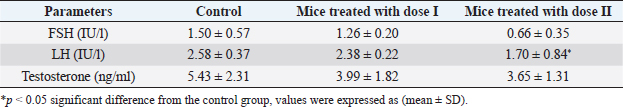| Research Article | ||
Open Vet. J.. 2023; 13(7): 873-878 Open Veterinary Journal, (2023), Vol. 13(7): 873-878 Original Research Sperm abnormality and infertility in male mice treated with the recommended dose of dimethoate and its doubleSamira Musa Sasi1*, Nagia Musa Alghoul2, Nuri Awayn3, Ahmed Elghoul4 and Ragil Angga Prastiya51Zoology Department, Faculty of Science, University of Tripoli, Tripoli, Libya 2Cell and Tissue Culture Department, Biotechnology Center, Tripoli, Libya 3Department of Chemistry, Faculty of Science, University of Tripoli, Tripoli, Libya 4Chemistry Department, Ministry of Interior, Tripoli, Libya 5Department of Reproduction, School of Health and Life Sciences, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Samira Musa Sasi. Zoology Department, Faculty of Science, University of Tripoli, Tripoli, Libya. Email: Samira_18_3 [at] yahoo.com Submitted: 25/02/2023 Accepted: 21/06/2023 Published: 31/07/2023 © 2023 Open Veterinary Journal
AbstractBackground: Dimethoate (DM) is one of the most important organophosphate insecticides used for controlling many pests which affect vegetables, fruits, and agricultural crops, its persistence in soils and crops could cause a health hazard to humans as well as other non-target organisms. Aim: This study was conducted to evaluate the effect of the recommended dose and its double of DM on sex hormones, sperm morphology, and fertility of adult male mice. Methods: Twenty-seven Swiss albino adult male mice were divided into three groups of nine animals each: control group received distilled water only, while other groups received DM orally at doses (0.1 and 0.2 ml DM/100 ml distilled water) for 20 days, at the end of the treatment, six mice from each group were sacrificed. The sperm morphology was evaluated and sex hormones were measured. Three mice from each group were allowed to mate with untreated females (1:2). Result: The results revealed a decrease in luteinizing hormone levels in mice treated with (0.2 ml DM/100 ml distilled water) compared with the control group while the levels of follicle-stimulating hormone and testosterone did not record any significant differences. Also, the results demonstrated a significant increase in abnormal sperm morphology such as head and tail. The fertility was reduced and the average number of dead embryos increased while the average number of live embryos decreased. Conclusion: This current study confirmed that DM has detrimental effects on sperm morphology, fertility, and the embryos; therefore, more efforts should be exerted to protect ourselves and our environment from the harmful effects of this pesticide. Keywords: Dimethoate, Male mice, Reproduction, Sex hormones. IntroductionPesticides are used in agriculture to enhance food production, especially in developing countries (Prakasam et al., 2001) and controlling insects and disease vectors (Farag et al., 2007). They can be harmful to humans and animals (Khogali et al., 2005) and cause environmental contamination (Tuzmen et al., 2008). Organophosphate insecticides (OPI) constitute one of the most widely used classes of pesticides for pest control (Selimen et al., 2014). They have toxic effects on many organs and systems such as the immune system, reproductive system, and nervous system (Yasin and Sharma, 2013), and can alter the genetic material particularly chromosomes in mammalian culture (Jamil et al., 2004). In addition, several physiological and behavioral dysfunctions occurred after exposure to light doses of OPI (Ambali et al., 2011; Ngoula et al., 2014). Dimethoate (DM) is one of the most used OPI for controlling many pests as botflies in livestock (Abouamer et al., 2013), the insects affecting vegetables, fruits and agricultural crops (Mandal et al., 2002, 2008; Abouamer et al., 2013). It can affect humans and wildlife in their natural habitats (Ngoula et al., 2014). Moreover, DM products are moderately toxic to higher vertebrates by ingestion, inhalation, and dermal absorption (Verma and Mohanty, 2009). Several studies