for embryo growth from laboratory animals and livestock (Boediono et al., 1995; Verschoor et al., 2003). In preparing the compound medium, there are basic things that must be considered, namely the source of water used as a solvent, and physical properties such as osmolarity and pH, and the composition of the media that is adjusted based on the needs for embryo growth and development. The most important benefit and application of double vitrification are related to the right time of embryonic development to be transferred to each patient. For example, if there is an excess number of zygotes in an IVF program patient, these zygotes can be frozen immediately or cultured in vitro until the blastocyst stage of development, and then frozen again for future transfer. Pregnancy failure due to OHSS can be overcome by repeating the transfer of previously frozen embryos after the patient’s uterus is in normal condition. The indicator of the success of vitrification is the viability of the post-vitrified embryo. Embryo viability includes survival (survival rate) and successful development of each stage of the embryo (development rate). The research results of Murakami et al. (2011) showed that double vitrification in human embryos with cryotop containers still had high viability, which was around 98.1%, and resulted in normal live births. The purpose of this study was to analyze the embryonic development of mice (Mus musculus L.) and hamsters using culture and vitrification medium. Materials and MethodsThis review uses a Systematic Review approach that is based on the Preferred Report Items for Systematic Reviews and Meta-analysis. The literature search was carried out in August to September 2022, in several databases, namely PubMed, ScienceDirect, NCBI, and Elsevier, with an article publication time span of 1973–2022. The inclusion criteria consisted of articles reporting “development of mouse embryos, (Mus musculus L.), hamsters, culture media, vitrification.” Based on the search results, 37 articles were found which were then included in the review results in this article. Then, the writing of this manuscript reviews articles based on abstracts and full texts for further description in order to find similarities and differences in each study and draw conclusions. ResultsBased on the results of a search conducted in PubMed, ScienceDirect, NCBI, and Elsevier, a total of 700 articles were obtained which then entered the elimination stage, resulting in 37 articles. Figure 1 showed the disintegration of the zona pellazida of 8-cell embryos with 0.25% pronase in the drop of KSOMaa medium. Figure 2 showed the preparation of embryo pairs ready for aggregation in KSOMaa culture medium. Figure 3 showed the aggregation of embryos in KSOMaa culture medium. Figure 4 showed the morphology of the blastocyst in the freezing process with double vitrification. On the other side, this result of this research also showed the percentage of embryonic quality of mice (M. musculus) before and after vitrification and thawing (Table 1), the percentage of zygotes able to pass cell block on M16 and HTF medium (Table 2), and development of 8-cell stage mouse and hamster embryos after 48 hours of culture (Table 3). DiscussionEmbryo aggregation in 8-cell division stage on KSOMaa culture mediumEmbryo aggregation or embryo fusion to produce embryos with high viability values can be carried out at an early stage of embryonic development until before the embryo reaches the compact morula stage (Kelly et al., 1978). In this study, the embryos used in the aggregation process were embryos with an 8-cell division stage and were proven to be capable of producing embryos with high viability values. The blastomeres of the 8-cell stage embryo usually form a loose configuration with plenty of space between the blastomeres. After the blastomeres undergo the next division, the blastomeres will undergo dramatic physiological changes to grow and develop into the next stage. During the growth and development stage, the agglomerated embryos continuously undergo changes, namely an increase in diameter, transcriptional activity, and cytoplasmic content. During cytoplasmic maturation, molecular and structural changes occur, namely a rapid increase in the number and size of organelles such as ribosomes, fat globules, Golgi apparatus, and mitochondria. Based on the research that has been done, it has been proven that the KSOMaa medium is able to stimulate the growth and development of the embryo at the stage of 8-cell division in the aggregation process so that it can produce aggregated embryos with high viability (Nieminen et al., 2004).
