| Original Article | ||
Open Vet. J.. 2022; 12(1): 33-43 Open Veterinary Journal, (2022), Vol. 12(1): 33-43 Original Research Epidemiology and laboratory diagnosis of very virulent infectious bursal disease virus in vaccinated chickens in Khartoum, SudanMohammed Gasim Omer1, and Abdelmalik Ibrahim Khalafalla1,2*1Department of Microbiology, Faculty of Veterinary Medicine, University of Khartoum, Khartoum, Sudan 2Veterinary laboratories Division, Abu Dhabi Agriculture and Food Safety Authority, Abu Dhabi, United Arab Emirates *Corresponding Author: Abdelmalik Ibrahim Khalafalla. Abu Dhabi Agriculture and Food Safety Authority, Abu Dhabi, United Arab Emirates. Email: abdokhlf [at] yahoo.co.uk Submitted: 23/10/2021 Accepted: 12/12/2021 Published: 09/01/2022 © 2022 Open Veterinary Journal
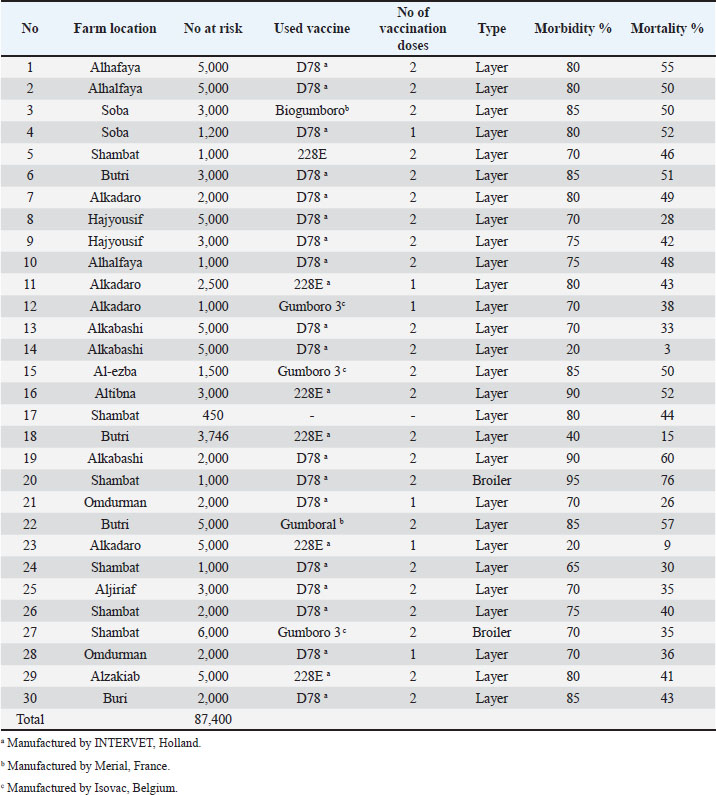
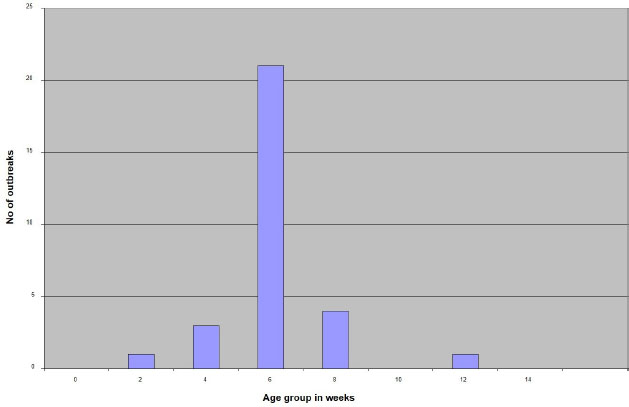
AbstractBackground: Infectious Bursal Disease (IBD, Gumboro disease) has become more severe than in early outbreaks in the 1980s. The present research aims to study the epidemiology of IBD in Khartoum state and compare some commonly used laboratory techniques for diagnosis. Method: We collected epidemiological data from 30 farms that showed signs suggestive of IBD, estimated the morbidity and mortality rates, and interviewed the owners about the type and the doses of the used vaccines. We collected bursas of Fabricius for virus assays and histopathology. Samples positive in the agar gel immunodiffusion (AGID) test were inoculated onto chicken embryo fibroblast cell culture and embryonated chicken eggs. Twenty-two-day-old chicks were infected experimentally with three selected isolates, and morbidity and mortality rates were compared. Results: The results showed that 70% of outbreaks occurred between 6 and 8 weeks of age, and the mean mortality rate was 51%. Epidemiologic, clinical, gross, and histopathological findings were characteristic of the severe disease caused by the very virulent IBDvirus (vvIBDV). The farms that used intermediate or the intermediate plus vaccines had lowered mortality compared with the farms that used intermediate vaccines. The AGID was found more sensitive than the counter-immuno-electrophoresis (CIEP) since it detected 83.4% of the IBDV antigen in the samples while the CIEP detected 66.7% of the samples. The reverse transcriptase polymerase chain reaction (RT-PCR) was found to be rapid, specific, and was more sensitive detecting 100% of the tested samples. Virus isolation in embryonated eggs and cell culture was not successful. Conclusion: A vvIBDV is responsible for the recent outbreaks of the disease in Sudan, resulting in a mean high mortality rate of 51%, even in vaccinated flocks. The RT-PCR and AGID are the best methods for laboratory confirmation. Keywords: Infectious bursal disease, Sudan, Epidemiology, Laboratory techniques. IntroductionInfectious bursal disease (IBD) is a highly contagious viral disease of young chickens that causes significant economic losses in the poultry industry worldwide. The disease is caused by the Infectious bursal disease virus (IBDV), which belongs to the genus Avibirnavirus of the family Birnaviridae. Birnaviridae is a family of viruses with bi-segmented dsRNA genomes with a total of about 6 kb forming icosahedral, non-enveloped virions (Delmas et al., 2019). The first observation of the disease was made by Cosgrove (1962) in the USA at Gumboro area and was named Gumboro disease. Since that time, the disease has been reported in many countries, including Sudan in 1982 (Shuaib et al., 1982; Arafat et al., 2017; Eladl et al., 2020; Mosad et al., 2020). The clinical disease often occurs between 3 and 6 weeks of age. Severe outbreaks are characterized by a sudden onset of depression in susceptible flocks. Based on the virus neutralization test (VNT), IBDV is classified into serotypes I and II (Jackwood, 2004). Serotype I viruses are pathogenic to chickens, while serotype II viruses (isolated from turkeys) are non-pathogenic to chickens. Several studies have reported the evolution of IBDV in some geographical locations worldwide with the emergence of an antigenic variant and recombinant, reassortant, and distinct strains of the virus (Aliyu et al., 2021). The first outbreak in Sudan was observed at El Obied (North Kordofan State) in 1981 (Shuaib et al., 1982). Since that time, the disease has been reported in many parts of Sudan and has become a serious problem facing the poultry industry in Sudan (Hajer and Ismail, 1988). In recent years, IBD has become the most devasting disease of chicken in Sudan, with mortality rates that exceed 50% even in vaccinated flocks. The IBDV has become more virulent, and the picture of the disease has changed and become more severe than in early outbreaks in the 1980s. It is of significance to determine the epidemiology of the disease in the field to provide data that can be used to develop effective control measures. Differential diagnosis of acute IBD should consider other diseases that can induce sudden death in young chickens, with either hemorrhages, nephritis, or bursal lesions. These include infectious diseases such as Newcastle disease, chicken anemia, and infections by