| Case Report | ||
Open Vet. J.. 2023; 13(5): 668-676 Open Veterinary Journal, (2023), Vol. 13(5): 668–676 Case Report Successful steroid tapering and partial treatment of suspected immune-mediated haemolytic anaemia in a dog with equine placenta extract supplementation: A case reportSachiko Kotoku1, Kentarou Tahara2, and Eiichi Hirano2*1Kotoku Animal Hospital, Gifu, Japan 2Medical Affairs Department, Japan Bio Products Co. Ltd., Tokyo, Japan *Corresponding Author: Eiichi Hirano. Medical Affairs Department, Japan Bio Products Co. Ltd., Shibuya, Tokyo, Japan. Email: ehirano [at] placenta-jbp.co.jp Submitted: 07/03/2023 Accepted: 11/04/2023 Published: 27/05/2023 © 2023 Open Veterinary Journal
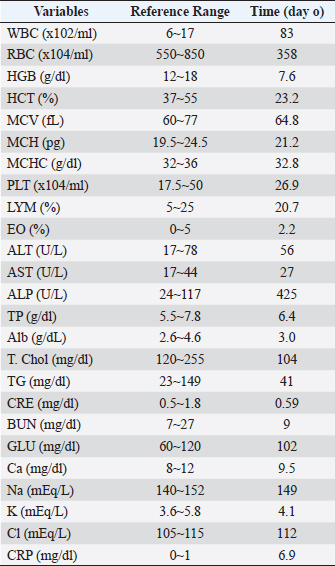
AbstractBackground: We report on the clinical management and outcome of an 11-year-old dog diagnosed with suspected refractory immune-mediated anemia (IMHA) and treated with equine placental extract supplementation. Case Description: The patient had received standard treatment with subcutaneous infusion of prednisone (2 mg/kg) and oral administration (1.3 mg/kg semel in die [sid]), with limited success as hematocrit (HCT) values continued to fall rapidly, and the patient continued to have severe symptoms of fatigue. The patient was then put on equine placental extract supplements, after which the patient’s physical exhaustion was improved, and although the HCT level initially continued to fall, it eventually began to rise and remained near normal for approximately 2 years. A significant reduction in prednisone use was achieved with placental supplementation. Conclusion: Equine placental supplementation may be useful as a new complementary therapy for suspected refractory IMHA. Keywords: Suspected refractory immune-mediated anemia, Steroid tapering, Partial treatment, Placenta extract supplementation, Case report. IntroductionSince its recognition as a canine disease in 1957, canine immune-mediated hemolytic anemia (IMHA) has been highly prevalent and continues to claim the lives of up to half of the affected dogs (Weinkle et al., 2005; Piek, 2011; Piek et al., 2011; Swann and Skelly, 2011). Standard treatment involves controlling the autoimmune response by targeting red blood cell (RBC) antigens through nonspecific immunosuppression with steroids (Swann and Skelly, 2011, 2013). Immunosuppressive therapy carries potential risks associated with several side effects that can cause increased patient morbidity, depending on the drugs used and the duration of treatment. Therefore, many second-line drugs, such as azathioprine, cyclosporine, and mofetil mycophenolate, are now used in the routine practice to treat IMHA in dogs to augment immunosuppression, control the initial disease more rapidly, and taper off steroids. Therefore, many second-line agents, such as azathioprine, cyclosporine, and mycophenolate mofetil, are now used in the routine practice of dogs with IMHA to augment immunosuppression, control initial disease, and taper off steroids more rapidly. However, in clinical practice, we are sometimes encountering cases in which such standard treatments do not deliver desired results. In such cases, standard treatment may be supplemented by the use of Oriental medicines and supplements such as Chinese herbal medicine, to ease and maintain symptoms. However, studies on the effectiveness of complementary therapies are rare. Placenta extract has been reported to exhibit a wide variety of physiological activities, such as modulation of an immune response, protection and regeneration of hepatocytes, regulation of hormonal balance, anticoagulation, promotion of wound healing, and anti-fatigue (Liu et al., 1998; Sur et al., 2003; Tiwary et al., 2006; Kong et al., 2008; Han et al., 2013). We present a case of suspected IMHA that was refractory to standard therapy, and placental extract supplementation was implemented as a complementary therapy. The placenta extract supplementation enabled the patient to maintain good physical condition for a long period despite suspected IMHA, besides a significantly reduced dose of steroids. Case DetailsAn 11-year-old neutered, male miniature dachshund weighing 5.6 