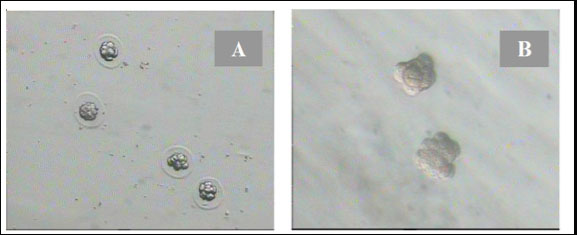
Fig. 1. Disintegration of the zona pellazida of 8-cell embryos with 0.25% pronase in the drop of KSOMaa medium. (A): 8-cell-stage embryos with the decay of the zona pellucida under the influence of pronase. (B): 8-cell stage of embryos without zona pellucida that were ready to be aggregated (Nieminen et al., 2004).
Fig. 2. Preparation of embryo pairs ready for aggregation in KSOMaa culture medium (Nieminen et al., 2004).
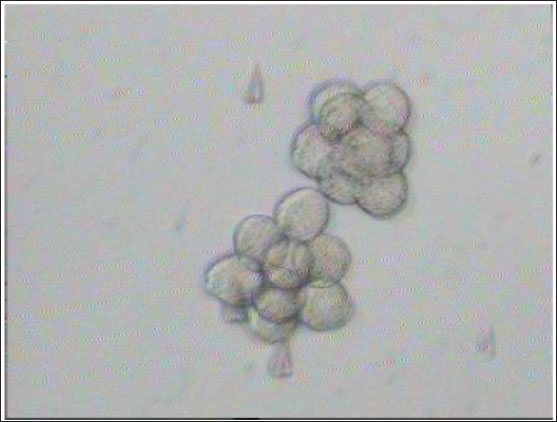
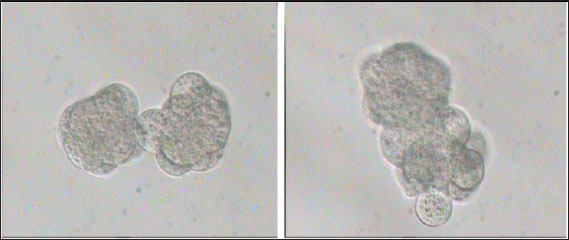
Fig. 3. Aggregation of embryos in KSOMaa culture medium. Left: early aggregation. Right: advanced aggregation (Nieminen et al., 2004). Viability of mice (M. musculus albinus) embryo after cryopreservation with double vitrificationThe success of vitrification cannot be separated from the optimization of each vitrification stage, starting from equilibration, vitrification, and heating, to in vitro culture. Equilibration of embryos in cryoprotectants prior to freezing was carried out to withdraw water and replace water with intracellular cryoprotectants. In mouse embryos, water and intracellular cryoprotectants will seep slowly by diffusion through the aquaporin protein on the plasma membrane (Kasai and Edashige, 2007) so that the perivitelline space will appear looser, the period and temperature of equilibration depending on the cryoprotectant used, taking into account the factors to minimize toxicity and osmotic pressure caused by cryoprotectants. The percentage of survival after multiple vitrification obtained in this study was 92.5%. Double vitrification has been proven to be successful because its viability is more than 80% (Saftiany, 2011), although, it is lower than the study of Murakami et al. (2011) who performed double vitrification with a cryotop container in embryos at the pronuclear development stage and continued at the blastocyst stage at 98.1%.
