| Research Article | ||
Open Vet. J.. 2023; 13(7): 826-833 Open Veterinary Journal, (2023), Vol. 13(7): 826–833 Original Research Immunogenicity of a freeze-dried combined vaccine against Rift Valley fever and bovine ephemeral fever in cattleNaglaa Ebrahim Aly1, Mohamed Hassan Atwa2, Amany Mohamed Abbas3, Diana Mohamed Abulmagd2*, Zeinab Taha Salem1 and Taradi Abdelfattah Sayed21Department of Pet Animal Vaccine, Agriculture Research Center (ARC), Veterinary Serum and Vaccine Research Institute, Abasia, Cairo, Egypt 2Department of Rift Valley Fever, Agriculture Research Center (ARC), Veterinary Serum and Vaccine Research Institute, Abasia, Cairo, Egypt 3Agriculture Research Center (ARC), Central Laboratory for Quality Control of Veterinary Biologics, Abasia, Cairo, Egypt *Corresponding Author: Diana Mohamed Abulmagd. Department of Rift Valley Fever, Agriculture Research Center (ARC), Veterinary Serum and Vaccine Research Institute, Cairo, Egypt. Email: diana2010mohamed [at] gmail.com Submitted: 05/03/2023 Accepted: 05/06/2023 Published: 31/07/2023 © 2023 Open Veterinary Journal
AbstractBackground: The target of vaccination is to encourage a strong, covering and long-lasting immune response against antigens. For achieving these objectives; effective adjuvant and new vaccine strategies are demanded to make the vaccine sufficiently immunogenic to instigate a powerful immune response Aim: This study was completed for elaboration and evaluation of freeze-dried combined vaccine against both Rift Valley fever (RVF) and bovine ephemeral fever (BEF) viruses using different stabilizers. Methods: Three formulae were prepared from such vaccine including: formula (1): stabilized with a mixture of 5% Lactalbumin Hydrolysate and 2.5% sucrose, formula (2): stabilized with a mixture of 50% the previous stabilizer and 50% of 1% Carbopol and formula (3): stabilized with 1% Carbopol solution. Samples of the three vaccine formulae were reconstituted on the time of experimental animal vaccination using saponin diluent which acts as an adjuvant for both RVFv and BEFv and as an inactivator BEF virus. The ratio between both viruses in all vaccine formulae was 1:1. Results: All vaccine batches were proved to be free of any foreign contaminants and unharmed for experimentally vaccinated animals. Each of the three groups of calves was vaccinated S/C with 2 ml of a reconstituted vaccine formula and their immune response was evaluated using serum neutralization test. The gained results revealed that the prepared combined freeze-dried vaccine with Carbopol elicited a better humoral immune response than the other two vaccine formulae. Conclusion: It could be recommended to use Carbopol as a stabilizer for the preparation of the aimed vaccine. Keywords: Rift Valley fever, Bovine ephemeral fever, Neutralization test, Cytopathic effect. IntroductionRift Valley fever (RVF) is a zoonosis viral disease that mainly affects animals and human beings. It was initially specified in 1931 in a sheep epidemic in the Rift Valley in Kenya. Even after, RVF outbreaks were reported in sub-Saharan Africa and Egypt. Cases were assured in Saudi Arabia and Yemen in 2000, and alarmist that it could expand to other areas of Asia and Europe. Mainly infections in humans, result from handling the blood and organs related to the infected animals (WHO, 2020). Mainly, the disease affected small and large ruminants, camels and humans causing general weakness, fever, salivation, fetid diarrhea, and reduction in milk yield with a storm of abortions in pregnant animals (FAO, 2003). The outbreak of RVF was associated with heavy rainfalls due to mosquito spreading (Nguku et al., 2006). The preferable device for protecting animal inhabitance and indirectly humane beings is vector hold and vaccination by using secure and potent vaccines (Abdel Ghaffar et al., 1979). RVF vaccines are two types, live attenuated Smithburn vaccine and adjuvanted inactivated vaccine (Faburay et al., 2017). The limitation of applying live RVF vaccine is due to teratogenic or abortogenic action (Hubbard et al., 1991). So, the inactivated RVF vaccine is mainly used in Egypt with two doses for inducing high protection levels of antibody titer and long period (OIE, 2016). On the other side, bovine ephemeral fever (BEF), or 3-day sickness (Akakpo, 2015) is a non-contagious epizootic arthropod-borne Rhabdovirus disease (mostly likely mosquitoes) mainly affects cattle and water buffalo, coming across tropical and subtropical zones of Asia, Australia, and Africa. The disease is marked by a short fever, shivering, lameness, and muscular stiffness, followed by inability to stand, reluctance to move, anorexia, ocular and nasal discharge, and excessive salivation. The disease may cause serious economic losses through deaths, decreased milk production mortality is usually low when diseased animals receive compatible medical attendance (George et al., 1984; Uren et al., 1992). The disease’s importance is related to economic losses, as it causes a marked drop in milk output with deprivation in beef cattle conditions (Walker, 2005; Akakpo, 2015). Vaccination of calves with steady boosts and quality vaccines usually induces efficient protection (Nandi and Negi, 1999). The current used vaccine against RVFv is the