reported that DM has harmful effects on liver (Heikal et al., 2011; Saafi et al., 2011; Al-Ali et al., 2016), ovary (Farag et al., 2007), brain (Astiz et al., 2009), blood parameters (Yasin and Sharma, 2013), the embryos (Sasi et al., 2013) and also induce DNA damage in mice bone marrow (Ayed-Boussema et al., 2012). Many studies have shown that male mice treated with DM caused a decrease in sperm motility and an increase in the percentage of abnormal sperm (Jallouli et al., 2015; Sasi et al., 2018a, 2018b), as well as structural alterations in testicular tissues (Bakir et al., 2020; Sasi and ELGhoul, 2021). The main objective of this study was to investigate the effect of the recommended dose of DM and its double on sex hormones, sperm morphology, and fertility in adult male mice. Materials and MethodsChemicalsDM (40% EC, good quality, Germany) which was used in this study was bought from Soliman Khater Market (Tripoli-Libya) in the shape of a volumetric bottle (250 ml). It was used to prepare the required doses. The working solution was prepared weekly and maintained in dark glass bottles at room temperature (25°C). Experimental animalsTwenty-seven Swiss albino male mice weighing between (20 and 27 g), aging between 8 to 12 weeks and free of any pathogen or external parasites. The mice were bred in the animal house of the Department of Zoology, Faculty of Science, University of Tripoli, Libya. They were housed in plastic cages containing wooden flakes which were changed weekly and kept under controlled temperature conditions (22°C ± 3°C) and a normal photoperiod of 12 hours dark/12 hours light. They were given a standard diet and water ad libitum. Experimental designThe animals were randomly divided into three groups of nine mice each and were put in separate cages and treated as follows: group I: control mice were orally given distilled water, while groups II and III received DM by gavage at the doses; dose I (0.1 ml DM/100 ml distilled water) and dose II (0.2 ml DM/100 ml distilled water), the experiments were continued for 20 days. Sperm suspension preparationAfter 24 hours of the last day of the treatment, six mice from each group were weighed prior to sacrifice. The mice were sacrificed by rapid cervical dislocation. Sperm samples were collected by squeezing vas deferens into 1 ml of physiological normal saline (0.9% NaCl) in a small dish. The sperm suspension was incubated for 10 minutes at 37°C to prevent sperm adherence, while the rest number of the mice from each group were used for a fertility test. Determination of sperm morphologySperm morphology observation was done by making smears from sperm suspension. One drop of sperm suspension was placed on a clean slide and smears were made and allowed to dry in air. Smears were stained with 1% eosin for 10 minutes. Sperm morphology was observed at 400× magnification using a light microscope, and then the normal and abnormal percentages of sperm were determined. Sperm with normal morphology and abnormalities in the head, mid-piece, and tail were assessed according to Otitoloju et al. (2010). Hormones measurementBlood samples were collected from the facial vein of control and treated animals. The samples were introduced into EDTA-containing tubes and were labeled and then sent for analysis. Luteinizing hormone (LH), follicle stimulating hormone (FSH), and testosterone were assayed using an ELISA hormone test kit. Fertility testAfter 24 hours of the last day of the treatment, each male treated with DM was mated with two untreated females overnight. Once the vaginal plug was observed, each female was caged separately. On 18 days of gestation, pregnant mice were killed by cervical dislocation, and the embryos were removed from the uterus. The number of live and dead embryos was determined and their body length was recorded. Table 1. Effect of DM on the level of sex hormones in adult male mice.
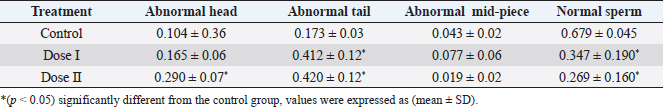
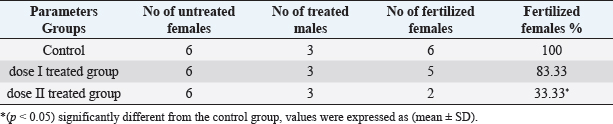
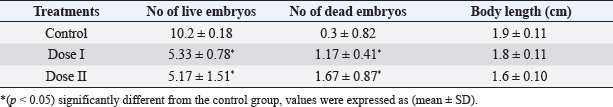
Table 2. Effect of different doses of DM on sperm morphology.
