| Original Article | ||
Open Vet. J.. 2023; 13(4): 433-442 Open Veterinary Journal, (2023), Vol. 13(4): 433–442 Original Research Comparative pathology and immunohistochemistry of Newcastle disease in domestic chicken (Gallus-gallus domesticus) and Alabio duck (Anas platyrhynchos Borneo)Etriwati Etriwati1*, Dewi Ratih Agungpriyono2, Surachmi Setiyaningsih3, Darniati Darniati4, Daud M. Ak4, Erwin Erwin5 and Ekowati Handharyani21Laboratory of Pathology, Faculty of Veterinary Medicine, Syiah Kuala University, Banda Aceh, Indonesia 2Division of Pathology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 3Division of Medical Microbiology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 4Laboratory of Microbiology, Faculty of Veterinary Medicine, Syiah Kuala University, Banda Aceh, Indonesia 5Laboratory of Clinic and Surgery, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia *Corresponding Author: Etriwati Etriwati. Laboratory of Pathology, Faculty of Veterinary Medicine, Syiah Kuala University, Banda Aceh, Indonesia. Email: etriwati.2102 [at] usk.ac.id Submitted: 27/12/2022 Accepted: 14/03/2023 Published: 11/04/2023 © 2023 Open Veterinary Journal
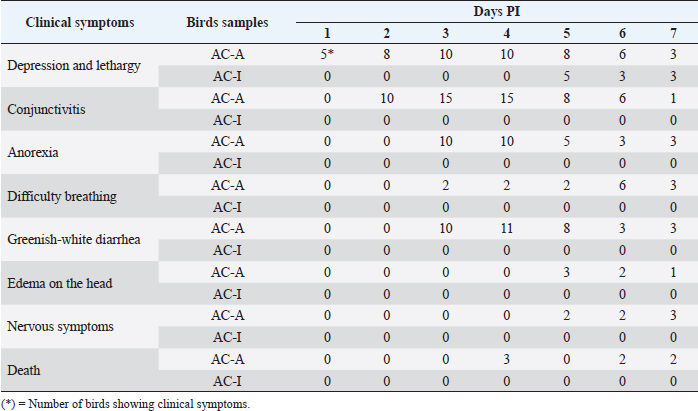
AbstractBackground: Newcastle disease is very pathogenic in chickens, whereas in ducks, the disease does not show any clinical symptoms. Aims: To compare the clinical symptoms features, pathological lesions, viral distribution, and apoptosis response caused by the Newcastle disease virus (NDV) in domestic chicken and Alabio duck. Methods: Forty domestic chickens and 40 Alabio ducks were separated into four groups: domestic chicken and Alabio duck treatment groups, where each was infected by NDV velogenic ducks/Aceh Besar_IND/2013/eoAC080721 in 106ELD50 dosage. The domestic chicken and Alabio duck control groups were each inoculated with Phosphate Buffer Saline. The infection route was intraorbital, 0.1 ml in volume. Symptoms were observed from day 1 until day 7 post-infection (PI). Necropsy was performed on days 1, 2, 3, 5, and 7 PI for organ collection. Results: Symptoms observed were disorders in the respiratory, gastrointestinal, and nervous systems, followed by 100% mortality in domestic chickens. In Alabio ducks, there were only depression and slight lethargy. The lesion in domestic chicken day 1 appeared by the lungs, thymus, Fabricius bursa, spleen, and kidney. On day 3 PI, lesions were also found in the heart, proventriculus, duodenum, and cecal tonsil. On days 5 and 7 PI, the trachea and brain lesions were found. In Alabio ducks, lesions were found in the lungs, thymus, spleen, and proventriculus on day 1. Afterward, on day 3, light lesions were found in the heart. On day 5, lesions were found in the trachea and brain; finally, on day 7, light lesions were only found in the thymus, spleen, and brain. Immunopositive reaction NDV in domestic chicken was highest in the proventriculus, duodenum, cecal tonsil, and lymphoreticular organs. In the Alabio duck, it was highest in the duodenum and cecal tonsil. The caspase-3 percentage in domestic chicken increased on day 3 PI; in Alabio ducks, on day 2 PI. Conclusion: Clinical symptoms and pathological lesions were faster and more severe in domestic chickens. The immunopositive reaction NDV in domestic chicken continued to increase, while in Alabio ducks, it decreased until the last observation day. Apoptosis percentage increased earlier in the Alabio duck than in domestic chicken. Keywords: Apoptosis, Caspase-3, Immunohistochemistry, Pathology, Newcastle disease. IntroductionNewcastle disease (ND) is highly pathogenic in reared chicken but less pathogenic in other birds that are important in spreading the Newcastle disease virus (NDV). It has high morbidity and mortality and can spread rapidly (OIE, 2021). NDV has widely infected bird species, such as chickens, ducks, geese, pigeons, parrots, and several other birds (Zhang et al., 2011). Ducks and geese act as a natural reservoir that does not show clinical symptoms but can transmit the deadly disease to chickens (Alexander and Senne, 2008). Dai et al. (2014) compared six breeds of ducks and found mallard ducks were the most susceptible and Pekin ducks were the more resistant; they found that the susceptibility of ducks to NDV decreased with age, and most deaths occurred between 15 and 30 days of age. Kencana et al. (2012) reported that 10 domestic chicken samples collected from field cases in 2008–2009 in Bali, Indonesia, were positive to be infected by the acute NDV. NDV infection in domestic chicken, broiler chicken, and waterfowl in Aceh, Indonesia, is dominated by virulent strains (Darniati et al., 2015; Daud et al., 2019). In addition, several pathogenic isolates have been isolated from reared ducks (Liu et al., 2010; Zhang et al., 2011; Panus et al., 2015). ND can cause damage to lymphoid tissue and macrophages. Previous studies reported that histopathologically ND causes lymphoid follicular depletion, necrosis, and apoptosis in the caecal tonsil, thymus, and bursa of Fabricius and spleen chicken (Nakamura et al., 2008; Anis et al., 2013; Etriwati et al., 2017a). Skeletal muscle congestion, mild intestinal erosion, and mild hemorrhagic cecal tonsils in ducks (Eze et al., 2014). Apoptosis is a