| Research Article | ||
Open Vet. J.. 2024; 14(7): 1625-1633 Open Veterinary Journal, (2024), Vol. 14(7): 1625–1633 Research Article Perioperative changes in plasma cardiac troponin I concentration during mitral valvuloplasty for severe mitral regurgitation in dogsTakeki Ando1,2, Kippei Mihara3, Isamu Kanemoto3 and Hideo Akiyoshi2*1Ando Animal Hospital, 8-20 Kuruma, Awaji, Hyogo 656-2311, Japan 2Laboratory of Veterinary Surgery, Graduate School of Veterinary Science, Osaka Metropolitan University, Osaka, Japan 3Chayagasaka Animal Hospital, 1-1-5 Shinnishi, Chikusa-ku, Nagoya, Aichi 464-0003, Japan *Corresponding Author: Hideo Akiyoshi. Laboratory of Veterinary Surgery, Department of Veterinary Clinical Medicine, Graduate School of Veterinary Science, Osaka Metropolitan University, Osaka, Japan. Email: h.akiyoshi [at] omu.ac.jp Submitted: 04/03/2024 Accepted: 09/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
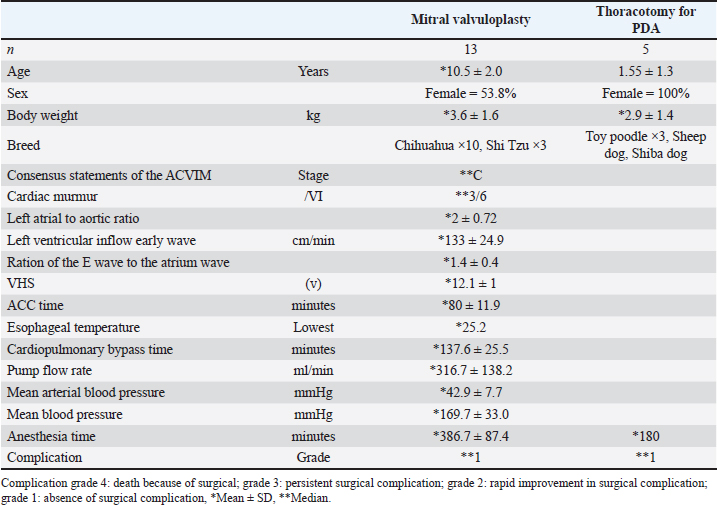
AbstractBackground: Mitral valvuloplasty (MVP) is a surgical procedure for treating severe mitral regurgitation in dogs. Although MVP is considered highly invasive, the extent of myocardial injury, postoperative complications, and recovery has not been evaluated. Aim: This study examined the degree of MVP invasiveness, the extent of myocardial damage, postoperative complications, cardiomyocyte recovery, and timing of hospital discharge. Methods: Cardiac troponin I (cTnI) was used to investigate the myocardial damage caused by cardiac arrest associated with a surgical approach to the myocardium in 13 patients with MVP and five controls with patent ductus arteriosus (PDA) who underwent similar anesthesia and thoracotomy. Results: The level of cTnI peaked 1 day after surgery and was significantly higher in the MVP group (median, 19.90 ng/ml) than in the control group (median, 1.50 ng/ml p < 0.001). At day 7, the cTnI level was significantly higher in the MVP group (1.9 ng/ml) than in the control group (0.1 ng/ml) (p < 0.001), and recovery to the preoperative level took 10 days in the MVP group but returned to the preoperative level at day 7 in the control group. Although the mean arterial pressure of cardiopulmonary bypass (CPB) at the time of use was 42.92 mmHg, the peak cTnI levels in the two patients who exhibited a temporary decrease of 20 mmHg or less (46.03 ng/ml) were significantly higher than in the other 11 patients (19.70 ng/ml) (p < 0.05). Preoperative cTnI levels were correlated with the severity of postoperative complications (P=0.03, F=0.71). Conclusion: The results showed that MVP caused temporary greater myocardial tissue damage than thoracotomy, but postoperative recovery was smoother. A high preoperative cTnI level requires relatively more careful postoperative management, and measuring the level of cTnI over time after surgery can provide information about the extent of myocardial damage and recovery from surgery and help determine the time of discharge. Keywords: Cardiac troponin I, Cardiopulmonary bypass, Invasiveness, Mitral valvuloplasty, Myxomatous mitral valve disease. IntroductionMitral valvuloplasty (MVP) is an invasive procedure that has been performed in some dogs with severe valvular heart disease (Kanemoto et al., 2021). However, the extent of myocardial injury, incidence of postoperative complications, and postoperative recovery associated with this procedure have not been evaluated. Although pharmacotherapy is the first choice for the treatment of heart diseases in dogs, surgery is required if drug therapy is ineffective. Myxomatous mitral valve disease (MMVD) is the most common cardiac disease in older small-breed dogs (Häggström, 2009). Diagnosis and treatment are guided by American College of Veterinary Internal Medicine (ACVIM) guidelines (Atkins et al., 2009; Keene et al., 2019). Although MMVD is mainly treated with medical therapy, surgical therapy may be chosen to improve clinical symptoms, improve the quality of life, or discontinue or reduce the medication dose in dogs over Stage B2. Recently, MVP with open-heart surgery has been proposed, showing a good short-term prognosis (Kanemoto, 2021). Unlike medical therapy for MMVD, these surgical therapies are now used by some experts as definitive treatment. However, no study has evaluated the degree of invasion and perioperative stages of MVP. MVP is a highly invasive surgical procedure that involves cardiac arrest, making it necessary to report on specific invasiveness, cardiomyocyte damage, and recovery for the widespread use of these treatments. Based on this information, clinical veterinarians can decide whether to choose MVP for the treatment of MMVD. Herein, we will report on the degree of MVP invasiveness, the extent of myocardial damage, postoperative complications, cardiomyocyte recovery, and the timing of discharge. Early diagnosis of MMVD during the asymptomatic stage can be tentatively made by checking for intracardiac murmurs through careful auscultation during routine health checkups, such as vaccinations, and by asking about increased respiratory rate at rest. A definitive diagnosis can be made by ultrasound examination (Atkins et al., 2009). The ACVIM guidelines indicate that the diagnostic criteria for MMVD stage B are a murmur intensity Levine of >3/6, left atrial/aortic (LA/Ao) of >1.6, LVIDDN of >1.7, and vertebral heart size (VHS) of >10.5v (Atkins et al., 2009; Gordon et al., 2017; Keene et al., 2019). Medical therapy is recommended during MMVD diagnosis regardless of the acute or chronic stage, which changes with the progression of the MMVD stages. Although these medical therapies require strict medication management and exercise restrictions, they enable the long-term survival of patients. However, MVP is recommended by some experts for patients with Stage B2 having difficulty in adhering to strict medication management and exercise restrictions (Keene et al., 2019; Gordon et al., 2022). Although previous studies have reported that MVP improves short-term outcomes (Griffiths et al., 2004; Matsuura et al., 2022; Pennington et al., 2024), pet owners are often provided with less information while choosing for an operation. MVP is a surgical treatment of choice because of its favorable impact on the quality of life, including reduction in MR, reduction in LA volume, and discontinuation or dose reduction (Kanemoto, 2021). Although cardiac surgery (Orton et al., 2001; Borgarelli and Haggstrom, 2010) can be fundamentally treated by stopping the heart by cardiopulmonary bypass (CPB), it is considered to be highly invasive surgery as it is highly affected by myocardial injury and myocardial ischemia associated with CPB and has a high risk of postoperative complications. A combination of CPB and deep surface-induced hypothermia (sHT) enabled circulatory arrest during cardiac surgery (Hikasa et al., 1967; Kanemoto et al., 2010). Stable and effective CPB has recently become possible even in small dogs (Kanemoto, 2021). Additionally, the discharge rates after CPB were improved in dogs (Kanemoto et al., 1990, 2010; Griffiths et al., 2004; Uechi et al., 2012; Yokoyama et al., 2017). Cardiac Troponin I (CTnI) is a biomarker that assesses myocardial injury and ischemic necrosis (Adams et al., 1994; Kociol et al., 2010; White, 2011). It is a useful tool for diagnosing myocardial infarction in humans (Thygesen et al., 2007) and predicting clinical outcomes by diagnosing cardiac disease in cats and dogs (Langhorn and Willesen, 2016). Furthermore, a CTnI value can be used to estimate the extent of myocardial ischemia or injury during open-heart surgery (Vermes et al., 2000; Blankenberg et al., 2019), which makes it suitable to assess the severity of myocardial and cardiomyocyte damage and its recovery (Wu et al., 2018). Levels of CTnI increase rapidly with cardiomyocytes after necrosis and ischemia, and they return to normal after 5 to 10 days of disappearance of these conditions (Goldmann et al., 2001; Burgener et al., 2006). In dogs anesthetized for surgery, the increases in CTnI levels induced by anesthesia are small (Cilli et al., 2010; Verbiest et al., 2013). The type of anesthesia has less influence on CTnI levels as shown by Saunders et al. (2009). During human cardiac surgery, an increase in cTnI levels was observed due to differences in the surgical approach to myocardial tissue (Immer et al., 1998). A peak value increase of cTnI by 35 µg/l or more was associated with the incidence of major postoperative complications (Immer et al., 1999). However, no studies have examined the relationship between open-heart surgery and cTnI levels in dogs (Pelander et al., 2008; Shih et al., 2009). To assess the degree of invasion in dogs with MVP, we evaluated cardiomyocyte damage, postoperative recovery, and complications using cTnI value, and compared it with the control group (TH group). Materials and MethodsAnimalsAll dogs in the MVP and TH groups underwent surgery at the owners’ request. An oral or written explanation of the study objectives was provided to the owners and oral or written informed consent was obtained from them. The present study complied with the animal testing regulations of Osaka Metropolitan University (Osaka Prefecture, Japan). The remaining plasma samples after treatment were used. MVP for mitral regurgitation was performed on 13 small dogs at the Chayagasaka Animal Hospital in 2015 (Aichi Prefecture, Japan). Altogether, five dogs underwent patent ductus arteriosus (PDA) thoracotomy (TH) at Ando Animal Hospital in 2016 (Hyogo Prefecture, Japan) AnesthesiaAll dogs received premedication with atropine (0.025 mg/kg SC), midazolam (0.2 mg/kg IV), and butorphanol (0.2 mg/kg IV) and anesthesia induction with alphaxalone (2–4 mg/kg IV) and propofol (0.4-PHR CD-2% isoflurane in 100% oxygen. Fentanyl hydrochloride (5–30 μg/kg/h), alphaxalone (20–70 μg/kg/h continuous infusion [CRI]), and intermittent rocuronium (9 μg/kg/min CRI) were used as needed) During extracorporeal circulation, 0.02 mg/kg/min of alphaxalone CRI, 9 μg/kg of rocuronium, 5 μg/kg/h of fentanyl, 0.3%–2% of isoflurane, and 100% oxygen were mixed with a membrane oxygenator from the extracorporeal circulation side (Kanemoto et al., 2010, 2021). Cardiac surgeryMVP was defined as artificial chordal reconstruction, suture valvuloplasty, and/or suture annuloplasty (Kanemoto et al., 2010, 2021). In MVP, the dog was placed in the right lateral position for a left-fifth intercostal thoracotomy (Yokoyama et al., 2017). Deep sHT was induced slowly by cooling the blood in a heat exchanger (Agua stream ASO4; JMS Co., Tokyo, Japan) to a minimum rectal temperature