| Research Article | ||
Open Vet. J.. 2023; 13(8): 955-964 Open Veterinary Journal, (2023), Vol. 13(8): 955-964 Original Research Comparison of coronary arteries morphometry and distribution in bovines with humans and other animal speciesFabián A. Gómez-Torres1*, Luz S. Cortés-Machado2 and Luis E. Ballesteros-Acuña11Department of Basic Sciences, School of Medicine, Universidad Industrial de Santander, Bucaramanga, Colombia 2Veterinary Medicine Faculty, Universidad Cooperativa de Colombia, Bucaramanga, Colombia *Corresponding Author: Fabián A. Gómez-Torres. Department of Basic Sciences, School of Medicine, Universidad Industrial de Santander, Bucaramanga, Colombia. Email: falegom [at] uis.edu.co Submitted: 05/05/2023 Accepted: 08/07/2023 Published: 31/08/2023 © 2023 Open Veterinary Journal
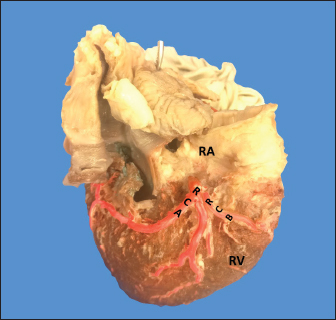
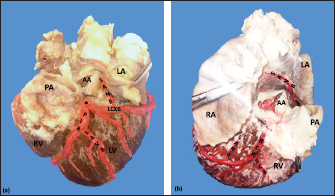
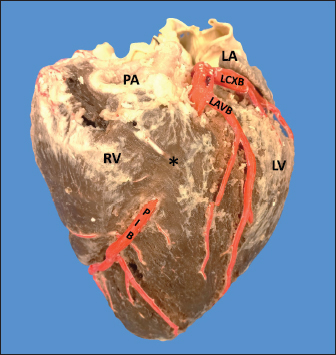
AbstractBackground: The left coronary artery (LCA) in the bovines is more developed than the right. Aim: The objective of the study is to describe the bovine coronary system from a morphological point of view, including the morphometry and its distribution. Methods: Arciform suture with 2.0 silk was applied around the sinus orifice and coronary ostium and a number 14 catheter was installed, to perfuse semi-synthetic polyester resin, consisting of a mixture of 85% GP40L palatal with 15% styrene with red color mineral. Results: The average weight of 28 bovine hearts used in our study was 1.534.1 kg. The right coronary artery had a proximal caliber of 5 +/− 0.9 mm. The LCA caliber and length were 9.4 +/− 1.2 and 18.3 +/− 4.8 mm, respectively. This artery was divided into two branches in 85.7% of the cases and trifurcated in 14.3%. The paraconal interventricular branch (PIB) ended more frequently in the apex (46.4%), and its proximal caliber was 6.4 +/− 1.4 mm. The left circumflex branch ended in 82.1% in the subsinusal interventricular sulcus, and its proximal caliber was 5.9 +/− 1.2 mm. The proximal calibers of the PIB and the left circumflex branch did not present statistically significant differences (p=0.137). The sinoatrial branch presented a dual origin (right and LCA) in 46.4% of the cases and a single origin from the LCA in 53.6% of the samples. In the evaluated hearts, left coronary dominance was observed in most cases (96.4%). Their presence of anastomosis between the branches of the coronary arteries was observed in 57.1% of cases. Conclusion: The presence of a myocardial bridge was found in six hearts (21.4%). In bovines, a high percentage of anastomosis was found, a protective factor in obstruction of the coronary arterial branches. Keywords: Anastomosis, Bovines, Coronary arteries, Coronary dominance, Myocardial bridges. IntroductionThe left coronary artery (LCA) in bovines is more developed than the right, which forms a unilateral or “left” vascularization and is responsible for generating the arterial branches that run through the paraconal interventricular sulcus (González and Rojo, 2010). The origin of the right coronary artery (RCA) is at the level of the upper border of the right leaflet of the aorta, it runs through the right atrioventricular groove without descending through the subsinusal interventricular sulcus. Its branches are to the right atrium and the underside of the right ventricle throughout its entire length. Additionally, it generates an arterial branch known as the sinoatrial node branch (SANB) (Martín-Roldán and Blanquez-Layunta, 1982; Deepak and Kisnha-Rao, 2010; González and Rojo, 2010). The LCA originates at the level of the free edge of the left aortic leaflet. There, it courses briefly between the left atrium and the origin of the pulmonary artery, then reaches the upper part of the anterior atrioventricular groove to divide where it divides into two branches, the paraconal interventricular branch (PIB) and the left circumflex branch (LCXB) (Martín-Roldán and Blanquez-Layunta, 1982; Deepak and Kisnha-Rao, 2010; González and Rojo, 2010). The PIB runs ventrally through the entire paraconal interventricular groove and ends at the level of the apex. Because this branch is short, it does not anastomose with the subsinusal interventricular branch (SIB). Throughout its course, it projects multiple branches for the left ventricle and two or three branches for the right ventricle. The main branch that is generated is very thick in most of its path (Martín-Roldán and Blanquez-Layunta, 1982; Deepak and Kisnha-Rao, 2010; González and Rojo, 2010). The LCXB runs through the coronary sulcus, around the left atrium to the subsinusal interventricular sulcus, descends through this sulcus and runs through almost its entire length, where it is called the SIB, reaching the cardiac apex. Throughout its entire length, it generates branches for the left atrium and for the posterior aspect of the left ventricle, and it also generates septal branches for the interventricular septum (Martín-Roldán and Blanquez-Layunta, 1982; Deepak and Kisnha-Rao, 2010; González and Rojo, 2010). In bovines, anatomical descriptions of the coronary vascular tree generally describe qualitative characteristics and some variant morphological expressions, with few studies describing the coronary system morphometrically in these animals (Martín-Roldán and Blanquez-Layunta, 1982; Deepak and Kisnha-Rao, 2010; González and Rojo, 2010). The description of myocardial bridges (MB), which correspond to myocardial bands that overlap and compress different segments of the branches of the coronary arteries, has not been evaluated in ruminants (Deepak and Kisnha-Rao, 2010; González and Rojo, 2010). Existing data on the anatomy of the coronary arteries in bovines are scarce, which makes access to information difficult (Bertho and Gagnon, 1964; Martín-Roldán and Blanquez-Layunta, 1982; Deepak and Kisnha-Rao, 2010), unlike in humans where there is a wide range of studies describing these conditions vascular structures. (James, 1965; Kalpana, 2003; Ballesteros et al., 2011). In addition, there is a reference to the study of the