| Research Article | ||
Open Vet. J.. 2023; 13(12): 1554-1561 Open Veterinary Journal, (2023), Vol. 13(12): 1554–1561 Original Research Specular microscopy of the corneal endothelial cells of bovines: an ex vivo studyMariane Gallicchio Azevedo*, Natália Pons Méndez, Luísa Soares Cargnin, Rafaella Silva Rocha, Maiara Poersch Seibel, Alessandra Fernandez da Silva and João Antonio Tadeu PigattoCollege of Veterinary, Federal University of Rio Grande do Sul, UFRGS, Porto Alegre, Brazil *Corresponding Author: Mariane Gallicchio Azevedo. College of Veterinary, Federal University of Rio Grande do Sul (UFRGS), Porto Alegre, Brazil. Email: mariane.gallicchio [at] gmail.com Submitted: 12/06/2023 Accepted: 16/11/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
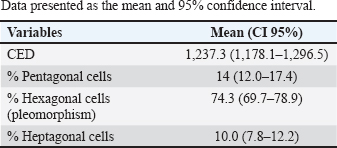
AbstractBackground: The endothelium is the most posterior layer of the cornea and is essential for maintaining corneal transparency. Due to variations in corneal endothelial parameters among different species, knowledge of the normal parameters for each species is crucial. Aim: To evaluate the corneal endothelium of bovines using contact specular microscopy. Methods: Twenty eyeballs from 10 male Brangus (Bos taurus) aged 24 months were evaluated. Contact specular microscopy was performed on the central corneal area. The analyzed parameters were endothelial cell density (ECD) and endothelial cell morphology. Results: The ECD in the central area was 1,277 cells/mm2. Regarding the morphology, mainly cells with six (74.3%), five (14.7%) and seven sides (10%) were found. There were no significant differences in ECD and morphology between left and right eyes. Conclusion: Contact specular microscopy facilitated the analysis and measurement of corneal endothelial parameters in bovines. The data obtained will serve as a reference for the analysis of bovine corneal endothelium. Keywords: Cornea, Endothelium, Morphology, Cattle. IntroductionThe endothelium constitutes the posterior layer of the cornea (Miller, 2001). During embryogenesis, it derives from the neural crest and is formed by a single layer of polygonal cells arranged in an organized mosaic; in most species, the predominant endothelial morphological pattern is hexagonal (Bahn et al., 1986; Tuft and Coster, 1990; Joyce, 2003). The main function of the endothelium is the preservation of corneal transparency via the regulation of stromal hydration through the endothelial barrier function (Taylor and Hunt, 1981; Tuft and Coster, 1990; Wen et al., 2001; Mimura et al., 2013). In most species, endothelial repair is limited. When endothelial cells are damaged, adjacent cells migrate toward the injury, resulting in hypertrophy and reestablishment of their function (MacCallum et al., 1983; Nishida et al., 2022). In humans, it is believed that the minimum cell density required for the functional maintenance of the corneal endothelium is 500 cells/mm2 (Zavala et al., 2013). Endothelial density is influenced by and declines due to factors related to aging, surgical or inflammatory trauma, and endothelial diseases. In cases of significant endothelial loss, where the remaining cells do not restore their function, endothelial decompensation occurs, causing corneal edema and visual deficits (Tuft and Coster, 1990; Abib and Barreto, 2001; Spinozzi et al., 2021). Endothelial evaluation through specular microscopy provides a non-invasive, accurate and reliable clinical analysis of quantitative and qualitative parameters of the corneal endothelium. Therefore, this technique is also employed for research purposes in the evaluation of intraocular drug toxicity and corneal surgical procedures (Abib, 2000; De Sanctis et al., 2006; Mccarey et al., 2008; Benetz and Lass, 2018; Coyo et al., 2018a, 2018b; Chaurasia and Vanathi, 2021). Endothelial parameters have been determined in several species, including humans, lagomorphs, alpacas, llamas, goats, horses, birds, pigs, dogs, and cats (Abib and Barreto, 2001; Pigatto et al., 2008; Tamayo-Arango et al., 