of 28°C (Kanemoto et al., 2021). Prior to releasing the aortic cross-clamp (ACC), blood was slowly warmed to an esophageal temperature of 37°C using a heat exchanger (Mihara et al., 2017). We inserted arterial and venous drainage cannulas through the left jugular vein and left carotid artery into the right atrium for CPB. It was then connected to a CPB open-circuit (Terumo Baby RXO5; Terumo Company, Tokyo, Japan) that included a rigid reservoir and a membrane oxygenator (Kanemoto et al., 2010, 2021; Mihara et al., 2017). Based on the clinical signs, the risk of postoperative surgical complications was categorized into four grades. Severity is based on the following criteria: severity 4, death due to surgical complications; severity 3, persistent surgical complications; severity 2, rapid improvement of surgical complications; and severity 1, no or mild complication and good postoperative course. As for the specific complications, the operators subjectively evaluated them as heart failure, infection or inflammation at the operative area (e.g., site of incision), infectious diseases of the urinary organs, thrombogenesis, renal failure, and pneumonia (Bilimoria et al., 2013). Ductus arteriosus ligation (TH) by thoracotomy without the surgical approach to the heart muscle wall was carried out for the PDA by thoracotomy for those dogs with low body weight (Goodrich et al., 2007). ExaminationAll animals underwent physical examinations, echocardiograms, and blood tests. Levine scale (Silverman and Wooley, 2008) was used during auscultation to assess for cardiac and respiratory conditions, including cardiac murmurs. A chest X-ray was performed to measure the VHS by using the vertebrae from the lateral view (Buchanan, 2000) and cardiothoracic ratio (CTR) from the dorsum of the abdomen (Hamlin, 1968). Three cardiac cycles were monitored by echocardiogram on each measurement, which were then averaged. Additionally, the LA/Ao ratio was calculated by comparing the left atrial and aortic inner diameters according to a two-dimensional short-axis view from the right parasternal-to-basal level (Rishniw and Erb, 2000). The E wave to A wave ratio (E/A) was calculated by measuring the early diastolic and atrial contraction velocities using a pulsed-wave Doppler technique based on a two-dimensional four-space image of the apical region from the left parasternal region at the approximate center of the mitral valve, where the mitral valve leaflet abuts (Bodh et al., 2019). The mitral regurgitant flow was assessed by calculating the time–velocity integral of the flow (Pedersen et al., 1999). A blood sample (1.0 ml) of plasma cTnI was collected from the femoral vein, placed in a heparinized tube, and centrifuged at 3,000 rpm for 5 minutes. The isolated plasma was analyzed rapidly using a bench-top-analyzer (Pathfast, Mitsubishi, Japan). A part of it was stored frozen at −20°C and measured after freezing. A benchtop chemiluminescent enzyme immunoassay system was used for testing (Kurihara et al., 2008). This test device uses chemiluminescent enzyme immunoassay, and the test system uses goat and mouse monoclonal antibodies. The measurement range was 0.02–50.00 ng/ml and the reference standard value for human was 0.028 ng/ml. A preliminary study of the time course of cTnI in MVP was performed in four dogs, and the cTnI levels were measured at 1, 2, 6, 12, 24, 48, 72, 96, 144, 168, and 240 h before and after surgery until the cTnI level had returned to the level measured before surgery. The cTnI levels were measured before and at 12, 24, and 168 h after surgery in 13 MVP and 5 TH dogs before and at 24 and 168 h after surgery. Statistical analysisDescriptive data are tabulated. A normal probability plot graph was visually evaluated and tested using the Kolmogorov–Smirnov test to test the normality of data. Normally distributed data were reported as mean (standard deviation) and non-normally distributed data as median (range). Wilcoxon signed-ranks test was used to test the difference in the pre-and postoperative cTnI levels, which were non-normalized values, between the two groups. A pair of normally distributed samples was tested using Student’s t-test. Mann–Whitney’s U test was used to test for differences in the hypotensive episodes during CPB between the two groups. A correlation between the severity of postoperative complications and variables was tested using Spearman’s rank correlation matrix. p <0.05 was determined to be significant. All statistical analyses were performed using the statistical package (Stata version 15, College Station, TX) Ethical approvalAll dogs in the MVP and TH groups underwent surgery at the owners’ request. An oral or written explanation of the study objectives was provided to the owners and oral or written informed consent was obtained from them. The present study complied with the animal testing regulations of Osaka Metropolitan University (Osaka Prefecture, Japan). The remaining plasma samples after treatment were used. ResultsCharacteristics of the study populationIn the study, 13 MVP dogs were enrolled, including 3 Shih Tzus and 10 Chihuahuas. These dogs had Stage C MR and received MVP as per the American Veterinary Society MR Severity Classification (Keene et al., 2019). The dogs’ mean age and weight at the time of surgery were 