| Case Report | ||
Open Vet J. 2023; 13(6): 801-806 Open Veterinary Journal, (2023), Vol. 13(6): 801-806 Case Report Efficacy and adverse events of anthracycline and propranolol combination in five dogs with stage 3 hemangiosarcomaMitsuhiko Terauchi1†, Yuji Fujii1,2†, Sho Goto1, Ryota Iwasaki1, Ryutaro Yoshikawa1 and Takashi Mori1,2*1Animal Medical Center, Faculty of Applied Biological Sciences, Gifu University, Gifu, Japan 2Joint Graduate School of Veterinary Sciences, Gifu University, Gifu, Japan †Contributed equally to this study. *Corresponding Author: Takashi Mori. Animal Medical Center, Faculty of Applied Biological Sciences, Gifu University, Gifu, Japan. Email: tmori [at] gifu-u.ac.jp Submitted: 27/02/2023 Accepted: 11/05/2023 Published: 25/06/2023 © 2023 Open Veterinary Journal
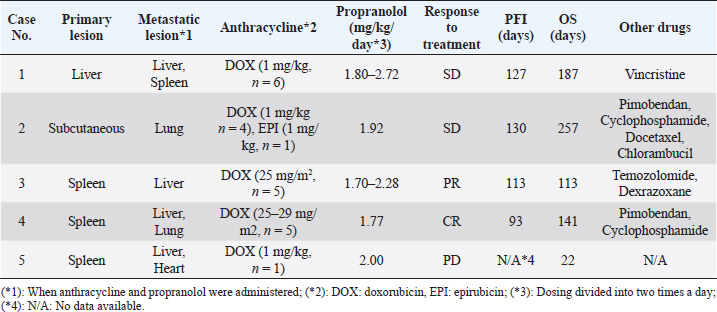
AbstractBackground: Canine hemangiosarcoma (HSA), which originates from endothelial cells, is one of the most common malignant neoplasms that frequently develop metastatic lesions. Although anthracycline-based HSA treatment strategies have been widely investigated, reliable therapy for dogs with clinically advanced-stage HSA (stage 3 HSA) has not been established yet. Recently, several studies have demonstrated that propranolol, a beta-adrenergic receptor antagonist, exhibits anti-tumor effects against tumors originating from vascular endothelial cells, indicating the possibility that propranolol is a candidate adjunctive agent for anthracycline-based therapy in dogs with stage 3 HSA. This study aimed to evaluate the clinical efficacy and adverse events (AEs) of anthracycline and propranolol combination in stage 3 HSA-affected dogs. Case Description: We retrospectively investigated five dogs diagnosed with stage 3 HSA which were administered with both anthracycline and propranolol during the same period between January 2020 and August 2021. Clinical benefit was observed in four of five HSA dogs (one of complete response, one of partial response, and two of stable disease) with gross metastatic lesions by anthracycline and propranolol combination. Notably, some or all of the metastatic lesions were reduced in two cases. In all five dogs administered with anthracycline and propranolol combination, no serious and irreversible AEs were observed. Conclusion: Our findings demonstrate the efficacy and safety of anthracycline and propranolol combination in stage 3 HSA-affected dogs. Further studies are needed to establish treatment protocols based on anthracycline and propranolol combination for dogs with advanced HSA. Keywords: Hemangiosarcoma, Stage 3, Dog, Anthracycline, Propranolol. IntroductionCanine Hemangiosarcoma (HSA) is one of the most major malignant neoplasm (Vail et al., 2019). HSA that originates from either endothelial cells or their precursors (Lamerato-Kozicki et al., 2006) can occur in any organ of the body, but most frequently in the spleen, heart, liver, and skin of affected dogs (Brown et al., 1985; Prymak et al., 1988; Griffin et al., 2021). HSA tumors can easily rupture due to their composition of abnormal blood and blood vessels, subsequently causing serious local problems, including hemorrhage in the body cavity (Griffin et al., 2021). On the other hand, HSA is known to be highly metastatic (Prymak et al., 1988). Thus, it is important to prevent the progression of metastatic lesions as well as surgical resection of the primary lesion in the treatment of dogs with HSA, especially those in the advanced clinical stage (stage 3 HSA). Various chemotherapy protocols have been investigated to prevent the progression of HSA (Sharun, 2019; Griffin et al., 2021). More specifically, doxorubicin (DOX) or epirubicin (EPI), which are anthracycline anti-tumor drugs, prolong the survival time of dogs with HSA compared to that of untreated dogs (Brown et al., 1985; Kim et al., 2007). Several studies have demonstrated the utility of treatment protocols that combine anthracyclines with various adjunctive chemotherapeutic agents, including cyclophosphamide and vincristine (Alvarez et al., 2013; Kahn et al., 2013; Finotello et al., 2017). These findings indicate that anthracycline-based chemotherapy is a useful option for preventing HSA progression. However, there is limited evidence of sufficient tumor shrinkage in these reports, and thus, the existing anthracycline-based protocols may remain palliative (Sharun, 2019). On the other hand, there is even more limited evidence to indicate the utility of chemotherapy for dogs with stage 3 HSA (Sharun, 2019). For instance, one previous study reported that the treatment protocol combining DOX, dacarbazine, and vincristine for dogs with nonresectable or stage 3 HSA showed a response rate of 47% (Dervisis et al., 2011). Another study revealed an 89% response rate in stage 3 HSA dogs receiving the combined protocol of DOX, vincristine, and cyclophosphamide (Alvarez et al., 2013). However, because both treatment protocols were aggressive chemotherapy, up to nearly half of the dogs required treatment deferral or dose reductions due to severe hematologic and