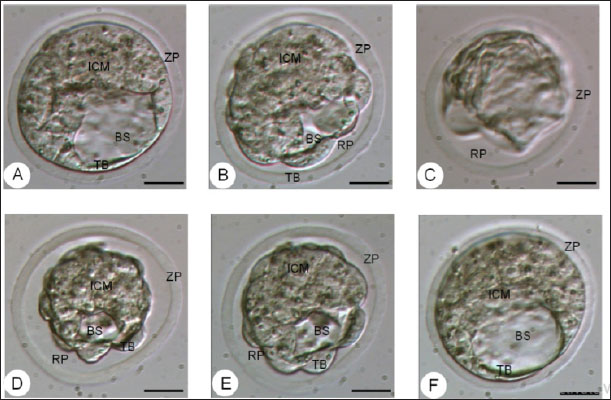
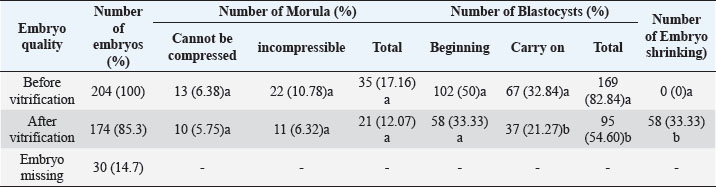
Fig. 4. The morphology of the blastocyst in the freezing process with double vitrification. (A): Blastocyst before vitrification. (B): Blastocyst exposed to equilibration medium, shrinkage occurs which is indicated by the widening of the perivitelline cavity (RP). (C): Blastocyst exposed to vitrified medium, maximal shrinkage occurs. (D–E): Blastocyst exposed to medium warming. (F): Blastocysts after two hours of in vitro culture. RP: Perivitelin Room; ZP: Zona Pellucida; BS: Blastosul; ICM: Inner Cell Mass; TB: Trophoblast (Bar: 20 μm). Table 1. Percentage of embryonic quality of mice (M. musculus) before and after vitrification and thawing (Kusumaningtyas et al., 2005).
Quality, implantation ability, and in-vivo viability of mice embryo (M. musculus) Swiss Webster strain after freezing by vitrification methodThe results of the study (Kusumaningtyas et al., 2005) showed a decrease in embryo quality after vitrification and thawing up to 33.33%. The decline in embryo quality is quite high compared to the research of Lopatáŕová on bovine embryos (Lopatářová et al., 2002). By using the medium added 16.5% EG + 16.5% DMSO + 0.5 M sucrose, Lopatarova showed a high recovery rate of around 92.9% for the bovine embryos used. In this experiment, Lopatarova used a longer incubation time of 72 hours, compared to the time we used in this study (24 hours) (Lopatářová et al., 2002). When the embryo is exposed to a vitrification medium, water will undergo exosmosis and a certain amount of ethylene glycol (EG) and dimethyl sulfoxide (DMSO) will enter the embryo cells. At the same time, sucrose will help the dehydration of cells, as a result, the cells will shrink (Saha et al., 1994). At the time of thawing the embryos were gradually exposed to some hypertonic medium containing sucrose. This hypertonic medium can prevent a very fast influx of water into the cells. At the same time, EG and DMSO will leave the cell thereby decreasing the concentration gradient between intracellular and extracellular. This flow of EG and DMSO can stop the movement of water across the cell membrane, thus preventing cell lysis during the diffusion of EG and DMSO outward (Ozkavukcu and Erdemli, 2002). The embryo expands again because water enters the cell and the cell will return to its normal size after the embryo is cultured for 1–2 hours in an M2 medium. Embryos that remain shriveled after vitrification and thawing are caused by the embryo not being able to return to its original state, due to large changes in osmotic pressure during vitrification and thawing. A large change in osmotic pressure will increase the intracellular ion concentration, cause damage to the plasma membrane and reduce the permeability of embryonic cells resulting in embryonic cells failing to expand (Lopatářová et al., 2002). Table 2. Percentage of zygotes able to pass cell block on M16 and HTF medium (Pusporini and Arifin, 2012).
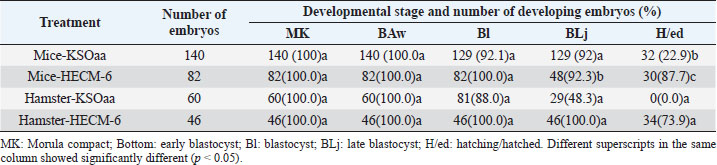
Table 3. Development of 8-cell stage mouse and hamster embryos after 48 hours of culture (Rosadi et al., 2008).