aluminum hydroxide gel inactivated RVF vaccine which can be administrated to all animals of all ages, and it requires repeated doses to induce and maintain protective immunity since an initial dose may only give protection for 7 months (El-Bagoury et al., 2013), while vaccination against BEFv is applied using SERVAC BEF attenuated vaccine which induce duration of immunity about 1 year (Elgendy et al., 2021) The current trend is using various microorganisms in a combined vaccine is highly sustain the protection against the diseases in livestock aside from conserving costs, efforts, and time during vaccination The target of immunization by vaccines is to encourage a potent, covering and prolonged immune response against the antigen. To achieve these objectives; effective adjuvant and new vaccine production are demanded to make the vaccine sufficiently immunogenic to instigate a powerful immune restraint (Fearon, 1997; Bomford, 1998). Carbopol is a synthetic polymer that has many implementations in pharmaceuticals. The aqueous Carbopol gel is compatible with many ingredients, thermostable, and flow easily during different routes of application (Islam et al., 2004). Carbopol has various advantages like high safety, nontoxic, and suspending agent (Ahuja et al., 1997). Carbopol can promote and animate cellular and humoral immunity in mammals (Gartlan et al., 2016). The feature of using aquatic Carbopol gel is its easy-flowing manner, its affinity with many active ingredients; and its thermal stability (Zhang et al., 2018). Saponins, extracted from Quillaia Saponaria Molina, it is widely used as adjuvants for several years and has been used in sundry veterinary vaccines. The adjuvants have the capability to modulate the cell-mediated immune system and promote antibody fabrication (Oda et al., 2000). It motivates a potent production of T-dependent as well as T-independent antigens, and cytokines such as interleukins and interferon that might intercede with their immune stimulant effects (Jie et al., 1984; Kensil, 1996). So, the definitive goal of this work is an elaboration of a combined inactivated RVF and BEF vaccine in a lyophilized form using high-quality stabilizer to increase its stability saving efforts and time consumed during vaccine production. Materials and MethodsExperimental animalsSwiss albino baby miceTen baby mice (3-4 days old) were used for safety tests to determine the complete inactivation of RVF virus. CalvesTwenty native breeds, non-vaccinated healthy calves, about 12 to 18 months old were tested and confirmed to be free from RVF and BEF antibodies as screened by serum neutralization test (SNT). They were classified into four groups, each one of the first three groups contains 6 animals vaccinated with one dose of one of the prepared combined vaccine formulae. A booster dose of vaccination was administrated to only three calves from each group after one month. The fourth group contains two calves kept as unvaccinated control group. All calves’ groups were kept under strict hygienic conditions in insect proof stables receiving balanced ration and adequate water with daily clinical examinations. In addition, another 10 calves were used to test the safety of the prepared vaccine formulae. VirusesVirulent RVFV (ZH 501) was supplied by the Department of Rift Valley Fever Vaccine Research; Veterinary Serum and Vaccine Research Institute (VSRI), Abasia Cairo, cultivated on BHK cells with final concentration 108 TCID50 / ml and used for the of lyophilized combined RVF and BEF vaccine. BEF virus strainBEFV/abasia/2000 (Soliman et al., 2001) was supplied by the Department of Pet Animal Vaccine Research, VSVRI; cultivated on BHK cells with final concentration 108 TCID50 / ml and employed for vaccine elaboration and SNT. Tissue cultureBaby hamster kidney cell line (BHK21) supplied by VSVRI were passaged and preserved according to Macpherson and Stocker (1962) and used for virus propagation, titration, vaccine elaboration and SNT. ChemicalsBinary ethylenimine0.1 M Binary ethyleneimine (BEI) stock solution prepared from 2 Bromo ethylamine hydrobromide (Aldrich Chemical Co., LTD) and 0.2 N NaOH, according to Bahnemann (1990) and Mark (2004) to be used for RVF virus inactivation process. CarbopolIt was supplied by Lubrizol as powder and dissolved in hot water to make 1% aqueous stock solutions (United States Pharmacopeial Convention Inc. 2022). The prepared solutions were exposed to heat sterilization by autoclaving at 121°C for 20 minutes, after that it was stored at 4°C until next use. SaponinSaponin was supplied by Sigma-Aldrich Labo Chemikalien Gm6H; Germany (cat. No.16109; lot.71500). It was prepared as watery solution in a concentration of 0.5 mg /ml in phosphate-buffered saline as described by Amoros et al. (1987) while was used as a diluent and to inactivate the live attenuated BEF virus vaccine just before vaccination. StabilizersA stabilizer formed from 5% lactalbumin hydrolysate (Sigma-Aldrich GmbH) with 2.5% sucrose was prepared according to Riyesh et al. (2011). Three stabilizers were used for elaboration of freeze-dried formulae of the RVF and BEF combined vaccine as follow: Stabilizer-1 composed of 5% Lactalbumin Hydrolysate and 2.5% sucrose Stabilizer-2 composed of a mixture of stabilizer-1 