infectious bronchitis viruses with nephron-pathogenic tendencies (OIE, 2008). Diagnosis of IBD is commonly made by the case history and clinical and postmortem (PM) examinations. However, as the disease symptoms are confused with many viral infections, laboratory confirmation is required. Recommended methods for IBD diagnosis include histopathological examination of bursae, virus detection in the bursa by immunoassays [Agar gel immunodiffusion (AGID), AC-ELISA, immunostaining], virus isolation and characterization, and virus detection by the reverse transcriptase-polymerase chain reaction reverse transcriptase polymerase chain reaction (RT-PCR) (OIE, 2008). The performance of these diagnostic methods needs to be evaluated in low-resource settings such as Sudan. The main objectives of this study are to determine the current situation of IBD in Khartoum state in terms of disease picture, morbidity, and mortality rates in non-vaccinated chickens in Sudan and compare the performance of RT-PCR with classical techniques of AGID, counter-immuno-electrophoresis (CIEP), histopathology and virus isolation. Materials and MethodsFiled investigationsField outbreaks of IBD were investigated in Khartoum State. Affected farms were visited, and through an interview with the owners we collected epidemiologic data including history of vaccination against IBD, the type of the used vaccine, and the frequency of vaccination. The morbidity was estimated, and the daily mortality was followed until the end of the disease course. Clinical presentation was recorded and affected birds photographed. A number of 8–10 sick birds were brought to the laboratory for PM examination. PM examinationThis was done in the PM room, Department of Pathology, Faculty of Veterinary Medicine, University of Khartoum, following standard techniques. PM was performed by using sterile scissors and forceps. Macroscopic lesions were recorded. Bursa of Fabricius and spleen were taken aseptically in sterile bottles and kept at −20°C till used. Additionally, bursas were taken in 10% neutral formalin for a histopathology examination. HistopathologyBursas were removed from birds that showed signs of IBD and fixed directly in 10% neutral formalin, kept at least 24–48 hours, and then processed according to the method of Drury and Wallington (1980). The paraffin wax-embedded tissue blocks were sectioned in 5-micron sick by rotary microtome, stained in slides with hematoxylin and eosin (H and E), and examined under the light microscope. Sample preparationBursa and spleen pieces were homogenized using sterile mortars and pestles with sterile sand and normal saline. 10% of suspensions were made and centrifuged at 1,000 rpm for 10 minutes. Supernatant fluids were collected into sterile bottles and treated with an antibiotic (1,000 IU penicillin—250 mg of streptomycin and 5,000 IU of Mycostatin/1 ml of sample). The supernatant fluids were left for ½ hour at 4°C and then stored at −20°C till used. The AGID testSamples were prepared, and the AGID test was performed as described by OIE (2008) with some modifications. Using a pipette, 20 µl of bursal or spleen homogenates were mixed with 20 µl of 5% sodium deoxycholate, then 20 µl of the mixtures were put in the six around wells (each hole was 6 mm with 3 mm interspaces between holes) and 20 µl of the hyperimmune serum was put in the inner well. Then the gel [1.4% purified agar in phosphate-buffered saline (PBS)] was incubated in a humidified chamber at room temperature for 24–48 hours. Standard IBDV antigen and IBDV Hyperimmune serum (HIS) were used (Intervet International B. V., Boxmeer, Holland). The test was read against an illuminated chamber, and clear precipitin lines were recorded as positive results. Detection of IBDV antigens by CIEPThe test was performed according to Berg (1982). The anode-oriented wells were filled with the HIS, while the cathode-oriented wells were filled with infected bursal homogenate of field samples. The samples were electrophorized at a constant voltage of 5 V/cm for 45 minutes at room temperature. Reading was observed against indirect illumination. A positive reaction was shown by the formation of precipitin lines between the samples and the hyper immune serum wells. Inoculation of chicken embryonated eggsFertile eggs were obtained from the poultry farm of the Faculty of Veterinary Medicine, University of Khartoum from semi-isolated specific antibody-negative white Leghorn chickens as 1 day old and incubated at 37°C to the required days. Sample inoculation onto the yolk sac and the chorioallantoic membrane (CAM) was done as described in previous publications (Khalafalla, 1994; American Association of Avian Pathologists, 2008; OIE, 2008). The inoculated eggs were candled daily for 5 days, and those which died before 24 hours were discarded. Yolk sacs were harvested and homogenized in sterile mortar and pestle with normal saline and used as antigen in AGID against known positive hyperimmune serum. CAMs were removed and placed in a sterile Petri dish and examined for pock lesion formation and/or any other lesions under indirect illumination. Isolation attempts in chicken embryo fibroblast (CEF) cell culturePrimary CEF cells