10.5 ± 2.0 years and 3.6 ± 1.6 kg, respectively, with 53.8% females (Table 1). The assessment of dog heart murmur was Levine 3/6 ± 0.5, mean LA/Ao was 2 ± 0.72, mean E wave peak was 133 ± 24.9 cm/s, mean E/A was 1.4 ± 0.4, VHS was 12.1 ± 1v, and mean CTR was 67% ± 7.6% (Table 1). MVP had a mean anesthetic time of 386.7 ± 87.4 minutes and an ACC time of 80 ± 11.9 minutes. The average pump time, pump flow rate, mean arterial pressure (AP), and blood pressure during CPB were 137.6 ± 25.5 minutes, 316.7 ± 138.2 ml/ minutes, 42.9 ± 7.7 mmHg, and 169.7 ± 33.0 mmHg, respectively (Table 1). At the early stage of CPB, the mean aortic pressure was 20 mmHg or less for 5–10 minutes in two patients. All dogs underwent artificial chordal reconstruction, annuloplasty, and valvuloplasty, which reduced the mean time–velocity integral of mitral regurgitant blood flow by 90% and demonstrated no mitral regurgitation (Pedersen et al., 1999). All dogs were treated in an intensive care unit for 24 hours (room temperature, 26°C; humidity, 50%; oxygen concentration, 40%) before being transferred to a general ward, fed a regular diet, and underwent light walking training. A patient was discharged from the hospital on the 7th day after surgery. After discharge, the dogs were treated with oral medications and owners were instructed to have the dogs rest at home. Additionally, sutures were removed by the referring veterinarian 10 days after surgery. All five patients in the TH group underwent PDA ligation and closure. They included Toy Poodles (n=3; females), an Esha Ness Sheepdog (female), and a Sheba dog (female). The average age and weight of the dogs at the time of surgery were 18.6 ± 15.6 months and 2.6 ± 1.5 kg, respectively. After surgery, no dogs demonstrated a PDA shunt. The postoperative management for the TH group was similar to that of the MVP group. Table 1. Mitral valvuloplasty surgery patient data in mitral regurgitation and thoracotomy in patients with patent ductus arteriosus (PDA).
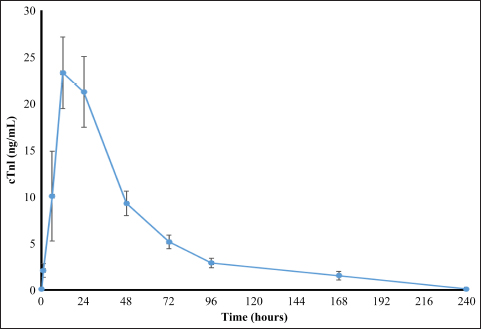
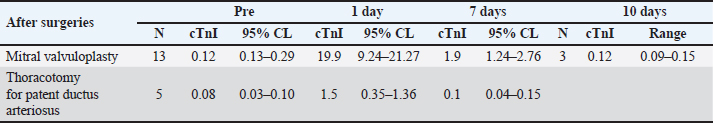
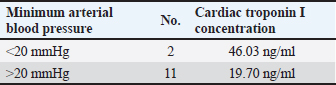
cTnI levelAmong patients who underwent MVP surgery, the changes in cTnI values during the perioperative period are shown in Figure 1 for four patients observed for up to 10 days after surgery. The median preoperative cTnI level was 0.12 ng/ml (95% CL 0.13–0.29 ng/ml), which increased significantly to a median of 19.9 ng/ml (95% CL 9.24–21.27 ng/ml) after one day of surgery. At 7 days after surgery, the median cTnI level was 1.9 ng/ml (95% CL 1.24–2.76 ng/ml), which was significantly higher than the preoperative level (p < 0.01). However, at 10 days after surgery, the median cTnI level value was 0.12 ng/ml, which returned to the preoperative level (Table 2). The median preoperative cTnI level in the TH group was 0.08 ng/ml (range 0.02–0.13 ng/ml). At day 1, this level increased significantly to 1.5 ng/ml (0.49–1.95 ng/ml) (p < 0.001). However, 7 days after surgery, the level decreased to 0.1 ng/ml (0.08–0.23 ng/ml), the same as the preoperative level, as shown in Table 2. Although there was no significant difference in preoperative cTnI levels between the MVP and TH groups, there was a significantly higher leakage of cTnI in the MVP group at day 1 postoperatively (Table 2). The peak cTnI value in the MVP group did not correlate with the grade of postoperative complications. However, a high preoperative cTnI level was significantly associated with the severity of postoperative complications (P=0.03 and F=0.71). Of the 13 MVPs who underwent CPB, 2 had significantly elevated peak cTnI levels (42.06 and 50 ng/ml) (p < 0.05). Although the mean arterial blood pressure during CPB was reduced to 20 mmHg or less during the initial 5–10 minutes of CPB, there were no other abnormal changes. The mean pump flow, mean AP, and mean AP measured at 5-minutes intervals were 316.7 ± 138.2, 42.9 ± 7.7, and 169.7 ± 33.0 mmHg, respectively, as shown in Figure 1. Among patients in the MVP group, the level of cTnI on the first postoperative day was significantly higher when the CPB was temporarily reduced to 20 mmHg or less (46.03 ng/ml) than when the mean CPB-AP remained stable at 20 mmHg or more (19.70 ng/ml) (p < 0.05) (Table 3). The dogs’ clinical symptoms improved postoperatively, with postoperative reductions in cTnI levels. The patients were discharged on postoperative day 7 (1.90 ng/ml) with continued cTnI level elevation (0.12 ng/ml). Ten days later preoperative levels were achieved (0.12 ng/ml) (Table 2). Conversely, the TH group, which did not undergo myocardial tissue surgery, already demonstrated cTnI levels similar (0.10 ng/ml) to preoperative levels (0.08 ng/ml) and was discharged 7 days after surgery. DiscussionOur results confirm that, in dogs, MVP surgery causes more severe myocardial tissue damage than thoracotomy for PDA based on the cTnI levels. However, the postoperative recovery was smooth, and dogs returned to their preoperative levels 10 days after surgery. As the measurement of concentration of cTnI in the perioperative stage can estimate preoperative evaluation of cardiomyocytes in cardiac surgery, the estimation of cardiomyocyte damage during operation, and recovery of cardiomyocytes after operation becomes an important item for examination.