significant factor in viral pathogenesis, especially in the mechanism of clearance virus by the immune system (Myers et al., 2012). NDV causes apoptosis in the spleen of domestic chickens (Kommers et al., 2002), chicken embryo fibroblast cells (Ravindra et al., 2009), and chicken macrophages (Lam, 1996). There are many things yet unknown on the cause of why ducks are more resistant to ND disease compared to chickens. Therefore, this study was designed to compare the clinical symptoms features, pathological lesions, viral distribution, and apoptosis response due to NDV in domestic chickens and Alabio ducks. Materials and MethodsResearch procedureOne-day-old domestic chickens (Gallus gallus domesticus) and 1-day-old Alabio duck (Anas platyrhynchos Borneo) were reared until 6 weeks in semi-isolated cages in groups. Feed and drinking water were provided ad libitum. The treatment groups are: AC-A (Domestic chicken group, n =20) and AC-I (Alabio duck group, n=20), were infected by NDV velogenic isolate Ducks/Aceh Besar_IND/2013/eoAC080721 under 106ELD50 dosage. The K-A (Domestic chicken control group, n=20) and K-I (Alabio duck control group, n=20) were inoculated by PBS. All inoculations were performed via intraorbital as much as 0.1 ml. Before being infected by NDV, the domestic chickens and Alabio ducks tested negative for NDV antibody by hemaglutinin inhibition test. Clinical symptoms and gross anatomy observationClinical symptoms were observed from day 1 until day 7 post-infection (PI). Three individuals from each group were necropsied on days 1, 2, 3, 5, and 7 PI. Gross pathological changes in the proventriculus, duodenum, ceca tonsil, trachea, lung, heart, thymus, Fabricius bursa, spleen, kidney, and brain were observed. All organ samples were cut into 1 × 1 × 0.5 cm sizes and fixed in neutral buffered formalin 10% for a minimum of 24 hours to be made into histopathology preparations in paraffin blocks. Histopathology examinationEach organ was trimmed into 5 mm size and put inside a tissue cassette, then put into an automatic tissue processor for dehydration, clearing, paraffin infiltration, embedding, and paraffin blocking. Finally, the blocks were cut into 5 µm with a rotary microtome to be stained with hematoxylin staining or immunohistochemistry. Hematoxylin and eosin stainingHematoxylin and eosin staining starter by deparaffinization by xylol and ethanol rehydration. Staining was performed by submerging preparations inside Mayer's hematoxylin stain, followed by eosin. The tissues were then dehydrated with ethanol 96% and absolute ethanol 2. The clearing was performed by submerging the tissue in xylol. The last process was mounting using gum and cover glass. Histopathology observation was performed by examining the lesion severity. The following criteria had determined: if the lesion was spread locally, multifocally, or diffuse, the seriousness in that order was light, moderate, or severe. The examination was conducted under 100 times magnification with five fields of view repetition. Immunohistochemistry stainingThe immunohistochemistry staining referred to the procedures recommended in the catalog from Dako, North America Inc. (Dako) with several modifications. Tissue slices attached to poly-L-Lysine 1% spread object glass was deparaffinized by xylol and then rehydrated by ethanol. The antigen retrieval process was performed by boiling the preparations in citrate buffer at 100°C for 15 minutes. Blocking of endogenous activity was performed by submerging the preparations in H2O2 3% for 35 minutes at room temperature and washing them with PBS in three repetitions for 5 minutes each. Blocking of non-specific protein bonds was conducted using normal fetal bovine serum 10% for 35 minutes at room temperature and then washed once more with PBS in three repetitions for 5 minutes each. Each tissue was given drops of primary antibody rabbit anti-NDV polyclonal antibody (1:250 in PBS), and for caspase-3, was given drops of primary antibody Polyclonal Anti-Casp3 (HPA002643, Sigma-Aldrich; 1:250 in PBS). The preparations were then incubated overnight at −5°C. The preparations were washed with PBS in three repetitions for 5 minutes each at room temperature. The preparations were then dropped with secondary antibody Dako REAL™ envision™/HRP, Rabbit/Mouse (K5007) for 40 minutes at room temperature, then washed with PBS in three repetitions 5 minutes each at room temperature. The preparations were then given Dako REAL™ DAB+chromogen in Dako REAL™ substrate buffer (K5007) for 40 seconds at room temperature and then washed with flowing water for 10 minutes and washed by PBS in three repetitions for 5 minutes each at room temperature. Mayer’s Hematoxylin was used as counterstaining. The examination was regarded as positive if, during preparation, reading brown stained antigen was found and regarded as negative if all preparation appeared blue. The immunopositivity against NDV on each organ was scored with light severity (1–10 immunopositive cells), moderate (11–20 immunopositive cells), and severe (more than 20 immunopositive cells) (Etriwati et al., 2017b). The immunohistochemistry results against NDV and caspase-3 were examined under 400 times magnification with five field view repetitions. Data analysisThe data