were derived from 10-day-old chicken embryos and cultured in a Dulbecco’s modified Eagle’s medium (Gibco, Carlsbad, CA) containing 10% fetal calf serum by standard procedures (Cunningham, 1973). After removing the media, 50 µl of bursal homogenates were inoculated on confluent cells grown in six well plates or 100 µl in a 25 ml tissue culture flask. Plates and flasks were incubated for 1 hour at 37°C in a CO2 incubator. Then the inoculum was aspirated, and the cells were washed with sterile PBS before the addition of maintenance media. The plates and the flasks were monitored for 7 days for cytopathic effect (CPE). If no, then passages were made with cells disturbed by freezing and thawing three times and harvested. The harvests were reinoculated onto a new cell culture flask and monitored for any changes in cell morphology. Reverse transcriptase polymerase chain reactionRNA extraction200 mg of bursal tissue was homogenized in sterile mortar and pestle using 2 ml TRIzol® reagent (GIBCO-BRL, Gaithersburg, MD) according to the manufacturer’s instructions. One freeze-dried ampule of vaccine strain D78 (Intervet International, Boxmeer, Holland) was dissolved in 1 ml sterile double distilled water (DDW), and 200 µl was taken for RNA extraction by TRIzol method as control positive as described before. RNA prepared from normal bursa of Fabricius (tested with AGID) and DDW were used as control negative. PCR procedureThe sequences of oligonucleotide primers used for RT-PCR were previously selected from the highly conserved VP2 genome region derives from the published sequence (Barlič-Maganja et al., 2002). Primer IBDV-F (5ʹ-ACAGGCCCAGAGTCTACACCAT AA-3ʹ) and primer IBDV-R (5ʹ-ATCCTGTTGCCACTCTTTCGTAGG-3ʹ). RT-PCR was carried out using a one-tube RT-PCR kit (Invitrogen, USA) in a final volume of 50 µl including 19 µl DDW, 25 µl 2× Reaction mix, 1 µl IBDV-F, 1 µl IBDV-R, 3 µl template, 1 µl RT/Platinum Taq mix. The RT-PCR was carried out with the uninterrupted program in TP3 Thermocycler (BIOMETRA, Göttingen, Germany) under the following condition: 45 minutes at 48°C for reverse transcription and 2 minutes at 94°C for transcriptase inactivation, 40 cycles of 30 seconds at 94°C, 1 minute at 60°C and 2 minutes at 68°C for PCR, and extension step of 7 minutes at 68°C. The final step was an open time (pause) at 4°C. The reaction products were analyzed by electrophoresis in 1% agarose gel stained with ethidium bromide (1 µl/40 ml agarose). Electrophoresis was performed in a Mini gel electrophoresis (BIOMETRA) using 75 volt and 150 mA for 45 minutes after the gel was covered with tris-acetate-EDTA (TAE) buffer using Standard Power Pack P25 (BIOMETRA). The cDNA bands were visualized using the BIODOC ANALAYZ gel documentation system (BIOMETRA). Experimental infection of chicks with IBDV-positive field samplesThis experiment aims to study clinical signs, PM findings, morbidity, and mortality of three different IBDV-positive samples. Sera were collected from all experimental chicks and tested by the AGID, using standard IBDV antigen, for IBDV antibodies. Three groups of non-vaccinated chicks A, B, and C (35 chicks in each group) derived from eggs obtained from the Faculty of Veterinary Medicine, University of Khartoum, were inoculated orally by 10% bursal homogenate (0.2 ml/bird) at the age of 22 days. Three IBDV isolates were selected for experimental infection based on the difference in epidemiology and clinical picture. Group A was infected with Omdurman isolate (caused 26% morality rate in the field); group B was infected with Butri isolate (caused 37% mortality rate), and group C was infected with Alkabashi isolate (caused 60% mortality rate). Morbidity and mortality in experimental chicks were recorded daily for 10 days. The fourth group of 20 chicks was left as uninfected control (group D). Bursal samples were taken from infected groups and tested by AGID against positive known hyperimmune serum for IBDV. All sera collected from the chicks before the experiment tested negative for antibodies against IBDV. Ethical approvalEthical approval for this study was obtained from the Ethical Committee of the Faculty of Veterinary Medicine, University of Khartoum. Written informal consensus was obtained from all owners of the investigated poultry farm before taking samples. ResultsField investigationsMorbidity and mortality due to IBDThe overall morbidity in the investigated outbreaks was estimated to be between 70% and 90% with a mean of 80% (Table 1), while the mortality varied between 26% and 76% with a mean of 51% depending on the area, age at infection and probably the infecting virus (Table 1). The distribution of IBD outbreaks in different age groups is shown in Figure 1. Comparison between mortality rates in the 30 chicken farms vaccinated with different IBDV vaccines is shown in Table 2. Clinical signsIn all investigated flocks, the disease was characterized by a sudden onset of depression. The affected birds showed signs of dullness, ruffled feathers, reluctance to move, watery diarrhea, recumbency with the beak on the earth, off food, and pasted vent (Fig. 2). Table 1. The incidence of IBD outbreaks in Khartoum State 2003–2004 and vaccination program.