and 50% of 1%carbopol Stabilizer-3 composed of 1% Carbopol solution Virus titrationBoth of RVF and BEF viruses were titrated in BHK21 cell culture (Fig. 1a) using the microtiter method according to Rossiter et al. (1985) and the virus titer was calculated as log10 TCID50/ml according to Reed and Muench (1938). Virus inactivationRVF virus was inactivated by using BEI according to Bahnemann (1990) and Mark (2004) while BEF was inactivated with saponin at the time of vaccination according to Albehwar et al. (2010). Checking of complete RVF virus inactivation (Safety test)Inactivated RVF virus samples were tested for the existence of vigorous virus in tissue culture according to (OIE 2012) by inoculating BHK confluent monocell layer with the inactivated virus to be detected with daily microscopic examination and in baby mice according to El Nimr (1980) and Eman (1995) by inoculating baby mice (3-5 days old) with 0.03 ml of inactivated virus then mice were kept for 10 days with daily examination. Mice dying within the first 24 hours were discarded. The inactivated virus was approved as safe if there is no cytopathic effect (CPE) in tissue culture and if all inoculated mice survived for 10 days. Preparation of combined freeze-dried RVF and BEF vaccineAfter propagation and titration of both viruses, the virus titer of used RVF virus was 108 and BEF virus titer was 108 TCID50/ml. Live BEF virus was mixed altogether with inactivated RVF in equal volumes to prepare three formulae of the combined lyophilized vaccine: Formula-1 was prepared by mixing the virus mixture with an equal volume of stabilizer-1 (5% lactalbumin hydrolysate and 2.5% sucrose). Formula-2 was prepared by mixing the virus mixture with an equal volume of stabilizer-2 (50% {5%Lactalbumin Hydrolysate with 2.5% sucrose} and 50% of 1% Carbopol). Formual-3 was prepared by mixing the virus mixture with an equal volume of 1% CarbopolEach vaccine formula was dispensed in neutral glass vials (2.5 ml/vial) and exposed to the freeze drying process. Each vial of vaccine formula was reconstituted in 10 ml Saponin (0.5 mg/ml) according to Amoros et al. (1987) as adjuvant just before inoculation where each vaccinal dose contains 106 TCID50 /ml of RVF and 106 TCID50 /ml of BEF Quality control testsSterility testRandomly picked up samples from the virus fluids and the final products were checked for their liberty of odd contaminants (aerobic and anaerobic bacteria, fungi, and mycoplasma) on Nutrient agar medium; Thioglycolate medium (Oxford, England); Sabouraud dextrose agar medium and PPLO medium according to the recommendation of the Code of Federal Regulations (2005) and OIE (2015). Safety testVaccine safety test was carried out to test the safety of both RVF and BEF in calves (the target host). Each of the three calves was inoculated subcutaneously with 10 ml from each formula of the prepared combined lyophilized vaccine keeping a fourth calf without vaccination as control. All calves were observed daily for 2 weeks for detection of any post-vaccinal reactions. Potency testEach formula of the ready lyophilized combined RVF and BEF vaccine was employed to vaccinate a calves’ group followed by monitoring of their immune response to RVF and BEF viruses using SNT microtiter technique as described by Rossiter et al. (1985) while the titer of the obtained antibodies was elaborated according to Reed and Muench (1938).
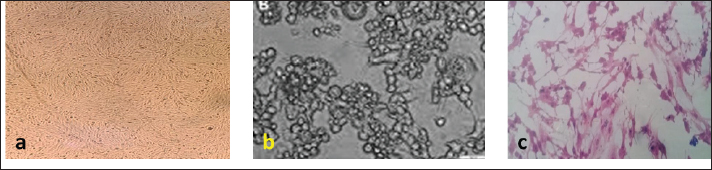
Fig. 1. (a) Uninfected BHK control cells. (b) CPE of RVFv in BHK. (c) CPE of BEFv in BHK. Schedule of calves’ vaccinationEach vaccine formula was reconstituted in 10 ml Saponin (0.5 mg/ml) as an adjuvant and inactivator to BEF virus at the time of inoculation as follows using vaccination dose of 2 ml/calf injected S/C in the neck side: Group (1): vaccinated with vaccine formula-1 Group (2): vaccinated with vaccine formula-2 Group (3): vaccinated with vaccine formula-3 Group (4): was kept as unvaccinated control. Three calves from each of the first three groups received a booster dose at the first month post vaccination (MPV) with the corresponding vaccine formula SamplesBlood samples were collected from all calves’ groups in sterilized, dry and clean screw capped bottles incubated at 37°C for 30 minutes then Kept overnight in a refrigerator. The formed serum was separated and centrifuged at 3,000 rpm for 10 minutes and kept at –20°C till subjected to serological test. Such samples were collected before vaccination then after 2 weeks before booster and then on monthly intervals for evaluation of the induced neutralizing BEF and RVF antibodies. Ethical approvalThe utilize of animals and their care were donated by the medical and Veterinary Research Ethics Board at the National Research Center in Egypt. ResultsProliferation and titration of RVF virus (ZH501) in tissue cultureUnder Biosafety measures; the virus (107.5 TCID50 / ml) was generated and proliferated in BHK cells for three successive times to excess the virus output and the virus titer reached after the three successive passages to 108 TCID50 / ml. A notable CPE of RVF virus showed as cell rounding and accumulation in clusters like grapes like aggregation as shown in Figure 1b and Table 1. Table 1. Titers of RVF virus (ZH501) and BEF virus in BHK cells.