kg presented with a reduced appetite for a week. Specifically, loss of appetite was observed about a week prior, and although the initially ate only snacks, the patient ceased eating altogether. No vomiting was observed. The patient drank fluids well, and his stools were slightly firm. The left eye was cloudy and drooled from the left mouth. The patient had no relevant medical history. Table 1 shows the blood test findings at the time of the initial visit, with a white blood cell (WBC) value of 4.9 times the upper reference limit. RBC, hemoglobin (HGB), and hematocrit (HCT) were 35%, 37%, and 37% lower than the lower reference limit, respectively. No other abnormalities were seen on the complete blood count test results. Hepatic function tests showed that alkaline phosphatase (ALP) was 3.6 times the upper limit of the reference value and the total cholesterol (T. Chol) was 13% lower than the lower reference limit. Other liver function test results were within normal parameters. The renal function values were normal for creatinine (CRE), blood urea nitrogen (BUN), and glucose (Glu) levels. Calcium (Ca), sodium (Na), potassium (K), and chlorine (Cl) levels were normal, and C-reactive protein (CRP) was 6.9 times the upper reference limit. A tentative diagnosis of IMHA was suspected based on the characteristic elevated WBC count, decreased RBC count, HGB and HTC, and elevated CRP levels. Besides the above insights, since the echographic measurements showed a somewhat mixed pattern of the thymus, we recommended the owner visit a larger institutional hospital to clarify whether the suspected IMHA symptoms were neoplastic. However, since the owner preferred symptomatic treatment at our hospital, we administered: ampicillin sodium (20 mg/kg), prednisone (2 mg/kg), thiamine chloride hydrochloride, sodium riboflavine phosphate, pyridoxine hydrochloride, and a nicotinamide combined drug (0.5 ml). The patient was also given glycyrrhizin, glycine, and a cysteine combined drug (0.5 ml) in a 500 ml sodium L-lactate ringer solution administered subcutaneously. Subcutaneous infusions of these drugs were also administered between days 0 and 4. In addition, prednisone and orbifloxacin were also orally administered at 1.3 mg/kg bid and 7.1 mg/kg eod, respectively. Table 1. Hematological and serum biochemical parameters at the initial visit.
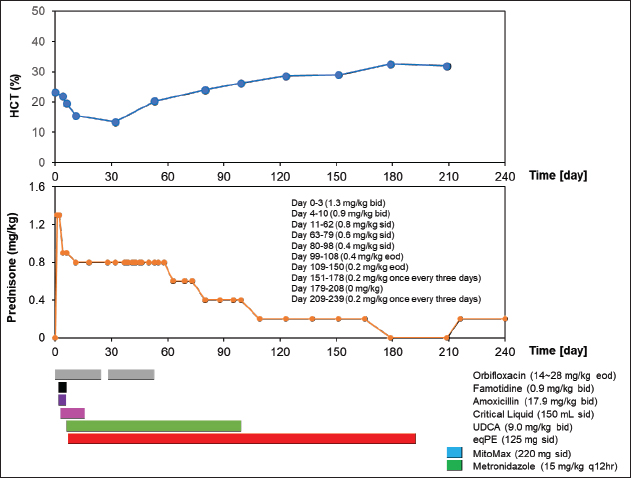
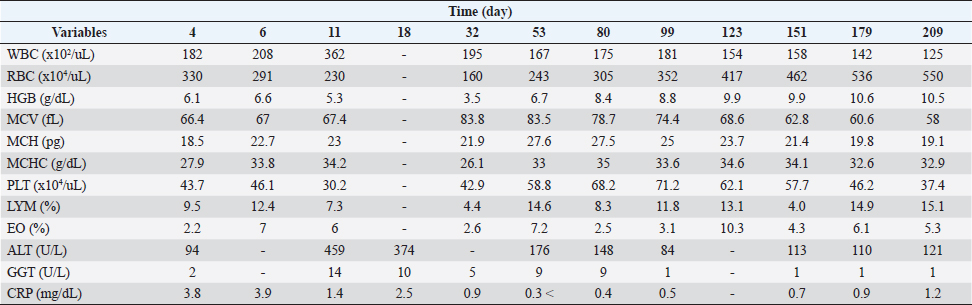
Figure 1 and Table 2 show the history of therapeutic and supplement use, and changes in blood test values over time between days 0 and 209. On day 1, the patient was observed to be in good health and consumed a small amount of meat, including a small amount of white meat. However, since the patient vomited a little saliva early in the morning and did not have food at night, which affected treatment compliance for famotidine (0.9 mg/kg bis in die: bid) and amoxicillin (17.9 mg/kg bid) as they were orally administered. On day 2, since it was observed that the patient could eat only his favorite snacks, critical liquid (150 ml, semel in die: sid) was administered orally. On day 4, the patient could eat pet food and had an increase in appetite. Blood test findings showed that WBC levels were 2.2 times higher than those at the initial visit, while RBC, HCT, HGB, MCH, and MCHC were 7.8%, 5.6%, 19.7%, 12.7%, and 14.9% lower, respectively. However, MCV, PLT, LYM, and EO levels were normal. Although the CRP level had decreased to approximately 45% of the level at the time of the initial visit, it was 3.8 times the upper limit of the reference value. Based on these results, the oral dose of prednisone was tapered from 1.3 mg/kg bid to 0.9 mg/kg bid. Gamma-glutamyl transpeptidase (GGT) levels were within the reference limits, while the ALT levels were found to be 68% higher than those at the initial visit, resulting in the administration of ursodeoxycholic acid (UDCA) at 9 mg/kg bid to assist with liver function. On day 6, compared to the previous examination on day 4, the WBC count increased by 14.3%. Additionally, EO increased by 218.2%, moving from the baseline to above the baseline, while RBC count and HCT decreased by 