Fig. 1. Perioperative cTnI levels in three dogs in the MVP group that were observed up to 10 days after surgery. Table 2. Patient data based on perioperative cardiac troponin I concentrations in mitral valvuloplasty and thoracotomy surgeries.
Table 3. Relationship between minimum blood pressure and concentration of cardiac troponin I during cardiopulmonary bypass.
The preoperative cTnI concentration did not differ significantly between the MVP (median, 0.12) and TH groups (median, 0.08). However, the value was slightly higher in the MVP group as it included older patients with ACVIM stage C who had chronically worsened MMVD in relatively elderly patients, whereas the TH group underwent surgery at a relatively young age before severe symptoms developed. Three patients with high preoperative cTnI levels (median 0.66 ng/ml, range 0.44–0.12 ng/ml) were more likely to have serious postoperative complications (long-term heart failure, P=0.03; F=0.71). As cases with high preoperative cTnI concentration may indicate preoperative myocardial tissue damage, the preoperative cTnI measurement seems to be effective for preoperative evaluation of the operation. Peak cTnI levels were observed 12 and 24 hours after cardiac-related surgery. The median cTnI levels were 27.06 ng/ml (range 9.71–50 ng/ml) and 19.9 ng/ml (range 8.64–50 ng/ml), respectively, with no significant difference between the two-time points (P=0.55). Therefore, it is reasonable to use the 24-hours postsurgery cTnI level for comparison of peak cTnI levels in dogs undergoing cardiac-related surgery (Shih et al., 2009). Adverse effects of cardiac-related surgery on cardiomyocytes can be attributed to anesthesia, surgical damage to the myocardium, and cardiac arrest at CPB (Ailawadi and Zacour, 2009). Peak cTnI levels at surgery were significantly higher in the MVP group (median 19.9 ng/ml) than in TH group (median 1.5 ng/ml) (p < 0.001), indicating cardiomyocyte injury. MVP surgery involves a large incision in the left atrial wall and a surgical approach to the left ventricular papillary muscle, resulting in frequent physical damage to myocardial cells and high cTnI values. Conversely, the surgical approach in the TH group did not involve heart muscle, and only aorta and pulmonary artery were approached, resulting in less damage to the heart muscle tissue and significantly lower peak cTnI levels. Two patients in the MVP group had significantly higher peak cTnI levels (42.06, 50 ng/ml) than others (median 19.9 ng/ml; P=0.04). In the MVP group, the mean AP during CPB was 42.9 ± 7.7 mmHg, measured at a 5-minutes interval. In patients with high cTnI levels, the mean AP decreased to 20 mmHg or less within 5–10 minutes during the early PCB exposure period, suggesting transient myocardial ischemia occurred, resulting in abnormally increased (Canty, 2022). The median cTnI level (19.7 ng/ml) was significantly lower at stable conditions with a CPB mean-AP of 20 mmHg or more. Based on these findings, postoperative cTnI measurement is beneficial for evaluating cardiomyocyte damage during surgery. Perioperative general anesthesia has minimal effects on perioperative cTnI levels and cardiomyocytes, as per previous studies (Goldmann et al., 2001; Pelander et al., 2008; Saunders et al., 2009; Cilli et al., 2010). The anesthesia protocol used in this study was similar to previous studies on MVP and TH (Pelander et al., 2008; Shih et al., 2009; Kanemoto et al., 2021). The changes in cTnI values in the TH group were consistent with previous reports (Pelander et al., 2008; Saunders et al., 2009; Shih et al., 2009; Cilli et al., 2010; Verbiest et al., 2013), indicating that anesthesia had minimal effects on cardiomyocytes in this study due to optimal maintenance of anesthesia. As for the TH group, the cTnI level was lowered to the preoperative level (0.08 ng/ml) 7 days after surgery (median: 0.1 ng/ml) and patient was discharged. Although the MVP group’s mean level remained significantly higher (P=0.001) than preoperative level (0.12 ng/ml) at 7 days (1.9 ng/ml), it decreased to the preoperative level after 10 days (0.12 ng/ml). This is consistent with a typical temporary cTnI leak that normalizes between days 5 and 10 (Goldmann et al., 2001; Burgener et al., 2006), indicating a smooth recovery. A decrease in cTnI levels was observed during postoperative recovery (Shih et al., 2009), which suggests that the appropriate time for discharge is 10 days after cardiomyocyte stabilization. Although cardiac surgery in children and infants was associated with major postoperative complications when peak cTnI levels increased to 35 μg/l or higher (Immer et al., 1999), only one out of three cases of major complications (heart failure) occurred in the MVP group in this study where the peak cTnI level exceeded 35 ng/ml. The other two cases experienced no adverse effects. There was no correlation between