coronary tree in other types of ruminants such as the European bison (Kupczyńska et al., 2015; Barszcz et al., 2020), sheep (Bertho and Gagnon, 1964; Besoluk and Tipirdamaz, 2001; Aksoy et al., 2009; Gómez et al., 2019) and goats (Lipovetsky et al., 1983). The objective of the study was to describe the bovine coronary system from the morphological point of view, making a description of the morphometry and its distribution, through the injection of the blood vessels with polyester resin, and in a context of comparative anatomy, contrasting the findings obtained with the distribution of these arteries in other animal species and humans. Materials and MethodsThis is a cross-sectional descriptive study that will evaluate the coronary circulation of 28 bovine hearts ethically obtained from animals that are destined for slaughter at the Minerva Foods slaughterhouse in the city of Bucaramanga, with an average age of 24–30 months and weighing between 450 and 500 kg. The work methodology was as follows: once the fresh hearts have been received from the processing plant, they were frozen so that the pieces are kept in an adequate state of conservation. When working on bovine hearts, they were placed for 6 hours in a container with running water to thaw and perform complete exsanguination of the organs, so that the vascular structures were permeable. Arciform suture with 2.0 silk was made around the sinus orifice and coronary ostium and a number 14 catheter was installed, to perfuse semi-synthetic polyester resin, consisting of a mixture of 85% GP40L palatal with 15% styrene with red color mineral. Subsequently, a partial corrosion process was conducted on the hearts with 15% Potassium Hydroxide to eliminate the subepicardial fat located in the interventricular and atrioventricular grooves. Consecutively, the coronary arteries and their branches were dissected from their origin to their distal segments, recording their trajectories, shapes, calibers, anastomosis, and the presence of anatomical variations. The external diameter of these vessels was measured at 0.5 cm from their respective origins with a digital caliper (Mitutoyo®). Digital photographs were taken of each heart of the study to support the recorded observations. The findings were recorded in a pre-established format and included in an Excel file for data management. The terminations of the right and LCA were evaluated, and the type of dominance was established in the samples using Schlesinger’s criteria (Schlesinger, 1940). The extent of left coronary dominance was determined according to Didio and Wakefield’s criteria (Didio and Wakefield, 1975), with a subgroup I, when both the right and LCA arteries reach the cardiac crux and end as parallel SIBs; subgroup II, when the left ventricle and the entire interventricular septum is irrigated by the LCA, there is only one SIB that originates from the LCXB, branch of the LCA; and subgroup III, if the SIB and the right posterior ventricular branch(es) originate from the LCA, thus supplying the left ventricle, the entire interventricular septum, and the part of the posterior wall of the right ventricle. The presence of MB was evaluated with its level in each coronary artery and their branches. MBs were classified as type I if a single MB were found in a vessel, type II for two MBs in the same vessel, and type III for two or three MBs in different vessels (Kosiński et al., 2010) Statistical analysisThe significance level for the statistical test was p < 0.05. Descriptive statistics and hypothesis tests were performed using SPSS 20 software (SPSS, Chicago, IL, USA) and Microsoft Excel 2013. Continuous variables were expressed as mean and 95% confidence interval. Descriptive statistics were calculated for each morphometric parameter and the Kolmogorov-Smirnov normality test was performed for each sample. For quantitative variables, when comparing two independent groups, the student’s test was used. The chi-square test was used to compare dichotomous qualitative variables, such as anastomosis and its variations. In the case of quantitative variables after normal distribution for groups of regions, the ANOVA test was used. Data were expressed as mean and SD for all measured lengths. Ethical approvalThe procedures were in accordance with the Ethics Committee of the Universidad Cooperativa de Colombia (N° 014-2018). Additionally, they comply with Law 84 of 1989 of national scope, corresponding to Chapter VI of the "National Statute for the Protection of Animals", on the use of animals in experimentation and research. ResultsThe average weight of the 28 bovine hearts used in our study was 1,534.1 kg. The RCA presented calibers of 5 ± 0.9 mm for the proximal third, 3.4 +/− 0.8 mm in the middle third and 2.3 ± 0.6 mm in the lower third (Fig. 1). The first segment of this artery presented an average of three arterial branches and the second segment had four branches. The RCA ended between the right margin and the crux cordis in 15 cases (53.6%), in 10 specimens (39.3%) it ended in the crux cordis, and in 7.1% in the middle third of the right atrioventricular sulcus (Fig. 2a). When analyzing its branches, we found the right marginal branch (RMB) in all the hearts, with a proximal caliber of 2.7 ± 0.6 mm and ending in 20 cases in the middle third (71.4%) (Table 1). No significant differences were found between the caliber of the RMB that ended in the middle third compared to those that ended in other segments of the right edge of the heart (p=0.925). The right cone branch (RCB) originated in all cases from the RCA, without finding the presence of a third coronary artery. Its proximal caliber was 2.2 ± 0.5 mm, and its distance from the origin of the aorta was 21.6 ± 8.4 mm. RCB ended on the surface of the conus arteriosus in 20 specimens (71.4%) (Table 1) (Fig. 1). In one case, we observed the presence of anastomosis between RCB and left conus branch (LCB). The atrioventricular node branch (AVNB) had a caliber of 1.3 ± 0.6 mm and originated from the RCA in 25% of the cases and from the LCXB in 75% of the samples (Fig. 2b).