Statistical analysisAll data were analyzed using SPSS (version 20) by one-way analysis of variance (ANOVA) followed by post-hoc Duncan, s test for multiple comparisons. ANOVA was used to determine the level of significance between the control and the treated groups, p < 0.05 was considered statistically significant. The data were expressed here as mean ± standard deviation (mean ± SD). Ethical approvalEthical approval for the experimental protocol of the study was obtained from the Ethics Committee of University of Tripoli (Ref No; SREC 17-2022). ResultsEffect of different doses of DM on the level of sex hormonesNo animals died during the experimental period. The results in (Table 1) showed a significant decrease (p < 0.05) in LH level in the mice treated with dose II of DM in comparison with the control and dose I treated group. Levels of FSH and testosterone hormone declined but this reduction was not significant (p > 0.05) among the treated groups and the control group. Effect of different doses of DM on sperm morphologyMorphological analysis of sperm in this study revealed a significant decrease (p < 0.05) in the percentage of spermatozoa with normal morphology in all treated groups compared to the control group. A significant increase (p > 0.05) of sperm with abnormal heads was recorded in the dose II treated group as compared to the control and dose I treated groups. A significant increase (p < 0.05) in the percentage of sperm with abnormal tails was observed in all treated groups compared to control (Table 2). DM had no effect on the percentage of sperm with abnormal mid-piece (p > 0.05) (Table 2). The types of abnormal sperm in this study included sperm with abnormal tails and sperm with abnormal heads compared to normal morphology of sperm (Figs. 1–3). Effect of different doses of DM on the fertilityThe results showed that fertility reduced significantly with treated males when mated with normal virgin females (Table 3). Effect of different doses of DM on embryos of untreated females impregnated by DM-treated malesThe results revealed a significant increase (p < 0.05) in the average number of dead embryos of females impregnated by treated males with dose II of DM and a significant decrease (p < 0.05) in the average number of live embryos of females impregnated by treated males. The body length of embryos of females impregnated by males received DM did not record any significant differences (p > 0.05) when compared to embryos of females impregnated by males of the control group (Table 4). DiscussionThis study was conducted to assess the effects of oral administration of DM on sperm morphology, sex hormones, fertility, and embryos. Our results demonstrated that administration of the recommended dose and the double recommended dose of DM pesticide to male mice caused a significant decline in LH level in the group treated with dose II and non-significant changes in the levels of FSH and testosterone in the treated groups as compared to the control. Similar results were done by Choudhary et al. (2008) and Joshi et al. (2007) revealed that DM led to a decrease in LH level. Ali and Ibrahim (2018) reported that exposure of male mice to pesticide malathion resulted in a significant decline in LH hormone. The reduction in LH level may be attributed to the effect of organophosphorus pesticides including DM on the pituitary-testicular axis (Tarmura et al., 2001; Verma and Mohanty, 2009), or by disrupting the central nervous system (Colborn, 2006) via suppression of releasing of brain’ hormones which stimulate FSH and LH secretion (Tarmura et al., 2001). Our study contradicted previous studies indicating that exposure of male rats to malathion pesticides (Ali and Ibrahim, 2018) and dichlorvos pesticide (Ezeji et al., 2015) caused a drop in testosterone levels. Zidan (2009) stated that treatment of male rats with mixed pesticides (chlorpyrifos, diazinon, and profenofos) added to powdered food at concentrations of 5 and 50 ppm of each pesticide for 65 days, led to a decrease in the serum testosterone in all treated groups and FSH level decreased with the highest concentration of the tested pesticides. This controversy in results among different studies may be attributed to the type of pesticide, the period of treatment, route of administration.
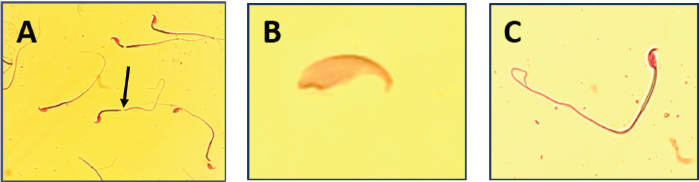
Fig. 1. Normal sperm with a hook (400×).
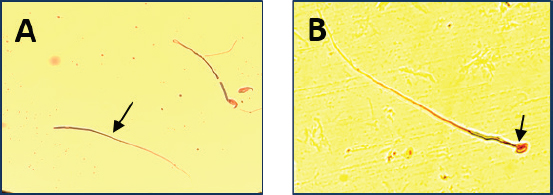
Fig. 2. Microscopic observation of sperm tail abnormalities (400×). (A) Irregular tail; (B) tailless head; and (C) coiled tail.