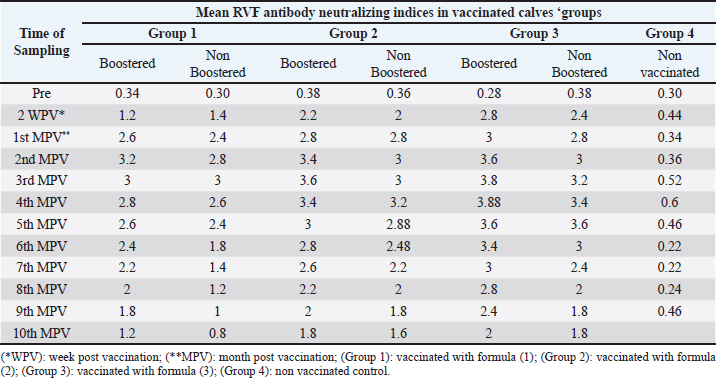
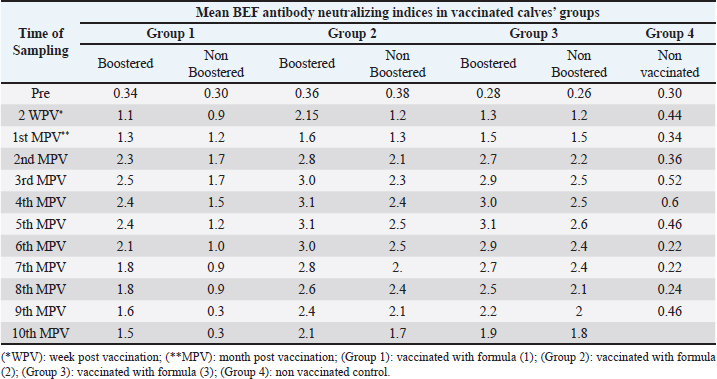
Proliferation and titration of BEF virus in tissue cultureThe virus (106.8 TCID50 / ml) was generated and proliferated in BHK cells for three consecutive times to rise the virus product till reached 108 TCID50 / ml. A notable CPE of BEF virus in BHK cells appeared as cell degeneration that end with the splitting of cells from the culture surface leaving hollow spaces. These stages of cellular changes take place within the initial 24 hours post infection and end usually within 72 hours post infection as shown in Figure 1c and Table 1. Determination of complete RVF virus inactivationInoculation of baby mice and in tissue culture with the inactivated RVF virus revealed its safety showing no mortalities or any abnormal signs of sickness in inoculated mice through 10 days post inoculation observation period and no CPE was observed in inoculated BHK cells, Quality control testing of the three prepared combines inactivated RVF and BEF vaccine formulae, revealed that all of them were free from foreign contaminants; safe in inoculated calves showing no any abnormal local or systemic clinical signs, and efficient inducing good levels of particular RVF and BEF antibodies in vaccinated calves as shown in Table 2. Monitoring of RVF neutralizing antibody levels in vaccinated calves using SNT showed that the peak NI was recorded on the second month (3.2) in Boostered calves in group-1 (vaccinated with formula-1) and on the third month in non-Boostered calves in the same group. Boostered calves in group-2 (vaccinated with formula-2) showed their peak of NI (3.6) on the third MPV while non-Boostered calves showed their peak NI (3.2) by the fourth MPV. The third calves ‘group had a peak of NI (3.88) in Boostered calves by the fourth (MPV) and 3.6 by the fifth MPV in non-Boostered calve while the non-vaccinated group showed non-protective. The recorded RVF neutralizing antibodies remained within the protective levels up to 9 and 10 MPV in Boostered calves in group-1 and other groups respectively as tabulated in Table 3. SNT achieved on serum samples gained from various vaccinated calves ‘groups showed that BEF neutralizing antibody levels in vaccinated calves showed that their peak of NI on the third MPV (2.5 and 1.7) in Boostered and non-Boostered calves respectively in group-1(vaccinated with formula-1). Boostered calves in group-2 (vaccinated with formula-2) showed their peak of NI (3.1) on the fourth MPV while non-Boostered calves showed their peak NI (2.5) by the fifth MPV. The third calves’ group had a peak of NI (3.1 and 2.6) in Boostered and non-Boostered calves respectively by the fifth MPV as shown in Table 4. Also, this table indicates that BEF neutralizing antibodies still remained within their protective levels up to the 9th and 10th MPV in Boostered calves in group-1 and other vaccinated groups respectively. Table 2. Quality control measures of Freeze-dried combined RVF and BEF vaccine.