from clinical symptoms and pathological lesions examinations were analyzed descriptively. The immunohistochemistry results against the NDV were scored based on the immunopositive cells’ level, while for caspase-3, the immunopositive reaction was analyzed based on the positive area percentage against caspase-3. Ethical approvalThe use and treatment of experimental animals in this study have approval from the animal ethics committee of the Institute for Research and Community Service, Bogor Agricultural University. ResultsClinical symptoms observationOn day 1, domestic chicken suffered from depression and lethargy; on day 2, PI conjunctivitis appeared; on day 3, PI had difficulty breathing, greenish-white diarrhea, and anorexia appeared; on days 5 and 7, PI nervous symptoms such as muscle tremor, difficulty in standing, and wings dropping along with light edema on the head appeared. Death in domestic chickens started to appear on day 4 PI (Table 1). Alabio duck groups only seemed to be depressed and lightly lethargic starting from day 5 PI. Control domestic chicken and Alabio duck group did not show any clinical symptoms. Gross anatomy observationThe control domestic chicken and Alabio duck group showed no gross anatomy lesions. Proventriculus, duodenum, and cecal tonsil did not show lesions on day 1 PI in domestic chickens, but in Alabio ducks, proventriculus appeared to have diffuse catarrhal exudation on the mucosal layer. Proventriculus on day 3 PI showed multifocal petechiae. On days 5 and 7, PI showed diffuse hemorrhage on domestic chickens (Fig. 1A). Alabio duck proventriculus on days 3 and 5 PI showed diffuse catarrhal exudation, and no lesion appeared on day 7 PI (Fig. 1A). Duodenum on day 3 until day 7 PI appeared hemorrhagic with multifocal necrosis on domestic chickens. In contrast, in Alabio ducks, no lesion appeared on all observation days. Cecal tonsil on day 3 PI until the last observation day seemed to have a multifocal hemorrhage in domestic chicken, but in Alabio ducks, no lesion appeared on all observation days (Fig. 1A). On day 5 PI, the trachea suffered multifocal diffuse congestion on domestic chickens and focal congestion on Alabio ducks (Fig. 2A). Lungs on days 1 and 3 PI appeared abnormal from multifocal congestion. On every following observation day, the lesions spread diffusely on domestic chickens, whereas, on Alabio ducks, multifocal congestion only appeared on day 1 PI. Table 1. Clinical symptoms of the NDV of the domestic chicken (AC-A) and Alabio duck (AC-I) infected with ducks/Aceh Besar_IND/2013/eoAC080721 isolate.
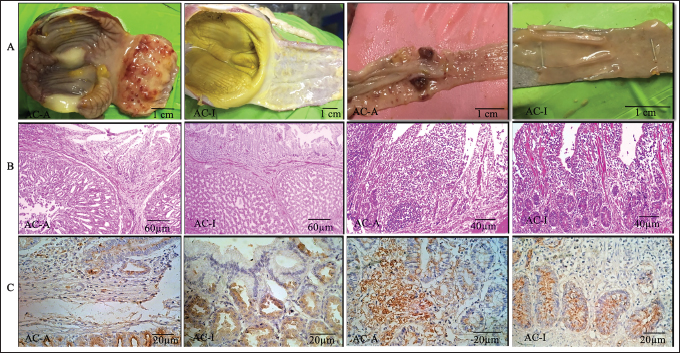
Fig. 1. Proventriculus and ceca tonsil on 5th-day pi. (A). Anatomical pathology; (B). Histopathology; (C). Immunohistochemistry; AC-A (Domestic chicken) and AC-I (Alabio ducks). Infected with isolate ducks/Aceh Besar_IND/2013/eoAC080721. Histopathology with hematoxylin and eosin. Immunohistochemical using anti-NDV antibody.
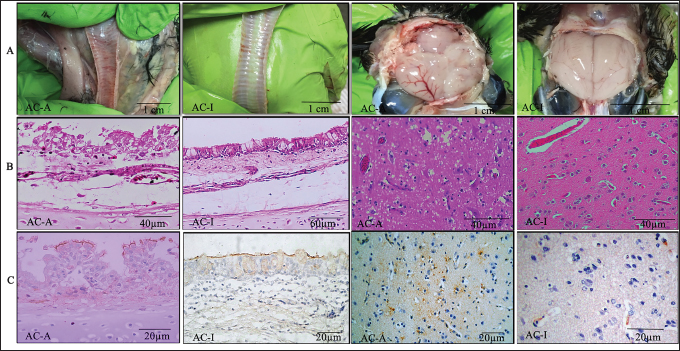
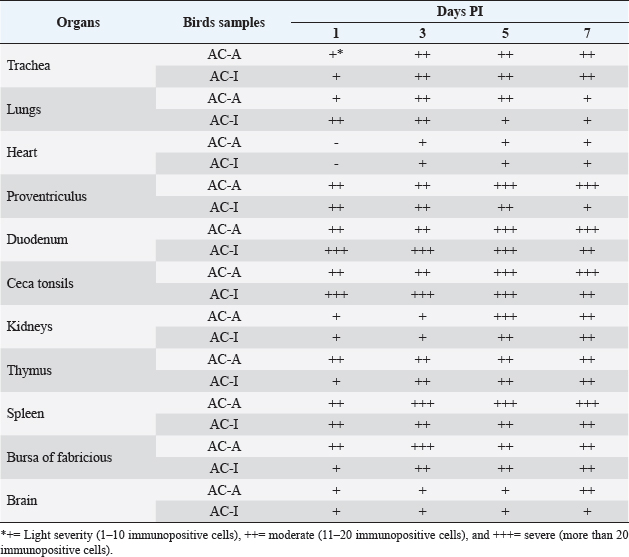
Fig. 2. Trachea and brain on the 5th-day pi. (A). Anatomical pathology; (B). Histopathology; (C). Immunohistochemistry; AC-A (Domestic chicken) and AC-I (Alabio ducks). Infected with isolate ducks/Aceh Besar_IND/2013/eoAC080721. Histopathology with hematoxylin and eosin. Immunohistochemical using anti-NDV antibody On all observation days, the Thymus of domestic chicken appeared to have half of its lobes atrophied and had petechiae/hemorrhage. In contrast, in Alabio ducks, part of the lobus showed petechiae on all observation days. The spleen of domestic chickens on days 1, 3, and 5 PI showed congestion, swelling, and multifocal necrosis, while the days after were followed by atrophy. In Alabio ducks, the spleen showed swelling and multifocal necrosis only during day 3 PI. The Fabricius bursa of domestic chicken on day 1 PI appeared to be swelling with focal hemorrhage. On the rest of the observation days, it was followed by atrophy and diffuse hemorrhage, whereas in Alabio ducks, no lesion was found on all observation days. Starting from day 3 PI, the domestic chicken heart showed swelling, while the Alabio duck heart showed no gross pathology changes on every observation day. Domestic chicken kidneys on all observation days appeared swelled with multifocal paleness, while in Alabio ducks, no lesion was seen on any observation day. Domestic chicken brain from day 5 PI started to show edema and multifocal congestion, while in Alabio duck, the lesion was focal (Fig. 2A) Histopathology examinationThe control domestic chicken and Alabio duck group showed no histopathology lesions. Domestic chicken proventriculus on day 1 PI showed congestion, focal mononuclear proliferation on the mucosal layer, necrosis, and focal epithelial cell desquamation on proventriculus glands. On day 3 PI, the lesions spread in a multifocal pattern; on days 5 (Fig. 1B) and 7 PI epithelial cells showed necrosis and diffuse hemorrhage by the muscle layer and multifocal epithelial cell desquamation as well as necrosis, congestion, and multifocal mononuclear cell infiltration by the proventiculus gland. Alabio duck proventriculus on day 1 PI showed focal epithelial cell desquamation; on days 3 and 5 PI showed congestion, epithelial cell