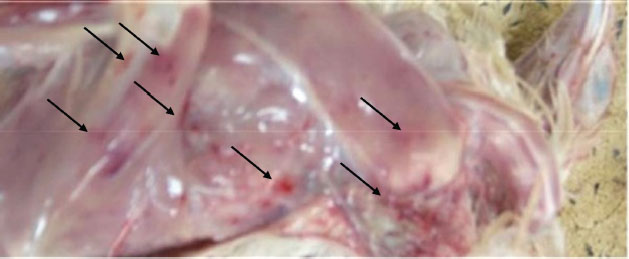
PM findingsPM examination of the affected birds revealed dehydration of carcasses, hemorrhages in skeletal muscles (thigh and pectorals muscles) (Fig. 3). In some cases, there were hemorrhages in the junction between the proventriculus and the gizzard. Kidneys showed enlargement with distended tubules containing urates. The bursa of Fabricius enlarged (two to three times) and became edematous, yellowish, and sometimes hemorrhagic. Histopathological findingsMost of the pathological lesions occurred in the bursa of Fabricius. Severe lymphoid necrosis and depletion occurred in both the medulla and cortex of the follicles. Severe edema with heavy infiltration of heterophils and macrophages was also observed (Fig. 4).
Fig. 1. A diagram showing distribution of IBD outbreaks in different age groups in Khartoum State, 2003 to 2004. Table 2. Comparison between mortality rates in 30 chicken farms vaccinated with different IBDV vaccines in the Sudan.
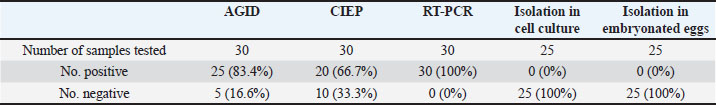
Laboratory diagnosisAgar gel immunodiffusionIBDV was identified in the 10% bursal homogenates of 25 samples out of 30 by AGID (83.3%). The samples gave precipitin lines with the known antisera against IBDV, indicating the presence of IBDV antigen in the bursal homogenate. CIEP testIBDV antigens were identified in bursal homogenates by using CIEP test in 18 samples out of 30 with clear precipitin lines (60%). Reverse transcription polymerase chain reactionRT-PCR was conducted on RNA samples extracted from infected bursas collected from field outbreaks using the TRIzol method. The RT-PCR was done with primer IBDV-F and IBDV-R. No amplification products were detected when the control negative (RNA prepared from normal bursa and DDW) were used as templates while the control positive (D78 vaccine) gave positive results. All the RNA samples extracted from the infected bursas collected from the 30 outbreaks gave positive amplification the VP2 gene (100%). The bands were detected in the ethidium bromide-stained gel that corresponds exactly to the expected cDNA band size of the control positive 479 bp (Fig. 5). Comparison between the diagnostic techniques used for diagnosis of IBD is displayed in Table 3. Virus isolationInoculation of embryonated eggsNo lesions were produced by inoculation of the CAM and the yolk sac of chicken embryonated eggs with 10% bursal homogenate of all AGID-positive samples (n=25). Three blind passages were carried out, and no lesions were seen. Each passage was tested by AGID against known antisera. No precipitin lines were seen between the yolk and the homogenized CAMs and the HIS. Cell cultureAll AGID positive samples (n=25) were inoculated onto the CEF cell culture. Each virus passage was tested using AGID against known HIS. No CPEs were seen on CEF cell cultures after inoculation, even after three blind passages.
Fig. 2. Chicks infected with IBDV day 3 after the onset of the disease. Insert: close view of an affected bird; note, ruffled feathers and depression of affected birds.
