| Research Article | ||
Open Vet. J.. 2023; 13(7): 854-863 Open Veterinary Journal, (2023), Vol. 13(7): 854-863 Original Research Effect of clove buds powder supplementation on hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity of broilersKhaled M. Ben Naser1, Bashir M. Sherif1, Siham M. Othman1* and Abdulatif A. Asheg21Department of Animal Production, Faculty of Agriculture, University of Tripoli, Tripoli, Libya 2Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya *Corresponding Author: Siham M. Othman. Department of Animal Production, Faculty of Agriculture, University of Tripoli, Tripoli, Libya. Email: s.otman [at] uot.edu.ly Submitted: 16/01/2023 Accepted: 14/06/2023 Published: 31/07/2023 © 2023 Open Veterinary Journal
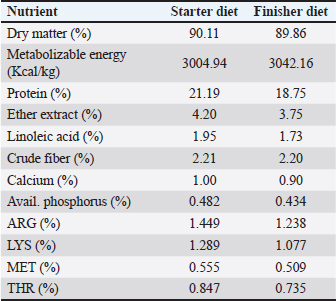
AbstractBackground: Phytogenic feed additives are products derived from plants used to improve the performance and health of animals. Nowadays, this type of phytogenic feed additive is widely used as an alternative to antibiotic growth promoters in poultry feed, and clove bud is one of the most effective medicinal herbs that has caught the attention of researchers. Aim: This experiment was conducted to evaluate the effects of adding clove bud powder to the broiler chicken’s feed on the hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity. Method: A total of 360 unsexed chicks (Ross 308) were allotted to three groups. All chicks were raised under the same normal management conditions from 1 day to 6 weeks of age. The first group was fed a basal diet and the other two groups were assigned to add 0.5% and 1.0% of clove bud powder to the basal diet. The studied traits were total leukocyte counts (TLC), differential leukocyte counts (DLC), blood cholesterol level, blood liver enzymes [aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase (ALP) enzymes], the weight of lymphoid organs (bursa of Fabricius and spleen), and cell-mediated immunity. Results: Despite the significant and varied changes (p ≤ 0.05) that resulted from adding clove buds powder to the broiler feed in the second and third weeks, the results at the end of the experiment indicated that there was no negative effect of adding clove powder on the TLC and DLC, as well as the heterophils/lymphocytes ratio. In addition, feeding on a diet containing clove buds powder had no significant effect on the level of cholesterol and liver enzymes in the blood, except for (ALP), which showed a significant increase (p ≤ 0.01) in comparison to the control group. Moreover, the results showed in the second and fifth weeks a significant increase (p ≤ 0.05) in the relative weight of the spleen, but, in general, there were no significant effects at the end of the experiment on the relative weight of the lymphoid organs. Furthermore, the broiler chickens that consumed clove bud powder at a rate of 0.5% showed a highly significant (p ≤ 0.01) cellular immune response. Conclusion: This study concluded that the addition of clove bud powder had no negative effect on leukocyte counts or differentiated leukocyte counts. The addition also raised the spleen weight and improved the level of blood alkane phosphatase activity and cellular immune response in broiler chickens during the growth stages. Keywords: Broiler, Clove, Hematological profile, Biochemical parameters, Immunity. IntroductionAntibiotics have been used in animal production as non-nutritive additives because of their positive effect on nutritional efficiency and to control infectious diseases. The use of antibiotics in the poultry industry as growth promoters has been banned in many countries around the world due to increasing levels of microbial resistance to drugs (Castanon, 2007). Currently, research is directed toward natural and healthy alternatives to improve the productivity and health of poultry. Ever since the day medicinal plants are considered a natural and safe alternative to improve the performance and health of broilers (Amad et al., 2013), they have been attracting a great deal of research in the field of developing poultry feed. Cloves (Syzygium aromaticum) have several therapeutic properties and contain many biologically active compounds such as eugenol, eugenyl acetate, and beta-caryophyllene (Alma et al., 2007). The major component in clove is eugenol (Barceloux, 2008). Clove also contains hydrolyzable tannins (Dibazar et al., 2014), which have antimicrobial activity (Leite et al., 2007; Shrivastava et al., 2014), antiviral (Hussein et al., 2000), antifungal (Campaniello et al., 2010), anti-inflammatory, and antioxidant properties (Wati et al., 2015). Cloves have also been used as a natural growth enhancer (Sjafani et al., 2022), and this may be due to the significant improvement of the morphological condition of the intestine and increased intestinal absorption surface (Agostini et al., 2012; Othman et al., 2022). Also, dietary supplementation with clove stimulates the immune response (Gandomani et al., 2014). This experiment aimed to investigate the effect of adding different levels of clove bud powder to broiler chicken feed on the hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity. Materials and MethodsThe experiment was conducted at the Poultry Research Station, Faculty of Agriculture, University of Tripoli, Libya. A total of 360 1-day-old- unsexed chicks (ROSS 308) were distributed to three treatment groups. Every group was replicated 6 times with 20 chicks/replicate, one chick was randomly taken from each replicate at the end of each week of the experiment to measure traits. The treatments included the control group that fed on the basal diet (Table 1) and the second group that fed on (the basal diet + 0.5% clove bud powder) and the third group that fed on (the basal diet + 1.0% clove bud powder). A starter feed was provided from 1 to 21 days and a finisher feed from 22 to 42 days of the experiment (Table 1), and water was available ad libitum. The chicks were raised under normal management conditions on floor pens with a wood-shaving floor (1.5 × 1.3 m), and the temperature was gradually decreased from 35°C to 21°C till the end of the experiment (42 days). All chicks were vaccinated according to the vaccination program implemented by National Center for Animal Health, Libya. Table 1. Nutrient content of basal diet over different periods of production.