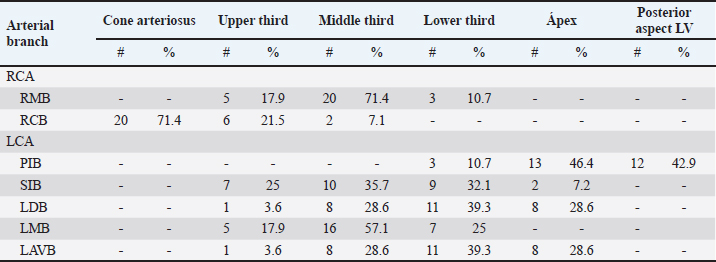
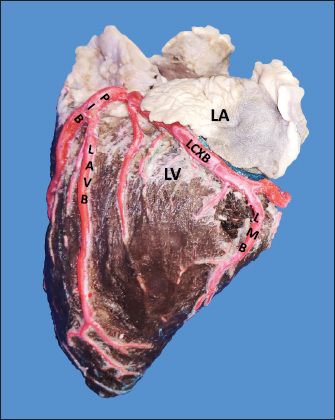
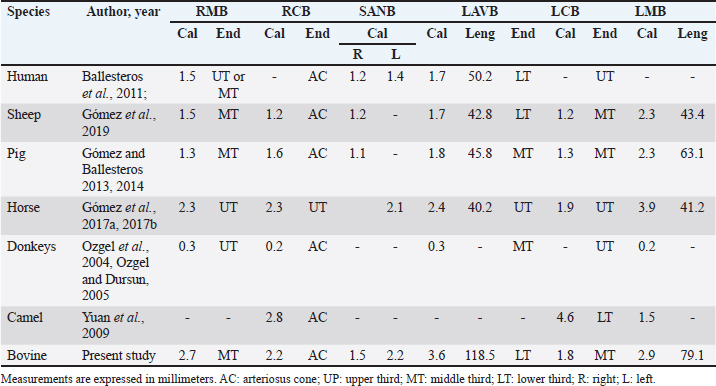
Fig. 1. Anterosuperior view of the heart. (RA): Right atrium; (RV): Right ventricle; (RCA): Right coronary artery; (RCB): Right conus branch. The LCA caliber and length were 9.4 ± 1.2 and 18.3 ± 4.8 mm, respectively. This artery was divided into two branches (PIB and LCXB) in 24 (85.7%) of the cases and trifurcated into PIB, LCXB, and left diagonal branch (LDB) in four (14.3%) specimens (Fig. 3). The LDB presented a caliber of 3.4 ± 0.8 mm, a length of 106.8 ± 40.3 mm, and ended in the same percentage in the upper and lower third and ended in the same percentage in the upper and lower third of the obtuse face of the heart (Table 1) (Fig. 3). The PIB ended in 13 (46.4%) cases in the apex, followed by 42.9% in the posterior aspect of the left ventricle and to a lesser extent in the lower third of the paraconal interventricular sulcus (10.7%). The PIB presented five right branches on average and six left branches. The proximal caliber of this main arterial branch was 6.4 ± 1.4 mm, the intermediate caliber was 4.6 ± 1.1 mm, and the distal caliber was 2.9 ± 0.7 mm. Its length was 202 ± 25.2 mm (Fig. 3). The LCXB ended in 23 specimens (82.1%) in the subsinusal interventricular sulcus and in 17.9% in the posterior aspect of the right ventricle. Its length was 149.1 ± 12.6 mm, and the calibers were as follows: proximal caliber was 5.9 ± 1.2 mm, the intermediate was 4.8 ± 0.9 mm, and the distal was 3.8 ± 0.9 mm. (Fig. 3). The proximal calibers of the PIB and the LCXB did not present statistically significant differences (p=0.137). The length between the PIB and the LCXB presented statistically significant differences (p < 0.001), being greater in the PIB and covering more territory of the left ventricle. The SANB presented a dual origin (RCA and LCA) in 46.4% of the cases and a single origin from the LCA in 15 (53.6%) specimens. When the SANB branched from the left coronary circulation, it originated from the LCXB in 23 (82.1%) of the hearts and directly from the LCA in 17.9%. The proximal caliber of the SANB was 1.9 ± 0.5 mm. The caliber of the SANB that originated from the LCA was 2.2 ± 0.4 mm and the caliber when it originated from the RCA was 1.5 ± 0.3 mm, being statistically significant (p < 0.001). The distance to the origin of the SANB when it was a branch of the LCA was 16.9 ± 8.2 mm and when it originated from the RCA it was 17.8 ± 9.4 mm (Fig. 4).