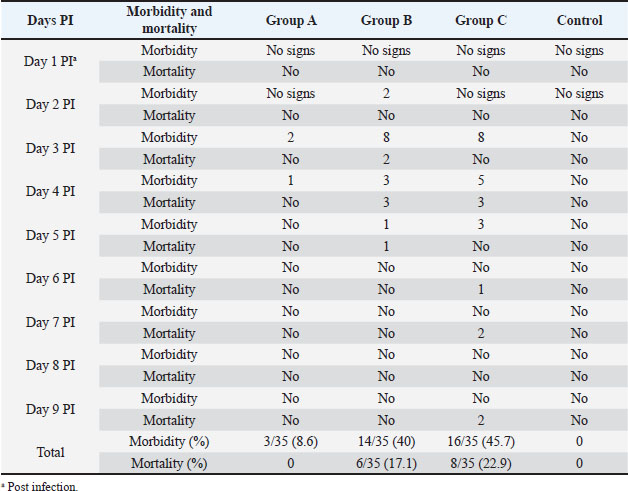
Fig. 3. Hemorrhages in skeletal muscles in IBD affected bird (arrows). Experimental infection of susceptible chicks with IBD positive samplesThe incubation period of IBD in experimentally infected chicks was found to be 2–3 days. Experimentally, only 6% of inoculated birds show signs of depression, dullness, and ruffled feathers after 36 hours post infection (PI). Table 4 shows the morbidity and mortality rate in the three groups inoculated with different IBDV isolates. The AGID is of low sensitivity, and a false negative result may happen. The virus isolation is laborious, nonspecific, and time-consuming. These methods have disadvantages like the inability to detect low levels of IBDV in tissues; besides, the recent very virulent strains of IBDV cannot be grown in cell culture. DiscussionImmunosuppressive viral diseases have been a significant concern for the poultry industry for several years. IBD or Gumboro is an acute, highly contagious viral disease of young chickens that leads to immunosuppression and significant economic losses (Mittal et al., 2005). Indeed, the reemergence of IBDV, in particular, the highly virulent forms, has been the cause of substantial losses in poultry (Paul et al., 2004). The two main objectives of this study were to determine the epidemiology of IBD infection in chicks in Khartoum State and to compare RT-PCR with classical techniques for its diagnosis. Field outbreaks of IBDV (n=30) that occurred between February 2003 and June 2004 in Khartoum State were investigated. The clinical signs of IBD were similar to those observed after experimental infection of susceptible chicks. The symptoms were characterized by sudden onset of depression, dullness, ruffled feathers, reluctance to move, watery diarrhea, recumbency with the beak on the earth, off food, and pasted vent. These signs are similar to those described in the early outbreaks of IBD by Cosgrove (1962) and also to those described by Parkhurst (1964) and Dey et al. (2019). The mortality ranged between 26% and 76%, with a mean of 51%. These mortality rates are closer to the percentages described by Chettle et al (1989), Nunoya et al. (1992), and Di Fabio et al. (1999) caused by the very virulent IBDV (vvIBDV). Overall, the epidemiologic picture, clinical manifestations, gross and histopathological findings reported in the present outbreaks were characteristic of the severe disease caused by the vvIBDV (Aliyu et al., 2016).
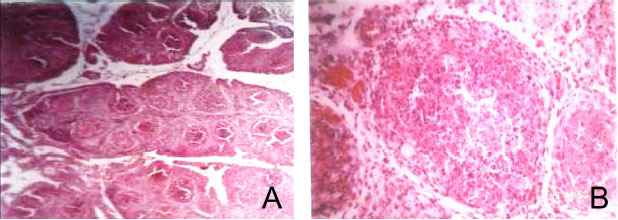
Fig. 4. Histopathological sections of bursa of Fabricius of IBD affected bird showing different sizes of follicles and cracking of follicles with increase of interfollicular tissue, (H and E, ×10) (Panel A) and degeneration of lymphocytes (Lymphoid depletion), (H and E, ×40) (Panel B).
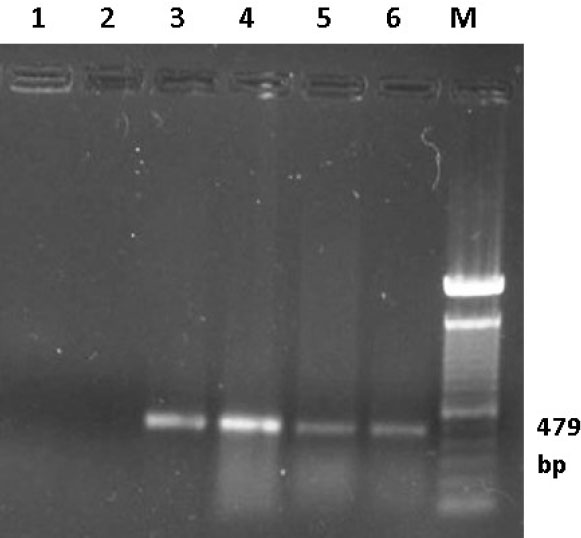
Fig. 5. Ethidium bromide-stained Agarose gel (1%). RT-PCR was carried out on RNA samples extracted from bursas of infected birds using primers IBDV-F/IBDV-R. Lane 1 control negative (DDW), lane 2 control negative (bursa from uninfected bird), lane 3 control positive (D78 IBD vaccine), lanes 4, 5 and 6 representative bursal samples from the field cases of IBD in Sudan, Lane M 100 bp marker. Table 3. Comparison between some diagnostic techniques used for diagnosis of IBD.
Table 4. Results of experimental infection of chicks with IBDV positive samples.
