| Case Report | ||
Open Vet. J.. 2023; 13(8): 1027-1031 Open Veterinary Journal, (2023), Vol. 13(8): 1027-1031 Case Report Dermatologic adverse effect of subcutaneous furosemide administration in a catChiara Mazzoldi, Francesca Aspidi and Giovanni Romito*Department of Veterinary Medical Sciences, Alma Mater Studiorum—University of Bologna, Ozzano dell’Emilia, Italy *Corresponding Author: Giovanni Romito. Department of Veterinary Medical Sciences, Alma Mater Studiorum—University of Bologna, Ozzano dell’Emilia, Italy. Email: giovanni.romito2 [at] unibo.it Submitted: 16/03/2023 Accepted: 12/07/2023 Published: 31/08/2023 © 2023 Open Veterinary Journal
AbstractBackground: Furosemide is a mainstay of treatment in congestive heart failure (CHF) and is widely prescribed to dogs and cats by several formulations, including the subcutaneous one. In canine and human medicine, dermatologic adverse effects of subcutaneous furosemide (SF) have been documented; conversely, no prior case has been published describing skin reactions to this therapeutic protocol in cats. In this report, we describe, for the first time in feline medicine, a suspected dermatologic adverse effect after SF in a cat. Case Description: A 2-year-old domestic shorthair cat was presented for CHF associated with lung edema and pleural effusion. Echocardiography revealed asymmetric left ventricular myocardial thickening and severe left atrial dilation. The cat was hospitalized and initially treated with oxygen, intravenous furosemide, and clopidogrel. After discharge, the route of administration of furosemide was switched from intravenous to oral. Within the following 2 weeks, the cat experienced two relapses of lung edema despite the progressive increase of the furosemide dose, the addition of spironolactone and adherence to the therapeutic protocol by the owners. The dose of furosemide was further increased and its route of administration at home was switched from oral to parental. As the owner was not able to administrate intramuscular injections, SF was prescribed. This allowed the prevention of further episodes of lung edema. However, although the cat had never presented skin problems before, multiple well-defined circular, crusted ulcerative cutaneous lesions associated with alopecia developed at the sites of furosemide injections 2 weeks later. After ruling out several differential diagnoses for these lesions, a rare side effect of furosemide, not yet described in cats but already known in canine and human medicine, was strongly suspected as the possible cause. Therefore, the ongoing injectable formulation of furosemide was interrupted and substituted with an alternative brand, maintaining the same dose and route of administration. Thanks to this change, the dermal ulcerations disappeared within 1 month. Subsequently, the cat experienced neither further skin problems nor a recurrence of lung edema. Conclusion: Although SF is sometimes prescribed in small animal practice, it should be noticed that this may lead to dermatologic adverse reactions in the cat. Keywords: Congestive heart failure, Diuretic resistance, Feline, Left ventricular myocardial thickening, Skin reaction. IntroductionFurosemide is a mainstay of treatment in congestive heart failure (CHF). In stable small animals, the chronic (home-based) management of CHF is usually based on oral furosemide, as this route of administration is relatively simple for the majority of owners and well tolerated by dogs and cats (Kittleson and Kienle, 1998; Plumb, 2008; Sabetti et al., 2022). However, as the bioavailability of oral furosemide is variably limited, in some cases, oral administration prevents good control of CHF, especially in advanced cardiac diseases or subjects affected by concomitant gastrointestinal disorders (Kittleson and Kienle, 1998; Plumb, 2008; Oyama and Adin, 2022). One way to favor the patient’s decongestion at home is to change the administration route from the oral to the parenteral one. This change aims to use a formulation of furosemide with a higher bioavailability and to deliver the medication via a route that may bypass the gastrointestinal tract, overcoming problems related to impaired absorption (Kittleson and Kienle, 1998; Plumb, 2008; Oyama and Adin, 2022). The intramuscular injection of furosemide is a cited option in veterinary literature (Kittleson and Kienle, 1998; Plumb, 2008); however, this route of administration requires specific technical skills of the owners (Baxter and Evans, 1973) and may not be tolerated by some cats. Therefore, although currently, subcutaneous furosemide (SF) is formally off-label in cats, this route of administration represents a valid option in small animal practice (Keene and Bonagura, 2009; Francey, 2009; Poissonnier et al., 2020) as it is feasible for many owners and less painful than the intramuscular one. At the same time, it is clinically important to know the possible drawbacks associated with SF, including potential adverse effects. Although adverse reactions to SF have been documented in canine (Scruggs and Rishniw, 2013) and human literature (Zacharias et al., 2011; Afari et al., 2019), no information is currently available regarding feline medicine. Therefore, this report aims to describe, for the first time, a suspected adverse skin reaction after SF in a cat. Case DetailsA 2-year-old, 4.5-kg neutered male