Fig. 3. Microscope observation of sperm head abnormalities (400×). (A) Headless tail and (B) amorphous head. Table 3. Effect of different doses of DM on fertility.
Table 4. Effect of different doses of DM on embryos of untreated females mated with treated males.
Treatment of male mice with DM for 20 days caused an increase in the percentage of abnormal spermatozoa compared with the control group, this was in concord with previous studies (Trivedi et al., 2010; Sasi et al., 2018a, 2018b). The results of the present study also support previous findings obtained by many other researchers. Kata (2018) stated that dichlorvos pesticide-induced a significant increase in sperm abnormalities in male mice treated intraperitoneal for 15 days. Akinwande et al. (2019) have reported that rats treated with chlorpyrifos pesticide for 3 weeks have a higher frequency of sperm abnormalities, especially at the tail and neck regions. Gaber et al. (2023) illustrated that long-term administration of malathion to male rats (3 times/week) for 60 days led to a significant increase in sperm abnormalities. These abnormalities in sperm may be attributed to the abnormal structure of microtubules in sperm that causes morphological abnormalities in the tail and to sperm DNA damage that causes morphological abnormalities in the head (Creasy, 2001; Trivedi et al., 2010), or a result of abnormal chromosomes (Narayana et al., 2002), or minor alteration in testicular DNA (Giri et al., 2002). Agunbiade et al. (2012) reported that abnormalities may be caused by errors in the spermatozoa differentiation process during spermatogenesis. Bruce and Heddle (1979) found that the chromosomal aberrations which occur during the packaging of genetic materials in sperm heads or point mutations in testicular DNA led to sperm abnormalities. Our results also revealed a decline in fertility. Similar results were also reported by Ngoula et al. (2014) and Petrelli et al. (2003). This reduction in fertility may be due to a decrease in sperm motility or an increase in sperm abnormalities (Jallouli et al., 2015; Sasi et al., 2018a, 2018b) or an increase in the level of malondialdehyde which affects fertility (Vasudeva et al., 2008). The current study demonstrated that untreated females impregnated by DM-treated males gave fewer number of viable embryos. The same result was obtained by Sasi et al. (2018b) and Sasi and EL-Ghoul (2021). The possible reason for the effect of DM on the embryos may be attributed to alterations in sperm DNA of male mice treated with DM by overproduction of free radicals (Navarro and Bustos, 2014; Bakir et al., 2020). ConclusionIn conclusion, the results of the present study have shown that administration of DM orally to adult male mice during 20 days affected sperm morphology, sex hormones, fertility, and also on the embryos. Therefore, it is better to avoid the increased risk of pesticide use and the application of an integrated control program that contributes significantly to reducing the number of different insect pests. AcknowledgmentsThe authors express their deepest appreciation to Prof. Dr. Nasser Salama at the Zoology Department, Faculty of Science, University of Tripoli, for the revision of this paper. Conflict of interestAll authors declare that there is no conflict of interest. FundingNone. Authors contributionSMS and NMA designed, organized, and conducted the study; SMS and NMA wrote the manuscript and performed the analysis of data; all other authors revised and approved the final manuscript. Availability of dataAll data supporting the findings of this study are available within the paper. ReferencesAbouamer, W., Abu-Shaeir, W. and Bakry, S. 