However, the mortality rates reported in this study are lower than those described by van den Berg et al. (1991), who reported a mortality rate between 70% and 100%. Moreover, the morbidity and mortality rates described in the present outbreaks are much higher than those reported during previous episodes of IBD in Sudan, which was described by Shuaib et al. (1982); Gaffar et al. (1988); Khalafalla et al. (1990). This mortality pattern points to the increased severity of IBDV in Sudan, or they may support the assumption of introducing the vvIBDV to the country. Research work that can genetically compare old and recent isolates of IBDV is highly needed to prove this hypothesis. In the investigated outbreaks, it was noticed that there was a high mortality rate despite vaccination against the disease (96.7% of flocks were vaccinated). This finding denotes a vaccination failure that might be due to the emergence of a vvIBDV that is antigenically different from the virus in the vaccines used (Snyder, 1990). The vvIBDV strains could establish infection in the presence of a high level of maternal antibodies that were protective against classical strains as indicated by Chettle et al. (1989) and cause up to 60%–100% mortality (van den Berg et al., 1991). The vaccination failure may also be due to improper timing of vaccination or faulty application of vaccines. In recent years, more and more mutations, recombination, and reassortment of IBDV were reported. Novel variant breakthrough in existing vaccine immunization has also been identified, and a new genotype classification scheme has been proposed (Wang et al., 2021). These points highlight the need for genetic comparison of the field and vaccinal virus genomes by partial or complete sequencing and cross serological tests by VNT or ELISA. In addition, for a vaccine, the antigen matching between the vaccine strain and the epidemic strains is also critical. It is worth mentioning that the mortality rate in birds vaccinated with the hot IBD vaccine (228E) was relatively lower (25%) than that recorded in birds vaccinated by the D78 and Gumboro 3 intermediate strains (39% and 38%, respectively). The hot vaccines are less attenuated and better able to cope with the field challenge by establishing strong “Immunity Cushing” in the infected areas (Mazariegos et al., 1990; Lukert and Saif, 1991). Histopathologically, the examined bursas showed infiltration by lymphocytes and necrosis of the plasma and the blast cells, which resulted in the destruction of all lymphoid tissue in the bursa, leaving the reticular structure of follicles. These results agree with that of Okoye and Shoyinka (1983), Lang et al. (1987), Lasher and Shane (1994). We collected bursal homogenates from three field outbreaks that caused different mortality rates (26%, 37%, and 60%) to infect susceptible 22-days-old chicks experimentally. Results showed comparatively lower mortality rates of 0%, 17%, and 23%, respectively. It is apparent from these results that the virulence of the field virus has remarkably reduced during this single chick passage. Considering that birds infected in the field outbreaks were vaccinated against the disease while the experimental chicks were not, it seems complicated to explain what happened. However, there are some possible explanations: the experimental chicks had a kind of transient exposure with a low virulent IBDV strain that resulted in some immunity. Low virulent strains of IBDV were previously reported in Sudan (Khalafalla et al., 1990), and subclinical IBD occurs when chickens are exposed to IBDV during the first 2 weeks post-hatch (McIlroy et al., 1989). However, resistance to infection could be due to effective local immunity, which is quite different from the humoral immune response. Another explanation could be that the IBD infection in the field depends on a combination of environmental and biological factors that are not considered in the experiment infection. According to Chang and Hamilton (1982), morbidity and mortality depend on the virulence of the challenge virus, the immune status and age of the infected birds, and other factors affecting the pathogenicity of IBDV like aflatoxins infection. A third explanation could be that clinical IBD results from genetic reassortment of more than virus strains that coexist in the environment. IBDV is a bi-segmented virus, and such genetic reassortment could occur in the field (Islam et al., 2001). Further research is needed to examine these possibilities. Clinical manifestations and PM findings in affected birds are characteristic that help diagnose a disease outbreak, but laboratory diagnosis is necessary for confirmation of the diseases. The classical technique that is commonly used in the diagnosis of IBD in Sudan is the AGID. The AGID test is highly specific for IBDV precipitins in that no false-positive reaction occurs, but it is less sensitive and may give false negatives (Nicholas et al., 1985). In the present study, classical techniques of AGID, CIEP, and virus isolation in embryonated eggs and cell culture were used for the diagnosis of field cases. Twenty-five samples out of 30 samples (83%) were clearly positive by the AGID test, and 18 samples out of 30 (60%) gave clear precipitin lines using the CIEP test. This result indicates that the AGID test is more sensitive than CIEP in detecting IBDV antigens. On the other hand, all the tested samples (n=300 were positive in the RT-PCR) indicating its high sensitivity (100%). The RT-PCR is rapid, specific, and highly sensitive, and there is no need to grow the virus even if it is in low levels