| Research Article | ||
Open Vet. J.. 2023; 13(6): 715-722 Open Veterinary Journal, (2023), Vol. 13(6): 715–722 Original Research Correlation of avian influenza-H9N2 with high mortality in broiler flocks in the southwest of Tripoli, LibyaAhmed Shaban Kassem Agha1, Imad Benlashehr2*, Khalid Mohammed Naffati1, Salah Abdulhadi Bshina3 and Ahmed Abdulmajed Khashkhosha11Department of Microbiology, Libyan Center for Biotechnology Research, Tripoli, Libya 2Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, University of Tripoli, Libya 3Department of Medicine, Faculty of Veterinary Medicine, Azzaytuna University, Tarhuna, Libya *Corresponding Author: Imad Benlashehr. Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, University of Tripoli, Libya. Email: i.benlashehr [at] uot.edu.ly Submitted: 06/02/2023 Accepted: 09/05/2023 Published: 08/06/2023 © 2023 Open Veterinary Journal
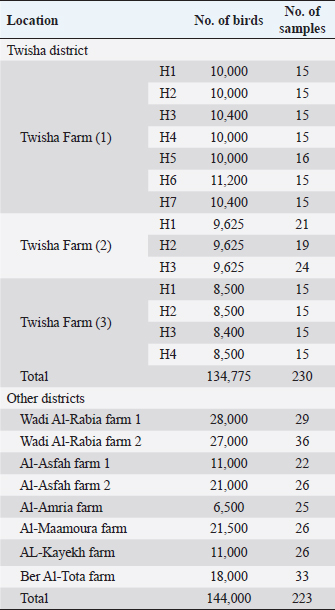
AbstractBackground: Low pathogenic H9N2 avian influenza (LPAI H9N2) caused by the influenza A virus which belongs to the family Orthomyxoviridae. It caused mild respiratory symptoms and a drop in egg production in poultry. Outbreaks of AI-H9N2 have occurred in poultry since the 1990s in many countries in USA, Europe, and Asia. Recently, outbreaks of H9N2 in commercial chicken were recorded in Morocco, Tunisia, Libya, and Egypt. Furthermore, numerous studies demonstrated that co-infection with AI H9N2 and other pathogens results in severe respiratory illness with high mortality in broiler chickens. Outbreaks of respiratory disease with variations in mortality rate were recorded in broiler flocks growing in the southwest of Tripoli in Libya. Aim: The present study was conducted to explain the variation of mortality rate on broiler flocks growing in the southwest area of Tripoli by detection of AI H9N2 antibodies and antigens. Methods: A total of 453 sera samples, 60 tracheal swabs, and 60 cloacal swabs were collected from unvaccinated broiler flocks against avian influenza. Specific avian influenza type A antibodies were detected by using the Elisa test, and specific AI-H9N2 antibodies were detected by using the HI test, whereas specific AI-H9N2 antigens were detected in tracheal and cloacal swabs by using One-Step RT-PCR (M gene) technique. Results: Respiratory diseases with high variations in mortality rate were recorded in broiler flocks growing in the southwest of Tripoli in Libya; the broiler mortality rate in Twisha farms was higher than other farms (62.2% and 11%, respectively). Whereas avian influenza type A antibodies were detected at a high level in Twisha and other farms (95.2%, and 76.7%, respectively). The positive samples for AI type A were tested for AI H9N2 using the HI test. Interestingly the percentage of AI-H9N2 antibodies was quite similar in high and low mortality regions (53.4% and 46.8%, respectively). Additionally, AI-H9N2 antigens were detected only in tracheal swabs in Twisha farm 3, Al-Maamoura, and Ber Al-Tota districts. Conclusion: This study confirmed the endemic of AI- H9N2 in broiler flocks in the southwest of Tripoli-Libya. Also, it clarified that AI-H9N2 was not responsible for the high mortality rate by itself in broiler flocks. Moreover, this study supported the presence of other subtypes of avian influenza in the studied area. Keywords: H9N2, Broiler flocks, Variation, Mortality rate, Libya. IntroductionAvian influenza (AI) is one of the important respiratory diseases in poultry caused by the influenza A virus which belongs to the family Orthomyxoviridae. Influenza A viruses have several subtypes based on hemagglutinin (HA) or neuraminidase antigens. So far, there are 18 (H1-H18) subtypes and 10 neuraminidase subtypes (N1-N11) that have been recognized (Fouchier et al., 2005; Tong et al., 2012). According to pathogenicity, Avian influenza viruses (AIVs) can be grouped as highly pathogenic avian influenza (HPAI) H5 and H7 subtypes, which result in heavy economic losses to the poultry industry and low pathogenic