Table 3. Mean RVF antibody neutralizing indices in calves vaccinated with the prepared formulae of the freeze dried combined RVF and BEF vaccine.
Table 4. Mean BEF antibody neutralizing indices in calves vaccinated with different formula of the prepared freeze-dried combined RVF and BEF vaccine.
DiscussionVaccines fundamentally improve the immune system’s capability to react rapidly against microorganisms after a second defiance and have been qualified as ‘weapons of mass protection’ (Cohen and Marshall, 2001; Curtiss, 2002). The target of vaccination is to invigorate a potent, protective and prolonged immune response to the administered antigen. To perform these goals, efficient adjuvant and vaccine strategies are needed to make the vaccine to an adequate degree immunogenic, for instigating a strong immune response (Fearon, 1997; Bomford, 1998). This study includes elaboration and evaluation of three different formulae of a freeze – dried combined vaccine against RVF and BEF. Concerning RVF and BEF virus titrations through the infectivity method in BHK cell line; Table 1 showed that the titer of RVF virus was 108 TCID50/ml with CPE represented by cell rounded and aggregated in clusters (Billecocq et al., 1996), while the titer of BEF was 108 TCID50/ml with CPE as degeneration and detachment from the culture surfaces (Azab et al., 2000). Determination of virus inactivation fulfillment in mice and tissue culture (Table 2), confirmed satisfactory results for 14 days post-inoculation with no clinical abnormalities or deaths in agreement with the recommendation of Code of Federal Regulations (2005). The intended lyophilized combined vaccine was confirmed to be sterile and free of mycoplasma, aerobic, anaerobic bacteria, and fungal contamination and safe for inoculated calves showing no elevation in body temperature which stayed within the physical levels for successive14 days post-vaccination without clinical abnormalities or deaths (Table 3) agreed with Wassel et al. (1996) and Code of Federal Regulations (2005) who stated that the eventual product should be free of foreign contaminants and safe in animals. The serum neutralization test as shown in Table 3, revealed that the RVF serum neutralizing antibody titers reached their protective NI (1.5) by the second WPVin group 2 and 3 and after 1 month in the first group showing an increasing till reaching its peak by the second MPV (3.2) when received the second doses, while in the non-Boostered group the level of antibodies reached its peak at the third MPV then decline to non-protective level after the ninth MPV (1.2). Booster animals in groups (2)and (3), exhibited the peak of their antibody titer by the third to fourth MPV and stayed within the covering grade till the end time of the experiment in agreement with Atwa et al. (2020) with little difference between the two groups, while the non-booster animals in group 2 and 3 had levels of RVF antibodies less than those in booster animals but higher than those in the sera of the vaccinated calves in the first group which although received two doses, and stayed also within the protective level till the ending of the experiment. These results agree with Atwa et al. (2020) who reported that the immune response in sheep vaccinated with lyophilized RVF vaccine using lactalbumin and sucrose as stabilizers and diluted with saponin, stayed within the protective level till the end of ninth MPV with two doses and they also mentioned that these results related to the using of saponin as adjuvant. Also, all the previous results come coincidence with Oda et al. (2000), who reported that Saponin based adjuvants can modify the cell mediated immune response as well as enhancement of antibody fabrication and have the merit that only a minimal dose is needed for adjuvant activity. BEF serum neutralizing antibody NI showed detectable levels (1.2–1.3) as mentioned by Kensil (1996) started from the second WPVin group 2 and 3 and reached the protective level after1 month in the first group which showed an increasing in their level till reach the peak (2.5) 3 MPV receiving 2 doses. The non-booster group exhibited the peak of BEF-NI at the second MPV then declined by the sixth MPV (1.0). In groups 2 and 3, the peak of antibody NI was recorded by the fourth to the fifth MPV and still