domestic shorthair cat was presented for acute-onset dyspnea. The past medical history was unremarkable. The cat lived indoors and was current on vaccinations and parasite prevention. On presentation, lung sounds were harsh, with no crackles. The cat exhibited inspiratory effort, with a respiratory rate of 44 bpm. The remaining physical findings were within normality. Thoracic radiographs identified moderate pleural effusion and lung edema. Echocardiography revealed asymmetric left ventricular myocardial thickening [interventricular septum end-diastolic thickness 5.7 mm (5 ± 0.7 mm) (Moise et al., 1986), left ventricular posterior wall end-diastolic thickness 7.5 mm (4.6 ± 0.5 mm) (Moise et al., 1986)] and severe left atrial dilation [left atrium-to-aortic root ratio 2.2 (1.29 ± 0.23) (Moise et al., 1986)]. The cat was hospitalized and treated with oxygen, furosemide (Dimazon 50 mg/ml, MSD Animal Health S.r.l., Segrate, Italy; dose: 1 mg/kg IV q6h), pimobendan (Vetmedin chew 1.25 mg, Boehringer Ingelheim Animal Health Italia S.p.A., Noventana, Italy; dose: 0.2 mg/kg PO q12h), and clopidogrel (Clopidogrel ACT 75 mg, BB FARMA Srl, Samarate, Italy; dose: 18.75 mag/cat PO q24h). During hospitalization, blood was collected to assess the cat’s systemic condition (i.e., complete blood count, biochemistry, test for feline leukemia and feline immunodeficiency virus), leading to unremarkable results. The cat was discharged after 4 days with oral furosemide (Dimazon 10 mg, MSD Animal Health S.r.l., Segrate, Italy; dose: 1 mg/kg PO q12h) and an unchanged dose of pimobendan and clopidogrel. One week after discharge, the cat was represented with a recurrence of lung edema, despite the regular administration of drugs by the owners. Therefore, the dose of furosemide was increased (2 mg/kg PO q12h), and spironolactone was added (Prilactone next 10 mg, CEVA salute animale S.p.A., Agrate Brianza, Italy; dose: 2 mg/kg PO q24h). Despite increasing diuretic therapy and adherence to the therapeutic protocol by the owners, the cat presented an additional relapse of lung edema the following week. Suspecting an ongoing diuretic resistance, the dose of furosemide was further increased, and its route of administration was switched from oral to parental. As the owners were not able to administrate the drug intramuscularly, the subcutaneous administration of furosemide was considered. In light of such a change, owners were carefully instructed about the technique of subcutaneous administration. Only when it was conclusively certified that owners were able to administrate SF (Dimazon 50 mg/ml, MSD Animal Health S.r.l., Segrate, Italy) properly, this therapeutic protocol was started at the dose of 2 mg/kg SC q8h. Other cardiac therapies were continued at the previously reported doses. Thanks to the introduction of SF, the cat no longer presented a recurrence of lung edema. However, although the cat had never presented skin problems before and owners administrated properly SF, multiple well-defined circulars, crusted ulcerative cutaneous lesions (0.5–1 cm) associated with alopecia developed at the sites of furosemide injections (i.e., dorsal area of thoracic region) 2 weeks later (Fig. 1). At that time, spot-on treatment with fluralaner and moxidectine (Intervet International B. V., Boxmeer, Holland) was prescribed, albeit without clinical improvement. Accordingly, a dermatological consultation was subsequently obtained. On physical inspection, neither signs of self-trauma due to pruritic allergy nor signs of trauma due to improper administration of SF were identified. Trichoscopic evaluation at the level of the aforesaid areas showed neither alteration of the hair shaft nor the presence of spores. Scraping and culture for dermatophytes tested negative. Cytological examination of impression smears and fine-needle aspiration obtained from crusted areas showed an inflammatory pattern characterized by neutrophils, eosinophils, and rare macrophages, without evidence of microorganisms. Owners declined skin biopsies. Given the above and considering that several differential diagnoses for inflammatory ulcerated lesions were unlikely in the light of anamnestic and clinical findings (e.g., thermal/electrical/chemical burns, skin trauma/infection/neoplasia/autoimmune disorder), it was hypothesized that the dermal lesions were primarily associated with the administration of SF. This hypothesis was further supported by the use of the Naranjo Algorithm Adverse Drug Reaction Probability Scale, which led to a score of 6/13 (i.e., “probable” reaction to the drug) (Naranjo et al., 1981). Nevertheless, considering the severity of the cardiac condition and the previous relapses of lung edema, we preferred not to return to oral furosemide. Rather, we decided to interrupt the ongoing injectable formulation and substitute it with an alternative brand (Lasix fiale 20 mg/2 ml, Sanofi S.r.l., Milano, Italy), maintaining the same dose. Moreover, the cutaneous lesions were treated locally with a lenitive local product for 1 week (Douxo S3 CALM pads, CEVA salute animale S.p.A., Agrate Brianza, Italy). The dermal ulcerations disappeared within 1 month (Fig. 2). Subsequently, the cat experienced neither further skin problems nor recurrence of lung edema, and he is still alive at the time of manuscript writing (13 months after the initial presentation).