11.8% and 11%, respectively, which were still below the baseline values. Blood HGB increased by 8.2%, which remained below the baseline. The MCV, PLT, and LYM values were within the reference values. The MCH and MCHC increased by 22.7% and 21.2%, respectively, and were converted to be within the reference values. Based on these results, the oral dose of prednisone was changed from 1.3 mg/kg bid to 0.9 mg/kg bid. Although the patient could eat food as long as it was a snack, a recurrent loss of energy and appetite was observed. Based on these observations, equine placenta extract (eqPE) was administered at 125 mg sid with the aim of reducing physical exhaustion and enhancing anti-inflammatory effects. In addition, ampicillin sodium (20 mg/kg) was replaced with enrofloxacin (5 mg/kg) by intravenous/subcutaneous injection under the same conditions as those on the first day. On day 11, a marked increase in appetite, without vomiting, was observed. Compared with day 6, the WBC count increased by 74%, which was above baseline. RBC, HCT, and HGB levels decreased by 21%, 20.5%, and 19.7%, respectively, which were still below the baseline values. The MCV, MCH, MCHC, PLT, and LYM levels were within the normal range. The EO level decreased by 14.3%, which was still below the normal range. The ALT level was 388.3% higher than that on day 4 and was still above baseline. GGT levels increased by 600% and changed from substandard to normal. Although the CRP level decreased by 64.1% from day 6, it was still above the baseline. Based on these results, the oral dose of prednisone was slightly tapered from 0.9 mg/kg bid to 0.8 mg/kg bid. ALT levels were 388% higher than those on day 4. GGT levels remained within the normal range. On day 18, a marked increase in appetite, without vomiting, was observed. The patient was also observed to be in good physical condition. Compared to day 11, ALT decreased by 18.5% but was still above the reference level. The CRP level increased by 8.6% and remained above the baseline. GGT level was within the reference range. On day 28, because the good physical condition of this case was confirmed to have been maintained, the prescription of orbifloxacin was deemed unnecessary and was discontinued.
Fig. 1. Clinical course of treatment with oral administration of drugs and supplements between day 0 and day 209. Changes in HCT values from day 0 to day 209 of the treatment period (upper). Variation in oral prednisone doses from day 0 to 240 of the treatment period (middle). Amount and duration of oral medications and supplements used from day 0 to day 240 of treatment (lower). On day 32, good physical condition was maintained. Compared to day 18, the WBC count decreased by 46.1% but remained above baseline. RBC, HCT, and HGB decreased by 30.4%, 13.6%, and 34%, respectively, but remained below the baseline. The MCV increased by 24.3%, which was converted from within reference to the above reference. The MCH and PLT were normal. MCHC and LYM decreased by 23.7% and 39.7%, respectively, and were converted from within reference to the reference below. The EO decreased by 56.7%, which was converted from the above reference to within reference. GGT was normal. CRP decreased by 64%, which was converted from the above reference to the within reference. Based on these results, orbifloxacin was deemed unnecessary and thus discontinued. The owner of the patient reported mobile forelimbs, but the patient could not stand on. The owners also reported a tremor, hard and infrequent, stools, and an apparently tense abdomen, and ascites were excluded as a differential on day 37. Orbifloxacin (14–28 mg/kg) was resumed orally from day 38. On day 53, the patient’s physical condition was maintained. Compared to day 32, WBC and MCV decreased by 14.4% and 0.4%, respectively, but remained above the baseline. The RBC, HCT, and HGB levels increased by 51.9%, 51.5%, and 91.4%, respectively, but remained below the baseline. MCH and PLT increased by 26% and 37%, respectively, which were converted from within reference to the above reference. MCHC and LYM increased by 26.4% and 231.8%, respectively, from below the reference values. EO increased by 