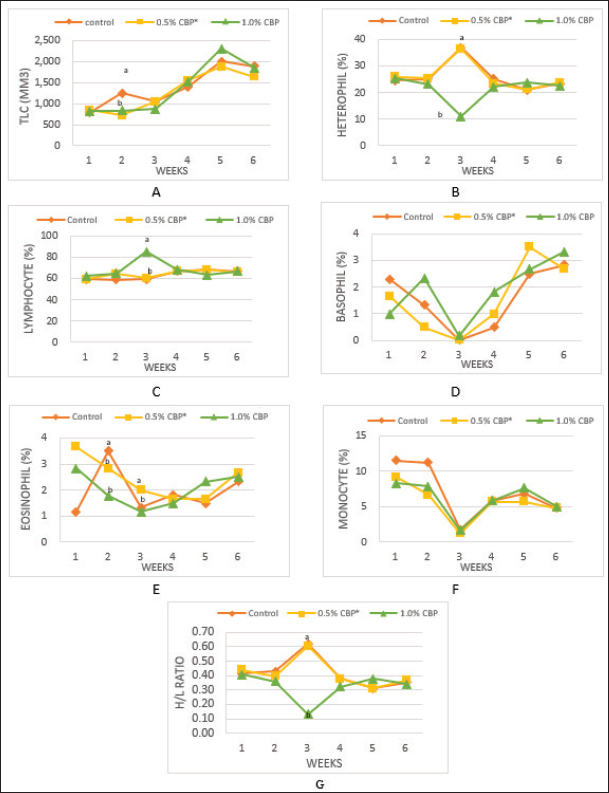
The experiment was designed according to a completely randomized design. Analysis of variance was implemented by using Statistical Analysis System (SAS, 2002), and Duncan’s multiple range tests were used to determine significant differences between treatment means (Duncan, 1955). The studied traits included blood constituents, biochemical parameters, the determination of immune organ masses, and cell-mediated immunity. The blood constituent traits were total leukocyte counts (TLC), differential leukocyte counts (DLC), and heterophil/lymphocyte ratio (H/L). The biochemical parameters were serum cholesterol level (mg/dl) (Allain et al., 1974) and Liver enzymes in the blood: aspartate aminotransferase (AST), alanine aminotransferase (ALT) (Schumann et al., 2002), and alkaline phosphatase (ALP) (IU/l) (Wenger et al., 1984). The determination of immune organ masses was the spleen/body weight ratio and the bursa of Fabricius/body weight ratio (Heckert et al., 2002). The effect of clove in chicken broiler feed on cell-mediated immunity was assessed by the hypersensitivity response of the chicken’s skin to dinitrochlorobenzene (DNCB) as per the method described by Tiwary and Goel (1985). On day 28, six chickens were randomly selected from each group, marked by different colors and sensitized by applying 250 μl of 1% DNCB dissolved in acetone on the side of the right thigh. On day 42 (after 14 days), the same chickens were challenged by topical application of 0.5% DNCB dissolved in a 4:1 mixture of acetone and olive oil on the same sensitized site (sike of the right thigh) and calculated as per the method of Chowdhury et al. (2005). Ethical approvalThis study was approved by the Graduate School of the University of Tripoli, Faculty of Agriculture, Department of Animal Production. All animal welfare protocols were followed. ResultsThe results in Figure 1A show that adding cloves at a rate of 0.5% and 1% to the broiler diet had no significant effect on the TLC compared with the control group at the end of the experiment (6 weeks of age). Nevertheless, the statistical analysis of the second week of the experiment showed a significant decrease (p ≤ 0.05) in the TLC (716.7/mm3 and 833.3/mm3) in the two groups that fed on a diet supplemented with clove powder at a rate of 0.5% and 1%, respectively, compared to the control (1,250/mm3). The results of the statistical analysis of DLC (heterophil, eosinophil, basophil, lymphocyte, and monocyte) in Figure 1B–F show that there was no significant effect of adding clove powder to the broiler diet in the end of the experiment among the experimental groups. While the weekly statistical analysis of the experimental data showed the presence of some significant changes, the results showed a highly negative effect (p ≤ 0.01) on the percentage of heterophil in the third week in the broilers of the third group, which was (11%) compared to the control group (37%). Similarly, there was a significant decrease (p ≤ 0.05) in the second week in the percentage of eosinophils in the second and third groups (2.83% and 1.83%, respectively) compared with the control group (3.50%). On the contrary, the addition of clove powder to the diet of broilers had a highly positive significant (p ≤ 0.001) effect in the second and third weeks on the percentage of lymphocytes, and the increase was the highest in the third group (85.17%) compared with the broilers of the control group (59.83%) in the third week of the experiment. In addition to the above, the results in Figure 1G indicated that supplementation with clove powder caused a significant increase in the H/L-ratio in the third week, while there was no significant effect in the sixth week of the experiment.