Vitrification of morula and blastocyst stage mouse embryos using VABEDS medium (15% EG, 15% DMSO, and 0.5 M sucrose) exposed to room temperature (± 27°C) for ± 1 minute proved to be successful. This technology can be used to support reproductive technology programs with or without embryo engineering. Reproductive technology in the form of embryo vitrification, thawing, and embryo transfer is indispensable in overcoming cases of infertility in humans. In the future, embryo vitrification (freezing) technology, thawing, and embryo transfer can be developed to store gamete cells (sperm and egg cells) as well as embryos of rare mammals such as jungle cats, tigers, Javan rhinoceros, Sumatran rhinos, deer and deer whose population is large, and getting smaller (Kusumaningtyas et al., 2005). Development of mice embryo cultured in M16 medium and human tubal fluidThe medium used for embryo culture is arranged based on the purpose of using tissue or organ culture medium, while embryo tissue until the blastocyst stage is relatively more homogeneous as long as other basic needs for culture are met (El-Sohaimy and Hafez, 2010). M16 medium and HTF medium used as culture medium can meet the basic needs of gamete cells to develop into embryos so as not to affect their morphology. In general, embryos require the same environment as other cultured mammalian cells. The elements needed for in vitro culture of embryos contained in M16 and HTF medium include a substrate, air, water, and temperature. The substrate in the M16 culture medium and HTF medium consisted of organic and inorganic chemicals. The amount of substrate added to the culture medium is adjusted to the composition of the substances in the Fallopian tube fluid where the embryo develops. The substrate consists of nutrients and other substances in dissolved form and the nutrients provided are a source of energy and protein (Summers et al., 2005; Lloyd et al., 2009). In M16 medium and HTF medium, the added energy sources are glucose, pyruvate, and lactate. The energy in the substrate is added to support the development of the preimplantation embryo from the single-cell stage to the blastocyst stage. In general, the pH in the cell culture medium was 7.4 beyond that pH the cells did not grow well, although the optimum pH for growth had little variation among different cell types. Ca, Mg, K, and PO4 ions contained in M16 and HTF medium are required for enzyme activity and maintaining the osmolarity of single-cell mouse embryos ranging from 250 to 280 mOsm and for two cells ranging from 272 to 280 mOsm (Biggers et al., 2000). Carbon dioxide is an important and beneficial element in embryonic development. Carbon dioxide is associated with bicarbonate buffer, which is very important to regulate the pH balance, so to regulate the balance of CO2 pressure inside and outside the medium can be given 5% CO2 in the air. The balance between CO2 carbonate which is related to pH balance is also related to temperature. Temperature can affect pH by increasing the solubility of CO2 at low temperatures and possibly through changes in ionization and pH of the buffer. The balance between these three things must be maintained because they have a direct influence on cell growth (Steeves et al., 2001; Feil et al., 2006). Water in cell culture is used as a solvent for chemicals that make up the medium, besides that water is also a medium for introducing substances needed for embryos to develop. The chemical elements that make up the culture medium if mixed with metals or other elements that can be present in the water will be toxic to the culture. Another element added in M16 medium and HTF medium is BSA. Serum is a biological fluid that is proven to support cell growth outside the body. Serum functions to provide a