7.2%, which was converted from within reference to the above reference. ALT decreased by 61.7% compared to that on day 11 but remained above baseline. GGT and CRP levels remained at reference values. On day 63, since the patient continued to feel well, oral orbifloxacin administration was deemed unnecessary and discontinued. In addition, oral prednisone was changed from 0.8 mg/kg sid to 0.6 mg/kg sid thereafter. Between days 80 and 209, the patient’s physical condition continued to be maintained. Although the WBC count remained above the reference level, it showed a decreasing slope as the days passed. The RBC count remained below the reference value, but gradually increased over days and then reached within the reference value on day 209. However, HCT and HGB remained below the standard values and exhibited a gradual upward movement with passaging days. Although MCV, MCH, and PLT were above the reference values, they gradually decreased over time and converted to reference values on days 99, 123, and 179, respectively. The MCHC remained within the reference value. LYM was temporarily below the reference value on day 151, but otherwise remained within the reference values. The EO level was within and above the reference level between days 80 and 209. Although ALT was above the reference level, it showed a decrease with passaging days and then remained constant at around 110 (U/L). Although GGT was within the standard value, it decreased over time and then remained below the standard value after day 99. CRP levels remained normal but slightly exceeded the standard value on day 209. Under these trends of improvement in blood tests, oral prednisone was tapered from 0.6 to 0.4 mg/kg sid on day 80, tapered from 0.4 mg/kg sid to 0.4 mg/kg eod on day 99, and tapered from 0.4 to 0.2 mg/kg eod on day 99, tapered to 0.4 mg/kg sid to 0.4 mg/kg eod on day 109, and tapered to 0.2 mg/kg eod to 0.2 mg/kg once every 3 days on day 151. Oral prednisone was temporarily discontinued on day 179, but oral dosing (0.2 mg/kg once every 3 days was resumed on day 209. On day 216, diarrhea was observed on day 214, and MitoMax (220 mg sid) and metronidazole (15 mg/kg q12 hours) were administered orally. Diarrhea resolved after several days. Table 2. Hematological and serum biochemical parameters after drugs and supplements administration between day 4 and day 209.
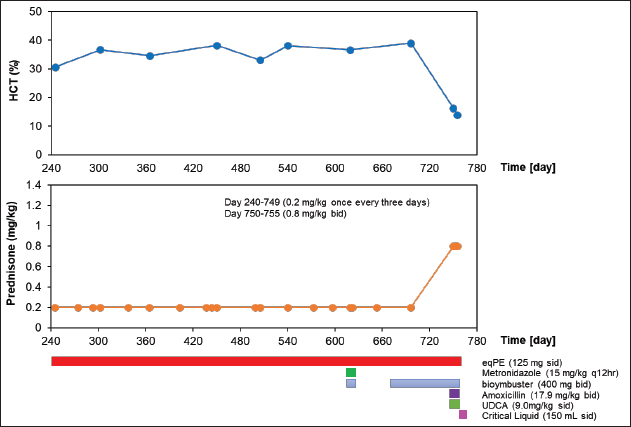
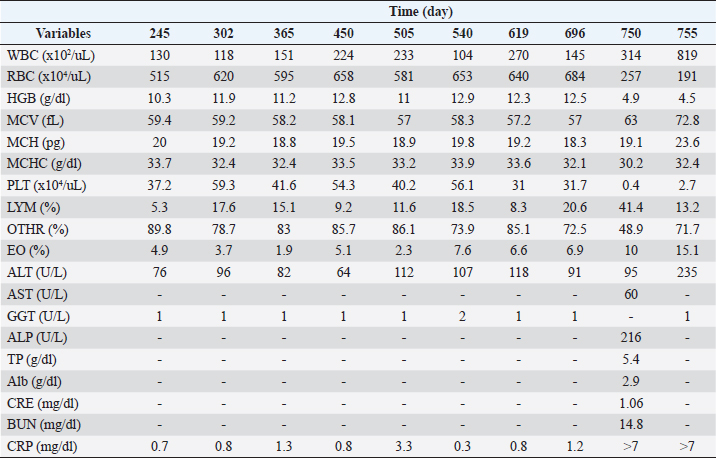
Figure 2 and Table 3 show the history of therapeutic and supplement use, and changes in blood test values over time between days 245 and 755. On day 245, the patient’s physical condition continued to improve. The WBC count was above the baseline and 4% higher than that on day 209. RBC, HCT, HBG, and MCV were lower than the reference values by 6.4%, 4.1%, 1.9%, and 2.4%, respectively, than those on day 209. The MCH increased by 4.7% from day 209 and returned to baseline levels. The MCHC, PLT, and LYM levels remained within the baseline values. The EO decreased by 7.6% from day 209 and returned to baseline levels. ALT and CRP levels were 37.2% and 41.7% lower, respectively, than those on day 209, and returned to baseline values. GGT level was within the reference range. On day 302, the patient remained in good physical condition. Compared with day 245, the WBC count decreased by 9.2%, however, it was still above the reference value. Compared with day 24, the RBC count increased by 20.4%, reaching the reference value. Between days 303 and 696, the patient’s physical condition and appetite were good. WBC exceeded the reference value, whereas RBC, MCHC, and LYM maintained the reference values. HCT was consistently slightly below the lower limit of the reference value, but it reached normal levels only on day 540. HGB and MCH consistently showed values slightly below the lower limit of the reference value, and occasionally showed values within the reference value. The MCV values were slightly below the lower limit of the reference value and did not reach the reference values. The PLT, EO, ALT, GGT, and CRP levels were repeatedly above and below the reference values. On day 622, diarrhea was observed in the