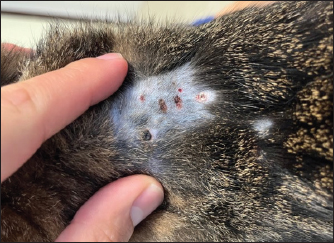
Fig. 1. Physical examination of a 2-year-old neutered male domestic shorthair cat treated with furosemide due to CHF. The picture has been obtained 2 weeks after the prescription of the first brand of SF. Note the well-defined circular, crusted ulcerative cutaneous lesions (0.5–1 cm) associated with alopecia at the level of the sites of furosemide injections (i.e., dorsal area of thoracic region).
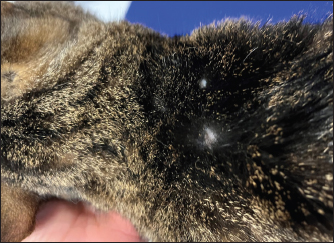
Fig. 2. Image from the same cat in Figure 1. The picture has been obtained during a recheck approximately 3 weeks after the interruption of the first brand of injectable furosemide and the introduction of an alternative brand. Note that, despite the new brand of furosemide was administrated subcutaneously, the cutaneous lesions disappeared and the alopecia was almost completely resolved. DiscussionPotential adverse effects should be considered when furosemide is prescribed. The most common are pre-renal azotemia and electrolyte disturbances (Kittleson and Kienle, 1998; Plumb, 2008; Sabetti et al., 2022), whereas skin reactions related to SF have rarely been reported both in humans and animals. In human medicine, the dermatologic adverse effects are typically mild (including stinging and burning sensations at the injection site, erythema, and skin swelling) and reversible after changing the site of injection (Zacharias et al., 2011; Afari et al., 2019). In veterinary medicine, only one case report has been published (Scruggs and Rishniw, 2013). It described the case of a dog with CHF that initially received SF without any side effects for some weeks. Then, 2 weeks after the change of the brand of furosemide, multiple ulcerative lesions developed at the injection site. The discontinuation of the new brand of furosemide and the reinstitution of the initial one led to the resolution of skin lesions within 1 week (Scruggs and Rishniw, 2013). The authors hypothesized that the dermatological reaction was mainly due to the different pH values of the new brand of furosemide as this was more alkaline (pH 8–9.3) than the initial formulation of furosemide (pH 7–7.8) (Scruggs and Rishniw, 2013). In support of their hypothesis, it should be noted that, in humans, alkaline solutions of injectable furosemide (pH usually ranging from 8.5 to 9) can cause skin irritation (Afari et al., 2019). In our report, skin lesions appeared associated exclusively with one injectable formulation of furosemide and then resolved after the introduction of a new brand of injectable furosemide. According to the particular temporal evolution of the adverse effect, we excluded that it was caused by an immune-mediated reaction to the molecule (as the cat received uneventfully furosemide orally before the onset of cutaneous lesions and continued to receive furosemide also later, after the introduction of the new brand of injectable furosemide, without further relapses of skin reactions) or by a simple mechanical injury/irritation due to the subcutaneous injections (as the cat continued to receive uneventfully subcutaneous injections over the following months). Moreover, we considered it unlikely that the differences in the pH between the two brands of furosemide had caused the adverse effect as these formulations have a similar pH (i.e., 8.3–8.9 and 9, respectively). We hypothesized that the cause of the skin lesions could be related to differences in the excipients contained in the two brands. Indeed, apart from furosemide and water for injections, the first brand contained benzyl alcohol, disodium edetate dihydrate, and sodium sulfite anhydrous, whereas the second brand only contained sodium chloride and sodium hydroxide. Two constituents of the first brand, namely benzyl alcohol and sodium sulfite, are widely used preservatives for injectable preparations as they have preservative properties and do not interfere with the bioavailability of a broad range of medications to which they are added (Shmunes, 1984; García-Gavín et al., 2012). However, both constituents are potential sources of allergic dermatitis in humans (Shmunes, 1984; García-Gavín