avian influenza (LPAI) like H9N2 which usually causes minimal clinical signs and drop in egg production. Nevertheless, co-infection of H9N2 with other pathogens may result in severe respiratory disease and cause mortality of up to 65% in broiler chickens and up to 70% drop in egg production in layers and breeders (Alexander et al., 2009; Capua et al., 2009; Abdel-Moneim et al., 2012; Seifi et al., 2012; Azizpour et al., 2014; Kammon et al., 2015). LPAI H9N2 became endemic globally in many countries like Germany, Italy, Ireland, South Africa, the USA, and Korea since the 1990s. (Arafa et al., 2012; Al-Garib et al., 2016). Also, H9N2 infections have been reported in Asia (Hong Kong, China, Korea, Pakistan, Japan, India, and Vietnam), the Middle East (United Arab Emirates, Saudi Arabia, Kuwait, Lebanon, Iraq, and Sultanate of Oman), and Africa (South Africa, Tunisia, Egypt, Morocco, and Algeria), causing widespread outbreaks in commercial chickens (Butt et al., 2010; Al-Garib et al., 2016; Kaboudi et al., 2019; Peacock et al., 2019). In several of these countries, the vaccine has been utilized to control the disease. Nevertheless, H9N2 infection has become endemic in commercial poultry in a significant number of countries. LPAI H9N2 has not only been detected in poultry but also in some human cases, being a real to human health and a global concern for public health (Body et al., 2015; Sikht, 2022). In Libya, the incidence and severity of respiratory disease in commercial chicken flocks have increased due to the intensification of the poultry industry, and in mid-2005 LPAI H9N2 subtype changed, believed to be one of the essential reasons for chicken respiratory diseases in Libya, as indicated by field reports (Al-Garib et al., 2007; Fares et al., 2010). Seroprevalence results showed a positive correlation between a high mortality rate and the detection of specific antibodies against AIV H9N2 (Al-Garib et al., 2007). Furthermore, the seroprevalence of AI (H5, H7, and H9) in broiler and layer flocks in the northwest of Libya revealed the presence of only antibodies against the LPAI H9 subtype (Fares et al., 2010). The last outbreaks of respiratory signs with a high mortality rate in Libyan broiler and layer hens during 2013 were described by the Co-infection of H9N2 with NDV (Kammon et al., 2015). The present study was conducted to explain the variation of mortality rate on broiler flocks in the southwest area of Tripoli by detection of AI H9N2 antibodies and antigens. Materials and MethodsField surveyTwenty-two commercial broiler flocks were studied in seven districts (Twisha, Al-Asfah, Al-Amria, Al-Maamoura, and AL-Kayekh) from Al-Jafara municipality and two districts (Wadi Al-Rabia, and Ber Al-Tota) from Tajoura municipality. All flocks were vaccinated against NDV, IBV, and (IBDV). By contrast, all the previous flocks were not vaccinated against AI. Furthermore, Poultry sheds were of low quality and suffered from insufficient ventilation in Twisha farms compared with other farms. Serological assaysSerological assays (Elisa and HI tests) were conducted at the National Center of Animal Health in Libya. Collection of serum samples for serological Elisa test and HI testFour hundred fifty-three blood samples were collected randomly from different districts in the southwest of Tripoli-Libya as recommended by WHO (2002) and described by Spackman (2007) (Table 1). ELISA test to detect AI type A antibodies in serum was conducted according to the manufacturer’s (X-OvO Limited, United Kingdom) instructions. An ELx800 ELISA reader with a 550 nm filter (BIO-TEK Instruments, Inc. Winooski, VT, USA) was used. The HA and HI were carried out according to the procedure described by Alexander et al. (1983), Alexander (2009), and OIE (2009). The HI assay has to end up completed using 96 ‘U’-well microtiter plates, doubling dilution in PBS, 1 % v/v crimson blood cells, and 4 HA devices of AIV antigen. Table 1. Blood samples from Twisha district and other districts at age 38 days.