the peak cTnI values in the group with and without postoperative complications (16.1 and 30.05 ng/ml, respectively). Although postoperative cTnI levels may be lower than preoperative levels if MVP resolves MR, improves MMVD symptoms, and reduces preoperative cardiac burden, this study did not confirm this finding. There are several limitations to this study. First, the small sample size in the MVP group may have hindered our ability to detect statistically significant differences. Additionally, the cTnI value was measured on day 7 after surgery in many animals, and long-term observations were not done to confirm the effect and recovery time of the operation. Furthermore, pathological examination was not possible, and necrosis and ischemia of the cardiomyocyte could not be proven either. With the accumulation of cases in the future, the cTnI value will be a useful index for assessing the damage and recovery of cardiomyocytes during open-heart surgery in dogs. Additionally, a detailed examination of the changes in cTnI levels over time may provide useful information for determining the time of discharge. As cTnI measurement is cumbersome, alternative test methods have been proposed and are expected to become widely available in the future (Mahmoud et al., 2024). Moreover, CPB should be managed to maintain the mean aortic pressure at ≥20 mmHg. Conclusively, MVP places a heavy burden on the myocardial tissue and the canines undergoing such procedure may take longer to recover after the operation. Furthermore, the generation risk of the postoperative complications was higher in the dogs with high preoperative cTnI levels. After surgery, the cTnI level may provide an estimate of the extent and recovery of damage of cardiomyocytes and may help determine the time of discharge. Therefore, a favorable CPB was suggested to be effective in protecting the cardiomyocytes. AcknowledgmentsThe authors are grateful to Enago (www.enago.jp) for their review of English. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsTakeki Ando wrote the paper; Kippei Mihara and Isamu Kanemoto provided guidance and performed some of the surgeries essential for this research; Hideo Akiyoshi provided research guidance. FundingThis research received no specific grant. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAdams, J.E., 3rd, Schechtman, K.B., Landt, Y., Ladenson, J.H. and Jaffe, A.S. 1994. Comparable detection of acute myocardial infarction by creatine kinase MB isoenzyme and cardiac troponin I. Clin. Chem. 40(7 Pt 1), 1291–1295. Ailawadi, G. and Zacour, R.K. 2009. Cardiopulmonary bypass/extracorporeal membrane oxygenation/left heart bypass: indications, techniques, and complications. Surg. Clin. North Am. 89(4), 781–796. Atkins, C., Bonagura, J., Ettinger, S., Fox, P., Gordon, S., Haggstrom, J., Hamlin, R., Keene, B., Luis-Fuentes, V. and Stepien, R. 2009. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J. Vet. Intern. Med. 23(6), 1142–1150. Bilimoria, K.Y., Liu, Y., Paruch, J.L., Zhou, L., Kmiecik, T.E., Ko, C.Y. and Cohen, M.E. 2013. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J. Am. Coll. Surg. 217(5), 833–842. Blankenberg, S., Wittlinger, T., Nowak, B. and Rupprecht, H.J. 2019. [Troponins as biomarkers for myocardial injury and myocardial infarction]. Herz. 44(1), 4–9. Bodh, D., Hoque, M. and Saxena, A.C. 2019. Echocardiographic study of healthy Indian Spitz dogs with normal reference ranges for the breed. Vet. World 12(6), 740–747. Borgarelli, M. and Haggstrom, J. 2010. Canine degenerative myxomatous mitral valve disease: natural history, clinical presentation and therapy. Vet. Clin. North Am. Small Anim. Pract. 40(4), 651–663. Buchanan, J.W. 2000. Vertebral scale system to measure heart size in radiographs. Vet. Clin. North Am. Small Anim. Pract. 30(2), 379–393. Burgener, I.A., Kovacevic, A., Mauldin, G.N. and Lombard, C. W. 2006. Cardiac troponins as indicators of acute myocardial damage in dogs. J. Vet. Intern. Med. 20(2), 277–283. Canty, J.M., Jr. 2022. Myocardial injury, troponin release, and cardiomyocyte death in brief ischemia, failure, and ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol. 323(1), H1–H15. Cilli, F., Alibhai, H.I., Armitage-Chan, E., Boswood, A., Hammond, R.A., Jasani, S. and Brodbelt, D.C. 2010. Incidence of elevation of cardiac troponin I prior to and following routine general anaesthesia in dogs. Vet. Anaesth. Analg. 37(5), 409–416. Goldmann, B.U., Christenson, R.H., Hamm, C.W., Meinertz, T. and Ohman, E.M. 2001. Implications of troponin testing in clinical medicine. Curr. Control Trials Cardiovasc. Med. 2(2), 75–84. Goodrich, K.R., Kyles, A.E., Kass, P.H. and Campbell, F. 2007. Retrospective comparison of surgical ligation and transarterial catheter occlusion for treatment of patent ductus arteriosus in two hundred and four dogs (1993–2003). Vet. Surg. 36(1), 43–49. Gordon, S.G., Saunders, A.B. and Wesselowski, S.R. 2017. Asymptomatic canine degenerative valve disease: current and future therapies. Vet. Clin. North Am. Small Anim. Pract. 