patient, and metronidazole (15 mg/kg q12hours) and bio-ester (400 mg bid) were administered. (400 mg bid) was administered orally, and this symptom improved after several days. On day 696, although the patient was feeling well but occasionally had loose stools, oral administration of bio-ester (400 mg bid) was resumed. On day 750, the patient could not stand on his back, breathing heavily, and leak stools. Compared to day 696, the WBC count and EO were elevated by 116.6% and 44.9%, respectively, which were 36 and 2 times the upper reference limits, respectively. HCT decreased by 58.5%, a level that varied from above to below the upper reference limit. RBC, HGB, MCHC, and PLT counts decreased by 62.4%, 60.8%, 5.9%, and 98.7%, respectively, a level that varied from within the reference to below the reference limit. The MCV and MCH increased by 10.5% and 4.4%, respectively, and were within the reference values. LYM increased by 101%, which was four times the upper reference limit. In addition, it varied from within the reference limit to the upper reference limit. ALT levels increased by 4.4%, which was still above the baseline level. AST and ALP showed 1.4 and 1.9 times the upper limit of reference values, respectively, in the current examination, although the most recent examination had not been performed. At this examination, TP was 1.8% lower than the lower reference limit, whereas Alb, CRE, and BUN levels were within the reference values. CRP levels were over seven times the upper limit of the reference values. Based on these results, the oral prednisone dose was changed from 0.2 mg/kg once every 3 days to 0.8 mg/kg bid, and oral amoxicillin (17.9 mg/kg bid) and UDCA (9.0 mg/kg sid) were started. Although the patient was slightly more energetic on day 751, his energy level decreased again on day 752. Furthermore, on day 753, the patient’s appetite had decreased. Based on these results, a critical liquid (150 ml sid) was orally administered. On day 755, compared to day 750, WBC and EO increased by 160.8% and 51%, respectively, which corresponded to 48.2 and three times the upper reference limit, respectively. RBC, HCT, and HGB levels decreased by 25.7%, 14.2%, and 8.2%, respectively, and were below the standard values. The MCV and MCH were within the reference values. The MCHC increased by 7.3% and was converted from below the reference to within the reference. Although the PLT increased by 575%, it remained below the reference value. LYM was reduced by 68.1% and converted to normal values. ALT levels increased by 147.4%, which corresponded to three times the upper limit of normal. GGT was normal. CRP levels were over seven times the upper limit of the reference values. Approximately 10 days after day 755, the owner informed us that the dog in question had died. DiscussionThe main treatment for IMHA, as in the present case, is generally drug therapy, mainly using immunosuppressive drugs such as steroids. However, numerous cases of refractory IMHA are difficult to treat or maintain with steroids, such as prednisone alone (Yuki et al., 2004). In such cases, immunosuppressive agents, such as azathioprine, danazol, cyclosporine, cyclophosphamide, bovine HGB solution, and human immunoglobulin, are used in combination with prednisone (Grundy and Barton, 2001; Yuki et al., 2004). However, even with these combination therapies, some cases remain untreatable. Therefore, owners of senior dogs with IMHA are reluctant to treat them with drugs. In our clinic, we provide not only Western medical treatment but also Oriental medical treatments such as acupuncture and moxibustion, Chinese herbal medicine, and laser therapy to reduce the treatment burden on the animals. In the present case, the patient experienced significant wasting caused by suspected IMHA, and we attempted to improve this by combining Western medical treatment with placental extract, an Oriental medical treatment. To the best of our knowledge, this is the first reported case in which eqPE administration as a supplement contributed to clinical improvement in a canine suspected IMHA case that was not readily amenable to treatment with surface Western medicine alone.