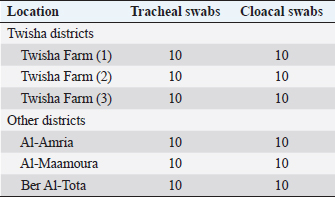
Molecular technique assays to detection of avian influenza type A antigenHighly sensitive monoclonal antibodies rapid test One hundred twenty tracheal and cloacal swabs were collected for Rapid Tests from broilers suffering from mild to severe clinical symptoms (Table 2). Antigen Rapid AIV Ag Test Kit was used according to the manufacturer’s (BIO NOTE, KOREA) instructions. Positive samples of rapid tests were confirmed by one-step RT-PCR (M gene). Virus antigen detection by molecular technique was done at the Institute of Veterinary Research of Tunisia, 20 Rue Djebel Lakhdar La Rabta 1006 Tunis. Individual plastic swabs for molecular technique were collected from the wall of the trachea and cloacal, according to Spackman (2007) and Spackman et al. (2002). Swab immerse in viral transport media tube containing brain–heart infusion broth with penicillin G (10,000 Units/ml), streptomycin (2 mg/ml), kanamycin (0.6 mg/ml), gentamicin (1 mg/ml), and amphotericin B (0.02 mg/ml). Swabs were eliminated using a disinfectant solution, and transport media tubes were Labeled and stored separately at -70°C for the following analysis. RNA was extracted from tracheal and cloacal swabs of individual birds according to the manufacturer’s instructions (Qiagen, GmbH - Germany) using QIAamp viral RNA mini Kit. One-step RT-PCR was performed using an Access RT-PCR System kit (Qiagen, Germany) according to the manufacturer’s procedure. Universal primers were used to determine the presence of influenza A viruses (Table 3). PCR was carried out in a final reaction volume of 25 μl using a 0.2 ml thin wall PCR tube. A master mix of 5 samples was prepared and liquated in 20 μl quantities in each PCR tube. A 5 μl sample of RNA was added in each tube to make the final volume of 25 μl. The amplified segments were separated by electrophoresis on 1.5% agarose gel (Applichem, Germany, GmbH) using 1× Tris/borate/ethylenediaminetetraacetic acid buffer at room temperature at gradients of 5 v/cm. For gel analysis, 15 μl of the products were loaded in each gel slot. 100 and 1,000 bp ladder (Qiagen, GmbH – Germany) was used to determine the fragment sizes. The gel was photographed by a gel documentation system (Alpha Innotech, Biometra). Table 2. Describes the rapid test specimen at age 38 days.
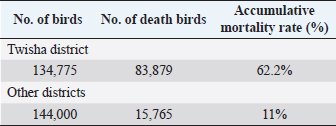
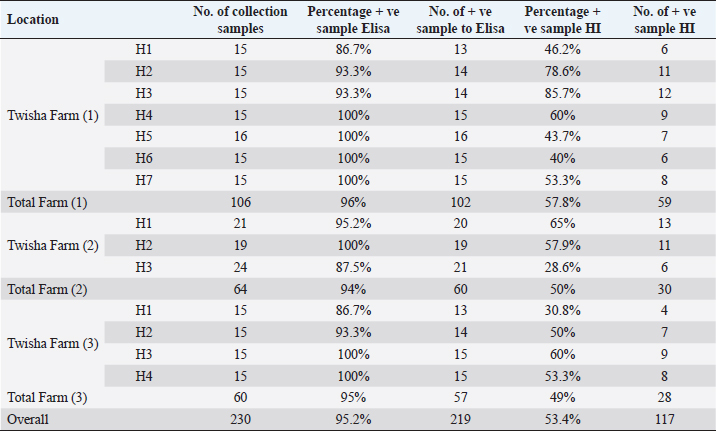
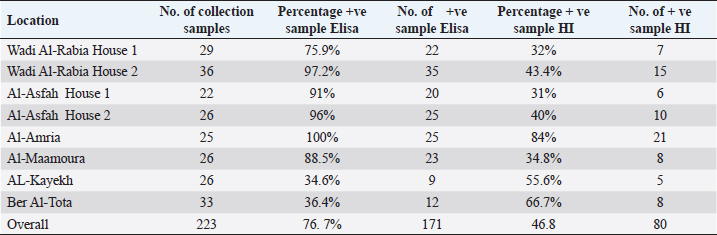
Statistical analysisData were managed and analyzed using SPSS, version 17.0 (SPSS Inc., Chicago, IL). Data were performed using analysis of variance (ANOVA). Statistical significance was designated for differences with p-values less than or equal to 0.05. Ethical approvalAll samples were collected according to the local and international standards of the care and use of animals in research. ResultsClinical signs and postmortemThe samples were collected prissily from broiler farms suffering from severe to mild respiratory signs, but the severity of respiratory signs varied from farm to farm. By contrast, postmortem examination did not provide clear evidence of a specific disease. Only the trachea and upper respiratory tract were congested with an accumulation of little mucus. The lung was also congested. The kidney and spleen were quite an enlargement. The thoracic and abdominal air sacs were slightly thickened and whitish. Mortality ratesA high broiler mortality rate (62.2%) was recorded on Twisha farms at Al-Jafara municipality. By contrast, a low broiler mortality rate (11%) was recorded on Al-Asfah, Al-Amria, Al-Maamoura, and AL-Kayekh farms at Al-Jafara and municipality, and Wadi Al-Rabia, and Ber Al-Tota farms at Tajoura municipality (Table 4). Serological assayTwo hundred thirty blood samples from Twisha farms