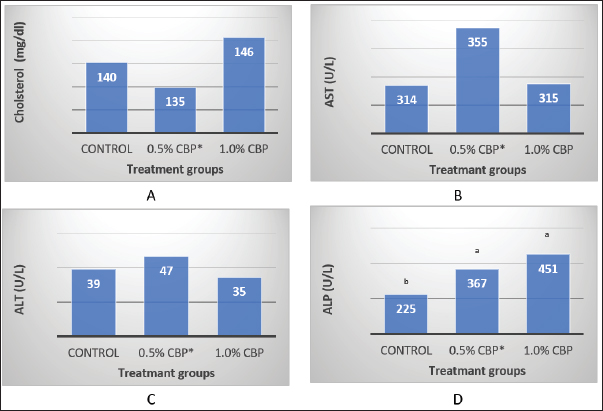
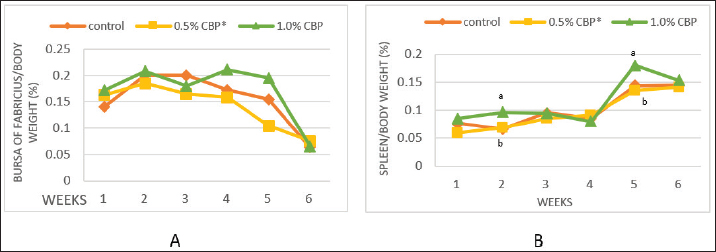
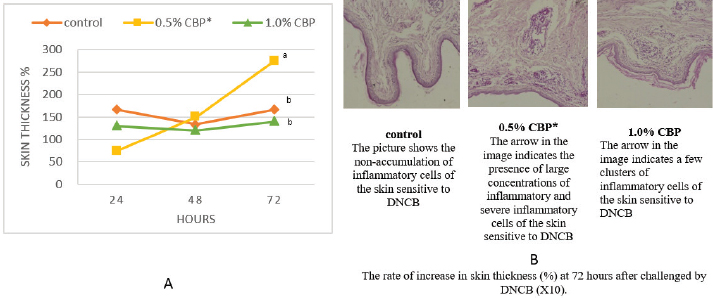
Fig. 1. Effect of adding clove buds powder to broiler feed in different levels on leukocyte counts and DLC. (*): Clove Buds Powder. Different letters within a treatment indicate a significant difference between the means at the level of probability differences between the means at the level of probability (p < 0.05). The effect of adding clove powder to broiler feed on the level of cholesterol and liver enzymes in the blood serum was shown in Figure 2A–D. It is clear from the statistical analysis that adding cloves at a rate of 0.5% and 1% did not have a significant effect on the level of cholesterol in the blood compared with the control group. Similarly, the addition of clove powder had no significant effects on the enzymes ALT and AST in the blood serum compared with the control group. On the other hand, the results indicated a highly significant increase (p ≤ 0.01) in ALP enzyme with an average of (366.85 and 450.63 U/l) when fed with 0.5% and 1%, respectively, clove powder compared to the control group, whose average enzyme level was (224.58 U/l). Figure 3 show the effect of the inclusion of clove powder in broiler feed on the weight of lymphoid organs. Through the experimental data analysis, Figure 3A showed that adding clove powder had no significant effect on the relative weight of the spleen at the end of the experiment (6 weeks of age). It can also be noticed that the high relative weight of the spleen was observed in the second week (p ≤ 0.001) and the fifth week (p ≤ 0.05) of the experiment (0.096% and 0.180%, respectively) compared to the control group, in which the mean relative weight of the spleen was (0.066%) in the second week and (0.143%) in the fifth week of the experiment. The results of the experiment in Figure 3B also indicated that the inclusion of clove powder in broiler feed had no significant effect on the bursa of Fabricius/body weight ratio either at the end of the experiment or through the weekly analysis of the experimental data. The effect of adding clove powder to broiler feed on the cellular immune response is illustrated in Figure 4A and B. The results showed that the cellular immune response did not change significantly on hours 24, and 48-post challenge with DNCB in the second and third experimental groups compared with the control group. But on hours 72-post challenge with DNCB, a significant response (p ≤ 0.05) was recorded in the broilers of the second group that were fed a diet containing 0.5% clove powder, the percentage of skin thickness was 275% compared with the broilers of the control group 166.67%. On the other hand, there was no significant cellular immune response in the birds in the third group after 72 hours of the injection. The results of the statistical analysis were supported by the microscopic images of the skin tissue sections, which showed a large concentration of inflammatory cells as a result of the injections in the broilers of the second group that were fed 0.5% clove powder.
Fig. 2. Effect of adding clove buds powder to broiler feed in different levels on Serum cholesterol level and Liver enzymes at 42 days of the experiment. (*): Clove Buds Powder. Different letters within a treatment indicate a significant difference between the means at the level of probability differences between the means at the level of probability (p < 0.01).
Fig. 3. Effect of adding clove buds powder to broiler feed in different levels on lymphoid organs weight in broiler chickens. (*): Clove Buds Powder. Different letters within a treatment indicate a significant difference between the means at the level of probability differences between the means at the level of probability (p < 0.05).