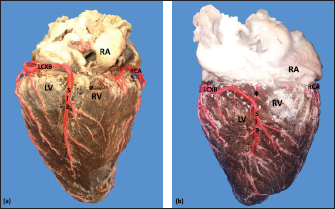
Fig. 2. Right surface of the heart. Left coronary dominance, subgroup II (a). Left coronary dominance, subgroup III (b). (LV): Left ventricle; (RA): Right atrium; (RV): Right ventricle; (LCXB): Left circumflex branch; (SIB): Subsinusal interventricular branch; (RCA): Right coronary artery; (*):AVNB. Table 1. Ending of the branches of the coronary arteries in bovines.
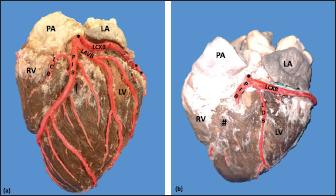
Fig. 3. Left surface of the heart. Division into two branches of the LCA (*) (a); division into three branches of the LCA (b). (LA): Left atrium; (LV): Left ventricle; (RV): Right ventricle; (PA): Pulmonary artery; (PIB): Paraconal interventricular branch; (LCXB): Left circumflex branch; (LDB): Left diagonal branch; (LCB): Left cone branch; (**): Left marginal branch; (LAVB): First left anterior ventricular branch; (#):MB.
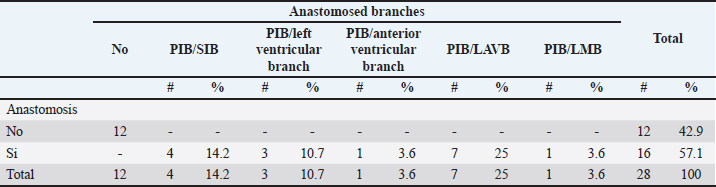
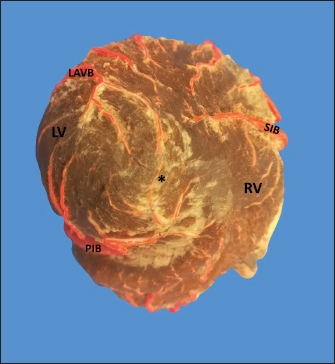
Fig. 4. Anterosuperior view of the heart. Origin of the SANB from the LCXB (a); dual origin of the SANB (b). (RA): Right atrium; (RV): Right ventricle; (LA): Left atrium; (LV): Left ventricle; (RCA): Right coronary artery; (RCB): Right conus branch; (PIB): Paraconal interventricular branch; (LCXB): Left circumflex branch; (*): LCA; (LAVB): First left anterior ventricular branch; (PA): Pulmonary artery; (AA): Aorta artery. The LCB, a branch of the PIB, presented a caliber of 1.8 ± 0.5 mm and a distance to the origin of the coronary ostium of 16.9 ± 8.2 mm (Fig. 3). The first left anterior ventricular branch (LAVB) of the PIB had a proximal caliber of 3.6 ± 1.1 mm and a distal caliber of 2.1 ± 0.8 mm. Its length was 118.5 ± 43.4 mm and the distance to the origin of the PIB was 19 ± 7.7 mm. It ended in the lower third of the left ventricular wall in 11 (39.3%) cases (Table 1) (Fig. 3). The left marginal branch (LMB), a branch of the LCXB, had a proximal caliber of 2.9 ± 0.7 mm, a distal caliber of 1.9 ± 0.6 mm, and a length of 79.1 ± 26.2 mm. Its ending was observed in the middle third of the obtuse face of the heart (57.1%) (Table 1). The proximal caliber of the LMB was greater among the branches that ended in the middle third compared with those in the upper third (p=0.003). This branch presented a distance to the apex of 76.4 ± 30.3 mm (Fig. 5). The SIB terminated between the middle and lower thirds (67.8%) (Table 1), presented a termination distance to the apex of 62.9 ± 22.2 mm, and an average of three right and two left branches were observed. The SIB originated mainly from the LCXB and had a proximal caliber of 3.6 ± 0.9 mm, an intermediate caliber of 2.8 ± 0.7, and a distal caliber of 1.9 ± 0.6 (Fig. 2a and b). In the hearts evaluated, 27 (96.4%) showed left coronary dominance. Within this group, the origin of the SIB was observed from the LCXB (subgroup II) (Fig. 2a) in 18 cases (67%), while in 9 cases (33%) it also originated from the SIB of the LCXB, and this last branch ended in the posterior face of the right ventricle (subgroup III) (Fig. 2b). There was only one case (3.6%) of mild right coronary dominance. The study found a 57.1% presence of anastomosis among the branches of the coronary arteries. There are significant statistical differences between the PIB-LAVB anastomoses (25%) compared to the other types of anastomoses (p < 0.001) (Table 2) (Fig. 6).