gastrointestinal toxicities (Dervisis et al., 2011; Alvarez et al., 2013). Therefore, it is necessary to establish novel anti-tumor strategies with higher efficacy and safety in dogs with stage 3 HSA. Recently, there has been accumulating evidence that propranolol, a beta-adrenergic receptor antagonist, has an anti-tumor effect against cancers originating from vascular endothelial cells (Wagner et al., 2018). For instance, propranolol is effective against benign infantile hemangiomas in human patients (Banavali, 2015; Daguzé et al., 2016; Pasquier et al., 2016). Furthermore, a more recent study using cultured cells showed that propranolol treatment enhances the effects of DOX on HSA affecting several animals, including dogs (Saha et al., 2021). These findings suggest that propranolol is a potential adjunctive agent in the anthracycline-based therapy of dogs with HSA in advanced clinical stages. However, no studies have examined the clinical application of propranolol in dogs with stage 3 HSA. In this study, we retrospectively evaluated the efficacy and adverse events (AEs) of anthracycline and propranolol (AP) in dogs with stage 3 HSA. Case DetailsCase selection and evaluationWe retrospectively evaluated dogs diagnosed with stage 3 HSA that were administered both anthracyclines and propranolol simultaneously between January 2020 and August 2021. The diagnosis of HSA was based on cytological and/or histopathological examination. HSA staging was performed according to the World Health Organization staging system (Vail et al., 2019). We evaluated only HSA cases with complete records of signalment, history, chief complaint, physical examination findings, abdominal and thoracic cavity imaging, treatment with AP combination for at least 3 weeks, and appropriate follow-up including documentation of AEs. Dogs with insufficient data were excluded from this study. We evaluated the treatment response, endpoints, and AEs in dogs with stage 3 HSA treated with AP combination. The treatment response was classified into four categories (complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD)) according to the canine Response evaluation criteria for solid tumors (Nguyen et al., 2015) during the AP administration period. Endpoints were evaluated using the progression-free interval (PFI) and overall survival (OS). Specifically, PFI was defined as the number of days from the initiation of AP combination therapy to tumor growth or development of new lesions. OS was defined as the number of days from the initiation of AP combination therapy to death. AEs during the treatment period were retrospectively evaluated according to VCOG-CTCAE (LeBlanc et al., 2021). Ethical approvalThe authors confirm that the ethical policies of the journal have been followed and that no ethical approval was required for this study. Case informationWe examined five dogs with stage 3 HSA selected based on the above-mentioned criteria (Table 1). The dogs belonged to the breeds Golden Retriever (n=1), French Bulldog (n=1), Maltese (n=1), Miniature Dachshund (n=1), and mixed breed (n=1). Four dogs were female (one intact and three neutered) and one was male (one intact). The median age at diagnosis was 10 years (range, 8–14 years). The median weight was 4.26 kg (range, 2.76–23.5 kg). In all cases, computed tomography (CT) was performed at diagnosis, and the primary and metastatic lesions were identified (Table 1). The primary lesions were found in the spleen (n=3), liver (n =1), and subcutaneous tissue (n=1). At the initiation of AP combination therapy, metastatic lesions were found in the liver (n=4), lung (n=2), heart (n=1), and spleen (n=1). Treatment and clinical courseIn all five dogs, chemotherapy was initiated after surgical resection of the primary lesion (Table 1). All the dogs were treated with anthracycline, DOX, or EPI. Specifically, the treatment protocol was DOX (1 mg/kg or 25–29 mg/m2) or EPI (1 mg/kg), administered every 3–4 weeks. Anthracycline therapy was administered 1–6 times (median, 5 times; total, 22 times). Additionally, all dogs were treated with propranolol [0.85–1.36 mg/kg, every 12 hours (q12h), orally]. In two dogs (case 2 and 4), a low dose of propranolol (0.44–0.63 mg/kg, q12h, orally) was preliminarily administered before starting the AP therapy, and AEs, especially those on blood pressure, were not observed (data not shown). Other drugs included pimobendan (n=2), cyclophosphamide (n=2), vincristine (n=1), docetaxel (n=1), chlorambucil (n=1), temozolomide (n=1), and dexrazoxane (n=1), and all of them were administered according to existing protocols. The details of each case are as follows. Case 1 was treated with DOX six times combined with propranolol. During AP combination therapy, there was no evidence of tumor progression. Thereafter, the dog was treated with propranolol alone. On day 127, PD was observed in the muscles, liver, and lungs, and vincristine treatment was initiated. After three cycles of vincristine administration, the dog died on day 187 owing to tumor progression. Table 1. Case information and outcomes.