protective till the end time of the experiment (10 MPV) with a little difference between the two groups receiving two doses of the vaccine with 1 month interval. The non-booster animals in group 2 and 3 had level of antibodies less than that in booster animals but still higher than that in the sera of calves in the first group which although received 2 doses. These findings agree with Gartlan et al. (2016) who mentioned that Carbopol (carbomer) enhances and activates cellular and humoral immunity in mammals and the same conclusions were reported by Mair et al. (2015) and Aly et al. (2020). In addition, Albehwar et al. (2010) stated that the use of a live BEF vaccine which was inactivated at the time of administration induced high levels of specific BEF neutralizing antibodies where the diluent of such vaccine contains saponin which acts as a virus in activator and immune stimulant. ConclusionThe present obtained results showed the possibility of elaboration of the combined RVF and BEF lyophilized vaccine with Carbopol for eliciting a good level of protective immunity against the two diseases in cattle with accepted duration of immunity. AcknowledgementsThe authors would like to thank the staff members of the departments of RVF and Pet Animal Vaccine Research, VSRI , Abasia- Cairo and for their support. Authors contributionsNaglaa Ebrahim Aly: Research idea; Preparation of lyophilized vaccine; Collection of blood and serum samples in order to track the level of blood immunity in calves after vaccination; Participating in writing the research. Mohamed Hassan Atwa: Conducting a test for the efficiency and effectiveness of the prepared vaccine; Participating in writing and publishing the research. Amany Mohamed Abbas: Participating in preparation of lyophilized vaccine; Participating in publishing the research. Diana Mohamed Abulmagd: Conducting a test for the efficiency and effectiveness of the prepared vaccine; Following up the induced immunoglobulins throughout the experiment; Evaluation and analysis the results; Writing the research; Participating publishing the research. Zeinab Taha Salem: Participating in preparation of lyophilized vaccine; Evaluation and analysis the results; Participating in publishing the research. Taradi Abdelfattah Sayed: Evaluation and analysis the results; Participating in writing the research. Conflict of interestThe authors declare that they have no competing interests. FundingThe study did not receive any external fund. Availability of dataThe data that support the findings of this study are available from the corresponding author upon reasonable request. ReferencesAbdel Ghaffar, S., Mohsen, A., Ayoub, N., Elnimr, M. and Fathia, M. 1979. Rift valley fever in Egypt III. Diagnosis and vaccination.14th Arab. Vet. Cong. Cairo, Egypt, March 24–29, 1979, pp 1–11. Ahuja, A., Khar, R.K. and Ali, J. 1997. Mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 23, 489–505. Akakpo, A.J. 2015. Three-day fever. Rev. Sci. Tech. OFF Int. Epiz. 34(2), 533–538. Albehwar, A.M., Nermein, G., Shafeik Saad, M.A., Ibrahim, M.M., Magda, S., Mohamed, A.A. and Khodeir, M.H. 2010. Comparative evaluation of the potency of traditionally inactivated bovine ephemeral fever vaccine and that one inactivated on the time of vaccination. 14th Sci. Cong., Fac. Vet. Med. Assiut University, Asyut, Egypt, 2010, pp 51–61. Amoros, M., Fauconnier, B. and Girre, R.L. 1987. In vitro antiviral activity of a saponin from Anagallisarvensis, Primulaceae, against herpes simplex virus and poliovirus. Antiviral Res. 8(1), 13–25. Atwa, M.H., Maha, R., Abdelfadeel, H., Khafagy, A., Al-Kholy, A., Kotb, E.Z. and Mahmoud, M.F. 2020. Efficacy of freeze-dried inactivated Rift Valley fever vaccine. J. Appl. Vet. Sci. 5(2), 33–39. Azab, A.M., Khodeir, M.H., Attyat, M. and El- Gallad, S.B. 2002. Susceptibility of different cell culture to bovine ephemeral virus. 6th Vet. Med. Conf. Hurghada, Egypt, 2002, pp 41–55. Bahnemann, H.G. 1990. Inactivation of viral antigens for vaccine elaboration with particular reference to the application of binary ethyleneimine. Vaccine 8, 299–303. Billecocq, A., Vialat, P. and Bouloy, M. 1996. Persistent infection of mammalian cells by Rift Valley fever virus. J. Gen. Virol. 77(Pt 12), 3053–3062. Bomford, R. 1998. Will adjuvants be needed for vaccines of the future? Develop. Biol. Standard. 