2013. Dimethoate induced intrauterine growth retardations in mice. Am-Eurasian. J. Toxicol. Sci. 5(4), 85–93. Agunbiade, S.O., Okonko, I.O., Alimba, C.G., Folarin, A.C. and Anugweje, K.C. 2012. Effects of a carbonaceous bottling plant effluent on albino mice sperm morphology and testes histopathology. Nat. Sci. 10(8), 154–160. Akinwande, D.V., Adeyem, J.A., Olawayi, S.T., Akinola, B.K. and Adedire, C.O. 2019. Protective effects of Camella sinensis on Syzygium aromaticum or chloropyrifos-induced reproductive toxicity in male wistar rats. J. Basic. Appl. Zool. 80(84), 2–9. Al-Ali, A.A., Kata, F.S. and Hussein, S.M. 2016. Biochemical and histopathological changes of dimethoate in some organs of laboratory mice Mus muculus. Basrah. J. Agri. Sci. 29(2), 162–186. Ali, R.I. and Ibrahim, M.A. 2018. Malathion induced testicular toxicity and oxidative damage in male mice: the protective effect of curcumin. Egypt. J. Forensic. Sci. 8(70), 1–13. Ambali, S.F., Shittu, M., Ayo, J.O., Esievo, K.A.N. and Ojo, S.A. 2011. Vitamin C alleviates chronic chlorpyrifos induced alterations in serum lipids and oxidative parameters in male wistar rats. Am. J. Pharmacol. Toxicol. 6(4), 109–118. Astiz, M., de Alaniz, M.J. and Marra, C.A. 2009. Effect of pesticides on cell survival in liver and brain rat tissue. Ecotoxicol. Environ. Saf. 72(7), 2025–2032. Ayed-Boussema, I., Rjiba, K., Mnasri, N., Moussa, A. and Bacha, H. 2012. Genotoxicity evaluation of dimethoate to experimental mice by micronucleus, chromosome aberration tests and comet Assay. Int. J. Toxicol. 31(1), 78–85. Bakir, E., Sariozkan, S., Endirlik, B.U., Kili, A.B., Yay, A.H., Tan, F.C., Eken, A. and Turk, G. 2020. Cherry laurel fruit extract counters dimethoate induced reproductive impairment and testicular apoptosis. Art. Hig. Rada. Toksikol. 71, 329–338. Bruce, W. and Heddle, J. 1979. The mutagenicity of 61 agents as determined by the micronucleus, Salmonella and sperm abnormality assays. Can. J. Cytol. Genet. 21, 319–334. Choudhary, N., Goyal, R. and Joshi, S.C. 2008. Effect of malathion on reproductive system of male rats. J. Environ. Biol. 29(2), 259–262. Colborn, T. 2006. A case for revisiting the safety of pesticides: a closer look at neurodevelopment. Environ. Health. Prospect. 114(1), 10–17. Creasy, D.M. 2001. Pathogenesis of male reproductive toxicity. Toxicol. Pathol. 29(1), 64–76. Ezeji, E.U., Ogueri, D.O., Udebuani, A.C., Okereke, J.N. and Obasi, K.O. 2015. Effect of dichlorvos on fertility of male albino rats. Nat. Sci. 13(12), 1–5. Farag, A.T., El-Aswad, A.F. and Shaaban, N.A. 2007. Assessment of reproductive toxicity of orally administered technical dimethoate in male mice. Reprod. Toxicol. 23(2), 232–238. Gaber, M.A., Saleem, T.H., Nassar, A.Y., Hozyen, H.F., Mahmoud, M.G. and Ali-Hussein, H.A. 2023. Effect of long-term malathion administration on testosterone, oxidative stress and sperm characteristics in rats. Assiut. Vet. Med. J. 69(176), 55–64. Giri, S., Prasad, S.B., Giri, A. and Sharma, G.D. 2002. Genotoxic effect of malathion: an organophosphorus insecticide, using three mammalian bio assays in vivo. Mutat. Res. 514, 223–231. Heikal, T.M., Ghanem, H.Z. and Soliman, M.S. 2011. Protective effect of green tea extracts against dimethoate induced DNA damage and oxidant/antioxidant status in male rats. Bio. Health. Sci. Bull. 3(1), 1–11. Jallouli, M., EI Bini Dhouib, I., Dhouib, H., Gharbi, N. and EI fazzaa, S. 2015. Effects of dimethoate in male mice reproductive parameters. Regulatory. Toxicol. Pharmacol. 73, 853–858. Jamil, K., Shaik, A.P., Mahboob, M. and Krishna, D. 2004. Effect of organophosphorus and organochlorine pesticides (monochrotophos, chlorpyriphos, dimethoate, and endosulfan) on human lymphocytes in-vitro. Drug. Chem. Toxicol. 