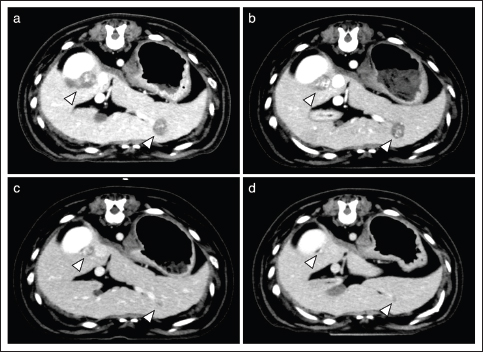
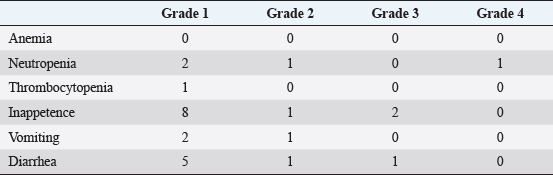
Case 2 was treated continuously with cyclophosphamide after the surgical removal of the primary lesion. AP combination therapy was initiated 80 days after surgery. More specifically, five cycles of anthracyclines (4 and 1 times of DOX and EPI, respectively) were administered with propranolol. During the AP combination therapy, there was no evidence of tumor progression. Thereafter, the dog was treated with propranolol alone. On day 130, PD was observed in the subcutaneous tissue, and docetaxel treatment was initiated. However, because of unacceptable AEs (gastrointestinal toxicities, including inappetence, vomiting, and diarrhea) with docetaxel, the treatment was replaced with chlorambucil. Finally, new metastatic lesions were successively identified in the lungs and spleen, and the dog died on day 257 because of intraperitoneal rupture of the tumor. Case 3 was untreated for 1 year after surgical resection of the primary lesion, but metastatic lesions were newly identified in the liver. Therefore, the dog was treated with temozolomide 48 days before the initiation of AP combination therapy. However, because of disease progression, the dog was treated with five doses of DOX and concomitant propranolol. After the first dose of DOX, some tumor lesions showed marked shrinkage and largely disappeared (Fig. 1). While the disease remained in remission, the dog died on day 113 because of aspiration pneumonia. Case 4 was treated with DOX five times combined with propranolol. After the first dose of DOX, all tumor lesions showed significant shrinkage and were undetectable on day 71. However, new lesions were identified in the lungs on day 93, and cyclophosphamide was administered in combination with DOX and propranolol. The dog died on day 141 due to tumor progression. Case 5 was treated with DOX and propranolol. However, the dog died on day 22 because of tumor progression before any response to treatment was achieved. Response to treatmentAP combination therapy resulted in clinical benefits (CB) in four of the five dogs with HSA (80%; 1 CR, 1 PR, 2 SD) with gross metastatic lesions (Table 1). The median PFI and OS were 120 (range, 93–130) and 141 (range, 22–257) days, respectively. Adverse eventsAEs observed during AP combination are shown in Table 2. The most observed AE was inappetence (Grade 1: 8/22 anthracycline administration). Severe neutropenia (Grade 4) was observed in one dog (case 2), whereas it was transient and not accompanied by clinical signs including fever. Evaluation of cardiac function revealed reduced fractional shortening (FS) in two dogs (cases 2 and 4). In case 2, a reduction in the FS to 29% was observed on day 109. In case 4, a reduction of FS to 21% was observed after the second DOX treatment (day 29). In both cases, additional administration of pimobendan resulted in prompt improvement of FS. None of the patients developed bradycardia [<60 beats per minute (bpm)] or tachycardia (>150 bpm) during the treatment period. For blood pressure that could be affected by propranolol, although continuous medical record descriptions were unavailable, we did not identify any abnormal findings (data not shown). DiscussionTo the best of our knowledge, this is the first report to evaluate the efficacy and AEs of AP combination therapy in dogs with stage 3 HSA. Our results demonstrate that AP combination therapy provides CB in the majority of cases of HSA (80%) (Table 1). In addition, AEs