desquamation, and multifocal mononuclear cell proliferation, while proventriculus glands showed multifocal necrosis, epithelial cell desquamation, and focal congestion. On day 7 PI, no lesion was seen by the proventriculus. On days 1 and 3 PI, domestic chicken duodenum showed hemorrhage, congestion, and focal crypt epithelial cell necrosis. On all following observation days, multifocal hemorrhage with intestine villus desquamation, diffuse crypt epithelial cell necrosis, and multifocal proliferation of crypt epithelial cells in lamina propia was observed. Alabio duck duodenum from days 3 to 5 PI showed multifocal crypt epithelial necrosis, congestion, hemorrhage, and focal goblet cell proliferation. On day 7 PI, there were multifocal crypt epithelial cell necrosis and focal mononuclear cell proliferation in lamina propria. The cecal tonsil of domestic chicken on all observation days histopathologically showed congestion, hemorrhage, necrosis (karyopicnosis) of crypt epithelial cells, mononuclear cell proliferation on the lamina propria, and lymphocyte cell depletion inside the lymphoid follicle, which spread in the multifocal pattern. The Alabio duck cecal tonsil on days 1 and 3 PI showed congestion, necrosis (Karyopicnosis) of crypt cells, and multifocal mononuclear cell proliferation by the lamina propria. On days 5 and 7, multifocal mononuclear cell proliferation in lamina propria and depletion of lymphocyte cells in lymphoid follicles were seen (Fig. 1B). Domestic chicken trachea on day 1 PI showed congestion, epithelial cell desquamation, focal inflammatory cell infiltration, and diffuse edema. On the following observation days, the lesions spread in multifocal patterns. Alabio duck trachea on all observation days showed congestion, epithelial desquamation, focal inflammatory cell infiltration, goblet cell proliferation, and diffuse edema (Fig. 2B). Domestic chicken lung on day 1 PI showed hemorrhage, congestion, edema, and multifocal mononuclear cell proliferation. On all following observation days, the lesion spread in a diffuse pattern. Alabio duck lungs on all observation days showed congestion, edema, hemorrhage, and multifocal mononuclear cell proliferation. The domestic chicken's thymus, Fabricius bursa, and spleen on days 1 and 3 PI showed lymphoid depletion, congestion, and multifocal vasculitis. On days 5 and 7, the lesion spread in a diffuse pattern accompanied by cyst formation, lysing cells, and part of the tissue being replaced by connective tissue. The ducks' Thymus, Fabricius bursae, and spleen on day 1 appeared to suffer lymphoid depletion, congestion, and focal vasculitis. On days 3 and 5 PI, the lesions were spread in a multifocal pattern, and on day 7, the lesions were spread in a focal pattern. The heart of domestic chicken on day 3 PI started to show edema and focal degeneration; on day 5 PI, it was accompanied by hemorrhage, congestion, and focal mononuclear cell infiltration. On day 7, the lesions spread in a multifocal pattern accompanied by pericarditis and endothelial hypertrophy. However, in Alabio duck, the lesions were only spread in a focal pattern without pericarditis. The kidney of domestic chicken on day 1 PI appeared to suffer from edema, congestion, and focal hemorrhage. On day 3 PI, the lesions were spread in a multifocal pattern. Every following observation day, the lesions spread diffusely, followed by multifocal mononuclear cell infiltration by the interstitial area of the kidney. Alabio duck kidney appeared to suffer from edema and focal congestion on all observation days, and focal mononuclear infiltration by kidney interstitial started to appear from day 3 PI. Domestic chicken brain on days 1 and 3 PI showed neuron degeneration, congestion, edema, and multifocal endothelial hypertrophy. On days 5 and 7 PI, they were followed by neuron cells necrosis, multifocal gliosis, and focal perivascular cuffing (Fig. 2B). The Alabio duck brain on every observation day appeared to suffer from congestion, edema, endothelial cell hypertrophy, and multifocal gliosis. NDV distributionImmunohistochemistry staining showed that immunopositive reaction against NDV was found in all treatment groups (Table 2) from day 1 until the last observation day, with severity ranging from light to severe, while on all control groups, immunonegative. The immunopositive location against NDV was not different between domestic chicken and Alabio duck. A positive reaction was found in epithelial cells and mononuclear inflammation in the intestinal organs (Fig. 1C). Cilia epithelial cells, mucosal layer mononuclear cells, and in the spaces of tracheal goblet calls (Fig. 2C). Parabronchi epithelial cells, pneumocystis, and inflammatory cells in lung alveoli. Heart blood vessel endothelial cells and the urinary. Reticular epithelial cells at the medulla layer and mononuclear cells by the thymus cortex. Lymphoid cells of white pulp and lymphoid cells inside the Fabricius bursa lymphoid follicle. The plica epithelial cells and mononuclear cells are suffering from depletion within the lymphoid follicle of the Fabricius bursa: the Virchow-Robin endothelial cell, necrotic glial cells, and neurons in the brain (Fig. 2C). The caspase-3 expression in lymphoreticular organsThe percentage of caspase-3 