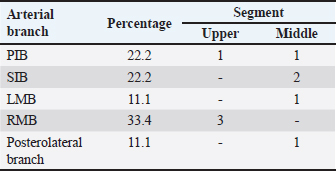
Fig. 5. Left surface of the heart. (LA): Left atrium; (LV): Left ventricle; (PIB): Paraconal interventricular branch; (LCXB): Left circumflex branch; (LMB): Left marginal branch; (LAVB): First left anterior ventricular branch. The presence of MB was found in six hearts of the study (21.4%). MB was found mainly in the RMB (33.4%) and its location in the middle third (55.5%) (Table 3). The length of the MB was 21.9 ± 13.9 mm, and for the instances of types of MB, six were type I MB (66.7%), one was type II (11.1%), and two were type III (22.2%) (Figs. 3b and 7). DiscussionThe proximal caliber of the RCA in our study of bovine hearts presented a mean value to that described in distinct species (Table 4). In ungulates, such as pigs and horses, the presence of 2–4 arterial branches has been described as emerging from the first and second segments of the RCA (Gómez and Ballesteros, 2013; Gómez et al., 2017a, 2019), which is consistent with our findings. The ending of the RMB in our series in bovines is like most studies (Table 5). Our observation of the RCB ending in the cone arteriosus was also observed in the majority of the reports (Table 5). The presence of a third coronary artery, which corresponds to the RCB emerging directly from the aorta, has been described in a small number of cases in some species such as elephants, pigs, and sheep (Cave, 1936; Sahni et al., 2008; Gómez and Ballesteros, 2013; Gómez et al., 2019), which was not observed in our research. In humans, this condition is present in a wide range of cases (25%–35%) (James, 1965; Kalpana, 2003; Ballesteros et al., 2011). AVNB has been reported to originate from the right circumflex branch of the RCA in camels, pigs, and horses in a range between 85% and 100% (Ghazi and Tadjalli, 1993; Crick et al., 1998; Sahni et al., 2008; Gómez and Ballesteros, 2013; Gómez et al., 2017a). In other ruminants such as sheep, an origin from branches of the RCA has been reported in 60.3% and emerging from the LCXB in 39.7% of the cases (Gómez et al., 2019). In our study in bovines, we also observed the origin of the AVNB from the two arterial branches described in sheep, but with a higher percentage of emergence from the LCXB (75%). Table 2. Anastomosis between the different branches of the coronary arteries in bovines.
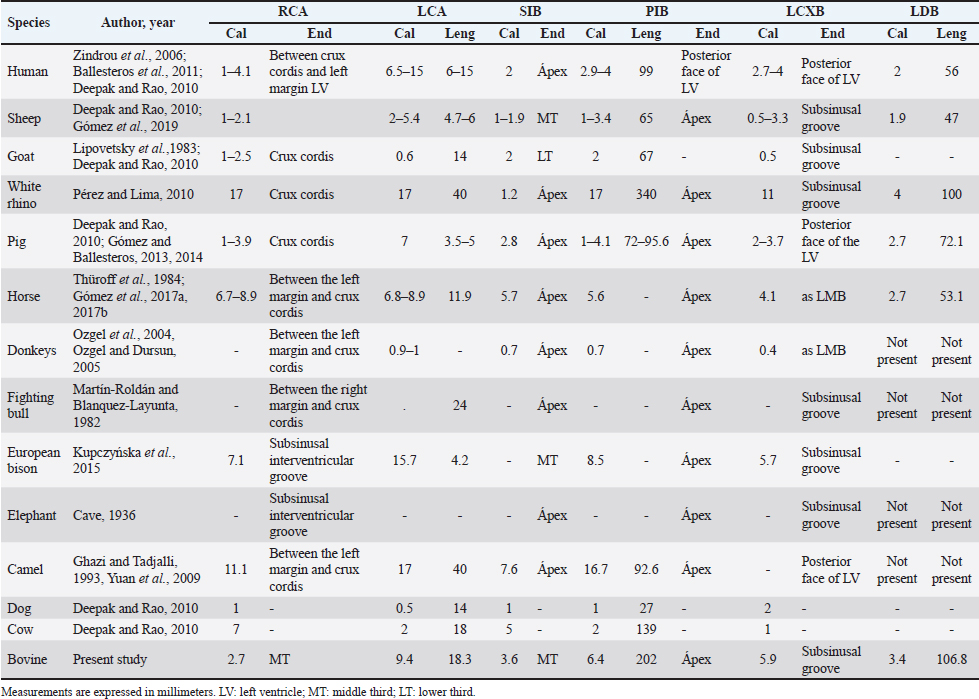
Fig. 6. Anastomosis between branches of the PIB and the first LAVB. (LV): Left ventricle; (RV): Right ventricle; (SIB): Subsinusal interventricular branch; (*): Anastomosis on the apex of the heart. LCA caliber has been reported to be larger than RCA caliber in humans, sheep, cows, goats, and pigs (Table 4), being consistent with our study in bovines. In turn, it has been observed that the caliber of the two arteries is similar in horses and white rhinos (Thüroff et al., 1984; Pérez and Lima, 2010; Gómez et al., 2017a, 2017b). In the fighting bull, it is described that the caliber of the RCA is twice that found in the LCA (Martín-Roldán and Blanquez-Layunta, 1982). The length of the LCA has been described in different animal species (Table 4), which in our study agrees with that reported in cows and is one of the longest arteries in domestic and wild animals. In pigs, some studies have reported a bifurcation pattern of the LCA in all cases, giving rise to PIB and LCXB (Crick et al., 1998; Sahni et al., 2008), and another study has described trifurcation of this artery in 20% of the samples, giving rise to an LDB (Gómez and Ballesteros, 2014). In humans, the greatest variation is described in terms of the branches emitted by the LCA, where bifurcation was found in 40%–70%, trifurcation in 9%–55%, and tetrafurcation in 5%–7% (Ortale et al., 2005; Ballesteros and Ramírez, 2008). In elephants, camels, fighting bulls, and donkeys, only one bifurcation pattern is reported (Cave, 1936; Martín-Roldán and Blanquez-Layunta, 1982; Ghazi and Tadjalli, 1993; Ozgel et al., 2004; Yuan et al., 2009; Deepak and Kisnha-Rao, 2010). In white rhinos (100%), horses (5.8%), sheep (8.1%), and European bison (7.1%), trifurcation of the LCA is reported (Pérez and Lima, 2010; Kupczyńska et al., 2015; Gómez et al., 2019), as in the present study in cattle where trifurcation was observed in 14.3% of samples. The caliber and length of LDB in our series in bovines are the highest reported of all the species in which this branch has been described (Table 4). Table 3. MB and their location in the different coronary arterial branches.