Fig. 2. Clinical course of treatment with oral administration of drugs and supplements between day 240 and day 755. Changes in HCT values from day 240 to day 755 of the treatment period (upper). Variation in oral prednisone doses from days 240 to 755 of the treatment period (middle). Amount and duration of oral medications and supplements used from day 240 to day 755 of treatment. (lower). The HCT value was the lowest on day 32, which was borderline life threatening. However, even under these conditions, the patient’s appetite was enhanced, the oral prednisone dose could be further tapered off, and subcutaneous prednisone infusion could be discontinued after eqPE administration. Whether these results can be attributed to eqPE administration requires verification in a randomized controlled trial. However, the possibility that eqPE administration contributed to the above improvements cannot be ruled out, since placental extract has been reported to regulate fatigue by improving immune levels and reducing fatigue-related factors in mice with protein energy malnutrition-induced fatigue (Han et al., 2013). In this case, the severe decline in HCT gradually increased after eqPE administration and remained normal for 28 days from day 151 to day 179, and remained nearly normal for 545 days from day 151 to day 696 until suspected IMHA recurred. These results suggest that eqPE, in combination with low-dose prednisone, can maintain the decreased HCT at normal levels in suspected IMHA for a long period. Although detailed verification of these factors is needed, we speculate that the anti-inflammatory effect of eqPE may have substituted for the anti-inflammatory effect of prednisone and that the anti-fatigue effect may have contributed to the improvement of physical exhaustion. In this case, diarrhea and forelimb failure were observed during treatment, including eqPE administration. Diarrhea was unlikely to have been caused by eqPE administration, since it was 210 days after eqPE administration and the eqPE dose was constant throughout the treatment period. Although prednisone administration was discontinued on day 179 in this patient with mild suspected IMHA, prednisone was still required on day 209. Diarrhea symptoms were observed on day 214, suggesting that the resumption of prednisone administration may have affected the side effects of prednisone. To the best of our knowledge, there was no association with suspected IMHA in our literature review. The cause of forelimb failure, in this case, is unknown, but since the patient was 12 years old at the onset of forelimb failure, aging may be a contributing factor. These results suggested that the occurrence of adverse events because of eqPE administration was negative. Table 3. Hematological and serum biochemical parameters after drugs and supplements administration between day 245 and day 755.
The dose of eqPE used in this study was not accurately determined. In our previous study, we reported that eqPE administered (112 mg/dog sid) to an elderly dog with nocturnal ringing and dementia showed improvement in nocturnal ringing, and no adverse events were observed (Amano et al., 2022). In the current study, the eqPE dose was 125 mg/dog sid, a 13.6% increase compared to a previous report. However, no notable adverse events were observed, suggesting that the resulting dose of eqPE was within the range. Because of the rarity of research papers on placenta extract supplementation in dogs, it is extremely difficult to rely on previous studies based on dose settings. However, there are several studies on the administration of placental extract supplements in rodent models (Takuma et al., 2012; Yamauchi et al., 2019; deToledo et al., 2021), in which doses of 1000 mg/kg to 2000 mg/kg, with a maximum of 5,000 mg/kg has been administered, and no adverse events have been observed in these studies. No adverse events were observed in any of these studies. The dose of eqPE in the present study was 700 mg/kg when compared to previous studies using simple body weight conversions of cases, which supports that the dose was reasonable. However, the dosage of eqPE requires further investigation. In this case, although a wide variety of Western medicine drugs, such as prednisone and antibiotics, were used in combination with eqPE throughout the treatment period, no notable adverse events were observed. These results suggest that Western medical treatment and eqPE may not interfere with each other and do not induce adverse effects. In addition, this patient developed occasional poor forelimb conditions and was treated with laser therapy from days 38 to 151 and from days 750 to 755. Although laser therapy and eqPE administration overlapped during the suspected IMHA treatment period, no adverse events were observed. It has been suggested