in lymphoreticular organs (Fig. 3) peaked earlier in the Alabio duck group, which was on day 2 PI, compared to the domestic chicken group, which on average, occurred after day 3 PI. The caspase-3 expression (Fig. 4) in domestic chicken and Alabio duck thymus was more dominant in the medullar area and rarely by the cortex. The caspase-3 expression in domestic chicken and Alabio duck Fabricius bursa by the plica epithelial and lymphoid follicle, while in the cecal tonsil by the mucosa epithelial cell, lamina propria inflammatory cells, and crypt epithelial cells. The caspase-3 expression of domestic chicken spleen was more dominant around the germinal center, whereas caspase-3 expression in Alabio ducks was more dominant in the germinal center. DiscussionThe chains of pathological changes occurring in the respiratory, circulation, gastrointestinal, urinary, and nervous systems are closely related to the clinical symptoms. For example, Conjunctivitis appearing on day 2 PI occurred because the infection route was intraorbital, causing a local immune response by the eye region. Depression, lethargy, difficulty breathing, and catarrhal exudation in the nose on AC-A align with the gross pathological changes, which were congestion and catarrhal exudation by the trachea. This is related to the increase of goblet cells initiated by viruses attaching to the epithelial cells via the use of sialic acid on the host cells as receptors. Clinical symptoms such as greenish-white diarrhea, nervous disorder, death, and head edema only appeared in domestic chickens but were not found in Alabio ducks. Diarrhea and anorexia on day 3 PI in domestic chicken were in line with gross pathological changes in skeletal muscle, which looked pale and emaciated. Anorexia was indicated by depression in the chicken period. The low feed and drinking water consumption was due to the chicken feeling sick from septicemia or viremia. The sickness in domestic chicken continued after day 3 PI and caused death starting from day 4 PI, followed by nervous symptoms on day 5 PI. Death with the nervous disorder is a clinical manifestation of neuron degeneration, edema, congestion, thickening blood vessel walls, perivascular cuffing, the proliferation of glial cells (gliosis), and necrosis in the brain. The presence of NDV in the brain can cause vascular and neuron damage, further causing an inflammatory response. The replication of NDV in internal organs initiated the pathogenesis of ND in the gastrointestinal tract. The NDV was distributed from the respiratory to the gastrointestinal system, possibly through the circulatory system or directly into the chicken’s internal organs. NDV replication in the gastrointestinal system is indicated by catarrhal exudation to widespread hemorrhage by the viscera due to blood vessel damage. The gross anatomy lesion that appeared by the gastrointestinal tract is in line with a histopathological lesion found: congestion, edema, epithelial cell necrosis and desquamation, the proliferation of mononuclear cells, and goblet cell hyperplasia from light to severe level. The findings matched a previous report by Igwe et al. (2014) about goblet cell hyperplasia and severe desquamation of intestinal epithelial cells. Necrosis was in the form of karyorrhexis debris and ulceration of intestine epithelial cells (Brown et al., 1999). The NDV immunopositive reaction in gastrointestinal organs was also distributed in severe severity. The immunopositive response in the gastrointestinal tract matched the previous report by Diparayoga et al. (2016) on inflammatory and gastrointestinal system epithelial cells. Adi et al. (2012) reported in the duodenum, proventriculus, and heart. Piacenti et al. (2006) said that in the esophagus, crop, pancreas, and proventriculus. Hamid et al. (1990) reported in the cecal tonsil. Kidney lesions also occur due to viremia, which allows NDV to be spread from the respiratory or gastrointestinal tract via the blood circulation system to the kidney. We assume that one of the reasons Alabio ducks are more resistant than local chickens is the lack of severe structural and histological abnormalities in the Alabio duck kidney. However, we are still unsure of the mechanism that causes the absence of these lesions. The immunopositive reaction in the kidney is similar to a previous report by Nakamura et al. (2008) about chicken kidney tubules epithelial cells and in the cytoplasm and nucleolus of duck kidney tubules cells (Njagi et al., 2012). The pathogenesis of a disease is also closely related the lymphoreticular organ damage as they produce immunity compounds to eliminate infectious agents. This research showed that all lymphoreticular organs generally suffered from changes in gross pathology and histopathology. The gross pathology and histopathology lesions generally spread lightly in Alabio duck groups, different from immunohistochemistry results whose distribution was light to severe. This data showed that although no severe lesion was found according to gross pathology and histopathology, the viral concentration within the lymphoreticular organ was high. Table 2. Distribution of the NDV of the domestic chicken (AC-A) and Alabio duck (AC-I) infected with ducks/Aceh Besar_IND/2013/eoAC080721 isolate.