number of growth factors, contains hormones and provides binding proteins that carry and bind small elements. Serum is also a source of various fats which are generally needed by cells to live and develop (Leese et al., 2001) (Summers et al., 2005). From the results of the study, in both M16 and HTF medium, the fertilization rate and the ability of the zygote to pass through the cell block were not significantly different, this was because the basic composition of M16 and HTF medium was almost the same. The ability of the zygote to pass through the cell block is seen by the ability of the zygote to divide into two cell stages. In both treatment groups, the number of embryos dividing into two-cell stages was quite high. Therefore, HTF medium can be used for culturing mouse embryos because of the similarity in the basic composition of the medium (Pusporini and Arifin, 2012). Development of mice and hamster embryos in KSOMaa and HECM-6 mediumSchini and Bavister (1988) found that in hamster embryos the 8-cell stage of phosphate alone did not cause inhibition of embryonic development, but at an earlier stage, low concentrations of up to 800 nM inhibited the development of hamster embryos (Schini and Bavister, 1988; Ludwig et al., 2001). A similar phenomenon occurs in mouse embryos (Lawitts and Biggers, 1991). KSOMaa was quite effective in supporting the growth of 8-cell stage (T1) mouse embryos but not sufficient for hamster (T3) embryos until advanced blastocyst stage. This medium is known to be able to support high blastocyst acquisition from mouse zygotes with strains undergoing 2-cell block. The number of cells obtained was more than using other media, even equivalent to the number of blastocyst cells produced in vivo (Erbach et al., 1994; Summers et al., 1995). Amino acids added to this medium stimulate embryonic cell proliferation, especially ICM and ICM differentiation (Summers et al., 2005). What is interesting from this study is the ability of HECM-6 to support the development of 8-cell stage mouse embryos. This medium does not contain glucose and sodium lactate is used as an energy source. Embryos that hatched were higher (p < 0.08) than those cultured using the KSOMaa medium. The absence of the KH2PO4 component in the medium was thought to reduce the negative effect of phosphate on the organization of mitochondria in the cytoplasm, thereby improving the metabolic process. The high hatching rate indicates that the embryo has sufficient energy to break down and exit the zona pellucida. It is not known whether this is related to the use of lactate as an energy source. Hamster embryos cultured in HECM-6 medium also had a high hatching rate (73.9%). The high hatching success indicates the metabolism is running well, and the composition of the medium can fully support the development of the embryo (Rosadi et al., 2008). ConclusionBased on the results of a search conducted in PubMed, ScienceDirect, NCBI, and Elsevier, a total of 700 articles were obtained which then entered the elimination stage, resulting in 37 articles. Further studies are needed, especially the identification of the embryonic development of mice (M. musculus L.) and hamsters using various culture mediums and the development of vitrification methods. ReferencesBiggers, J.D., McGinnis, L.K. and Raffin, M. 2000. Amino acids and preimplantation development of the mouse in protein-free potassium simplex optimized medium. Biol. Reprod. 63(1), 281–293. Boediono, A., Saha, S., Sumantri, C., Suzuki, T. and Rajamahendran, R. 1995. Effect of the presence of a CL in the ovary on oocyte number, cleavage rate and blastocyst production in vitro in cattle. Theriogenology 1(43), 169. El-Sohaimy, S.A. and Hafez, E.E. 2010. Biochemical and nutritional characterizations of date palm fruits (Phoenix dactylifera L.). J. Appl. Sci. Res. 6(6), 1060–1067. Erbach, G.T., Lawitts, J.A., Papaioannou, V.E. and Biggers, J.D. 1994. Differential growth of the mouse preimplantation embryo in chemically defined media. Biol. Reprod. 