and 223 blood samples from different farms were exanimated by using the ELISA technique to detect AI type A antibodies. The percentage of positive antibodies against AI type A in Twisha farms was 95.2%, whereas in different farms was 76.7% (Table 5). Similar rates of antibody titration against AI type A were recorded in Twisha farms 1, 2, and 3 (96%, 94%, and 95%, respectively). Also, in AL-Asfah, farm (1) was 91%, and farm (2) was 96% (table 5). By contrast, different percentages of antibody titration against AI type A with significant differences (p ˂ 0.05) were recorded in the Ber Al-Tota farm 36.4%, Wadi Al-Rabia farm (1) 75.9%, Al-Amria farm 100%, Al-Maamoura farm 88.5%, and AL-Kayekh farm 34.6% (Table 5 and 6). The positive samples for AI type A were tested for subtypes H9N2 using the HI test. H9N2 antibodies were detected in 53.4% of samples collected from Twisha farms and 46.8% of samples collected from other farms. This result refers to a significant decrease (p < 0.05) by two folds in the number of positive samples to AI H9N2 antibodies compared with positive samples to AIV type A antibodies in the studied area (Tables 5 and 6). Table 3. Polymerase chain reaction primers used for the influenza A virus and H9 subtype.
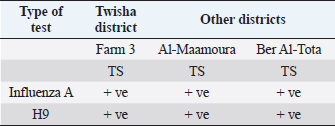
Virological testsRapid test to detect AIV type (A): Tracheal and cloacal swabs were collected from farms with positive serology to AIV type A at age 38 days. All swabs collected from Twisha farms were negative to AIV type A, except tracheal swabs collected from farm 3 were positive to AIV type A. By contrast, all tracheal and cloacal swabs collected from Al-Amria, Al-Maamoura, and Ber Al-Tota district were positive to AIV type A. Table 4. The mortality rates of broiler birds at 38 days old.
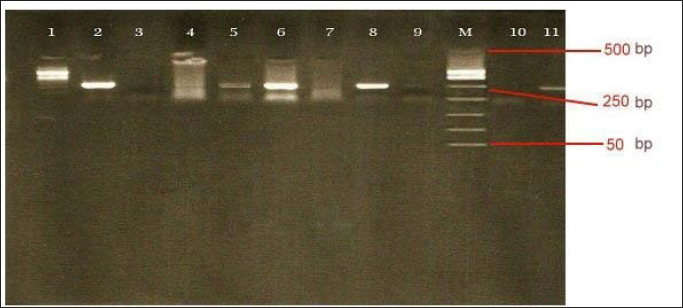
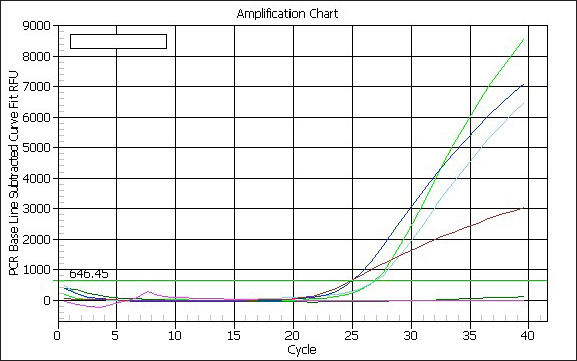
Molecular diagnosis of avian influenzaThe positive rapid test samples were confirmed by using a one-step PCR technique to detect AI H9N2. As illustrated in (Table 7). Only tracheal swabs collected from Twisha-farm (3), Al-Maamoura, and Ber Al-Tota districts were positive for AI type A and the H9N2 subtype by rRT-PCR. At the same time, tracheal swabs collected from Al-Amria districts were negative for AI type A and AI H9N2. Moreover, all cloacal swabs were negative for AI type A and H9N2 using the same technique (Table 7; Figs. 1 and 2). DiscussionThis study appears a high variation in broiler mortality rate between Twisha farms compared with other farms (62.2% and 11%, respectively). At the same time, all broiler flocks suffer from mild to severe respiratory signs. Furthermore, the postmortem examination does not refer to specific diseases that can explain the difference in broiler mortality rate between flocks. The seroprevalence of AI types A antibodies are detected at a high level in the Twisha district, suffering from a high mortality rate, and different districts suffering from a low mortality rate (95.2% and 76.7%, respectively) using the Elisa test. This result is in accordance with Al-Natour et al. (2005) in Jordan who indicated that the seroprevalence of AI antibodies is 71%; Barbour et al. (2006) in Lebanon reports that seroprevalence of AI antibodies detected on 19 out of the 24 farms investigated. Furthermore, the wide distribution of AI type A in all studied flocks is a strong indicator of low biosecurity at the farm level in the studied area. Moreover, this result supports previous data published in Libya by Al-Garib et al. (2007), Fares et al. (2010), and Kammon et al. (2015). A positive correlation between a high mortality rate and the detection of specific antibodies against AI-H9N2 has been reported in Libya (Al-Garib et al., 2007). Moreover, several studies demonstrated that AI-H9N2 