Fig. 4. The effect of adding clove buds powder to the broiler’s diet in different levels clove buds powder on skin thickness in broiler chickens on hours 24, 48, and 72-post challenge with DNCB. (*): Clove Buds Powder. Different letters within a treatment indicate a significant difference between the means at the level of probability differences between the means at the level of probability (p < 0.05). DiscussionEugenol is the main component of clove extract (70%–90%) (Barceloux, 2008), and it is found in many other plants such as basil, cinnamon, nutmeg, and bay leaf. Eugenol is a volatile phenolic constituent of clove characterized by many beneficial effects, whether on the animal’s productivity or health performance. Therefore, the measurement of blood parameters is one of the most efficient screening assessments to evaluate their effect on the immune system. The results obtained from our data (Fig. 1A) showed a limited effect of adding clove bud powder on the TCL, as well as the DCL. The non-appearance of adverse effects as a result of adding clove bud powder to TCL is considered an encouraging matter if compared with the improvement of other productive traits or physiological parameters. The same conclusion was observed by Toghyani et al. (2010) who mentioned that feeding broilers thyme supplementation had no adverse effect on their blood profile. Additionally, supplementation of some Libyan medicinal plants in the broiler’s diet had no significant effect on TCL (Asheg et al., 2015). On the contrary, Abdel Fatah et al. (2020) reported a decrease in WBC in broilers fed clove powder (2%) in the diet. It can be noticed that the high concentration of clove may cause undesirable effects. It was mentioned that herbal medicines may have side effects or be ineffective (George, 2011), or be toxic (Nasri and Shirzad, 2013). Some previous studies reported that the inclusion of phytogenic feed additives increased WBC counts (Amad et al., 2013; Wati et al., 2015). Moreover, adding medicinal plants or their oils, such as thyme and cinnamon (Al-Kassie, 2009), thyme oil (Adam et al., 2020), or bay leaf (Mohammed et al., 2020) increased the WBC significantly. It is clear that adding cloves or other medicinal plants or their extracts had a positive effect on WBC counts. The superior effect of medicinal herbs on WBC may be due to the phytochemicals ability to stimulate immune systems and lymphoid organs and reduce metabolic stress in the body, which enhances better utilization of nutrients in the body to build up blood cells. Regarding the differential WBC count findings in Figure 1B and C, our results agreed with what was concluded by Al-Asheq et al. (2015). They indicated that supplementation with aromatic plants in broilers’ diet significantly decreased the heterophils percentage and increased the lymphocytes percentage in the third week. While there was no significant effect at the end of the experiment (the sixth week) on the percentage of heterophils or lymphocytes. In addition, our results indicate that there was no significant change in the H/L ratio at the end of the experiment, which is in agreement with the inference of Toghyani et al. (2010). Furthermore, Najafi and Torki (2010) found that adding cloves did not have a significant effect on the lymphocyte percentage or heterophil percentage. In general, most studies reported that aromatic plants had a beneficial effect on differentiated WBC, and this agreed with the results of the third week of our study as shown in Figure 1G. Along the same lines, Gandomani et al. (2014) pointed out that the dietary inclusion of clove bud powder decreased the heterophil percentage and increased the lymphocyte percentage, which in turn reduced the H/L ratio. Additionally, supplementation with phytogenic feed additives (fennel, melissa balm, peppermint, anise, oak, clove, and thyme) significantly contributed to increasing the lymphocyte percentage and decreasing the heterophil percentage and the H/L ratio (Wati et al., 2015). The decrease in Heterophil percentage and increase in lymphocytes percentage is attributed to the antioxidant and anti-inflammatory effects of clove buds (Wati et al., 2015). Likewise, Gandomani et al. (2014) stated that clove bud had a strong antioxidant effect, which reduced oxidative stress in immune cells and increased the number of lymphocytes. The H/L ratio is a good signal of the health status of birds, as a higher ratio indicates that the birds are under severe stress (Al-Darraji, 1995). Therefore, aromatic herbs as antioxidants play an important biological role in improving immunity by lowering the H/L ratio in the blood of birds (Ojala et al., 2000), and this was approved by Naderi et al. (2014) when feeding broilers on cinnamon and turmeric , as well as when feeding on laurel leaves (Mohammed et al., 2020). The result of our study related to the serum total cholesterol in Figure 2A was in agreement with some other studies. Broiler chicks fed on a basal diet containing clove essential oils did not show a significant change in plasma cholesterol (Najafi and Torki, 2010; Azadegan et al., 2014). Other studies conducted on dietary supplementation with anise seed (Soltan et al., 2008), thyme powder (Toghyani et al., 2010), or basil essential oil (Riyazi et al., 2015) indicated that there is no significant effect on plasma cholesterol. In addition to the above, feed additives may have hypolipidemic activity. Venkadeswaran et al. (2014) found that eugenol inclusion reduced total cholesterol, LDL-cholesterol, and triglyceride levels. Moreover, dietary clove powder in broilers (Mahrous et al., 2017) and clove oil in laying hen diets (Şehitoğlu and Kaya, 2021) significantly lowered serum total cholesterol. Besides, supplementation with cinnamon, thyme, and Alchornea cordifolia leaf meal significantly decreased total cholesterol concentration