50(5), 1027–1033. Feil, D., Lane, M., Roberts, C.T., Kelley, R.L., Edwards, L.J., Thompson, J.G. and Kind, K.L. 2006. Effect of culturing mouse embryos under different oxygen concentrations on subsequent fetal and placental development. J. Physiol. 572(1), 87–96. Kasai, M. and Edashige, K. 2007. Vitrification in animal reproduction: 4B vitrification of embryos using conventional straws with an ethylene glycol-based solutions. Vitrification in assisted reproduction: a user’s manual and trouble-shooting guide, pp: 75. Kelly, S.J., Mulnard, J.G. and Graham, C.F. 1978. Cell division and cell allocation in early mouse development. J. Embryol. Exp. Morphol. 48, 37–51. Kurniawati, D. 2006. Perbandingan tingkat keberhasilan perkembangan embrio hasil fertilisasi in vitro pada oosit mencit (Mus musculus l.) Strain swiss webster dengan menggunakan spermatozoa epididimis dan spermatozoa hasil kriopreservasi. Availble via https://digilib.uns.ac.id/dokumen/detail/6441/Perbandingan-tingkat-keberhasilan-perkembangan-embrio-hasil-fertilisasi-in-vitro-pada-oosit-mencit-mus-musculus-l-Strain-swiss-webster-dengan-menggunakan-spermatozoa-epididimis-dan-spermatozoa-hasil-kriopreservasi Kusumaningtyas, H., Boediono, A. and Sumarsono, S.H. 2005. Kualitas, kemampuan implantasi dan viabilitas in-vivo embrio mencit (Mus musculus) galur swiss webster setelah pembekuan dengan metode vitrifikasi. Available via https://repository.ipb.ac.id/handle/123456789/53658 Lawitts, J.A. and Biggers, J.D. 1991. Optimization of mouse embryo culture media using simplex methods. J. Reprod. Fertil. 91(2), 543-556. Leese, H.J., Tay, J.I., Reischl, J. and Downing, S.J. 2001. Formation of Fallopian tubal fluid: role of a neglected epithelium. Reproduction 121(3), 339–346. Lloyd, R.E., Romar, R., Matás, C., Gutiérrez-Adán, A., Holt, W.V. and Coy, P. 2009. Effects of oviductal fluid on the development, quality, and gene expression of porcine blastocysts produced in vitro. Reproduction 137(4), 679–687. Lopatářová, M., Čech, S., Havlíček, V. and Holý, L. 2002. Effect of vitrification in open pulled straws on survival of bovine embryos from superovulated cows. Acta Vet. Brno. 71(1), 93–99. Ludwig, T.E., Squirrell, J.M., Palmenberg, A.C. and Bavister, B.D. 2001. Relationship between development, metabolism, and mitochondrial organization in 2-cell hamster embryos in the presence of low levels of phosphate. Biol. Reprod. 65(6), 1648–1654. Murakami, M., Egashira, A., Murakami, K., Araki, Y. and Kuramoto, T. 2011. Perinatal outcome of twice-frozen-thawed embryo transfers: a clinical follow-up study. Fertil. Steril. 95(8), 2648–2650. Nieminen, P.-M., Aho, M., Kananen-Anttila, K., Reinikainen, E. and Halmekytö, M. 2004. Handmade cloning in trans-species NT: Culture medium has an effect on the ability of reconstructed bovine-murine embryos to develop beyond the 8-cell stage. Reprod. Fertil. Develop. 17(2), 179. Ozkavukcu, S. and Erdemli, E. 2002. Cryopreservation: basic knowledge and biophysical effects. J. Ankara Med. School. 24(4), 187–196. Pusporini, S.E. and Arifin, M.Z. 2012. The comparison of mice fertility rate and embryonic development cell block when cultured in M16 and Human Tubal Fluid media. J. Vet. 13(3), 227–234. Rosadi, B., Setiadi, M.A., Sajuthi, D. and Boediono, A. 2008. Development of mice and hamster embryos in Ksomaa and HECM-6 medium. J. Vet. 9(4), 157–162 Rosenkrans Jr, C.F. and First, N.L. 1994. Effect of free amino acids and vitamins on cleavage and developmental rate of bovine zygotes in vitro. J. Anim. Sci. 72(2), 434–437. Saftiany, R. 2011. Viabilitas embrio mencit (Mus musculus albinus) setelah kriopreservasi ganda dengan metode vitrifikasi pada tahap pembelahan dan blastosis. Available via http://repository.ipb.ac.id/handle/123456789/47480. Saha, S., Takagi, M., Boediono, A. and Suzuki, T. 1994. Direct rehydration of in vitro fertilised bovine embryos after vitrification. Vet. Rec. 134(11), 276–277. Schini, S.A. and Bavister, B.D. 1988. Two-cell block to development of cultured hamster embryos is caused by phosphate and glucose. Biol. Reprod. 