observed in this study, including myelotoxicities, gastrointestinal toxicities, and cardiac dysfunctions (Table 2), were comparable to those by anthracycline therapy in previous studies (Alvarez et al., 2013; Finotello et al., 2017; Sharun, 2019; Griffin et al., 2021). Notably, none of these AEs were serious and irreversible, and no dogs required treatment deferral or dose reductions due to adverse effects. These findings indicate the potential of propranolol as an effective and safe adjunctive agent to anthracycline-based therapy for dogs with stage 3 HSA. Interestingly, the tumor sizes of the metastatic lesions were reduced in two of the four dogs in which CBs were observed (Table 1). In case 3, the tumor shrank after the initiation of AP combination therapy, even though the tumor had not been responsive to conventional therapy (Fig. 1). Considering such a clinical course, it seems reasonable to conclude that AP combination therapy showed a remarkable anti-tumor effect in these dogs with HSA. Although previous studies have shown that conventional anthracycline-based therapies may contribute to prolonged survival time of dogs with HSA (Alvarez et al., 2013; Finotello et al., 2017; Sharun, 2019; Griffin et al., 2021), there are few reports demonstrating a significant reduction of tumor lesions in dogs with advanced HSA based on these treatments. Additionally, the high CB rate (80%) in the present study is comparable only to that of the aggressive chemotherapy protocols using vincristine, DOX, and cyclophosphamide with severe adverse effect risks (e.g., sepsis) (Alvarez et al., 2013). Considering the safety described above, our findings on anti-tumor responses indicate the possibility that AP combination therapy may contribute to the establishment of useful and well-tolerated treatment strategies in dogs with stage 3 HSA. Despite showing that propranolol may exhibit beneficial effects in combination with anthracycline in dogs with HSA, the present study had several limitations. More specifically, because the present study was retrospective and included a limited number of cases, further investigation is warranted to determine whether the tumor shrinkage observed in this study, which is likely to be clinically beneficial, plays a role in the prognosis of dogs with advanced HSA. Additionally, it is also important to comprehensively examine whether other drugs, including chemotherapeutic agents that are traditionally used for HSA (Alvarez et al., 2013; Finotello et al., 2017; Sharun, 2019; Griffin et al., 2021), provide synergistic effects or AEs in combination with AP in larger studies. Finally, the effects on cardiac function, a possible AE with propranolol (Hengst et al., 2015), will need to be cautiously evaluated, although there were no detailed medical record descriptions and no clinical problems identified in the cases in this study. More especially, as described in the previous study (Loizzi et al., 2013), the evaluation of blood pressure, heart rate, and blood glucose levels in a prior propranolol dose trial would be an important consideration. In the future, these issues need to be verified in order to establish effective treatment strategies based on AP combination for dogs with stage 3 HSA.
Fig. 1. Transition of liver metastatic lesions (Case 3). CT images are shown before (a), and on day 22 (b), 49 (c), and 77 (d) after initiation of the AP combination therapy. Arrowheads indicate metastatic lesions. Table 2. AEs observed after anthracycline administration (n=22).