in serum (Najafi and Taherpour, 2014; Oloruntola et al., 2016). Serum AST and ALT concentrations are valuable parameters to evaluate liver status. Studies conducted by Abdel Fatah et al. (2020) and Şehitoğlu and Kaya (2021) agreed with our results in Figure 2B and C whereas they noted that supplemental clove in broiler diets did not affect the levels of AST and ALT in the blood serum. In the same line, findings by Soltan et al. (2008); Gowda et al. (2009); and Mustafa (2016) indicated that the addition of other phytogenic feed additives had no significant effect on the level of serum liver enzymes. Moreover, some studies showed the hepatoprotective action of medicinal and aromatic plants. Soltan et al. (2008) indicated that the addition of anise seeds to broiler feed reduced ALT levels in the blood. Also, Oladokun et al. (2021) stated that chicks that received clove essential oil recorded the highest significant reduction in plasma AST level. In general, increasing AST and ALT are associated with liver damage (hepatocellular degeneration). Thus, the reduction of AST and/or ALT may have been considered evidence of the hepatoprotective activity of phytogenic feed additives (Langhout, 2000). In addition, Gowda et al. (2009) showed that active compounds in aromatic plants possessing antioxidant properties have a role in protecting liver cells from oxidative damage. From the previous results, it could be concluded that clove and other aromatic plants are safe for chickens and do not cause any adverse effects on the liver. In opposition, Al-Mufarrej et al. (2019) listed that clove powder supplementation at rates of 5% and 6% significantly increased AST, which means that high levels of clove may adversely affect liver function. Our study recorded a significant increase in serum ALP concentration compared to the control group (Fig. 2D). Consistent with our results, Wati et al. (2015) reported that serum ALP was higher in the broiler group fed with plant feed additives and showed that a higher rate of ALP might be an indicator of osteoclast activity. Additionally, Şehitoğlu and Kaya (2021) revealed that supplemental oil clove in laying hens’ diets significantly increased serum ALP. Otherwise, the Inclusion of clove powder (Al-Mufarrej et al., 2019), anise seed (Soltan et al., 2008), clove essential oil (Oladokun et al., 2021), and the mixture of essential oils (Mustafa, 2016) did not affect the plasma ALP concentration. On the contrary, clove oil supplementation caused a reduction in liver AST, ALT, and ALP (Asimi and Sahu, 2016; Gashlan and Al-Beladi, 2017). These variations in the literature’s results may be due to the different levels, sources, and types of phytogenic feed additives and different animal species that were used in each study. Our findings linked to the lymphoid organs (spleen and bursa of Fabricius) in Figure 3A and B correspond to many investigations conducted on clove and other aromatic plants. Some scientists found that the relative weight of the spleen, bursa of Fabricius, and thymus were not influenced by clove supplementation (Chowdhury et al., 2005; Najafi and Torki, 2010; Al-Mufarrej et al., 2019). Besides, Sadeghi et al. (2012) found that cinnamon, thyme, and turmeric supplementation had no significant effect on the relative weights of the spleen and bursa. Differently, Gandomani et al. (2014) exhibited that the addition of clove bud powder to layer diets caused an increase in the relative weight of the spleen. This inconsistency may be attributed to the differences in the sources of the clove, the levels of clove, diet composition or environmental conditions. Also, Gandomani et al. (2014) suggested that the finding might be associated with the likely positive interaction effects of bioactive compounds of clove bud and fatty acids on cell proliferation in such peripheral lymphoid organs as the spleen. Eugenol was assured as an efficient antimicrobial compound, and earlier studies confirmed the bioactive effects of eugenol as antibacterial (Leite et al., 2007), antifungal (Campaniello et al., 2010), and antiviral (Hussein et al., 2000). Moreover, the anti-inflammatory activity of eugenol has been proven (Magalhaes et al., 2010). Therefore, all these beneficial activities can enhance the immune system, which supports our results that clove buds (that are rich in eugenol) can improve the cellular immune response. In the same vein, Wang et al. (1998) mentioned that as a result of the antibacterial action of eugenol, clove essential oils improved immunity. Additionally, herbal plants and their essential oils may have antioxidant activity (Gumus et al., 2017; Elwan et al., 2019) and have a positive effect on immunity (Ghanima et al., 2020). Our previous result in Figure 1C showed an increase in lymphocytes in the second and third weeks, which is an indicator of improvement in the cellular immune system. This improvement could result in a reduction of oxidative stress in immune cells due to the antioxidant activity of the clove plant. Likewise, the antioxidant properties of medicinal plants might be responsible for increased antibody production (Najafi and Torki, 2010). In line with our findings in Figure 4A and B, Farhath et al. (2013) determined that the essential oils (geranial, geranial acetate, gingerol, and eugenol) significantly improved the specific and non-specific immunity and enhanced the proliferation of lymphocytes. Their data showed an increase in foot pat thickness due to the high production of antibodies and phagocytic functions of macrophage cells and non-specific immunity. Correspondingly, an earlier study by Carrasco et al. (2009) showed that clove essential oil augmented leukocyte counts and enriched delayed-type hypersensitivity responses while restoring cellular and humoral immune responses. In addition, Mahrous et al. (2017) stated that clove