Fig. 7. Left surface of the heart. (LA): Left atrium; (LV): Left ventricle; (RV): Right ventricle; (PA): Pulmonary artery; (PIB): Paraconal interventricular branch; (LCXB): Left circumflex branch; (LAVB): First left anterior ventricular branch; (*): MB. In our study, we found that the caliber of PIB was medium distance compared with the studies in the different animal species (Table 4). The length of this branch in our research was one of the longest observed in the animals that have been described and the termination site in the apex agrees with our series (Table 4). The caliber of LCXB in our study was one of the longest that has been described in the different domestic and wild species, and the termination of this branch is as long as in most of the reports (Table 4). In pigs, it has been reported that the length of the LCXB was 80 mm (Gómez and Ballesteros, 2014), being much lower than what was found in our study in bovines (149.1 mm). Table 4. Characterization of the coronary arteries and their main branches in bovines.
Table 5. Characterization of the minor branches of the coronary arteries in bovines.
Previous reports indicate that the SANB originates from the RCA in all cases in pigs and sheep (Crick et al., 1998; Gómez and Ballesteros, 2013) and in horses, this branch originates from the LCXB of the LCA in all specimens studied (Gómez et al., 2017b). In humans, SANB is reported to originate from the RCA in 50%–79% of the samples, from the LCXB in 30%–45%, and from both arteries in 3%–7% of the hearts (Pejković et al., 2008; Ballesteros et al., 2011). In our study, we found a similar origin of this branch as in humans, emerging mainly in a unique way directly from the LCA or from the LCXB, but with a significant percentage of dual emergence between the RCA and the LCA (46.4%), which guarantees excellent irrigation to the sinoatrial node and therefore its proper functioning in this species. The caliber of SANB and LCB reported in previous studies in distinct species is similar to our findings (Table 5). In our study, the first LAVB ended in the lower third of the left ventricle, which is like that observed in other species. The caliber and length of this branch were greater than that described in previous studies (Table 5). Different calibers, lengths, and completion of the LMB have been described in animal species, which differs in our research from most studies (Table 5). A vast variety is described in the proximal caliber of the SIB, some studies agree with our findings in bovines, in turn, the ending of this arterial branch is observed in most species in the cardiac apex and in others in the middle third as it happens in our series (Table 4). In our study, we observed left coronary dominance in almost 100% of the cases, with the formation of the SIB from the LCXB. These findings are consistent with most ruminants where mainly left dominance is described (sheep, goats, and European bison) (Lipovetsky et al., 1983; Deepak and Kisnha-Rao, 2010; Kupczyńska et al., 2015; Gómez et al., 2019). In other species, right coronary dominance is described in most cases, such as in humans, white rhinos, horses, camels and elephants (Bertho and Gagnon, 1964; Ghazi and Tadjalli, 1993; Pérez and Lima, 2010; Gómez et al., 2017a). In humans and pigs, despite showing mostly right dominance, other types of dominance can also be observed in parallel (left and balanced) (Nerantzis et al., 1996; Sahni et al., 2008; Ballesteros et al., 2007; Gómez and Ballesteros, 2015a, 2015b). The presence of anastomosis is a morphological expression of protection in the heart when there is an obstruction of an arterial branch that limits the flow of blood to certain areas of the heart, and allows adequate irrigation to these obstructed territories. The anastomosis between the PIB and the SIB in the cardiac apex has been described in different species, among them are the pigs that describe the presence of this trait in 7.6% of the cases (Gómez and Ballesteros, 2013), in sheep in 12.7% (Gómez et al., 2019), and in the fighting bull this anastomosis is described between the PIB and a small branch of the SIB without mentioning the percentage of presentation (Martín-Roldán and Blanquez-Layunta, 1982). In humans, the presence of this type of anastomosis is of vital importance, when an obstruction of one of the two interventricular branches occurs, its irrigation may be compensated thanks to this type of anatomical expression, which otherwise would cause a heart attack at the lower segment of the left or right walls of the heart (Ballesteros and Ramírez, 2008). In our study in bovines, a high percentage of anastomosis was found (57.1%) and not only between the PIB and the SIB (14.2%), as has been described in other species, but we found that the PIB anastomoses frequently and at a high-rate percentage with other branches of the LCA, which would allow extra protection for the bovine heart and the low presence of myocardial infarctions in this species. In addition, it would serve as a study model of this anatomical condition in human medicine. The MB are myocardial bands that overlap the coronary arteries and their branches and can cause the decrease or total obstruction of blood flow to the territories after the presence of the MB and can cause areas of infarction. In pigs, it has been described in 42.4% of the samples (Gómez