39(5), 1183–1192. Steeves, C.L., Lane, M., Bavister, B.D., Phillips, K.P. and Baltz, J.M. 2001. Differences in intracellular pH regulation by Na+/H+ antiporter among two-cell mouse embryos derived from females of different strains. Biol. Reprod. 65(1), 14–22. Summers, M.C., Bhatnagar, P.R., Lawitts, J.A. and Biggers, J.D. 1995. Fertilization in vitro of mouse ova from inbred and outbred strains: complete preimplantation embryo development in glucose-supplemented KSOM. Biol. Reprod. 53(2), 431–437. Summers, M.C., McGinnis, L.K., Lawitts, J.A. and Biggers, J.D. 2005. Mouse embryo development following IVF in media containing either L-glutamine or glycyl-L-glutamine. Human Reprod. 20(5), 1364–1371. Verschoor, A., Brockman, M.A., Gadjeva, M., Knipe, D.M. and Carroll, M.C. 2003. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. J. Immunol. 171(10), 5363–5371. | ||
| How to Cite this Article |
| Pubmed Style Mafruchati M, Makuwira J. Analysis of mice (Mus Musculus L.) and hamster embryo development using culture and vitrification medium: Systematic review. Open Vet. J.. 2023; 13(2): 143-149. doi:10.5455/OVJ.2023.v13.i2.2 Web Style Mafruchati M, Makuwira J. Analysis of mice (Mus Musculus L.) and hamster embryo development using culture and vitrification medium: Systematic review. https://www.openveterinaryjournal.com/?mno=126149 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i2.2 AMA (American Medical Association) Style Mafruchati M, Makuwira J. Analysis of mice (Mus Musculus L.) and hamster embryo development using culture and vitrification medium: Systematic review. Open Vet. J.. 2023; 13(2): 143-149. doi:10.5455/OVJ.2023.v13.i2.2 Vancouver/ICMJE Style Mafruchati M, Makuwira J. Analysis of mice (Mus Musculus L.) and hamster embryo development using culture and vitrification medium: Systematic review. Open Vet. J.. (2023), [cited January 25, 2026]; 13(2): 143-149. doi:10.5455/OVJ.2023.v13.i2.2 Harvard Style Mafruchati, M. & Makuwira, . J. (2023) Analysis of mice (Mus Musculus L.) and hamster embryo development using culture and vitrification medium: Systematic review. Open Vet. J., 13 (2), 143-149. doi:10.5455/OVJ.2023.v13.i2.2 Turabian Style Mafruchati, Maslichah, and Jonathan Makuwira. 2023. Analysis of mice (Mus Musculus L.) and hamster embryo development using culture and vitrification medium: Systematic review. Open Veterinary Journal, 13 (2), 143-149. doi:10.5455/OVJ.2023.v13.i2.2 Chicago Style Mafruchati, Maslichah, and Jonathan Makuwira. "Analysis of mice (Mus Musculus L.) and hamster embryo development using culture and vitrification medium: Systematic review." Open Veterinary Journal 13 (2023), 143-149. doi:10.5455/OVJ.2023.v13.i2.2 MLA (The Modern Language Association) Style Mafruchati, Maslichah, and Jonathan Makuwira. "Analysis of mice (Mus Musculus L.) and hamster embryo development using culture and vitrification medium: Systematic review." Open Veterinary Journal 13.2 (2023), 143-149. Print. doi:10.5455/OVJ.2023.v13.i2.2 APA (American Psychological Association) Style Mafruchati, M. & Makuwira, . J. (2023) Analysis of mice (Mus Musculus L.) and hamster embryo development using culture and vitrification medium: Systematic review. Open Veterinary Journal, 13 (2), 143-149. doi:10.5455/OVJ.2023.v13.i2.2 |