significantly enhanced the immune response in broiler chickens. Other studies reported different outcomes, Kammon et al. (2010) reported that there was no significant effect on the thickness of the skin’s sensitivity to DNCB in chickens given chlorpyrifos. Mehr et al. (2014) stated that clove powder did not affect cutaneous basophil hypersensitivity. Halder et al. (2011) showed that the administration of clove oil decreased cell-mediated immunity. Dibazar et al. (2014) suggested that clove extracts could suppress T-cell cellular immunity. ConclusionThis study concluded that the addition of clove bud powder had no negative effect on leukocyte counts or differentiated leukocyte counts. The addition of clove bud powder also raised the spleen weight, stimulated the blood ALP activity, and improved the cellular immune response in broiler chickens during the growth stages. AcknowledgmentsThe authors thank Mr. Ahmed O. Bahij, the General Manager of the Golden Poultry Company for his financial support in providing chicks and feed to conduct this experiment. Conflict of interestThe authors declare that there is no conflict of interest. FundingThe experiment was conducted with the personal financial support of the author, Siham M. Othman, and the support of the Golden Poultry Company. Data availabilityAll data supporting the findings of this study are available within the manuscript. Any other data are available from the corresponding author upon reasonable request. Authors contributionsKhaled M. Ben Naser: Conception, design of the study and statistical data analysis. Bashir M. Sherif: Wrote the manuscript and design figures. Siham M. Othman: Corresponding author and funded the research. Abdulatif A. Asheg: Supervision and discussion of blood parameters results. ReferencesAbdel Fatah, G., Hassan, A., Saleh, R. and Amer, M. 2020. The efficacy of clove and thyme against experimentally induced candidiasis in broilers. Mansoura Vet. Med. J. 21(2), 25–31. Adam, A., Shihata, S., El-Hady, A. and Mohamed, A. 2020. Effect of thyme (Thymus Vulgaris) on productive performance, carcass characteristics, blood hematology and lipid profile of broiler chicks. Egypt. Poult. Sci. J. 40(3), 715–727. Agostini, P.S., Solà-Oriol, D., Nofrarías, M., Barroeta, A.C., Gasa, J. and Manzanilla, E.G. 2012. Role of in-feed clove supplementation on growth performance, intestinal microbiology, and morphology in broiler chicken. Livest. Sci. 147(1), 113–118. Al-Darraji, H.J. 1995. ‘Studying some physiological traits and thermal resistance for broilers chickens (Fawbro) and comparing it with some commercial broilers chickens.’ Master Thesis, College of Agriculture, University of Baghdad, Baghdad, Iraq. Al-Kassie, G.A. 2009. Influence of two plant extracts derived from thyme and cinnamon on broiler performance. Pak. Vet. J. 29(4), 169–173. Al-Mufarrej, S., Qaid, M., Fazea, E. and Al-Baadani, H.A.-B. 2019. Effects of clove powder supplementation on performance, blood biochemistry, and immune responses in broiler chickens. S. Afr. J. Anim. Sci. 49(5), 835–844. Allain, C.C., Poon, L.S., Chan, C.S., Richmond, W. and Fu, P.C. 1974. Enzymatic determination of total serum cholesterol. Clin Chem. 20(4), 470–475. Alma, H.M., Ertas, M., Nitz, S. and Kollmannsberger, H. 2007. Research on essential oil content and chemical composition of Turkish clove (Syzygium aromaticum L.). Bio. Resources. 2(2), 265–269. Amad, A.A., Wendler, K. and Zentek, J. 2013. Effects of a phytogenic feed additive on growth performance, selected blood criteria and jejunal morphology in broiler chickens. Emir. J. Food Agric. 90(12), 549–554. Asheg, A., El-Nyhom, S., Eissa, A., Kammon, A., Kanoun, A., Ben Naser, K., and Abouzeed, Y. 2015. Effect of some Libyan medicinal plants on hematological Profile, cholesterol level and immune status of broiler chicken. Res. J. Pharm. Biol. Chem. 6(2), 1164–1170. Asimi, O.A. and Sahu, N.P. 2016. Effect of antioxidant rich spices, clove and cardamom extracts on the metabolic enzyme activity of Labeo rohita. J. Fisheries Livest. Prod. 4(1), 16. Azadegan, M.M., Hassanabadi, A., NASIRI, M.H. and Kermanshahi, H. 2014. Supplementation of clove essential oils and probiotic to the broiler’s diet on performance, carcass traits and blood components. Iran. J. Appl. Anim. Sci. 4(1), 117–122. Barceloux, D.G. 2008. Medical toxicology of natural substances: foods, fungi, medicinal herbs, plants, and venomous animals. Hoboken, NJ: John Wiley & Sons. Campaniello, D., Corbo, M.R. and Sinigaglia, M. 2010. Antifungal activity of eugenol against Penicillium, Aspergillus, and Fusarium species. J. Food Prot. 73(6), 1124–1128. Carrasco, F.R., Schmidt, G., Romero, A.L., Sartoretto, J.L., Caparroz-Assef, S.M., Bersani-Amado, C.A. and Cuman, R.K.N. 2009. Immunomodulatory activity of Zingiber officinale roscoe, Salvia officinalis L. and Syzygium aromaticum L. essential oils: evidence for humor-and cell-mediated responses. J. Pharm. Pharmacol. 61(7), 961–967. Castanon, J. 2007. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 86(11), 2466–2471. Chowdhury, S.R., Smith, T.K., Boermans, H.J. and Woodward, B. 2005. Effects of feed-borne Fusarium mycotoxins on hematology and immunology of turkeys. Poult. Sci. 84(11), 1698–1706. Dibazar, S.P., Fateh, S. and Daneshmandi, S. 2014. Clove (Syzygium aromaticum) ingredients affect lymphocyte subtypes expansion and cytokine profile responses: an in vitro evaluation. J. Food Drug Anal. 22(4), 448–454. Duncan, D.B. 1955. Multiple range and multiple F tests. Biometrics. 11(1), 1–42. Elwan, H.A., Elnesr, S.S., Mohany, M. and Al-Rejaie, S.S. 2019. The effects of dietary tomato powder (Solanum lycopersicum L.) supplementation on the haematological, immunological, serum biochemical and antioxidant parameters of growing rabbits. J. Anim. Physiol. Anim. Nutr. (Berl). 