47(5), 955–975. Gordon, S.G., Saunders, A.B. and Wesselowski, S.R. 2022. Asymptomatic Canine degenerative valve disease: diagnosis and current and future therapies. Vet. Clin. North Am. Small Anim. Pract. 52(3), 819–840. Griffiths, L.G., Orton, E.C. and Boon, J.A. 2004. Evaluation of techniques and outcomes of mitral valve repair in dogs. J. Am. Vet. Med. Assoc. 224(12), 1941–1945. Häggström, J., Höglund, K. and Borgarelli, M. 2009. An update on treatment and prognostic indicators in canine myxomatous mitral valve disease. J. Small Anim. Pract. 50(Suppl. 1), 25–33. Hamlin, R.L. 1968. Analysis of the cardiac silhouette in dorsoventral radiographs from dogs with heart disease. J. Am. Vet. Med. Assoc. 153(11), 1446–1460. Hikasa, Y., Shiratani, H., Satomura, K., Saito, A. and Mori, T. 1967. [Surgical treatment for infantile heart diseases--with reference to open heart radical surgery]. Geka. Chiryo. 17(2), 209–220. Immer, F.F., Stocker, F., Seiler, A.M., Pfammatter, J.P., Bachmann, D., Printzen, G. and Carrel, T. 1999. Troponin-I for prediction of early postoperative course after pediatric cardiac surgery. J. Am. Coll. Cardiol. 33(6), 1719–1723. Immer, F.F., Stocker, F.P., Seiler, A.M., Pfammatter, J.P., Printzen, G. and Carrel, T.P. 1998. Comparison of troponin-I and troponin-T after pediatric cardiovascular operation. Ann. Thorac. Surg. 66(6), 2073–2077. Kanemoto, I., Mihara, K. and Sato, K. 2021. Open-heart techniques and mitral valve plasty for mitral regurgitation in toy- and small-breed dogs: a review. Open Vet. J. 11(1), 14–26. Kanemoto, I., Shibata, S., Noguchi, H., Chimura, S., Kobayashi, M. and Shimizu, Y. 1990. Successful mitral valvuloplasty for mitral regurgitation in a dog. Nihon. Juigaku. Zasshi. 52(2), 411–414. Kanemoto, I., Taguchi, D., Yokoyama, S., Mizuno, M., Suzuki, H. and Kanamoto, T. 2010. Open heart surgery with deep hypothermia and cardiopulmonary bypass in small and toy dogs. Vet. Surg. 39(6), 674–679. Keene, B.W., Atkins, C.E., Bonagura, J.D., Fox, P.R., Haggstrom, J., Fuentes, V.L., Oyama, M.A., Rush, J.E., Stepien, R. and Uechi, M. 2019. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 33(3), 1127–1140. Kociol, R.D., Pang, P.S., Gheorghiade, M., Fonarow, G.C., O'Connor, C.M. and Felker, G.M. 2010. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J. Am. Coll. Cardiol. 56(14), 1071–1078. Kurihara, T., Yanagida, A., Yokoi, H., Koyata, A., Matsuya, T., Ogawa, J., Okamura, Y. and Miyamoto, D. 2008. Evaluation of cardiac assays on a benchtop chemiluminescent enzyme immunoassay analyzer, PATHFAST. Anal. Biochem. 375(1), 144–146. Langhorn, R. and Willesen, J. L. 2016. Cardiac troponins in dogs and cats. J. Vet. Intern. Med. 30(1), 36–50. Mahmoud, N., Mohamed, W.R. and Mohamed, T. 2024. Femtosecond laser-induced fluorescence for rapid monitoring of cardiac troponin 1 as a cardiovascular disease biomarker. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 305, 123491. Matsuura, K., Yoshida, T., Yamada, S., Aboshi, Y., Yotsuida, H., Yaginuma, Y. and Hasegawa, M. 2022. The outcome of surgical mitral valve repair with loop-in-loop technique in dogs with different stage myxomatous mitral valve disease. J. Vet. Cardiol. 42, 74–82. Mihara, K., Kanemoto, I., Sato, K., Mori, T., Abe, H., Niimi, S., Yamada, K., Ohira, K., Andou, T. and Hirakawa, A. 2017. Open heart surgery with deep hypothermic cardiopulmonary bypass and more than 90 minutes of aortic cross clamp time in 10 small dogs. Vet. Sci. Dev. 7(1), 83–86. Orton, E.C., Mama, K., Hellyer, P. and Hackett, T.B. 2001. Open surgical repair of tetralogy of Fallot in dogs. J. Am. Vet. Med. Assoc. 219(8), 1089–1093, 1073. Pedersen, H.D., Häggström, J., Falk, T., Mow, T., Olsen, L.H., Iversen, L. and Jensen, A.L. 1999. Auscultation in mild mitral regurgitation in dogs: observer variation, effects of physical maneuvers, and agreement with color Doppler echocardiography and phonocardiography. J. Vet. Intern. Med. 13(1), 56–64. Pelander, L., Hagman, R. and Haggstrom, J. 2008. Concentrations of cardiac Troponin I before and after ovariohysterectomy in 46 female dogs with pyometra. Acta Vet. Scand. 50, 35. Pennington, C., Bristow, P., Navarro-Cubas, X. and Kurosawa, T.A. 2024. Improved owner quality of life following surgical repair of canine myxomatous mitral valve disease. J. Am. Vet. Med. Assoc. 262(4), 1–6. Rishniw, M. and Erb, H. N. 2000. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J. Vet. Intern. Med. 14(4), 429–435. Saunders, A.B., Hanzlicek, A.S., Martinez, E.A., Stickney, M.J., Steiner, J.M., Suchodolski, J.S. and Fosgate, G.T. 2009. Assessment of cardiac troponin I and C-reactive protein concentrations associated with anesthetic protocols using sevoflurane or a combination of fentanyl, midazolam, and sevoflurane in dogs. Vet. Anaesth. Analg. 