viruses have various pathogenic and mortality effects, from low to high, depending on several factors (Pazani et al., 2008). That is why avian AI H9N2 is implicated in inducing different mortality between broiler flocks growing southwest of Tripoli in Libya. On the other hand, the HI test in this study shows that nearly 50% of AIV type A antibodies are positive for AI-H9N2 in high and low motility rate flocks in the same and/or different regions. The present study interferes with data demonstrating the positive correlation between the presence of H9N2 and high motility in Libya broiler flocks by Al-Garib et al. (2007). Furthermore, AI-H9N2 antigen is detected in some studied flocks by using a one-step PCR technique. The detection of AI-H9N2 in Libya has been reported by Kammon et al. (2015). This result denies the responsibility of H9N2 by itself in the difference in mortality between broiler flocks in Twisha farms compared to other farms. Those results agree with studies demonstrating that AI-H9N2 viruses have various pathogenicity effects and mortality rates from low to high, depending on environmental conditions, the strain of chickens, age of the affected birds, the health status of commercial chickens, secondary infection, route of infection, as well as sub strains of AIV viruses (Zanella et al., 2001; Vasfi Marandi et al., 2002; Kianizadeh et al., 2006; Pazani et al., 2008). High mortality rates of up to 65% are recorded in chickens suffering from a combined infection of H9N2 with Mycoplasma gallisepticum and/ or Escherichia coli (Vasfi Marandi et al., 2002). Also, variable mortality ranging from 5% to 90% is reported in poultry depending on the age of birds, and the presence of secondary infections such as Pasteurella multocida, Ornithobacterium rhinotracheal, and Mycoplasmas was reported in Italy (Zanella et al., 2001). The different broiler mortality between Twisha farms and other farms could be due to the poultry sheds being low quality in Twisha farms, the presence of HPAI, virulent strains of H9N2, and mixed infection of H9N2 with high virulence viruses like APMV-1 (Newcastle disease) which is capable of elevating mortality up to 70% in unvaccinated flocks in Libyan poultry farms as mentioned by Kammon et al. (2015). The present results descend outbreaks on poultry fields in Libya. Therefore, continuous monitoring of the poultry flocks is required to avoid high mortality rates. The seroprevalence comparative of AI type A antibodies with AI H9N2 in the present study are strong indicators of the presence of another subtype of AIV type A (HPAI and LPAI) in studied areas (Al-Jafara and Tajoura municipalities). These concerns had been raised by Al-Garib et al. (2007); and Kammon et al. (2015). In terms of public health aspects, H9N2 may pose a threat risk to human health and life after being isolated from human flu-like illness in China in 1994, in Hong Kong in 2003, in Bangladesh in 2006, in Egypt in 2006, in Oman in 2009, and Pakistan in 2003 (Gomaa et al., 2015; Pusch and Suarez, 2018; Ali et al., 2019; Peacock et al., 2019; European Food Safety Authority et al., 2023). Table 5. Comparative between AIV type A detected by Elisa test and specific antibodies AI H9N2 detected by HI test in Twisha district.
Table 6. Comparative between AIV type A detected by Elisa test and specific antibodies AI H9N2 detected by HI test in different districts.
Table 7. Describe the results of tracheal swabs by RT-PCR test.
Fig. 1. Electrophoresis for avian influenza A virus. Lanes 2, 5, 6, 8=avian influenza A virus positive flocks (positive; band at 240 bp). Lane 1, 3,4,7,9=avian influenza A virus negative flocks. Lane M=Ladder marker. Lane 10=negative control. Lane 11=avian influenza A virus positive control (positive; band at 240 bp).