92, 13–17. Code of federal regulation: animal and animal products 9. 2005. Published by the office of federal register national archives and administration. Vet. Microbial. 16(2), 101–107. Cohen, J. and Marshall, E. 2001. Bioterrorism. Vaccines for biodefense: a system in distress. Science 294(5542), 498–501. Curtiss, R. 2002. Bacterial infectious disease control by vaccine development. J. Clin. Invest. 110(8), 1061–1066. El-Bagoury, G.F., El-Habbaa, A.S., Ibrahim, A.M. and Yousef, N.E.E. 2013. Evaluation of inactivated Rift Valley fever vaccine with paraffin oil adjuvant. Benha Vet. Med. J. 25(1), 157–164. Elgendy, I.A.M., aboezz, Z.R.A., Khodair, M., Kotb, A.M. and Sharawi, S.S.A. 2021. Comparative evaluation of three formulae of bovine ephemeral fever virus vaccines. Benha Vet. Med. J. 41, 34–38. El-Nimr, M.M. Studies on the inactivated vaccine against Rift Valley fever. Ph. D. Thesis (Microbiology). Fac. Vet. Assuit. Univ., Asyut, Egypt, 1980. Eman, M.S. Studies on Rift valley fever vaccine inactivated with binary. Ph.D. Thesis (Microbiology), Fac. of Vet. Med, Cairo Univ., Asyut, Egypt, 1995. Faburay, B., Labeaud, A.D., Mcvey, D.S. and William, C.W. 2017. The involving transmission pattern of Rift valley fever in the Arabian Peninsula. Acad. Sci. 969, 201–204. FAO. 2003. Rift Valley Fever. EMPRES transboundary animal disease. Bulletin, 2003,23. Fearon, D.T. 1997. Seeking wisdom in innate immunity. Nature. 388(6640), 323–324. Gartlan, K.H., Krashias, G., Wegmann, F., Hillson, W.R., Scherer, E.M., Greenberg, P.D., Eisenbarth, S.C., Moghaddam, A.E. and Sattentau, Q.J. 2016. Sterile inflammation induced by Carbopol elicits robust adaptive immune responses in the absence of pathogen-associated molecular patterns. Vaccine 34(19), 2188–2196. Hubbard, K.A., Baskerville, A. and Stephenson, J.R. 1991. Ability of a mutagenized virus variant to protect young lambs from Rift Valley fever. Am. J. Vet. Res. 52(1), 50–55. Islam, M.T., Rodríguez-Hornedo, N., Ciotti, S. and Ackermann, C. 2004. Rheological characterization of topical carbomer gels neutralized to different pH. Pharm. Res. 21(7), 1192–1199. Jie, Y.H., Cammisuli, S. and Baggliolini, M. 1984. Immunomodulatory effects of Panax ginseng CA Meyer in the mouse. Agents Actions. 15(3-4), 386–391. Kensil, C.R. 1996. Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 13(1-2), 1–55. Macpherson, J.A. and Stocker, N.G. 1962. Polyoma Transformation of hamster cell clones an investigation of hamster cell clones of genetic factors affecting all competence. Virology 16, 147. Mair, K.H., Koinig, H., Gerner, W., Hohne, A., Bretthauer, J. and Kroll, J.J. 2015. Carbopol improves the early cellular immune responses induced by the modified-life vaccine. Ingelvac PRRS((R)) MLV. Vet. Microbiol. 176, 352–357. Mark, W.M. 2004. Chemically inactivated EHV-1 kya virus; and an adjuvant which includes cross link unsaturated carboxylic acid polymer. Patents, publication number US 6803041 B2. Aly, N.I., El-Shamandy, O.A., Shendy, M.B., Warda, F.F. and Farouk, E.M. 2020. Efficacy of using carbopol as an adjuvant for tissue culture inactivated rabies vaccine. J. Appl. Vet. Sci. 5(3), 103–107. Nandi, S. and Negi, B.S. 1999. Bovine ephemeral fever: a review. Comp. Immunol. Microbiol. Infect. Dis. 22(2), 81–91. Nguku, P.M.1., Sharif, S.K., Mutonga, D., Amwayi, S., Omolo, J., Mohammed, O., Farnon, E.C., Gould, L.H., Lederman, E., Rao, C., Sang, R., Schnabel, D., Feikin, D.R., Hightower, A., Njenga, M.K. and Breiman, R.F. 2006. An investigation of a major outbreak of Rift Valley fever in Kenya. Am. J. Trop. Med. Hyg. 83(2), 5–13. Oda, K., Matsuda, H., Murakami, T., Katayama, S., Ohgitani, T. and Yoshikawa, M. 2000. Adjuvant and hemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 381(1), 67–74. OIE. 2012. RVF Chapter 2.1.15, pp: 334–343. OIE. 2015. Rift Valley Fever. Chapter 2.5.9, pp: 1–12. OIE Terrestrial Manual. 