36(5), 449–456. Shih, A.C., Maisenbacher, H.W., Barreirinha, A., Adin, D.B., Schmidt, M.K., Prosek, R. and Estrada, A.H. 2009. Effect of routine cardiovascular catheterization on cardiac troponin I concentration in dogs. J. Vet. Cardiol. 11(Suppl. 1), S87–92. Silverman, M.E. and Wooley, C.F. 2008. Samuel A. Levine and the history of grading systolic murmurs. Am. J. Cardiol. 102(8), 1107–1110. Thygesen, K., Alpert, J.S., White, H.D., Jaffe, A.S., Apple, F.S., Galvani, M., Katus, H.A., Newby, L.K., Ravkilde, J., Chaitman, B., Clemmensen, P.M., Dellborg, M., Hod, H., Porela, P., Underwood, R., Bax, J.J., Beller, G.A., Bonow, R., Van der Wall, E. E. and Al-Attar, N. 2007. Universal definition of myocardial infarction. Circulation 116(22), 2634–2653. Uechi, M., Mizukoshi, T., Mizuno, T., Mizuno, M., Harada, K., Ebisawa, T., Takeuchi, J., Sawada, T., Uchida, S., Shinoda, A., Kasuya, A., Endo, M., Nishida, M., Kono, S., Fujiwara, M. and Nakamura, T. 2012. Mitral valve repair under cardiopulmonary bypass in small-breed dogs: 48 cases (2006–2009). J. Am. Vet. Med. Assoc. 240(10), 1194–1201. Verbiest, T., Binst, D., Waelbers, T., Coppieters, E. and Polis, I. 2013. Perioperative changes in cardiac troponin I concentrations in dogs. Res. Vet. Sci. 94(3), 446–448. Vermes, E., Mesguich, M., Houel, R., Soustelle, C., Le Besnerais, P., Hillion, M. L. and Loisance, D. 2000. Cardiac troponin I release after open heart surgery: a marker of myocardial protection? Ann. Thorac. Surg. 70(6), 2087–2090. White, H. D. 2011. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J. Am. Coll. Cardiol. 57(24), 2406–2408. Wu, Q., Wang, T., Chen, S., Zhou, Q., Li, H., Hu, N., Feng, Y., Dong, N., Yao, S. and Xia, Z. 2018. Cardiac protective effects of remote ischaemic preconditioning in children undergoing tetralogy of fallot repair surgery: a randomized controlled trial. Eur. Heart J. 39(12), 1028–1037. Yokoyama, S., Kanemoto, I., Mihara, K., Ando, T., Kawase, K., Sahashi, Y. and Iguchi, K. 2017. Treatment of severe mitral regurgitation caused by lesions in both leaflets using multiple mitral valve plasty techniques in a small dog. Open Vet. J. 7(4), 328–331. | ||
| How to Cite this Article |
| Pubmed Style Ando T, Mihara K, Kanemoto I, Akiyoshi H. Perioperative changes in plasma cardiac troponin I concentration during mitral valvuloplasty for severe mitral regurgitation in dogs. Open Vet. J.. 2024; 14(7): 1625-1633. doi:10.5455/OVJ.2024.v14.i7.12 Web Style Ando T, Mihara K, Kanemoto I, Akiyoshi H. Perioperative changes in plasma cardiac troponin I concentration during mitral valvuloplasty for severe mitral regurgitation in dogs. https://www.openveterinaryjournal.com/?mno=153353 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i7.12 AMA (American Medical Association) Style Ando T, Mihara K, Kanemoto I, Akiyoshi H. Perioperative changes in plasma cardiac troponin I concentration during mitral valvuloplasty for severe mitral regurgitation in dogs. Open Vet. J.. 2024; 14(7): 1625-1633. doi:10.5455/OVJ.2024.v14.i7.12 Vancouver/ICMJE Style Ando T, Mihara K, Kanemoto I, Akiyoshi H. Perioperative changes in plasma cardiac troponin I concentration during mitral valvuloplasty for severe mitral regurgitation in dogs. Open Vet. J.. (2024), [cited January 25, 2026]; 14(7): 1625-1633. doi:10.5455/OVJ.2024.v14.i7.12 Harvard Style Ando, T., Mihara, . K., Kanemoto, . I. & Akiyoshi, . H. (2024) Perioperative changes in plasma cardiac troponin I concentration during mitral valvuloplasty for severe mitral regurgitation in dogs. Open Vet. J., 14 (7), 1625-1633. doi:10.5455/OVJ.2024.v14.i7.12 Turabian Style Ando, Takeki, Kippei Mihara, Isamu Kanemoto, and Hideo Akiyoshi. 2024. Perioperative changes in plasma cardiac troponin I concentration during mitral valvuloplasty for severe mitral regurgitation in dogs. Open Veterinary Journal, 14 (7), 1625-1633. doi:10.5455/OVJ.2024.v14.i7.12 Chicago Style Ando, Takeki, Kippei Mihara, Isamu Kanemoto, and Hideo Akiyoshi. "Perioperative changes in plasma cardiac troponin I concentration during mitral valvuloplasty for severe mitral regurgitation in dogs." Open Veterinary Journal 14 (2024), 1625-1633. doi:10.5455/OVJ.2024.v14.i7.12 MLA (The Modern Language Association) Style Ando, Takeki, Kippei Mihara, Isamu Kanemoto, and Hideo Akiyoshi. "Perioperative changes in plasma cardiac troponin I concentration during mitral valvuloplasty for severe mitral regurgitation in dogs." Open Veterinary Journal 14.7 (2024), 1625-1633. Print. doi:10.5455/OVJ.2024.v14.i7.12 APA (American Psychological Association) Style Ando, T., Mihara, . K., Kanemoto, . I. & Akiyoshi, . H. (2024) Perioperative changes in plasma cardiac troponin I concentration during mitral valvuloplasty for severe mitral regurgitation in dogs. Open Veterinary Journal, 14 (7), 1625-1633. doi:10.5455/OVJ.2024.v14.i7.12 |