Fig. 2. Real-time PCR amplification and cycling chart. ConclusionThe prevalence of AI type A virus antibody was ubiquitous and widely distributed in all investigated broiler flocks. It was 95.2% in the Twisha district and 76.7% in different districts. Furthermore, AI H9N2 was 53.4% in the Twisha district and 46.8% in different districts. According to RT-PCR results, AI-H9N2 antigen was detected in some studied flocks. All those results clarified that H9N2 became endemic in broiler flocks growing southwest of Tripoli-Libya, and it was not responsible for the high mortality rate by itself in broiler flocks. Moreover, this study supported the presence of other subtypes of AI in the studied area. Since direct contact with poultry is common in Libya, this suggests that transmission of the H9N2 influenza virus into humans is not impossible. ConclusionLibyan Veterinary Animal Health must develop intervention strategies through routine serological surveillance programs in poultry for early detection of the causative agent. Phylogenetic analysis studies must conduct to provide information on the origins of Libya isolates. Surveillance and epidemiological studies in different avian species must start to identify the incidence of AI disease. Developing a poultry vaccine program is necessary to combat AI infection. Exchange information with neighboring countries to eliminate the spread of disease. Animal Health Authority with the Ministry must collaborate with Human Health in Libya to prevent human infections. AcknowledgmentsI thank Dr. Al-Garib “may his soul rest in peace” for his generous attitude in providing information to carry out the research work. Special thanks to the staff of the serology laboratory at the National Center of Animal Health in Tripoli. ReferencesAbdel-Moneim, A.S., Afifi, M.A. and El-Kady, M.F. 2012. Isolation and mutation trend analysis of influenza a virus subtype H9N2 in Egypt. Virol. J. 9, 173–192. Alexander, D.J., Allan, W.H., Biggs, P.M., Bracewell, C.D., Darbyshire, J.H., Dawson, P.S., Harris, A.H., Jordan, F.T., MacPherson, I., McFerran, J.B., Randall, C.J., Stuart, J.C., Swarbrick, O. and Wilding, G.P. 1983. A standard technique for haemagglutination inhibition tests for antibodies to avian infectious bronchitis virus. Vet. Rec. 113(3), 64. Alexander, D.J. 2009: Avian influenza. In Manual of diagnostic tests and vaccines for terrestrial animals 2010, Chap. 2.3.4. Paris, France: World Organization for Animal Health. Al-Garib, S., Agha, A. and Al-Mesilaty, L. 2016. Low pathogenic avian influenza H9N2: world-wide distribution. World’s Poult. Sci. J. 72, 125–136. Al-Garib, S., Kammon, A., Asheg, A., Hamid, M., Fathalla, H. and Lawala, A. Detection of antibodies to avian influenza H9N2 in broiler flocks clinically expressing a respiratory disease syndrome. In Proceeding The 15th Congress & Exhibition of the World Veterinary Poultry Association, Beijing, China, 2007, Vol. 130, pp 1–108. Ali, M., Yaqub, T., Mukhtar, N., Imran, M., Ghafoor, A., Shahid, M.F., Naeem, M., Iqbal, M., Smith, G.J.D. and Su, Y.C.F. 2019. Avian influenza A (H9N2) virus in poultry worker, Pakistan, 2015. Emerg. Infect. Dis. 25(1), 136–139. Al-Natour, M.Q. and Abo-Shehada, M.N. 2005. Sero-prevalence of avian influenza among broiler-breeder flocks in Jordan. Prev. Vet. Med. 70, 45–50. Arafa, A.S., Hagag, N., Erfan, A., Mady, W., El-Husseiny, M., Adel, A. and Nasef, S. 2012. Complete genome characterization of avian influenza virus subtype H9N2 from a commercial quail flock in Egypt. Virus Genes 45(2), 283–294. Azizpour, A., Goudarzi, H. and Charkhkar, S. 2014. Experimental study on tissue tropism and dissemination of H9N2 avian influenza virus and Ornithobacterium rhinotracheale co-infection in SPF chickens. J. Anim. Plant Sci. 24, 1655–1662. Barbour, E.K., Sagherian, V.K., Sagherian Danker, S.K., Jaber, L.S., Usayran, N.N. and