that placenta extract supplementation and laser therapy may not interfere with each other. In this case, the HCT level continued to fall rapidly from days 0 to 11, even with subcutaneous and oral administration of prednisone, suggesting resistance to prednisone therapy, and the patient continued to suffer from severe anorexia and reduced physical fitness during this period. To ease this, the prednisone dose had to be tapered off, which may have contributed to the rapid decline in the HCT level. Critical liquid, a known appetite stimulant (Miyabe et al., 2019; Kikuchi et al., 2020), was orally administered to ease anorexia by tapering off the required prednisone. However, no improvements were observed. It has been reported that placental extract has anti-inflammatory and anti-fatigue effects, in addition to improving liver function (Liu et al., 1998; Sur et al., 2003; Han et al., 2013), and such multifunctional properties were presumed to be advantageous in this case. In fact, CRP levels continued to decrease after eqPE administration and returned to normal after day 32, and appetite increased on day 11. Although ALT levels temporarily increased on day 11, they continued to decrease thereafter and did not reach normal values until day 755, when suspected IMHA recurred. The patient’s ALT level did not increase again until day 755 when suspected IMHA recurred. These results suggested that eqPE administration had a multifaceted effect on this patient, as expected. In conclusion, in this study, eqPE supplementation in suspected refractory IMHA contributed to the conversion of HCT to near-normal values and the maintenance of stable HCT values for approximately 2 years, without causing any adverse effects, suggesting that eqPE supplementation may be effective in the treatment of suspected IMHA in combination with Western drugs such as steroids, and Oriental medicine techniques such as laser therapy, without interference. The case also suggests that eqPE contributed to a significant tapering of prednisone, which not only reduced the physical burden of drug but also contributed to a reduction in the owner’s financial burden. Therefore, eqPE may play an important role as a supplement in the treatment of suspected refractory IMHA, and may be useful as a new complementary technique. However, more detailed studies and case reports are required to confirm our findings. AcknowledgementWe are sincerely grateful to all the co-medical staff, dogs, and dog owners. We also appreciate Editage (www.editage.jp) for the English language editing. Author contributionsSK conceived and designed the clinical study, analysed and interpreted the data. KT analysed and interpreted the data. EH analysed and interpreted the data, and wrote the manuscript. Conflict of InterestKentarou Tahara and Eiichi Hirano are employees of the Japan Bio Products Co., Ltd. Sachiko Kotoku has no competing interests. Ethical approvalNo ethical approval was required for this case. ReferencesAmano, T., Ikeda, T., Yamaguchi, M., Kakehi, N., Hanada, K., Watanabe, T., Tahara, K. and Hirano, E. 2022. Equine placental extract supplement as a night barking remedy in dogs with cognitive dysfunction syndrome. Vet. Med. Sci. 8(5), 1887–1892. de Toledo, A., Nomoto, K., Hirano, E. and Tohda, C. 2021. Horse placental extract enhances neurogenesis in the presence of amyloid β. Nutrients. 13(5), 1672. Grundy, S.A. and Barton, C. 2001. Influence of drug treatment on survival of dogs with immune-mediated hemolytic anemia: 88 cases (1989–1999). J. Am. Vet. Med. Assoc. 15(218), 543–546. Han, N.R., Kim, K.Y., Kim, M.J., Kim, M.H., Kim, H.M. and Jeong, H.J. 2013. Porcine placenta mitigates protein-energy malnutrition-induced fatigue. Nutrition. 29(11–12), 1381–1387. Kikuchi, R., Fujii, K., Kamebayashi, Y., Tsukahata, K., Sato, M., Hayase, A. and Komiyama, N. 2020. Placement of subcutaneous ureteral bypass without fluoroscopic guidance in cats with ureteral obstruction. Jpn. J. Vet. Anesth. Surg. 51(2), 29–35. Kong, M.H., Lee, E.J., Lee, S.Y., Cho, S.J., Hong, Y.S. and Park, S.B. 2008. Effect of human placental extract on menopausal symptoms, fatigue, and risk factors for cardiovascular disease in middle-aged Korean women. Menopause. 15(2), 296–303. Liu, K.X., Kato, Y., Kaku, T. and Sugiyama, Y. 1998. Human placental extract stimulates liver regeneration in rats. Biol. Pharm. Bull. 21(1), 44–49. Miyabe, M., Saeki, K., Shimizu, Y., Shimizu, Y., Wada, Y., Ishikawa, M., Hoshi, F., Maeta, M., Yamazoe, K., Onishi, A., Asanuma, T., Kutara, K. and Kanda, T. 2019. A perioperative nursing case of a dog with cutaneous ureterostomy surgery. Vet. Nurs. 24(2), 31–35. Piek, C.J. 2011. Canine idiopathic immune-mediated haemolytic anaemia: a review with recommendations for future research. Vet. Q. 31(3), 129–141. Piek, C.J., van Spil, W.E., Junius, G. and Dekker, A. 2011. Lack of evidence of a beneficial effect of azathioprine in dogs treated with prednisolone for idiopathic immune-mediated hemolytic anemia: a retrospective cohort study. BMC. Vet. Res. 13(7). 