and Ballesteros, 2015b), in humans in 39% of the hearts (Ballesteros and Ramírez, 2008), and in other species such as horses and sheep they have not been found (Gómez et al., 2017a, 2017b, 2019). In our research on bovines, despite being an herbivorous animal, we found a significant percentage of this anatomical variation (21.4%), and more frequently in the RMB, unlike humans, which is located mainly in the anterior interventricular branch (PIB in animals). (Ballesteros and Ramírez, 2008) and in pigs in the SIB (Gómez and Ballesteros, 2015b). ConclusionIn bovines, it was found that a high percentage of anastomosis is present, which can be considered a protective factor in case of obstruction of the coronary arterial branches. Also, the double irrigation of the sinoatrial node from the RCA and the LCA could allow a better functioning of this structure. Additionally, bovines present a predominantly left coronary circulation in all cases and MB was observed in different coronary arterial branches. AcknowledgmentsTo the "Minerva Foods" bovine slaughter plant in the city of Bucaramanga-Colombia for the donation of cardiac specimens for the development of this research. Conflict of interestThe authors have no conflicts of interest to declare FundingThere was no funding source for this study, and it was all a contribution from the authors. Data availabilityAll data supporting the findings of this study are available within the manuscript. Any extra data needed are available from the corresponding author upon reasonable request. Authors contributionThis research was conceptualized by Fabian Gómez-Torres and Luis Ballesteros-Acuña. Injection of the vascular beds of the hearts and cleaning was performed by all authors. The photographs were taken by Luz Cortés-Machado. All authors contributed to the manuscript's reading, reviewing, revising, and approving the final version. ReferencesAksoy, G., Özmen, E., Kürtül, I., Özcan, S. and Karadağ, H. 2009. The venous drainage of the heart in the Tuj sheep. Kafkas. Univ. Vet. Fak. Derg. 15, 279–286. Ballesteros, L., Corzo, E. and Saldarriaga, B. 2007. Determinación de la Dominancia Coronaria en Población Mestiza Colombiana: un Estudio Anatómico Directo. Int. J. Morphol. 25, 483–491. Ballesteros, L.E. and Ramirez, L.M. 2008. Morphological expression of the left coronary artery: a direct anatomical study. Folia. Morphol. 67, 135–142. Ballesteros, L.E., Ramirez, L.M. and Quintero, I.D. 2011. Right coronary artery anatomy: anatomical and morphometric analysis. Rev. Bras. Cir. Cardiovasc. 26, 230–237. Barszcz, K., Polguj, M., Goździewska-Harłajczuk, K., Klećkowska-Nawrot, J., Olbrych, K., Haładaj, R. and Kupczyńska, M. 2020. Gross anatomy of coronary veins of the European bison (Bison bonasus). BMC. Vet. Res. 16, 38. Bertho, E. and Gagnon, G.A. 1964. Comparative study in three dimension of the blood supply of the normal interventricular septum in human, canine, bovine, procine, ovine and equine heart. Dis. Chest. 46, 251–262. Besoluk, K. and Tipirdamaz, S. 2001. Comparative macoanatomic investigations of the venous drainage of the heart in Akkaraman sheep and Angora goats. Anat. Histol. Embryol. 30, 249–252. Cave, A.J. 1936. On the cardiac arteries of the asiatic elephant. J. Anat. 71, 124–127. Crick, S.J., Sheppard, M.N., Ho, S.Y., Gebstein, L. and Anderson, R.H. 1998. Anatomy of the pig heart: comparisons with normal human cardiac structure. J. Anat. 193, 105–119. Deepak, S. and Rao, K. 2010. Comparative anatomy of the coronary arteries in case of humans with other mammals. Anatomica. Karnataka. 4, 35–41. Didio, L.J. and Wakefield, T.W. 1975. Coronary arterial predominance or balance on the surface of the human cardiac ventricles. Anat. Anz. 37, 147–158. Ghazi, S.R. and Tadjalli, M. 1993. Coronary arterial anatomy of the one-humped camel (Camelus dromedarius). Vet. Res. Commun. 17, 163–170. Gómez, F.A. and Ballesteros, L.E. 2013. Anatomic study of the right coronary artery in pigs: feature review in comparison with the human artery. Int. J. Morphol. 31, 1289–1296. Gómez, F.A. and Ballesteros, L.E. 2014. Morphologic expression of the left coronary artery in pigs. An approach in relation to human heart. Rev. Bras. Cir. Cardiovasc. 29, 214–220. Gómez, F.A. and Ballesteros, L.E. 2015a. Evaluation of coronary dominance in pigs; a comparative study with findings in human hearts. Arq. Bras. Med. Vet. Zootec. 67, 783–789. Gómez, F.A. and Ballesteros, L.E. 2015b. Characterization of myocardial bridges in pigs: a comparative anatomical analysis with the human heart. Folia. Morphol. 74, 395–398. Gómez, F.A., Ballesteros, L.E. and Estupiñán, H.Y. 2017a. Morphologic expression of the right coronary artery in horses. Comparative description with humans, pigs and other animal species. Austral. J. Vet. Sci. 49, 161–166. Gómez, F.A., Ballesteros, L.E. and Estupiñán, H.Y. 2017b. Morphological characterization of the left coronary artery in horses. Comparative analysis with humans, pigs, and other animal species. Ital. J. Anat. Embryol. 122, 137–146. Gómez, F.A., Cortés, L.S. and Ballesteros, L.E. 2019. Morphological characterization of the coronary arteries in African sheep (Ovis orientalis). Differential analysis with those of humans and other animal species. Folia. Morphol. 78, 63–70. González, E. and Rojo, C. 2010. Estudio del corazón. Morfología. Anatomía comparada. Dependencias cavitarias, valvulares y vasculares. Serie. Veterinaria. 