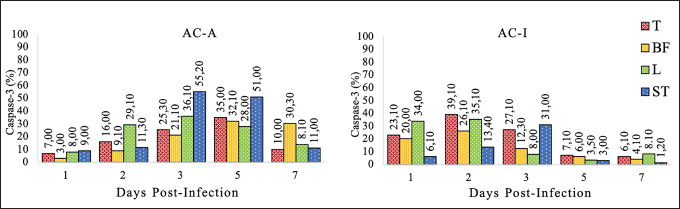
Fig. 3. Caspase-3% on the lymphoreticular organs. Domestic chickens (AC-A) and Alabio ducks (AC-I) infected with ducks/Aceh Besar_IND/2013/eoAC080721 isolate. T: thymus, BF: Bursa of fabricious, L: Spleen, ST: Ceca tonsils. The different bird species used in this research showed that the severe immunopositive reaction found in the gastrointestinal and lymphoreticular organs in Alabio duck proved that although Alabio ducks do not show ND clinical symptoms, inside their body, NDV was present in high concentration. The differences in clinical symptoms and lesion features that appeared on domestic chickens and Alabio ducks may be caused by different genes or protein expressions in domestic chickens and Alabio ducks that fight viral infection. According to Anis et al. (2013), the interferon-ß expression is earlier, stronger, and more intensive in duck tissue (Japanese commercial duck) compared to chicken (white leghorn SPF) upon infection by virulent NDV. In chicken, retinoic acid-induced gene-I (RIG-I) was reported to be absent. However, it was highly expressed in the duck spleen and heart (Chen et al., 2013, 2015). The absence of RIG-I is hypothesized to make chicken less resistant to the influenza virus than duck as its natural reservoir (Barber et al., 2010). Kang et al. (2015) explained that RIG-I is identified as a cytoplasmic censor against RNA virus, which is important in initiating the nonspecific immune response.
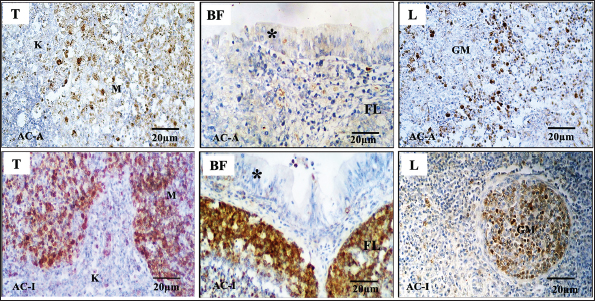
Fig. 4. Caspase-3 expression on the lymphoreticular organs. Domestic chickens (AC-A) and Alabio ducks (AC-I) infected with ducks/Aceh Besar_IND/2013/eoAC080721 isolate. T: thymus, BF: Bursa of fabricious, L: Spleen, K: Cortex, M: Medullar, (*): Plica epithelia, FL: Lymphoid follicle, GM: Germinal center. Immunohistochemical using anti-casp3 antibody. The caspase-3 expression as an apoptosis indicator in this research added information that the clinical expression and lesion differences in domestic chicken and Alabio duck infected by a local isolate from duck might be caused by the time difference of the highest progressive increase of apoptosis response, which was on day 2 PI in Alabio duck and day 3 PI in domestic chicken by the thymus, Fabricius bursa, and spleen; all of them crucial as primary and secondary systems in fighting infections. Ideally, when an infectious agent contacts host cells, an apoptosis process for clearance rapidly occurs to prevent viral aggression from continuing. The apoptosis percentage in the lymphoreticular organ continued to show a regressive decrease after reaching the peak, probably caused by two conditions. In domestic chickens, histopathologically, on days 1 and 3, PI lymphoid depletion, congestion, and multifocal vasculitis were found; on days 5 and 7, PI lesions were spread in diffuse accompanied by cells undergoing lysis and partly replaced by connective tissue. In the Fabricius bursa, the process was accompanied by cyst formation. It was different in Alabio ducks. In Alabio ducks, the apoptosis response decrease at the end of observation was probably caused by the rapid NDV clearance, which prompts earlier cell regeneration. Anis et al. (2013) stated that several duck organs showed increased mitosis compared to ducks, allowing rapid tissue regeneration. The lowering apoptosis responses in Alabio duck after reaching its peak align with the lowering lesion severity into focal in the thymus, Fabricius bursa, and spleen. This allows the lymphoreticular organ ability as the defensive organ to return to normal, so Alabio ducks turn healthy again until the last observation day. In closing, it can be said that generally, the difference in lesion pattern between domestic chickens and ducks infected by the velogenic NDV Ducks/Aceh Besar_IND/2013/eoAC080721 is that in domestic chickens until the last observation day, the lesion progressively turned severe, while in Alabio ducks the lesions showed improvements. In summary, the clinical symptoms, pathological lesions, and histopathology of ND are more severe in domestic chickens compared to Alabio ducks in the treatment group. The immunopositive reaction against the NDV in domestic chicken continued to increase, while in Alabio ducks, it decreased until the last observation day, especially in gastrointestinal tracts. The apoptosis response showed earlier in Alabio ducks compared to domestic chickens. AcknowledgmentsThe authors thank IPB University, Bogor, and Universitas Syiah Kuala, Banda Aceh, Indonesia, for their support during this research. In addition, the authors are also thankful to the U.S. Department of State (USDA/ARS/BEP/CRDF) grant NDV 31039 and Ministry of Education, Culture, Research, and Technology for the Letter of Agreement on the Assignment of Lector Research Implementation for Lektor Year 2022, Number: 294/UN11.2.1/PT.01.03/PNBP/2022, 14 February 2022. Conflict