27(2), 133–144. Joshi, S.C., Mathur, R. and Gulati, N. 2007. Testicular toxicity of chlorpyrifos (an organophosphate pesticides) in albino rat. Toxicol. Ind. Health. 23(7), 439–444. Kata, F.S. 2018. Effect of dichlorvos pesticide on fertility of laboratory male mice (Mus musculus L). Basrah. J. Vet. Res. 7(1), 9–18. Khogali, F.A., Sheikh, J.B., Abdel Rahman, S., Rahim, A.A. and Daghestani, M.H. 2005. Histopathological and hematological effects of dimethoate 40EC on some organs of albino mice. J. King. Saud. Univ. 18(2), 73–87. Mandal, D.M., Mandal, S. and Pal, N.K. 2002. Evaluation of bioremediation potential of organophosphorus pesticide dimethoate 30% EC by heavy metal and antibiotic resistant Proteus vulgaris isolated from Ganges at Sreerampore, India. Res. J. Chem. Environ. 6, 49–52. Mandal, D.M., Mandal, S., Pal, N.K. and Aich, A. 2008. Potential metabolites of dimethoate produced by bacterial degradation. World. J. Microbiol. Biotechnol. 24(1), 69–72. Narayana, K., D’Souza, U.J. and Seetharama Rao, K.P. 2002. Ribavirin-induced sperm shape abnormalities in wistar rat. Mutat. Res. 513(1–2), 193–196. Navarro, E. and Bustos, E. 2014. Effect of malathion on cellularity and sperm differentiation in testes and epididymis of adult rats. Int. J. Morphol. 32(1), 119–124. Ngoula, F., Watcho, P., Kenfack, A., N’zouk Manga, J., Fualefac Defang, H., Pierre, K. and Joseph, T. 2014. Effect of dimethoate (an organophosphate insecticide) on the reproductive system and fertility of adult male rat. Am. J. Pharmacol. Toxicol. 9(1), 75–83. Otitoloju, A.A., Obe, I.A., Adewale, O.A., Otubanjo, O.A. and Osunkalu, V.O. 2010. Preliminary study on the induction of sperm head abnormalities in mice, Mus musculus, exposed to radiofrequency radiations from global system for mobile communication base stations. Bull. Environ. Contam. Toxicol. 84, 51–54. Petrelli, G., Figa-Talamanca, I., lauria, L. and Mantovani, A. 2003. Spontaneous abortion in spouses of greenhouse workers exposed to pesticides. Environ. Health. Perspect. Med. 8(3), 77–81. Prakasam, A., Sethupathy, S. and Lalitha, S. 2001. Plasma and RBCs antioxidant status in occupational male pesticide sprayers. Clin. Chem. Acta. 310(2), 107–112. Saafi, E., Louedi, M., Elfeki, A., Zakhama, A., Najjar, M., Hammami, M. and Achour, L. 2011. Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Experi. Toxicol. Pathol. 63(5), 433–441. Sasi, S.M., AL-Ghoul, N.M. and AL-Shakshouki, F.M. 2018a. Effect of early exposure of dimethoate on reproductive potential in Swiss male mice. Syrian. J. Agri. Res. 5(2), 212–221. Sasi, S.M. and EL-Ghoul, N.M. 2021. Effect of the recommended dose of dimethoate and its double on the embryos and testicular tissues in male mice. J. Mar. Sci. Environ. Technol. 7(1), 29–35. Sasi, S.M., Sasi, N.M., Abdulaziz, A., Esa, M. and Almsri, M. 2018b. Effect of dimethoate (Insecticide) on reproductive function in adult male mice. J. Libyan. Studies. 2(15), 1–12. Sasi, S.M., Sasi, N.M. and Dawd, D.S. 2013. Effect of dimethoate on pregnant female mice and their embryos. J. Basic. Appl. Sci. 19(2), 33–51. Selimen, S., Saloua, F. and Najoua, G. 2014. Oxidative stress and cytotoxic potential of anticholinesterase insecticide, malathion in reproductive toxicology of male adolescent mice after acute exposure. Iran. J. Basic. Med. Sci. 17(7), 522–530. Tarmura, H., Maness, S.C., Reischmann, K., Dorman, D.C., Gray, L.E. and Gaido, K.W. 2001. Androgen receptor antagonism by the organophosphate insecticide fenitrothion. Toxicol. Sci. 60, 56–62. Trivedi, P.P., Kushwaha, S., Tripathi, D.N. and Jena, G. 2010. Evaluation of male germ cell toxicity in rats: correlation between sperm head morphology and sperm comet assay. Mutat. Res. Gen. Toxicol. En. 703(2), 115–121. Tuzmen, N., Candan, N., Kaya, E. and Demiryas, N. 2008. Biochemical effects of chlorpyrifos and deltamethrin on altered antioxidative defense mechanisms and lipid peroxidation in rat liver. Cell. Bio. Chem. Funct. 