103(2), 534–546. Farhath, S., Vijaya, P. and Vimal, M. 2013. Immunomodulatory activity of geranial, geranial acetate, gingerol, and eugenol essential oils: evidence for humoral and cell-mediated responses. Avicenna J. Phytomed. 3(3), 224–230. Gandomani, V.T., Mahdavi, A., Rahmani, H., Riasi, A. and Jahanian, E. 2014. Effects of different levels of clove bud (Syzygium aromaticum) on performance, intestinal microbial colonization, jejunal morphology, and immunocompetence of laying hens fed different n-6 to n-3 ratios. Livest. Sci. 167, 236–248. Gashlan, H.M. and Al-Beladi, A.B. 2017. Effects of clove oil on liver and antioxidant status of streptozotocin-induced diabetic rats. GARJMMS. 6, 103–110. George, P. 2011. Concerns regarding the safety and toxicity of medicinal plants-An overview. J. Appl. Pharm. Sci. 1(6), 40–44. Ghanima, M.M.A., Alagawany, M., Abd El-Hack, M.E., Taha, A., Elnesr, S.S., Ajarem, J., Allam, A.A. and Mahmoud, A.M. 2020. Consequences of various housing systems and dietary supplementation of thymol, carvacrol, and euganol on performance, egg quality, blood chemistry, and antioxidant parameters. Poult. Sci. 99(9), 4384–4397. Gowda, N.K., Ledoux, D.R., Rottinghaus, G.E., Bermudez, A.J. and Chen, Y.C. 2009. Antioxidant efficacy of curcuminoids from turmeric (Curcuma longa L.) powder in broiler chickens fed diets containing aflatoxin B1. Br. J. Nutr. 102(11), 1629–1634. Gumus, R., Ercan, N. and Imik, H. 2017. The effect of thyme essential oil (Thymus vulgaris) added to quail diets on performance, some blood parameters, and the antioxidative metabolism of the serum and liver tissues. Rev. Bras. Cienc. Avic. 19, 297–304. Halder, S., Mehta, A.K., Mediratta, P.K. and Sharma, K.K. 2011. Essential oil of clove (Eugenia caryophyllata) augments the humoral immune response but decreases cell mediated immunity. Phytother. Res. 25(8), 1254–1256. Heckert, R.A., Estevez, I., Russek-Cohen, E. and Pettit-Riley, R. 2002. Effects of density and perch availability on the immune status of broilers. Poult. Sci. 81(4), 451–457. Hussein, G., Miyashiro, H., Nakamura, N., Hattori, M., Kakiuchi, N. and Shimotohno, K. 2000. Inhibitory effects of sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytother. Res. 14(7), 510–516. Kammon, A., Brar, R., Sodhi, S., Banga, H., Nagra, N. and Singh, J. 2010. Ameliorating effect of vitamin C on immunological implications induced by chronic chlorpyrifos toxicity in broilers. LVMJ. J. 1, 164–180. Langhout, P. 2000. New additives for broiler chickens. World poult. 16(3), 22–27. Leite, A.M., Lima, E.d.O., Souza, E.L.d., Diniz, M.d.F.F.M., Trajano, V.N. and Medeiros, I.A.d. 2007. Inhibitory effect of beta-pinene, alpha-pinene and eugenol on the growth of potential infectious endocarditis causing gram-positive bacteria. RBCF. 43, 121–126. Magalhaes, C.B., Riva, D.R., DePaula, L.J., Brando-Lima, A., Koatz, V.L., Leal-Cardoso, J.H., Zin, W.A. and Faffe, D.S. 2010. In vivo anti-inflammatory action of eugenol on lipopolysaccharide-induced lung injury. J. Appl. Physiol. 108(4), 845–851. Mahrous, H.S., El-Far, A.H., Sadek, K.M. and Abdel-Latif, M.A. 2017. Effects of different levels of clove bud (Syzygium aromaticum) dietary supplementation on immunity, antioxidant status, and performance in broiler Chickens. Alex. J. Vet. Sci. 54(2), 29–39. Mehr, M.A., Hassanabadi, A., Moghaddam, H.N. and Kermanshahi, H. 2014. Supplementation of clove essential oils and probiotic on blood components, lymphoid organs and immune response in broiler chickens. Opin. Anim. Vet. Sci. 4(4), 218–223. Mohammed, A.K., Ali, N.A. and Mousa, C.R. 2020. Effect of adding different levels of crushed laurel leaves (Laurus nobilis) to the diet on some blood parameters for broiler chickens. EurAsian J. Biosci. 14(1), 401–405. Mustafa, D.B.M. 2016. ‘Effect of mixture of three herbal essential oils on performance, carcass yield and blood serum constituents of broiler chicks. Khartoum, Sudan: Sudan University of Science and Technology. Naderi, M., Akbari, M., Asadi-Khoshoei, E., Khaksar, K. and Khajali, F. 2014. Effects of dietary inclusion of turmeric (Curcuma longa) and cinnamon (Cinnamomum verum) powders on performance, organs relative weight and some immune system parameters in broiler chickens. Poult. Sci. J. 2(2), 153–163. Najafi, P. and Torki, M. 2010. Performance, blood metabolites and immunocompetaence of broiler Chickens fed diets included essentional oils of medicinal herbs. J. Anim. Vet. Adv. 9(7), 1164–1168. Najafi, S. and Taherpour, K. 2014. Effects of dietary ginger (Zingiber Ofjicinale), cinnamon (Cinnamomum), synbiotic and antibiotic supplementation on performance of broilers. J. Anim. Sci. Adv. 4(1), 658–667. Nasri, H. and Shirzad, H. 2013. Toxicity and safety of medicinal plants. J. Herb. Med. Pharmaco. 2(2), 21–22. Ojala, T., Remes, S., Haansuu, P., Vuorela, H., Hiltunen, R., Haahtela, K. and Vuorela, P. 2000. Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 73(1-2), 299–305. Oladokun, S., MacIsaac, J., Rathgeber, B. and Adewole, D. 2021. Essential oil delivery route: effect on broiler chicken’s growth performance, blood biochemistry, intestinal morphology, immune, and antioxidant status. Animals. 11(12), 3386. Oloruntola, O., Ayodele, S., Agbede, J. and Oloruntola, D. 2016. Effect of feeding broiler chickens with diets containing alchornea cordifolia leaf meal and enzyme supplementation. Arch. de Zootec. 65(252), 489–498. Othman, S.M., Ben Naser, K.M., Kanoun, A.H., Salim., A.A., Sherif , B.M. and Asheg, A.A. 2022. Effect of adding clove buds powder in feed on performance and jejunum morphology in broiler chickens. Open Vet. J. 12(6), 995–999. Riyazi, S., Ebrahimnezhad, Y., Hosseini, S., Meimandipour, A. and Ghorbani, A. 2015. Comparison of the effects of basil (Ocimum basilicum) essential oil, avilamycin and protexin on broiler performance, blood biochemistry and carcass characteristics. Arch. Anim. Breed. 