2016. Infection with Rift Valley Fever Virus, Chapter 2.1.18. Reed, L.J. and Muench, H. 1983. A simple method for estimating 50 percent endpoint. Am. J. Hyg. 27, 493. Riyesh, T., Balamurugan, V., Sen, A., Bhanuprakash, V., Venkatesan, G., Yadav, V. and Singh, R.K. 2011. Evaluation of efficacy of stabilizers on the thermostability of live attenuated thermos-adapted Pest des petits ruminants’ vaccines. Virol. Sin. 26(5), 324–337. Rossiter, P.B., Jesset, D.M. and Taylor, W.P. 1985. Microneutralization system for use with different strains of pest des petit ruminant’s virus and rinderpest virus. Trop. Anim. Health Prod. 17(2), 75–81. Soliman, S.M., Taha, M.M., Samir, S.S. and Daoud, A.M. 2001. Isolation and identification of BEF virus in Egypt. Beni-Suef Vet. J. 11(2), 24–32. St. George, T.D., Cybinski, D.H., Murphy, G.M. and Dimmock, C.K. 1984. Serological and biochemical factors in bovine ephemeral fever. Aust. J. Biol. Sci. 37, 341–349. United States Pharmacopeia. 2022. NF Monographs, carbomer 934p. Rockville, MD: USP-NF, pp: 1900–1911. Uren, M.F., St. George,T T.D. and Murphy, G.M. 1992. Studies on the pathogenesis of bovine ephemeral fever in experimental cattle. III. Virological and biochemical data. Vet. Microbiol. 30, 297–307. Walker, P.J. 2005. Bovine ephemeral fever in Australia and the world. Curr. Top. Microbiol. Immunol. 292, 57–80. Wassel, M.S., Aboulsaoud, S., Girgis, S., Hussein, A.Z. and Eman, El Rawy. 1996. Trials for elaboration and evaluation of a combined vaccine for IBR (IBR-V), (BVD), (PI-3V), P. Multocida and P. Haemolytica in Vet. Med. J. Assiut, Egypt, 1996, 35, 70. WHO. 2020. Rift valley fever. Mauritania. (Accessed 12 October 2020). Zhang, Y., Ng, W., Hu, J., Mussa, S.S., Ge, Y. and Xu, H. 2018. Formulation and in vitro stability evaluation of ethosomal carbomer hydrogel for transdermal vaccine delivery. Colloids Surf B. Biointerfaces. 163, 184–191. | ||
| How to Cite this Article |
| Pubmed Style Aly NE, Atwa MH, Abbas AM, Abulmagd DM, Salem ZT, Sayed TA. Immunogenicity of a freeze-dried combined vaccine against Rift Valley fever and bovine ephemeral fever in cattle. Open Vet. J.. 2023; 13(7): 826-833. doi:10.5455/OVJ.2023.v13.i7.3 Web Style Aly NE, Atwa MH, Abbas AM, Abulmagd DM, Salem ZT, Sayed TA. Immunogenicity of a freeze-dried combined vaccine against Rift Valley fever and bovine ephemeral fever in cattle. https://www.openveterinaryjournal.com/?mno=138162 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i7.3 AMA (American Medical Association) Style Aly NE, Atwa MH, Abbas AM, Abulmagd DM, Salem ZT, Sayed TA. Immunogenicity of a freeze-dried combined vaccine against Rift Valley fever and bovine ephemeral fever in cattle. Open Vet. J.. 2023; 13(7): 826-833. doi:10.5455/OVJ.2023.v13.i7.3 Vancouver/ICMJE Style Aly NE, Atwa MH, Abbas AM, Abulmagd DM, Salem ZT, Sayed TA. Immunogenicity of a freeze-dried combined vaccine against Rift Valley fever and bovine ephemeral fever in cattle. Open Vet. J.. (2023), [cited January 25, 2026]; 13(7): 826-833. doi:10.5455/OVJ.2023.v13.i7.3 Harvard Style Aly, N. E., Atwa, . M. H., Abbas, . A. M., Abulmagd, . D. M., Salem, . Z. T. & Sayed, . T. A. (2023) Immunogenicity of a freeze-dried combined vaccine against Rift Valley fever and bovine ephemeral fever in cattle. Open Vet. J., 13 (7), 826-833. doi:10.5455/OVJ.2023.v13.i7.3 Turabian Style Aly, Naglaa Ebrahim, Mohamed Hassan Atwa, Amany Mohamed Abbas, Diana Mohamed Abulmagd, Zeinab Taha Salem, and Taradi Abdelfattah Sayed. 2023. Immunogenicity of a freeze-dried combined vaccine against Rift Valley fever and bovine ephemeral fever in cattle. Open Veterinary Journal, 13 (7), 826-833. doi:10.5455/OVJ.2023.v13.i7.3 Chicago Style Aly, Naglaa Ebrahim, Mohamed Hassan Atwa, Amany Mohamed Abbas, Diana Mohamed Abulmagd, Zeinab Taha Salem, and Taradi Abdelfattah Sayed. "Immunogenicity of a freeze-dried combined vaccine against Rift Valley fever and bovine ephemeral fever in cattle." Open Veterinary Journal 13 (2023), 826-833. doi:10.5455/OVJ.2023.v13.i7.3 MLA (The Modern Language Association) Style Aly, Naglaa Ebrahim, Mohamed Hassan Atwa, Amany Mohamed Abbas, Diana Mohamed Abulmagd, Zeinab Taha Salem, and Taradi Abdelfattah Sayed. "Immunogenicity of a freeze-dried combined vaccine against Rift Valley fever and bovine ephemeral fever in cattle." Open Veterinary Journal 13.7 (2023), 826-833. Print. doi:10.5455/OVJ.2023.v13.i7.3 APA (American Psychological Association) Style Aly, N. E., Atwa, . M. H., Abbas, . A. M., Abulmagd, . D. M., Salem, . Z. T. & Sayed, . T. A. (2023) Immunogenicity of a freeze-dried combined vaccine against Rift Valley fever and bovine ephemeral fever in cattle. Open Veterinary Journal, 13 (7), 826-833. doi:10.5455/OVJ.2023.v13.i7.3 |