In conclusion, the present retrospective study demonstrated the efficacy and safety of the AP combination therapy in dogs with stage 3 HSA. Our data provide valuable insights into the development of novel treatment strategies for advanced HSA in dogs. AcknowledgmentsWe acknowledge the help and support of all the staff members at the animal medical center for the care and treatment of the dogs described in this article. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsConceptualization: Takashi Mori. Investigation: Mitsuhiko Terauchi and Yuji Fujii. Writing - the original draft and Visualization: Yuji Fujii, Data curation: Mitsuhiko Terauchi, Yuji Fujii, Sho Goto, Ryota Iwasaki, and Ryutaro Yoshikawa. Writing - review & editing: All authors. ReferencesAlvarez, F.J., Hosoya, K., Lara-Garcia, A., Kisseberth, W. and Couto, G. 2013. VAC protocol for treatment of dogs with stage III hemangiosarcoma. J. Am. Anim. Hosp. Assoc. 49, 370–377. Banavali, S., Pasquier, E. and Andre N. 2015. Targeted therapy with propranolol and metronomic chemotherapy combination: sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience 9, 499. Brown, N.O., Patnaik, A.K. and MacEwen, E.G. 1985. Canine hemangiosarcoma: retrospective analysis of 104 cases. J. Am. Vet. Med. Assoc. 186, 56–58. Daguzé, J., Saint-Jean, M., Peuvrel, L., Cassagnau, E., Quéreux, G., Khammari, A. and Dréno, B. 2016. Visceral metastatic angiosarcoma treated effectively with oral cyclophosphamide combined with propranolol. JAAD Case Rep. 2, 497–499. Dervisis, N.G., Dominguez, P.A., Newman, R.G., Cadile, C.D. and Kitchell, B.E. 2011. Treatment with DAV for advanced-stage hemangiosarcoma in dogs. J. Am. Anim. Hosp. Assoc. 47, 170–178. Finotello, R., Henriques, J., Sabattini, S., Stefanello, D., Felisberto, R., Pizzoni, S., Ferrari, R. and Marconato, L. 2017. A retrospective analysis of chemotherapy switch suggests improved outcome in surgically removed, biologically aggressive canine haemangiosarcoma. Vet. Comp. Oncol. 15, 493–503. Griffin, M.A., Culp, W.T.N. and Rebhun, R.B. 2021. Canine and feline haemangiosarcoma. Vet. Rec. 189(9), e585. Hengst, M., Oelert, M. and Hoeger, P.H. 2015. Blood pressure monitoring during the induction and maintenance period of propranolol therapy for complicated infantile hemangiomas: a prospective study of 109 infants. Pediatr. Dermatol. 32, 802–807. Kahn, S.A., Mullin, C.M., de Lorimier, L.-P., Burgess, K.E., Risbon, R.E., Fred, R.M., Drobatz, K. and Clifford, C.A. 2013. Doxorubicin and deracoxib adjuvant therapy for canine splenic hemangiosarcoma: a pilot study. Can. Vet. J. La Rev. Vet. Can. 54, 237–242. Kim, S.E., Liptak, J.M., Gall, T.T., Monteith, G.J. and Woods, J.P. 2007. Epirubicin in the adjuvant treatment of splenic hemangiosarcoma in dogs: 59 cases (1997-2004). J. Am. Vet. Med. Assoc. 231, 1550–1557. Lamerato-Kozicki, A.R., Helm, K.M., Jubala, C.M., Cutter, G.C. and Modiano, J.F. 2006. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp. Hematol. 34, 870–878. LeBlanc, A.K., Atherton, M., Bentley, R.T., Boudreau, C.E., Burton, J.H., Curran, K.M., Dow, S., Giuffrida, M.A., Kellihan, H.B., Mason, N.J., Oblak, M., Selmic, L.E., Selting, K.A., Singh, A., Tjostheim, S., Vail, D.M., Weishaar, K.M., Berger, E.P., Rossmeisl, J.H. and Mazcko, C. 2021. Veterinary Cooperative Oncology Group—common terminology criteria for adverse events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 19, 311–352. Loizzi, M., De Palma, A., Pagliarulo, V. and Quaranta, N. 2013. Propranolol as first-line treatment of a severe subglottic haemangioma. Eur. J. Cardiothorac. Surg. 43, 187–189. Nguyen, S.M., Thamm, D.H., Vail, D.M. and London, C.A. 2015. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 13, 176–183. Pasquier, E., André, N., Street, J., Chougule, A., Rekhi, B., Ghosh, J., Philip, D.S.J., Meurer, M., MacKenzie, K.L., Kavallaris, M. and Banavali, S.D. 2016. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine 6, 87–95. Prymak, C., McKee, L.J., Goldschmidt, M.H. and Glickman, L.T. 1988. Epidemiologic, clinical, pathologic, and prognostic characteristics of splenic hemangiosarcoma and splenic hematoma in dogs: 217 cases (1985). J. Am. Vet. Med. Assoc. 193, 706–712. Saha, J., Kim, J.H., Amaya, C.N., Witcher, C., Khammanivong, A., Korpela, D.M., Brown, D.R., Taylor, J., Bryan, B.A. and Dickerson, E.B. 2021. Propranolol sensitizes vascular sarcoma cells to doxorubicin by altering lysosomal drug sequestration and drug efflux. Front. Oncol. 10, 1–15. Sharun, K. 2019. Chemotherapeutic management of hemangiosarcoma in canines—an update. Cancer Ther. Oncol. Int. J. 14, 14–16. Vail, D.M., Thamm, D.H. and Liptak, J.M. 2019. Withrow and MacEwen’s small animal clinical oncology, 6th ed. Saunders. Wagner, M.J., Cranmer, L.D., Loggers, E.T. and Pollack, S.M. 2018. Propranolol for the treatment of vascular sarcomas. J. Exp. Pharmacol. 10, 51–58. | ||
| How to Cite this Article |
| Pubmed Style Terauchi M, Fujii Y, Goto S, Iwasaki R, Yoshikawa R, Mori T. Efficacy and adverse events of anthracycline and propranolol combination in five dogs with stage 3 hemangiosarcoma. Open Vet J. 2023; 13(6): 801-806. doi:10.5455/OVJ.2023.v13.i6.15 Web Style Terauchi M, Fujii Y, Goto S, Iwasaki R, Yoshikawa R, Mori T. Efficacy and adverse events of anthracycline and propranolol combination in five dogs with stage 3 hemangiosarcoma. https://www.openveterinaryjournal.com/?mno=144688 [Access: June 01, 2025]. doi:10.5455/OVJ.2023.v13.i6.15 AMA (American Medical Association) Style Terauchi M, Fujii Y, Goto S, Iwasaki R, Yoshikawa R, Mori T. Efficacy and adverse events of anthracycline and propranolol combination in five dogs with stage 3 hemangiosarcoma. Open Vet J. 2023; 13(6): 801-806. doi:10.5455/OVJ.2023.v13.i6.15 Vancouver/ICMJE Style Terauchi M, Fujii Y, Goto S, Iwasaki R, Yoshikawa R, Mori T. Efficacy and adverse events of anthracycline and propranolol combination in five dogs with stage 3 hemangiosarcoma. Open Vet J. (2023), [cited June 01, 2025]; 13(6): 801-806. doi:10.5455/OVJ.2023.v13.i6.15 Harvard Style Terauchi, M., Fujii, . Y., Goto, . S., Iwasaki, . R., Yoshikawa, . R. & Mori, . T. (2023) Efficacy and adverse events of anthracycline and propranolol combination in five dogs with stage 3 hemangiosarcoma. Open Vet J, 13 (6), 801-806. doi:10.5455/OVJ.2023.v13.i6.15 Turabian Style Terauchi, Mitsuhiko, Yuji Fujii, Sho Goto, Ryota Iwasaki, Ryutaro Yoshikawa, and Takashi Mori. 2023. Efficacy and adverse events of anthracycline and propranolol combination in five dogs with stage 3 hemangiosarcoma. Open Veterinary Journal, 13 (6), 801-806. doi:10.5455/OVJ.2023.v13.i6.15 Chicago Style Terauchi, Mitsuhiko, Yuji Fujii, Sho Goto, Ryota Iwasaki, Ryutaro Yoshikawa, and Takashi Mori. "Efficacy and adverse events of anthracycline and propranolol combination in five dogs with stage 3 hemangiosarcoma." Open Veterinary Journal 13 (2023), 801-806. doi:10.5455/OVJ.2023.v13.i6.15 MLA (The Modern Language Association) Style Terauchi, Mitsuhiko, Yuji Fujii, Sho Goto, Ryota Iwasaki, Ryutaro Yoshikawa, and Takashi Mori. "Efficacy and adverse events of anthracycline and propranolol combination in five dogs with stage 3 hemangiosarcoma." Open Veterinary Journal 13.6 (2023), 801-806. Print. doi:10.5455/OVJ.2023.v13.i6.15 APA (American Psychological Association) Style Terauchi, M., Fujii, . Y., Goto, . S., Iwasaki, . R., Yoshikawa, . R. & Mori, . T. (2023) Efficacy and adverse events of anthracycline and propranolol combination in five dogs with stage 3 hemangiosarcoma. Open Veterinary Journal, 13 (6), 801-806. doi:10.5455/OVJ.2023.v13.i6.15 |