26(1), 119–124. Vasudeva, K., Apurva, K.R. and Rajini, P.S. 2008. Dimethoate induced biochemical perturbations in rat’s pancreas and its attenuation by cashew nut skin extract. Pesticide. Biochem. Physiol. 90, 58–65. Verma, R. and Mohanty, B. 2009. Early-life exposure to dimethoate-induced reproductive toxicity: evaluation of effects on pituitary-testicular axis of mice. Toxicol. Sci. 112(2), 450–458. Yasin, M. and Sharma, P. 2013. Effect of dimethoate 30EC on some hematological parameters of albino mice following an oral exposure. Int. J. Recent. Sci. Res. 4(9), 1323–1326. Zidan, N.A. 2009. Evaluation of the reproductive toxicity of chlorpyrifos methyl, Diazinon and profenofos pesticides in male rats. Int. J. Pharm. 5(1), 51–57. | ||
| How to Cite this Article |
| Pubmed Style Sasi SM, Alghoul NM, Awayn N, Elghoul A, Prastiya RA. Sperm abnormality and infertility in male mice treated with the recommended dose of dimethoate and its double. Open Vet. J.. 2023; 13(7): 873-878. doi:10.5455/OVJ.2023.v13.i7.9 Web Style Sasi SM, Alghoul NM, Awayn N, Elghoul A, Prastiya RA. Sperm abnormality and infertility in male mice treated with the recommended dose of dimethoate and its double. https://www.openveterinaryjournal.com/?mno=143912 [Access: January 24, 2026]. doi:10.5455/OVJ.2023.v13.i7.9 AMA (American Medical Association) Style Sasi SM, Alghoul NM, Awayn N, Elghoul A, Prastiya RA. Sperm abnormality and infertility in male mice treated with the recommended dose of dimethoate and its double. Open Vet. J.. 2023; 13(7): 873-878. doi:10.5455/OVJ.2023.v13.i7.9 Vancouver/ICMJE Style Sasi SM, Alghoul NM, Awayn N, Elghoul A, Prastiya RA. Sperm abnormality and infertility in male mice treated with the recommended dose of dimethoate and its double. Open Vet. J.. (2023), [cited January 24, 2026]; 13(7): 873-878. doi:10.5455/OVJ.2023.v13.i7.9 Harvard Style Sasi, S. M., Alghoul, . N. M., Awayn, . N., Elghoul, . A. & Prastiya, . R. A. (2023) Sperm abnormality and infertility in male mice treated with the recommended dose of dimethoate and its double. Open Vet. J., 13 (7), 873-878. doi:10.5455/OVJ.2023.v13.i7.9 Turabian Style Sasi, Samira Musa, Nagia Musa Alghoul, Nuri Awayn, Ahmed Elghoul, and Ragil Angga Prastiya. 2023. Sperm abnormality and infertility in male mice treated with the recommended dose of dimethoate and its double. Open Veterinary Journal, 13 (7), 873-878. doi:10.5455/OVJ.2023.v13.i7.9 Chicago Style Sasi, Samira Musa, Nagia Musa Alghoul, Nuri Awayn, Ahmed Elghoul, and Ragil Angga Prastiya. "Sperm abnormality and infertility in male mice treated with the recommended dose of dimethoate and its double." Open Veterinary Journal 13 (2023), 873-878. doi:10.5455/OVJ.2023.v13.i7.9 MLA (The Modern Language Association) Style Sasi, Samira Musa, Nagia Musa Alghoul, Nuri Awayn, Ahmed Elghoul, and Ragil Angga Prastiya. "Sperm abnormality and infertility in male mice treated with the recommended dose of dimethoate and its double." Open Veterinary Journal 13.7 (2023), 873-878. Print. doi:10.5455/OVJ.2023.v13.i7.9 APA (American Psychological Association) Style Sasi, S. M., Alghoul, . N. M., Awayn, . N., Elghoul, . A. & Prastiya, . R. A. (2023) Sperm abnormality and infertility in male mice treated with the recommended dose of dimethoate and its double. Open Veterinary Journal, 13 (7), 873-878. doi:10.5455/OVJ.2023.v13.i7.9 |