of interestsThe authors declare that they have no conflict interests Authors’ contributionsEW, DR, and EH executed the analysis and interpretation of data from the tissue. EW, DD, MD, and SS performed the technic experiment and prepared the isolate. EW and EE executed the experiment. All authors interpreted and critically revised the manuscript for important intellectual contents and approved the final version. ReferencesAdi, A.A.A.M., Kardena, I.M., Astawa, N.M. and Matsumoto, Y. 2012. Immunohistochemical detection of viral antigen in tissue of chickens experimentally infected with Newcastle disease virus. J. Vet. 13, 278–283. Alexander, D.J. and Senne, D.A. 2008. Newcastle disease, other avian paramyxovirus and pneumovirus infections: Newcastle disease. In: Disease of poultry. Ed., Saif, Y. Ames, IA: Iowa State University, pp: 75–115. Anis, Z., Morita, T., Azuma, K., Ito, H. and Shimada, A. 2013. Histopathological alteration in immune organs of chickens and ducks after experimental infection with virulent 9a5b Newcastle disease virus. J. Comp. Path. 149, 82–93. Barber, M.R.W., Aldridge, J.R., Webster, R.G. and Magor, K.E. 2010. Association of RIG-I with innate immunity of ducks to influenza. Proc. Nat. Acad. Sci. U. S. Am. 107, 5913–5918. Brown, C., King, D.J. and Seal, B.S. 1999. Pathogenesis of Newcastle disease in chickens experimentally infected with viruses of different virulence. Vet. Pathol. 36, 125–132. Chen, Y., Huang, Z., Wang, B., Yu, Q., Liu, R., Xu, Q., Chang, G., Ding, J. and Chen, G. 2015. Duck RIG-I CARD domain induces the chicken IFN-β by activating NF-κB. BioMed. Res. Int. 2015, 1–6. Chen, Y., Zhang, Y. and Huang, Z. 2013. Molecular characterization, expression patterns, and subcellular localization of RIG-I in the Jinding duck (Anas platyrhynchos domesticus). Dev. Comp. Immunol. 41, 766–771. Dai, Y., Cheng, X., Liu, M., Shen, X., Li, J., Yu, S., Zou, J. and Ding, C. 2014. Experimental infection of duck origin virulent Newcastle disease virus strain in ducks. BMC. Vet. Res. 10, 1–9. Darniati, D., Setiyaningsih, S. and Indrawati, A. 2015. Molecular detection and diversity of Newcastle disease virus isolates from native chickens in Aceh. J. Kedokt. Hewan. 9, 178–184. Daud, A.K., Setiyaningsih, S. and Sudirman, I. 2019. Identification and molecular characterization of Newcastle disease virus circulates in some districts in Aceh. J. Kedokt. Hewan. 13, 10–14. Diparayoga, I.M.G., Astawa, N.M. and Adi, A.A.A.M. 2016. Effect of maternal antibodies on histopathogenesis of Newcastle disease virus in broiler chickens. Vet. Sci. Med. J. 4, 27–31. Etriwati, E., Ratih, D., Handharyani, E. and Setiyaningsih, S. 2017a. Histopathology studies on spleen and bursa of fabricius of Newcastle disease chickhens from field case. J. Vet. 18(4), 510–515. Etriwati, E., Ratih, D., Handharyani, E. and Setiyaningsih, S. 2017b. Pathology and immunohistochemistry study of Newcastle disease field case in chicken in Indonesia. Vet. World. 10, 1066–1071. Eze, C.P., Okoye, J.O.A., Ogbonna, I.O., Ezema, W.S., Eze, D.C., Okwor, E.C., Ibu, J.O. and Salihu, E.A. 2014. Comparative study of the pathology and pathogenesis of a local velogenic Newcastle disease virus infection in ducks and chickens. Int. J. Poult. Sci. 13(1), 52–61. Hamid, H., Campbell, R.S.F. and Lamichhane, C. 1990. The pathology of infection of chickens with the lentogenic V4 strain of Newcastle disease virus. Avian. Pathol. 19, 687–696. Igwe, O.A., Ezema, S.W., Eze, C.D. and Okoye, O.A.J. 2014. Experimental velogenic Newcastle disease can be severe and viscerotropic in chickens but moderate and neurotropic in Guinea fowls. Int. J. Poult. Sci. 13, 582–590. Kang, Y., Li, Y., Yuan, R., Feng, M., Xiang, B., Sun, M., Li, Y., Xie, P., Tan, Y. and Ren, T. 2015. Host innate immune responses of ducks infected with Newcastle disease viruses of different pathogenicities. Front. Microbiol. 6, 1283. Kencana, G.A.Y., Kardena, I.M. and Mahardika, I.G.N.K. 2012. Diagnosis confirmation of Newcastle disease on native chicken in Bali using RT-PCR method. J. Kedokt. Hewan. 6, 28–31. Kommers, G.D., King, D.J., Seal, B.C., Carmichael, K.P. and Brown, C.C. 2002. Pathogenesis of six pigeon-origin isolates of Newcastle disease virus for domestic chickens. Vet. Pathol. 39(3), 353–362. Lam, K.M. 1996. Newcastle disease virus-induced apoptosis in the peripheral blood mononuclear cells of chicken. J. Comp. Pathol. 114, 63–71. Liu, M., Wei, Y.Y., Dai, Y.B., Cheng, X., Zhou, S., Pan, Z.M., Xu, L.X. and Jiao, X.A. 2010. Isolation and preliminary identification of a virulent Newcastle disease virus isolate of duck origin. Chin. J. Anim. Infect. Dis. 18, 67–71. Myers, R.K., McGavin, M.C. and Zachary, J.F. 2012. Cellular adaptations, injury, and death: morphologic, biochemical, and genetic bases. In: Pathologic Basis of Veterinary Disease. Eds., McGavin, M.D. and Zachary, J.F. St Louis (US): Mosby Elsevier, pp: 2–59. Nakamura, K., Ohtsu, N., Nakamura, T., Yamamoto, Y., Yamada, M., Mase, M. and Imai, K. 2008. Pathologic and immunohistochemical studies of ND in broiler chickens vaccinated with ND: severe nonpurulent encephalitis and necrotizing pancreatitis. Vet. Pathol. 