2, 1–20. James , T.N. 1965. Anatomy of the coronary arteries in health and disease. Circulation 32, 1020–1033. Kalpana, RA. 2003. Study on principal branches of coronary arteries in humans. J. Anat. Soc. India. 52, 137–140. Kosiński, A., Grzybiak, M. and Kozłowski, D. 2010. Distribution of myocardial bridges in domestic pig. Pol. J. Vet. Sciences. 13, 689–693. Kupczyńska, M., Barszcz, K., Olbrych, K., Polguj, M., Wysiadecki, G., Topol, M. and Klećkowska-Nawrot, J. 2015. Coronary arteries of the European bison (Bison bonasus). Acta. Vet. Scand. 57, 82. Lipovetsky, G., Fenoglio, J.J., Gieger, M., Srinivasan, M.R. and Dobelle, W.H. 1983. Coronary artery anatomy of the goat. Artif. Organs. 7, 238–245. Martín-Roldán, R. and Blanquez-Layunta, M.J. 1982. Distribución de las arterias coronarias del toro de lidia. Zbl. Vet. Med. C. Anat. Histol. Embryol. 11, 182–189. Nerantzis, C.E., Papachristos, J.C., Gribizi, J.E., Voudris, VA., Infantis, G.P. and Koroxenidis, G.T. 1996. Functional dominance of the right coronary artery: incidence in the human heart. Clin. Anat. 9, 10–13. Ortale, J., Meciano-Filho, J.M., Paccola, A., Leal, J.G.P.G. and Scaranari, C.A. 2005. Anatomy of the lateral, diagonal and anterosuperior branches in the left ventricle of the human heart. Rev. Bras. Cir. Cardiovasc. 20, 149–158. Ozgel, O. and Dursun, N. 2005. The arterial vascularization of septum interventriculare in donkeys (Equs asinus L). Anat. Histol. Embryol. 34, 80–84. Ozgel, O., Haligur, A., Dursun, N. and Karakurum, E. 2004. The macroanatomy of coronary arteries in donkeys (Equus asinus L.). Anat. Histol. Embryol. 33, 278–283. Pejković, B., Krajnc, I., Anderhuber, F. and Kosutić, D. 2008. Anatomical aspects of the arterial blood supply to the sinoatrial and atrioventricular nodes of the human heart. J. Int. Med. Res. 36, 691–698. Pérez, W. and Lima, M. 2010. Distribución de las Arterias Coronarias en el Rinoceronte Blanco (Ceratotherium simum). Int. J. Morphol. 28, 811–814. Sahni, D., Kaur, G.D., Jit, H. and Jit, I. 2008. Anatomy and distribution of coronary arteries in pig in comparison with man. Indian. J. Med. Res. 127, 564–570. Schlesinger, M.J. 1940. Relation of anatomic pattern to phatologic conditions of the coronary arteries. Arch. Pathol. 30, 403–415. Thüroff, J.W., Hort, W. and Lichti, H. 1984. Diameter of coronary arteries in 36 species of mammalian from mouse to giraffe. Basic. Res. Cardiol. 79, 199–206. Yuan, G., Ma, J., Ye, W., Bai, Z. and Wang, J. 2009. Macroanatomy of coronary arteries in Bactrian camel (Camelus bactrianus). Vet. Res. Commun. 33, 367–377. Zindrou, D., Taylor, K.M. and Bagger, J.P. 2006. Coronary artery size and disease in UK South Asian and Caucasian men. Eur. J. Cardiothorac. Surg. 29, 492–495. | ||
| How to Cite this Article |
| Pubmed Style Gómez-torres FA, Cortés-machado LS, Ballesteros-acuña LE. Comparison of coronary arteries morphometry and distribution in bovines with humans and other animal species. Open Vet. J.. 2023; 13(8): 955-964. doi:10.5455/OVJ.2023.v13.i8.1 Web Style Gómez-torres FA, Cortés-machado LS, Ballesteros-acuña LE. Comparison of coronary arteries morphometry and distribution in bovines with humans and other animal species. https://www.openveterinaryjournal.com/?mno=152183 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i8.1 AMA (American Medical Association) Style Gómez-torres FA, Cortés-machado LS, Ballesteros-acuña LE. Comparison of coronary arteries morphometry and distribution in bovines with humans and other animal species. Open Vet. J.. 2023; 13(8): 955-964. doi:10.5455/OVJ.2023.v13.i8.1 Vancouver/ICMJE Style Gómez-torres FA, Cortés-machado LS, Ballesteros-acuña LE. Comparison of coronary arteries morphometry and distribution in bovines with humans and other animal species. Open Vet. J.. (2023), [cited January 25, 2026]; 13(8): 955-964. doi:10.5455/OVJ.2023.v13.i8.1 Harvard Style Gómez-torres, F. A., Cortés-machado, . L. S. & Ballesteros-acuña, . L. E. (2023) Comparison of coronary arteries morphometry and distribution in bovines with humans and other animal species. Open Vet. J., 13 (8), 955-964. doi:10.5455/OVJ.2023.v13.i8.1 Turabian Style Gómez-torres, Fabián A., Luz S. Cortés-machado, and Luis E. Ballesteros-acuña. 2023. Comparison of coronary arteries morphometry and distribution in bovines with humans and other animal species. Open Veterinary Journal, 13 (8), 955-964. doi:10.5455/OVJ.2023.v13.i8.1 Chicago Style Gómez-torres, Fabián A., Luz S. Cortés-machado, and Luis E. Ballesteros-acuña. "Comparison of coronary arteries morphometry and distribution in bovines with humans and other animal species." Open Veterinary Journal 13 (2023), 955-964. doi:10.5455/OVJ.2023.v13.i8.1 MLA (The Modern Language Association) Style Gómez-torres, Fabián A., Luz S. Cortés-machado, and Luis E. Ballesteros-acuña. "Comparison of coronary arteries morphometry and distribution in bovines with humans and other animal species." Open Veterinary Journal 13.8 (2023), 955-964. Print. doi:10.5455/OVJ.2023.v13.i8.1 APA (American Psychological Association) Style Gómez-torres, F. A., Cortés-machado, . L. S. & Ballesteros-acuña, . L. E. (2023) Comparison of coronary arteries morphometry and distribution in bovines with humans and other animal species. Open Veterinary Journal, 13 (8), 955-964. doi:10.5455/OVJ.2023.v13.i8.1 |