and the loss of virus infectivity does not affect the RT-PCR. No lesions were produced by inoculation of 15 samples onto the CAM and yolk sac of chicken embryonated eggs with 10% bursal homogenate of field samples and no lesions or morphological changes were seen in CEF cell culture after inoculation, even after three blind passages. These findings point to the inability to grow Sudanese isolates of IBDV in embryonated eggs and cell culture and render these techniques unsuitable for routine diagnosis. These findings are not unexpected since Bumstead et al. (1993) and Islam et al. (2001) reported similar results and argue that the vvIBDV does not grow in cell culture. Therefore, virus isolation is not recommended for the diagnosis of IBD as indicated in the present study. ConclusionA vvIBDV is responsible for the recent outbreaks of the disease in Sudan, resulting in a mean high mortality rate of 51%, even in vaccinated flocks. The introduction of PCR in Sudan is of great value in diagnosing diseases because it is a rapid, reliable, specific, and sensitive technique. Further research work should be carried out to molecularly characterized IBDV strains through sequencing, and attempts should be to produce an IBD vaccine locally through attenuation of a local isolate of the virus in cell culture or inactivation. We also recommend revising IBD vaccination programs in Sudan, taking into consideration the results of this study. AcknowledgmentsThe authors are grateful to our colleagues and the staff of the Virology Research Laboratory, Faculty of Veterinary medicine and to Prof. Ahmed A. Gameel, Department of Pathology, for the help in the histopathology. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAliyu, H.B., Hair-Bejo, M., Omar, A.R. and Ideris, A. 2021. Genetic Diversity of Recent Infectious Bursal Disease Viruses Isolated from Vaccinated Poultry Flocks in Malaysia. Front. Vet. Sci. 8, 643976. doi: 10.3389/fvets.2021.643976. Aliyu, H.B., Sa’idu, L., Jamilu, A., Andamin, A.D. and Akpavie, S.O. 2016. Outbreaks of virulent infectious bursal disease in flocks of battery cage brooding system of commercial chickens. J. Vet. Med. 2016, 8182160; doi: 10.1155/2016/8182160 American Association of Avian Pathologists. 2008. Chapter 43. In Laboratory manual for the isolation and identification of avian pathogens, 5th ed. Philadelphia, PA: AAAP, University of Pennsylvania, pp: 19348–1692. Arafat, N., Eladl, A.H., Mahgoub, H. and El-Shafei, R.A. 2017. Effect of infectious bursal disease (IBD) vaccine on Salmonella Enteritidis infected chickens. Vaccine 35(29), 3682–3689. Barlič-Maganja, D., Zorman-Rojs, O. and Grom, J. 2002. Detection of infectious bursal disease virus in different lymphoid organs by single-step reverse transcriptase polymerase chain reaction and microplate hybridization assay. J. Vet. Diag. Invest. 14, 243–246. Berg, N.W. 1982. Rapid detection of infectious bursal disease antibodies by counterimmunoelectrophoresis. Avian Pathol. 11(4), 611–614. Bumstead, N., Reece, R.L. and Cook, J.K.A. 1993. Genetic differences in susceptibility of chicken’s lines to infection with infectious bursal disease virus. Poult. Sci. 72, 403–410. Chang, C.F. and Hamilton, P.B. 1982. Increased severity and new symptoms of infectious bursal disease during aflatoxicosis in broiler chickens. Poult. Sci. 16, 1016–1068. Chettle, N.J., Stuart, J.C. and Wyeth, P.J. 1989. Outbreak of virulent IBD in East Anglia. Vet. Rec. 125(10), 271–272. Cosgrove, A.S. 1962. An apparently new disease of chickens: avian nephrosis. Avian Dis. 6, 27–30. Cunningham, C.H. 1973. A laboratory guide in virology, 7th ed. Minneapolis, MN: Burgess Publishing. Dey, S., Pathak, D.C., Ramamurthy, N., Maity, H.K. and Chellappa, M.M. 2019. Infectious bursal disease virus in chickens: prevalence, impact, and management strategies. Vet. Med. (Auckl), 10, 85–97. Drury, R.A.B. and Wallington, E.A. 1980. Histological technique, 5th ed. Oxford, UK; New York, NY: Oxford University Press. Delmas, B., Attoui, H., Ghosh, S., Malik, Y.S., Mundt, E. and Vakharia, V.N. 2019. ICTV virus taxonomy profile: Birnaviridae. J. Gen. Virol. 100(1), 5–6; doi: 10.1099/jgv.0.001185 Di Fabio, J., Rossini, L.I., Eterradossi, N., Toquin, D. and Gardin, Y. 1999. European-like pathogenic infectious bursal disease virus in Brazil. Vet. Rec. 145, 203–204. Eladl, A.H., Mosad, S.M., El-Shafei, R.A., Saleh, R.M., Ali, H.S., Badawy, B.M. and Elshal, M.F. 2020. Immunostimulant effect of a mixed herbal extract on infectious bursal disease virus (IBDV) vaccinated chickens in the context of a co-infection model of avian influenza virus H9N2 and IBDV. Comp. Immunol. Microbiol. Infect. Dis. 72, 101505. Gaffar, M.A.E., Khier, S.M.A., Tagadin, M.H. and Ahmed, A.I. 1988. Observation on infectious bursal disease and Newcastle disease in eastern region of the Sudan. Bull. Anim. Health Prod. Afr. 36, 304–308. Hajer, I. and Ismail, M.H. 1988. Proceeding of the symposium on performance of exotic poultry breeds (white and brown) under Sudan conditions (Khartoum, Sudan, 16–17 Oct.1988). pp: 95–103. Islam, M.R., Zierenberg, K. and Muller, H. 2001. The genome segment B encoding the RNA-dependent RNA polymerase protein VP1 of very virulent infectious bursal disease virus (IBDV) is phylogenetically distinct from that of all other IBDV strains. Arch. Virol. 