et al., 2012). Although data on skin reactions to these constituents are lacking in veterinary literature, their presence in the first brand of furosemide, their lack in the second brand of furosemide and their role in allergic dermatitis in humans are collectively in line with our etiological hypothesis. An additional consideration should be made on the lenitive product we employed, which is composed of different ingredients, including synthetic and natural substances, able to restore the skin barrier, improve skin hydration, reduce hypersensitivity reactions, and slow inflammatory processes (Pin et al., 2014). This product was primarily prescribed to prevent pruritus eventually elicited by the primary cutaneous disorder, with the aim of preventing further mechanical injury of the diseased skin area caused by scratching. As this product is not able to lead to resolution of skin disorders similar to the one described in the present case, in our opinion, the cat’s cutaneous lesions improved over time primarily due to substitution of the first injectable formulation of furosemide with the new brand (as previously described in a dog showing spontaneous recovery of dermatologic adverse effects of SF once the brand of furosemide was changed with a new one (Scruggs and Rishniw, 2013)). Similar to the report of Scruggs and Rishniw (2013), one limitation of the present report is the lack of a histopathological analysis of the cutaneous lesions. However, the findings from dermatological consultation associated with the cat’s clinical course observed before, during, and after the administration of the first brand of furosemide strongly support the assumption that the cutaneous lesions were due to the subcutaneous administration of a particular formulation of injectable furosemide. ConclusionThis report describes, for the first time in feline medicine, a suspected adverse skin reaction after SF in a cat. Although furosemide is sometimes administrated subcutaneously in small animal practice (Keene and Bonagura, 2009; Francey, 2009; Poissonnier et al., 2020), it is important to underline that its subcutaneous administration is formally off-label in cats, and the producers of the brands of furosemide employed in this report indicate as routes of administration exclusively the intravenous and the intramuscular ones. Therefore, SF should be considered cautiously in cats and prescribed in selected cases (e.g., when other routes are not feasible, tolerated, or efficient). AcknowledgmentsThis research did not receive any grants from funding agencies in the public, commercial, or not-for-profit sectors. Conflicts of interestThe authors declare that there is no conflict of interest. FundingNo sources of funding for the work presented here. Author contributionsMazzoldi, C, Aspidi, F. and Romito, G.: Case evaluation and clinical management, drafting and approval of the final manuscript. Romito, G.: Manuscript supervision. Data availabilityThe data that support the findings of this case report are available from the corresponding author upon reasonable request. ReferencesAfari, M.E., Aoun, J., Khare, S. and Tsao, L. 2019. Subcutaneous furosemide for the treatment of heart failure: a state-of-the art review. Heart. Fail. Rev. 24, 309–313. Baxter, J.S. and Evans, J.M. 1973. Intramuscular injection in the cat. J. Small. Anim. Pract. 14, 297–302. Francey, T. 2009. Diuretics. In Small animal critical care medicine. Eds., Silverstein, D.C. and Hopper, K. St. Louis, MO: Saunders Elsevier, pp: 770–774. García-Gavín, J., Parente, J. and Goossens, A. 2012. Allergic contact dermatitis caused by sodium metabisulfite: a challenging allergen: a case series and literature review. Contact Dermatitis 67, 260–269. Keene, B.W. and Bonagura, J.D. 2009. Management of heart failure in dogs. In Kirk’s current veterinary therapy XIV. Eds., Bonagura, J.D. and Twedt, D.C. St. Louis, MO: Saunders Elsevier, pp: 769–780. Kittleson, M.D. and Kienle, R.D. 1998. Management of heart failure. In Small animal cardiovascular medicine, 1st ed. Eds., Kittleson, M.D. and Kienle, R.D. St. Louis, MO: Mosby, pp: 149–194. Moise, N.S., Dietze, A.E., Mezza, L.E., Strickland, D., Erb, N.H. and Edwards, N.J. 1986. Echocardiography, electrocardiography, and radiography of cats with dilatation cardiomyopathy, hypertrophic cardiomyopathy, and hyperthyroidism. Am. J. Vet. Res. 47, 1476–1486. Naranjo, C.A., Busto, U., Sellers, E.M., Sandor, P., Ruiz, I., Roberts, E.A., Janecek, E., Domecq, C. and Greenblatt, D.J. 1981. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 30, 239–245. Oyama, M.A. and Adin, D. 2022. Toward quantification of loop diuretic responsiveness for congestive heart failure. J. Vet. Intern. Med. 37, 12–21. Pin, D., Bekrich, M., Fantini, O., Noel, G. and Vidémont E. 2014. An emulsion restores the skin barrier by decreasing the skin pH and inflammation in a canine experimental model. J. Comp. Pathol. 151, 244–254. Plumb, D.C. 2008. Furosemide. In Plumb’s veterinary drug handbook, 6th ed. Ed., Plumb, D.C. Ames, IA: Blackwell Publishing, pp: 413–415. Poissonnier, C., Ghazal, S., Passavin, P., Alvarado, M.P., Lefort, S., Trehiou-Sechi, E., Saponaro, V., Barbarino, A., Delle Cave, J., Marchal, C.R., Depré, B., Vannucci, E., Tissier, R., Verwaerde, P. and Chetboul V. 2020. Tolerance of torasemide in cats with congestive heart failure: a retrospective study on 21 cases (2016–2019). BMC Vet. Res. 16, 339. Sabetti, M.C., Fidanzio, F., Troìa, R., Perissinotto, L., Romito, G., Mazzoldi, C., Quintavalla, C., Crosara, S. and Dondi F. 2022. Effect of sampling time on urinary electrolytes following oral furosemide administration in dogs with myxomatous mitral valve disease. J. Vet. Cardiol. 41, 57–69. Scruggs, S.M. and Rishniw, M. 2013. Dermatologic adverse effect of subcutaneous furosemide administration in a dog. J. Vet. Intern. Med. 27, 1248–1250. Shmunes, E. 1984. Allergic dermatitis to benzyl alcohol in an injectable solution. Arch. Dermatol. 120, 1200–1201. Zacharias, H., Raw, J., Nunn, A., Parsons, S. and Johnson, M. 2011. Is there a role for subcutaneous furosemide in the community and hospice management of end-stage heart failure? Palliat. Med. 25, 658–663. | ||
| How to Cite this Article |
| Pubmed Style Mazzoldi C, Aspidi F, Romito G. Dermatologic adverse effect of subcutaneous furosemide administration in a cat. Open Vet. J.. 2023; 13(8): 1027-1031. doi:10.5455/OVJ.2023.v13.i8.9 Web Style Mazzoldi C, Aspidi F, Romito G. Dermatologic adverse effect of subcutaneous furosemide administration in a cat. https://www.openveterinaryjournal.com/?mno=146571 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i8.9 AMA (American Medical Association) Style Mazzoldi C, Aspidi F, Romito G. Dermatologic adverse effect of subcutaneous furosemide administration in a cat. Open Vet. J.. 2023; 13(8): 1027-1031. doi:10.5455/OVJ.2023.v13.i8.9 Vancouver/ICMJE Style Mazzoldi C, Aspidi F, Romito G. Dermatologic adverse effect of subcutaneous furosemide administration in a cat. Open Vet. J.. (2023), [cited January 25, 2026]; 13(8): 1027-1031. doi:10.5455/OVJ.2023.v13.i8.9 Harvard Style Mazzoldi, C., Aspidi, . F. & Romito, . G. (2023) Dermatologic adverse effect of subcutaneous furosemide administration in a cat. Open Vet. J., 13 (8), 1027-1031. doi:10.5455/OVJ.2023.v13.i8.9 Turabian Style Mazzoldi, Chiara, Francesca Aspidi, and Giovanni Romito. 2023. Dermatologic adverse effect of subcutaneous furosemide administration in a cat. Open Veterinary Journal, 13 (8), 1027-1031. doi:10.5455/OVJ.2023.v13.i8.9 Chicago Style Mazzoldi, Chiara, Francesca Aspidi, and Giovanni Romito. "Dermatologic adverse effect of subcutaneous furosemide administration in a cat." Open Veterinary Journal 13 (2023), 1027-1031. doi:10.5455/OVJ.2023.v13.i8.9 MLA (The Modern Language Association) Style Mazzoldi, Chiara, Francesca Aspidi, and Giovanni Romito. "Dermatologic adverse effect of subcutaneous furosemide administration in a cat." Open Veterinary Journal 13.8 (2023), 1027-1031. Print. doi:10.5455/OVJ.2023.v13.i8.9 APA (American Psychological Association) Style Mazzoldi, C., Aspidi, . F. & Romito, . G. (2023) Dermatologic adverse effect of subcutaneous furosemide administration in a cat. Open Veterinary Journal, 13 (8), 1027-1031. doi:10.5455/OVJ.2023.v13.i8.9 |