Farran, M.T. 2006. Avian influenza outbreak in poultry in the Lebanon and transmission to neighbouring farmers and swine. Vet. Ital. 42(2), 77–85. Body, M.H., Alrarawahi, A.H., Alhubsy, S.S., Saravanan, N., Rajmony, S. and Mansoor, M.K. 2015. Characterization of low pathogenic avian influenza virus subtype H9N2 isolated from free-living mynah birds (Acridotheres tristis) in the sultanate of oman. Avian Dis. 59, 329–334. Butt, A.M., Siddique, S., Idrees, M. and Tong, Y. 2010. Avian influenza a (H9N2): computational molecular analysis and phylogenetic characterization of viral surface proteins isolated between 1997 and 2009 from the human population. Virol. J. 7, 319. Capua, I. and Alexander, D.J. 2009. Avian influenza infection in birds. Poult. Sci. 88, 842–846. European Food Safety Authority, European Centre for Disease Prevention and Control, European Union Reference Laboratory for Avian Influenza, Adlhoch, C., Fusaro, A., Gonzales, J.L., Kuiken, T., Marangon, S., Mirinaviciute, G., Niqueux, É., Stahl, K., Staubach, C., Terregino, C., Broglia, A. and Baldinelli, F. 2023. Avian influenza overview December 2022 - March 2023. EFSA J. 21(3), e07917. Fares, A.R., Elhafi, G., Alzaletny, R. and Gerish, E. 2010. Seroprevalence of avian influenza in broilers and layers, Northwestern area of Libya. Hammamet, TN: Congres Veterinaire Maghrebin, pp 10–11. Fouchier, R.A., Munster, V., Wallensten, A., Bestebroer, T.M., Herfst, S., Smith, D., Rimmelzwaan, G.F., Olsen, B. and Osterhaus, A.D. 2005. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. Virol. J. 79, 2814–2822. Fouchier, R.A., Bestebroer, T.M., Herfst, S., Van Der Kemp, L., Rimmelzwaan, G.F. and Osterhaus, A.D. 2000. Detection of influenza A viruses from different species by PCR amplification of conserved sequences in the matrix gene. J. Clin. Microbiol. 38(11), 4096–4101. Gomaa, M.R., Kayed, A.S., Elabd, M.A., Zeid, D.A., Zaki, S.A., El Rifay, A.S., Sherif, L.S., McKenzie, P.P., Webster, R.G., Webby, R.J., Ali, M.A. and Kayali, G. 2015. Avian influenza A(H5N1) and A(H9N2) seroprevalence and risk factors for infection among Egyptians: a prospective, controlled seroepidemiological study. J. Infect. Dis. 211(9), 1399–1407. Kaboudi, K. 2019. Low pathogenic avian influenza virus subtype H9N2 in poultry in north Africa: current status. Vet. Sci. Res. Rev. 5(2), 73–79. Kammon, A., Heidari, A., Dayhum, A., Eldaghayes, I., Sharif, M., Monne, I., Cattoli, G., Asheg, A., Farhat, M. and Kraim, E. 2015. Characterization of avian influenza and newcastle disease viruses from poultry in Libya. Avian Dis. 59(3), 422–430. Kianizadeh, M., Pourbakhsh, S., Toroghi, R. and Momayez, R. 2006. Pathogenicity and hemagglutinin gene sequence analysis of Iranian avian influenza H9N2 viruses isolated during (1998–2001). Iranian J. Vet. Res. 7(3), 16. OIE. 2009. World Organization for animal health. Chapter 2.3.4 Avian influenza. Manual of diagnostic tests and vaccines for terrestrial. OIE. Pazani, J., Vasfi Marandi, M., Ashrafihelan, J., Hossein Marjanmehr, S. and Ghods, F. 2008. Pathological studies of A / chicken / tehran / ZMT - 173/99 (H9N2) influenza viruses in commercial broiler chickens of Iran. Int. J. Poult. Sci. 7(5), 502–510. Peacock, T.H.P., James, J., Sealy, J.E. and Iqbal, M. 2019. A global perspective on H9N2 avian influenza virus. Viruses. 11(7), 620. Pusch, E.A. and D.L. Suarez. 