45, 928–933. Njagi, L.W., Nyaga, P.N., Bebora, L.C., Mbuthia, P.G. and Ming, U.M. 2012. Effect of immunosuppression on Newcastle disease virus persistence in ducks with different immune status. ISRN. Vet Sci. 2012, 253809. OIE. 2021. Newcastle disease (chapter 2. 3.14). OIE Terrestrial Manual, Paris, France. Panus, A., Setiyaningsih, S. and Mayasari, N.L.P.I. 2015. Newcastle disease virus infection study on duck and chicken in Subang District. Indones. J. Anim. Vet. Sci. 20, 134–147. Piacenti, A.M., King, D.J., Seal, B.S., Zhang, J. and Brown, C.C. 2006. Pathogenesis of Newcastle disease in commercial and specific pathogen-free Turkeys experimentally infected with isolates of different virulence. Vet. Pathol. 43, 168–178. Ravindra, P.V., Tiwari, A.K., Sharma, B., Rajawat, Y.S., Ratta, B., Palia, S., Sundaresan, N.R., Chaturvedi, U., Kumar, G.B.A., Chindera, K., Saxena, M., Subudhi, P.K., Rai, A. and Chauhan, R.S. 2009. HN protein of Newcastle disease virus causes apoptosis in chicken embryo fibroblast cells. Arch. Virol. 153, 749–754. Zhang, S., Wang, X., Zhao, C., Liu, D., Hu, Y., Zhao, J. and Zhang, G. 2011. Phylogenetic and pathotypical analysis of two virulent Newcastle disease viruses isolated from domestic ducks in China. PLoS One 6, e25000. | ||
| How to Cite this Article |
| Pubmed Style Etriwati E, Agungpriyono DR, Setiyaningsih S, Darniati D, Ak DM, Erwin E, Handharyani E. Comparative pathology and immunohistochemistry of Newcastle disease in domestic chicken (Gallus-gallus domesticus) and Alabio duck (Anas platyrhynchos Borneo). Open Vet. J.. 2023; 13(4): 433-442. doi:10.5455/OVJ.2023.v13.i4.5 Web Style Etriwati E, Agungpriyono DR, Setiyaningsih S, Darniati D, Ak DM, Erwin E, Handharyani E. Comparative pathology and immunohistochemistry of Newcastle disease in domestic chicken (Gallus-gallus domesticus) and Alabio duck (Anas platyrhynchos Borneo). https://www.openveterinaryjournal.com/?mno=134079 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i4.5 AMA (American Medical Association) Style Etriwati E, Agungpriyono DR, Setiyaningsih S, Darniati D, Ak DM, Erwin E, Handharyani E. Comparative pathology and immunohistochemistry of Newcastle disease in domestic chicken (Gallus-gallus domesticus) and Alabio duck (Anas platyrhynchos Borneo). Open Vet. J.. 2023; 13(4): 433-442. doi:10.5455/OVJ.2023.v13.i4.5 Vancouver/ICMJE Style Etriwati E, Agungpriyono DR, Setiyaningsih S, Darniati D, Ak DM, Erwin E, Handharyani E. Comparative pathology and immunohistochemistry of Newcastle disease in domestic chicken (Gallus-gallus domesticus) and Alabio duck (Anas platyrhynchos Borneo). Open Vet. J.. (2023), [cited January 25, 2026]; 13(4): 433-442. doi:10.5455/OVJ.2023.v13.i4.5 Harvard Style Etriwati, E., Agungpriyono, . D. R., Setiyaningsih, . S., Darniati, . D., Ak, . D. M., Erwin, . E. & Handharyani, . E. (2023) Comparative pathology and immunohistochemistry of Newcastle disease in domestic chicken (Gallus-gallus domesticus) and Alabio duck (Anas platyrhynchos Borneo). Open Vet. J., 13 (4), 433-442. doi:10.5455/OVJ.2023.v13.i4.5 Turabian Style Etriwati, Etriwati, Dewi Ratih Agungpriyono, Surachmi Setiyaningsih, Darniati Darniati, Daud M Ak, Erwin Erwin, and Ekowati Handharyani. 2023. Comparative pathology and immunohistochemistry of Newcastle disease in domestic chicken (Gallus-gallus domesticus) and Alabio duck (Anas platyrhynchos Borneo). Open Veterinary Journal, 13 (4), 433-442. doi:10.5455/OVJ.2023.v13.i4.5 Chicago Style Etriwati, Etriwati, Dewi Ratih Agungpriyono, Surachmi Setiyaningsih, Darniati Darniati, Daud M Ak, Erwin Erwin, and Ekowati Handharyani. "Comparative pathology and immunohistochemistry of Newcastle disease in domestic chicken (Gallus-gallus domesticus) and Alabio duck (Anas platyrhynchos Borneo)." Open Veterinary Journal 13 (2023), 433-442. doi:10.5455/OVJ.2023.v13.i4.5 MLA (The Modern Language Association) Style Etriwati, Etriwati, Dewi Ratih Agungpriyono, Surachmi Setiyaningsih, Darniati Darniati, Daud M Ak, Erwin Erwin, and Ekowati Handharyani. "Comparative pathology and immunohistochemistry of Newcastle disease in domestic chicken (Gallus-gallus domesticus) and Alabio duck (Anas platyrhynchos Borneo)." Open Veterinary Journal 13.4 (2023), 433-442. Print. doi:10.5455/OVJ.2023.v13.i4.5 APA (American Psychological Association) Style Etriwati, E., Agungpriyono, . D. R., Setiyaningsih, . S., Darniati, . D., Ak, . D. M., Erwin, . E. & Handharyani, . E. (2023) Comparative pathology and immunohistochemistry of Newcastle disease in domestic chicken (Gallus-gallus domesticus) and Alabio duck (Anas platyrhynchos Borneo). Open Veterinary Journal, 13 (4), 433-442. doi:10.5455/OVJ.2023.v13.i4.5 |