58(2), 425–432. Sadeghi, G., Karimi, A., Padidar Jahromi, S., Azizi, T. and Daneshmand, A. 2012. Effects of cinnamon, thyme and turmeric infusions on the performance and immune response in of 1-to 21-day-old male broilers. Braz. J. Poult. Sci. 14, 15–20. SAS. 2002. Statistical analysis system 9.00, Cary, NC: SAS Institute Inc. Schumann, G., Bonora, R., Ceriotti, F., Férard, G., Ferrero, C.A., Franck, P.F., Gella, F.J., Hoelzel, W., Jørgensen, P.J., Kanno, T., Kessner, A., Klauke, R., Kristiansen, N., Lessinger, J.M., Linsinger, T.P., Misaki, H., Panteghini, M., Pauwels, J., Schiele, F., Schimmel, H.G., Weidemann, G. and Siekmann, L. International Federation of Clinical Chemistry and Laboratory Medicine. 2002. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. Part 4. Reference procedure for the measurement of catalytic concentration of alanine aminotransferase. Clin. Chem. Lab. Med. 40(7), 718–724. Şehitoğlu, M. and Kaya, H. 2021. The effect of clove oil supplementation in laying hen diets on performance, egg quality, some blood parameters, and Yolk TBARS. Turkish J. Agric. Food Sci. Technol. 9(12), 2213–2218. Shrivastava, K., Sahu, S., Mishra, S.K. and De, K. 2014. In vitro antimicrobial activity and phytochemical screening of Syzygium aromaticum. Asian J. Res. Pharma. Sci. 4(1), 12–15. Sjafani, N., Hasan, S. and Sapsuha, Y. 2022. The influence of clove leave extract (Syzygium aromaticum) on growth performance and bacterial population of broiler chickens raised in stressful conditions of high stocking density. J. Anim. Behav. Biometeorol. 10(2), 1–5. Soltan, M., Shewita, R. and El-Katcha, M. 2008. Effect of dietary anise seeds supplementation on growth performance, immune response, carcass traits and some blood parameters of broiler chickens. Int. J. Poult. Sci. 7(11), 1078–1088. Tiwary, B.K. and Goel, M.C. 1985. Contact sensitivity to DNCB in normal and cell-mediated-immunity deficient chickens: in vivo detection and correlation with lymphocyte transformation and graft-versus-host reaction. Vet. Immunol. Immunopathol. 8(4), 329–339. Toghyani, M., Tohidi, M., Gheisari, A.A. and Tabeidian, S.A. 2010. Performance, immunity, serum biochemical and hematological parameters in broiler chicks fed dietary thyme as alternative for an antibiotic growth promoter. Afr. J. Biotechnol. 9(40), 6819–6825. Venkadeswaran, K., Muralidharan, A.R., Annadurai, T., Ruban, V.V., Sundararajan, M., Anandhi, R., Thomas, P.A. and Geraldine, P. 2014. Antihypercholesterolemic and antioxidative potential of an extract of the plant, piper betle, and its active constituent, eugenol, in triton WR-1339-induced hypercholesterolemia in experimental rats. Evid. Based Compl. Alternat. Med. 2014, 478973. Wang, R., Li, D. and Bourne, S. 1998. Can 2000 years of herbal medicine history help us solve problems in the year 2000. Alltechs Ann. Symp. 14, 168–184. Wati, T., Ghosh, T. K., Syed, B. and Haldar, S. 2015. Comparative efficacy of a phytogenic feed additive and an antibiotic growth promoter on production performance, caecal microbial population and humoral immune response of broiler chickens inoculated with enteric pathogens. Anim. Nutr. 1(3), 213–219. Wenger, C., Kaplan, A., Rubaltelli, F. and Hammerman, C. 1984. Alkaline phosphatase. Clin. Chem. The C.V. Mosby Co. St Louis, Toronto. Princeton. 1094–1098. | ||
| How to Cite this Article |
| Pubmed Style Ben-naser KM, Sherif BM, Othman SM, Asheg AA. Effect clove buds powder supplementation on hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity of broilers. Open Vet. J.. 2023; 13(7): 854-863. doi:10.5455/OVJ.2023.v13.i7.7 Web Style Ben-naser KM, Sherif BM, Othman SM, Asheg AA. Effect clove buds powder supplementation on hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity of broilers. https://www.openveterinaryjournal.com/?mno=139975 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i7.7 AMA (American Medical Association) Style Ben-naser KM, Sherif BM, Othman SM, Asheg AA. Effect clove buds powder supplementation on hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity of broilers. Open Vet. J.. 2023; 13(7): 854-863. doi:10.5455/OVJ.2023.v13.i7.7 Vancouver/ICMJE Style Ben-naser KM, Sherif BM, Othman SM, Asheg AA. Effect clove buds powder supplementation on hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity of broilers. Open Vet. J.. (2023), [cited January 25, 2026]; 13(7): 854-863. doi:10.5455/OVJ.2023.v13.i7.7 Harvard Style Ben-naser, K. M., Sherif, . B. M., Othman, . S. M. & Asheg, . A. A. (2023) Effect clove buds powder supplementation on hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity of broilers. Open Vet. J., 13 (7), 854-863. doi:10.5455/OVJ.2023.v13.i7.7 Turabian Style Ben-naser, Khaled M., Bashir M. Sherif, Siham M. Othman, and Abdulatif A. Asheg. 2023. Effect clove buds powder supplementation on hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity of broilers. Open Veterinary Journal, 13 (7), 854-863. doi:10.5455/OVJ.2023.v13.i7.7 Chicago Style Ben-naser, Khaled M., Bashir M. Sherif, Siham M. Othman, and Abdulatif A. Asheg. "Effect clove buds powder supplementation on hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity of broilers." Open Veterinary Journal 13 (2023), 854-863. doi:10.5455/OVJ.2023.v13.i7.7 MLA (The Modern Language Association) Style Ben-naser, Khaled M., Bashir M. Sherif, Siham M. Othman, and Abdulatif A. Asheg. "Effect clove buds powder supplementation on hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity of broilers." Open Veterinary Journal 13.7 (2023), 854-863. Print. doi:10.5455/OVJ.2023.v13.i7.7 APA (American Psychological Association) Style Ben-naser, K. M., Sherif, . B. M., Othman, . S. M. & Asheg, . A. A. (2023) Effect clove buds powder supplementation on hematological profile, biochemical parameters, lymphoid organs, and cell-mediated immunity of broilers. Open Veterinary Journal, 13 (7), 854-863. doi:10.5455/OVJ.2023.v13.i7.7 |