2018. The multifaceted zoonotic risk of H9N2 avian influenza. Vet. Sci. 5(4), 82. Seifi, S., Asasi, K. and Mohammadi, A. 2012. Short paper: an experimental study on broiler chicken co-infected with the specimens containing avian influenza (h9 subtype) and infectious bronchitis (4/91 strain) viruses. Iranian J. Vet. Res. 13, 138–142. Sikht, F.Z., Ducatez, M., Touzani, C.D., Rubrum, A., Webby, R., El Houadfi, M., Tligui, N.S., Camus, C. and Fellahi, S. 2022. Avian influenza a H9N2 viruses in Morocco, 2018-2019. Viruses 14(3), 529. Spackman, E. 2007. Athens, GA, 2007. ISBN: 978-1-58829-939-0. Spackman, E., Senne, D.A., Myers, T.J., Bulaga, L.L., Garber, L.P., Perdue, M.L., Lohman, K., Daum, L.T. and Suarez, D.L. 2002. Development of a real-time reverse transcriptase PCR assay for type a influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 40(9), 3256–3260. Tong, S., Li, Y., Rivailler, P., Conrardy, C., Castillo, D.A.A., Chen, L.M., Recuenco, S., Ellison, J.A., Davis, C.T., York, I.A. and Turmelle, A.S. 2012. A distinct lineage of influenza a virus from bats. Proc. Natl. Acad. Sci. 109, 4269–4274. Vasfi Marandi, M. and Bozorgmehri Fard, M.H. 2002. Isolation of H9N2 subtype of avian influenza viruses during an outbreak in chickens in Iran. Iranian Biomed. J. 6, 13–17. WHO. 2002. Manual on animal influenza diagnosis and surveillance. World Health Organiztion. Geneva, Switzerland. Zanella, A., Dall’ara, P. and Martino, P.A. 2001. Avian influenza epidemic in Italy due to serovar H7N1. Avian Dis. 45, 257–261. | ||
| How to Cite this Article |
| Pubmed Style Kassem-agha AS, Benlashehr I, Naffati KM, Bshina SA, Khashkhosha AA. Correlation of avian influenza-H9N2 with high mortality in broiler flocks in the southwest of Tripoli, Libya. Open Vet. J.. 2023; 13(6): 715-722. doi:10.5455/OVJ.2023.v13.i6.6 Web Style Kassem-agha AS, Benlashehr I, Naffati KM, Bshina SA, Khashkhosha AA. Correlation of avian influenza-H9N2 with high mortality in broiler flocks in the southwest of Tripoli, Libya. https://www.openveterinaryjournal.com/?mno=142853 [Access: January 13, 2026]. doi:10.5455/OVJ.2023.v13.i6.6 AMA (American Medical Association) Style Kassem-agha AS, Benlashehr I, Naffati KM, Bshina SA, Khashkhosha AA. Correlation of avian influenza-H9N2 with high mortality in broiler flocks in the southwest of Tripoli, Libya. Open Vet. J.. 2023; 13(6): 715-722. doi:10.5455/OVJ.2023.v13.i6.6 Vancouver/ICMJE Style Kassem-agha AS, Benlashehr I, Naffati KM, Bshina SA, Khashkhosha AA. Correlation of avian influenza-H9N2 with high mortality in broiler flocks in the southwest of Tripoli, Libya. Open Vet. J.. (2023), [cited January 13, 2026]; 13(6): 715-722. doi:10.5455/OVJ.2023.v13.i6.6 Harvard Style Kassem-agha, A. S., Benlashehr, . I., Naffati, . K. M., Bshina, . S. A. & Khashkhosha, . A. A. (2023) Correlation of avian influenza-H9N2 with high mortality in broiler flocks in the southwest of Tripoli, Libya. Open Vet. J., 13 (6), 715-722. doi:10.5455/OVJ.2023.v13.i6.6 Turabian Style Kassem-agha, Ahmed Shaban, Imad Benlashehr, Khalid Mohammed Naffati, Salah Abdulhadi Bshina, and Ahmed Abdulmajed Khashkhosha. 2023. Correlation of avian influenza-H9N2 with high mortality in broiler flocks in the southwest of Tripoli, Libya. Open Veterinary Journal, 13 (6), 715-722. doi:10.5455/OVJ.2023.v13.i6.6 Chicago Style Kassem-agha, Ahmed Shaban, Imad Benlashehr, Khalid Mohammed Naffati, Salah Abdulhadi Bshina, and Ahmed Abdulmajed Khashkhosha. "Correlation of avian influenza-H9N2 with high mortality in broiler flocks in the southwest of Tripoli, Libya." Open Veterinary Journal 13 (2023), 715-722. doi:10.5455/OVJ.2023.v13.i6.6 MLA (The Modern Language Association) Style Kassem-agha, Ahmed Shaban, Imad Benlashehr, Khalid Mohammed Naffati, Salah Abdulhadi Bshina, and Ahmed Abdulmajed Khashkhosha. "Correlation of avian influenza-H9N2 with high mortality in broiler flocks in the southwest of Tripoli, Libya." Open Veterinary Journal 13.6 (2023), 715-722. Print. doi:10.5455/OVJ.2023.v13.i6.6 APA (American Psychological Association) Style Kassem-agha, A. S., Benlashehr, . I., Naffati, . K. M., Bshina, . S. A. & Khashkhosha, . A. A. (2023) Correlation of avian influenza-H9N2 with high mortality in broiler flocks in the southwest of Tripoli, Libya. Open Veterinary Journal, 13 (6), 715-722. doi:10.5455/OVJ.2023.v13.i6.6 |