146(12), 2481–2492. Jackwood, D.J. 2004. Recent trends in the molecular diagnosis of infectious bursal disease viruses. Anim. Health Res. Rev. 5(2), 313–316. Khalafalla, A.I. 1994. Isolation and characterization of lentogenic NDVs from apparently healthy chicken in the Sudan. Bull. Anim. Health Prod. Afr. 42, 179–182. Khalafalla, A.I., Mustafa, A., Abbas, Z., Hajer, I. and El Saman, S. 1990. Case report of a mild infection of infectious bursal disease in broiler chicks in the Sudan. Sudan J. Vet. Res. 10, 45–48. Lasher, H.N. and Shane, S.M. 1994. Infectious bursal disease. World Poult. Sci. J. 50, 133–166. Lang, H., Muller, H., Kaufer, I. and Becht, H. 1987. Pathogenic and structural properties of wild type infectious bursal disease virus (IBDV) and virus grown in vitro. Arch. Virol. 92, 187–196. Lukert, P.D. and Saif, Y.M. 1991. Infectious bursal disease. In Disease of poultry, 9th ed. Ed., Clanek, B.W. Ames, IA: Iowa state university press. pp: 648–663. Mazariegos, L.A., Lukert, P.D. and Brown, J. 1990. Pathogencity and immunosuppressive prosperities of IBDV intermediate strains. Avian Dis. 34(1), 203–208. McIlroy, S.G, Goodall, E.A. and McCracken, R.M. 1989. Economic effects of subclinical infectious bursal disease on broiler production. Avian Pathol. 18, 465–480. Mittal, N.D., Jindal, S.L., Gupta, R.S. and Tiwari, A.K. 2005. Detection of infectious bursal disease virus in field outbreaks in broiler chickens by reverse transcription-polymerase chain reaction. Int. J. Poult. Sci. 4(4), 239–243. Mosad, S.M., Eladl, A.H., El-Tholoth, M., Ali, H.S. and Hamed, M.F. 2020. Molecular characterization and pathogenicity of very virulent infectious bursal disease virus isolated from naturally infected Turkey poults in Egypt. Trop. Anim. Health Prod. 52(6), 3819–3831. Nicholas, R.A.J., Reed, N.E., Wood, G.W., Hebert, C.N., Muskett, J.C. and Thornton, D.H. 1985. Detection of antibodies against infectious bursal disease: a comparison of three serological methods. Res. Vet. Sci. 38, 189–192. Nunoya, T., Otaki, Y., Tajima, M., Hiraga, M. and Saito, T. 1992. Occurrence of acute infectious bursal disease with high mortality in Japan and pathogenicity of field isolates in specific pathogen free chickens. Avian Dis. 36, 597–609. OIE (World Animal Health Organization). 2008. Newcastle disease. Manual of diagnostic tests and vaccines for terrestrial animals. Paris, France: OIE, pp: 576–589. Okoye, J.O.A. and Shoyinka, S.V.O. 1983. Newcastle disease in a vaccinated flock which had experienced subclinical IBD. Trop. Anim. Health Prod. 15(4), 221–225. Parkhurst, R.T. 1964. On the farm studies on Gumboro disease in broilers. Avian Dis. 8, 254–256. Paul, B.K., Das, S.K., Badhy, S.C., Amin, M.R., Aminand, K.M.R. and Banik, S.C. 2004. Effect of existing and imposed vaccination on body weight against Gumboro in broiler under farm condition. Int. J. Poult. Sci. 3(10), 655–657. Shuaib, M.A., Salman, A., Mahmoud, A., Ginawi, A. and Sawi, S.A. 1982. Isolation of IBDV in the Sudan. Sudan J. Vet. Res. 4, 7–12. Snyder, D.B. 1990. Changes in the field status of infectious bursal disease virus. Avian Pathol. 19, 419–423. van den Berg, T.P., Gozne, M. and Meulemans, G. 1991. Acute IBDV in poultry: isolation and characterization of a highly virulent strain. Avian Pathol. 20, 133–143. Wang, Y.L., Fan, L.J., Jiang, N., Gao, L., Li, K., Gao, Y.L., Liu, C.J., Cui, H.Y., Pan, Q., Zhang, Y.P., Wang, X. and Qi, X. 2021. An improved scheme for infectious bursal disease virus genotype classification based on both genome-segments A and B. J. Integr. Agric. 20, 1372–1381. | ||
| How to Cite this Article |
| Pubmed Style Omer MG, Khalafalla AI. Epidemiology and Laboratory Diagnosis of Severe Infectious Bursal Disease in Vaccinated Chickens in Khartoum, Sudan. Open Vet. J.. 2022; 12(1): 33-43. doi:10.5455/OVJ.2022.v12.i1.5 Web Style Omer MG, Khalafalla AI. Epidemiology and Laboratory Diagnosis of Severe Infectious Bursal Disease in Vaccinated Chickens in Khartoum, Sudan. https://www.openveterinaryjournal.com/?mno=135189 [Access: January 25, 2026]. doi:10.5455/OVJ.2022.v12.i1.5 AMA (American Medical Association) Style Omer MG, Khalafalla AI. Epidemiology and Laboratory Diagnosis of Severe Infectious Bursal Disease in Vaccinated Chickens in Khartoum, Sudan. Open Vet. J.. 2022; 12(1): 33-43. doi:10.5455/OVJ.2022.v12.i1.5 Vancouver/ICMJE Style Omer MG, Khalafalla AI. Epidemiology and Laboratory Diagnosis of Severe Infectious Bursal Disease in Vaccinated Chickens in Khartoum, Sudan. Open Vet. J.. (2022), [cited January 25, 2026]; 12(1): 33-43. doi:10.5455/OVJ.2022.v12.i1.5 Harvard Style Omer, M. G. & Khalafalla, . A. I. (2022) Epidemiology and Laboratory Diagnosis of Severe Infectious Bursal Disease in Vaccinated Chickens in Khartoum, Sudan. Open Vet. J., 12 (1), 33-43. doi:10.5455/OVJ.2022.v12.i1.5 Turabian Style Omer, Mohamed Gasim, and Abdelmalik Ibrahim Khalafalla. 2022. Epidemiology and Laboratory Diagnosis of Severe Infectious Bursal Disease in Vaccinated Chickens in Khartoum, Sudan. Open Veterinary Journal, 12 (1), 33-43. doi:10.5455/OVJ.2022.v12.i1.5 Chicago Style Omer, Mohamed Gasim, and Abdelmalik Ibrahim Khalafalla. "Epidemiology and Laboratory Diagnosis of Severe Infectious Bursal Disease in Vaccinated Chickens in Khartoum, Sudan." Open Veterinary Journal 12 (2022), 33-43. doi:10.5455/OVJ.2022.v12.i1.5 MLA (The Modern Language Association) Style Omer, Mohamed Gasim, and Abdelmalik Ibrahim Khalafalla. "Epidemiology and Laboratory Diagnosis of Severe Infectious Bursal Disease in Vaccinated Chickens in Khartoum, Sudan." Open Veterinary Journal 12.1 (2022), 33-43. Print. doi:10.5455/OVJ.2022.v12.i1.5 APA (American Psychological Association) Style Omer, M. G. & Khalafalla, . A. I. (2022) Epidemiology and Laboratory Diagnosis of Severe Infectious Bursal Disease in Vaccinated Chickens in Khartoum, Sudan. Open Veterinary Journal, 12 (1), 33-43. doi:10.5455/OVJ.2022.v12.i1.5 |