15. Sur, T.K., Biswas, T.K., Ali, L. and Mukherjee, B. 2003. Anti-inflammatory and anti-platelet aggregation activity of human placental extract. Acta Pharmacol. Sin. 24(2), 187–192. Swann, J.W. and Skelly, B.J. 2011. Evaluation of immunosuppressive regimens for immune-mediated haemolytic anaemia: a retrospective study of 42 dogs. J. Small Anim. Pract. 52(7), 353–358. Swann, J.W. and Skelly, B.J. 2013. Systematic review of evidence relating to the treatment of immune-mediated hemolytic anemia in dogs. J. Vet. Intern. Med. 27(1), 1–9. Takuma, K., Mizoguchi, H., Funatsu, Y., Kitahara, Y., Ibi, D., Kamei, H., Matsuda, T., Koike, K., Nagai, T. and Yamada, K. 2012. Placental extract improves hippocampal neuronal loss and fear memory impairment resulting from chronic restraint stress in ovariectomized mice. J. Pharmacol. Sci. 120(2), 89–97. Tiwary, S.K., Shukla, D., Tripathi, A.K., Agrawal, S., Singh, M.K. and Shukla, V.K. 2006. Effect of placental-extract gel and cream on non-healing wounds. J. Wound Care. 15(7), 325–328. Yamauchi, A., Tone, T., Sugimoto, K., Lim, H.S., Kaku, T., Tohda, C., Shindo, T., Tamada, K., Mizukami, Y. and Hirano, E. 2019. Porcine placental extract facilitates memory and learning in aged mice. Food Sci. Nutri. 7(9), 2995–3005. Yuki, M., Suzuki, K., Sugimoto, N., Higuchi, T., Suzuki, H. and Ishikawa, K. 2004. Clinical study of tacrolimus administered to two dogs with immune-mediated hemolytic anemia. J. Jpn. Vet. Med. Assoc. 57(11), 721–724. Weinkle, T.K., Center, S.A., Randolph, J.F., Warner, K.L., Barr, S.C. and Erb, H.N. 2005. Evaluation of prognostic factors, survival rates, and treatment protocols for immune-mediated hemolytic anemia in dogs: 151 cases (1993-2002). J. Am. Vet. Med. Assoc. 226(11), 1869–1880. | ||
| How to Cite this Article |
| Pubmed Style Kotoku S, Tahara K, Hirano E. Successful steroid tapering and partial treatment of suspected immune-mediated haemolytic anaemia in a dog with equine placenta extract supplementation: A case report. Open Vet. J.. 2023; 13(5): 668-676. doi:10.5455/OVJ.2023.v13.i5.21 Web Style Kotoku S, Tahara K, Hirano E. Successful steroid tapering and partial treatment of suspected immune-mediated haemolytic anaemia in a dog with equine placenta extract supplementation: A case report. https://www.openveterinaryjournal.com/?mno=145618 [Access: December 04, 2025]. doi:10.5455/OVJ.2023.v13.i5.21 AMA (American Medical Association) Style Kotoku S, Tahara K, Hirano E. Successful steroid tapering and partial treatment of suspected immune-mediated haemolytic anaemia in a dog with equine placenta extract supplementation: A case report. Open Vet. J.. 2023; 13(5): 668-676. doi:10.5455/OVJ.2023.v13.i5.21 Vancouver/ICMJE Style Kotoku S, Tahara K, Hirano E. Successful steroid tapering and partial treatment of suspected immune-mediated haemolytic anaemia in a dog with equine placenta extract supplementation: A case report. Open Vet. J.. (2023), [cited December 04, 2025]; 13(5): 668-676. doi:10.5455/OVJ.2023.v13.i5.21 Harvard Style Kotoku, S., Tahara, . K. & Hirano, . E. (2023) Successful steroid tapering and partial treatment of suspected immune-mediated haemolytic anaemia in a dog with equine placenta extract supplementation: A case report. Open Vet. J., 13 (5), 668-676. doi:10.5455/OVJ.2023.v13.i5.21 Turabian Style Kotoku, Sachiko, Kentarou Tahara, and Eiichi Hirano. 2023. Successful steroid tapering and partial treatment of suspected immune-mediated haemolytic anaemia in a dog with equine placenta extract supplementation: A case report. Open Veterinary Journal, 13 (5), 668-676. doi:10.5455/OVJ.2023.v13.i5.21 Chicago Style Kotoku, Sachiko, Kentarou Tahara, and Eiichi Hirano. "Successful steroid tapering and partial treatment of suspected immune-mediated haemolytic anaemia in a dog with equine placenta extract supplementation: A case report." Open Veterinary Journal 13 (2023), 668-676. doi:10.5455/OVJ.2023.v13.i5.21 MLA (The Modern Language Association) Style Kotoku, Sachiko, Kentarou Tahara, and Eiichi Hirano. "Successful steroid tapering and partial treatment of suspected immune-mediated haemolytic anaemia in a dog with equine placenta extract supplementation: A case report." Open Veterinary Journal 13.5 (2023), 668-676. Print. doi:10.5455/OVJ.2023.v13.i5.21 APA (American Psychological Association) Style Kotoku, S., Tahara, . K. & Hirano, . E. (2023) Successful steroid tapering and partial treatment of suspected immune-mediated haemolytic anaemia in a dog with equine placenta extract supplementation: A case report. Open Veterinary Journal, 13 (5), 668-676. doi:10.5455/OVJ.2023.v13.i5.21 |