2009; Franzen et al., 2010; Coyo et al., 2018a, 2018b; Chaurasia and Vanathi, 2021). Due to variations in corneal endothelial parameters among different species, knowledge of the normal parameters for each species is essential. Such knowledge is also a prerequisite for recognizing pathological alterations related to this layer of the cornea. However, studies related to endothelial parameters of bovine species are scarce (Bahn et al., 1986). The aim of this study is to analyze and quantify the parameters of the corneal endothelium of a bovine species (Bos taurus) using contact specular microscopy. Materials and MethodsThe study was approved by the Research of Committee of the Federal University of Rio Grande do Sul. The eyes were donated by a local slaughterhouse. Twenty eyeballs from 10 Brangus breed male bovines aged 24 months were studied. Enucleation was made immediately after slaughtering, and the eyes were labeled and stored in a moist chamber until analysis under a specular microscope. All ocular bulbs underwent an ophthalmic examination which included biomicroscopy with a slit lamp (Kowa SL-15, Nagoya, Aichi, Japan) (Fig. 1) and a fluorescein test (1% sodium fluorescein, Allergan, Sao Paulo, Brazil) (Fig. 2). Only healthy eyes were selected. Only eyes with transparent corneas and eyes with a negative fluorescein test were included in the study. All analyses were carried out within 4 hours after slaughter. For endothelial evaluation, a contact specular microscope (Celmax, Medical Service, Sao Carlos, Brazil) was used. All evaluations were carried out by the same examiner. After being removed from the wet chamber, the eyes were placed on a support adapted to the contact specular microscope and lubricated with ophthalmic eye drops (Lacri, carboxymethylcellulose). The objective lens of the specular microscope was positioned in the central region of the cornea, at a 90° angle between the evaluated structure and the device (Figs. 3 and 4). From each cornea, we obtained one micrograph, and 30 endothelial cells were analyzed in each image. Cell density was calculated using software coupled to the specular microscope (Celmax® specular microscope). Endothelial morphology was obtained through manual assessment of the number of sides of each cell. The values obtained were the means of each identified parameter. Statistical analysisQuantitative variables were analyzed by entering the data into Excel and subsequently exporting them to IBM* SPSS v. 20.0 (Statistical Package for the Social Sciences) for statistical analysis. Endothelial cell density (ECD) and endothelial cell morphology were described by means and 95% confidence intervals. Comparison among eyes was performed using Student's t-test for paired samples. A significance level of 5% was considered for established comparisons.
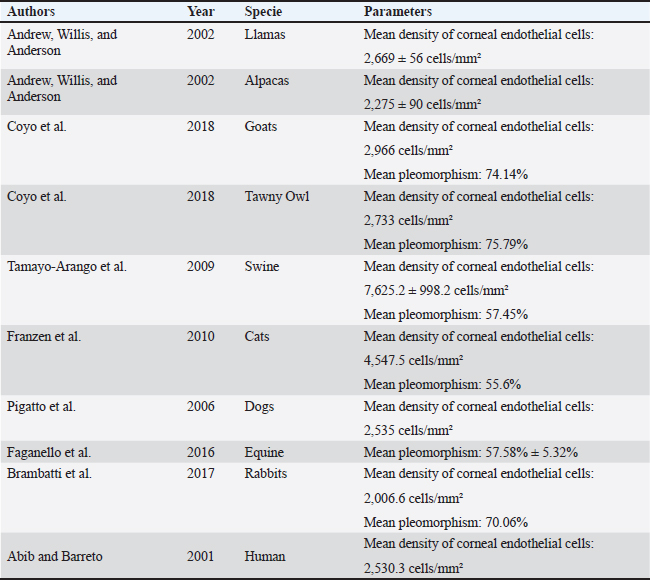
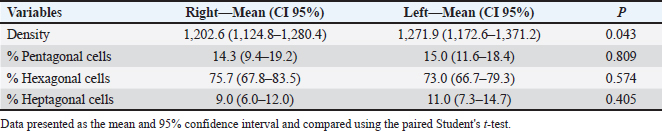
Fig. 1. Slit lamp examination of a bovine eye. Ethical approvalThe study was approved by the Research of Committee of the Federal University of Rio Grande do Sul. The eyes were donated by a local slaughterhouse. The animals were slaughtered for the production of meat and meat products and not for reasons related to the study. ResultsAll enucleated eyes were included in the study. All eyes had a transparent cornea. Using the contact specular microscope, it was possible to assess the corneal endothelium of the bovine eyes and obtain clear images of all analyzed eyes. A regular and continuous pattern of juxtaposed cells, with sharp borders, was observed in the central region of the bovine corneal endothelium (Fig. 5). The average values of cell density and endothelial morphology are different from the values found in other species already studied (Table 1).
Fig. 2. Bovine eye after fluorescein staining. It is observed that the dye did not adhere to the cornea.
Fig. 3. Image during contact specular microscopy of a bovine eye.
Fig. 4. Objective lens of the specular microscope in contact with the cornea of a bovine eye.
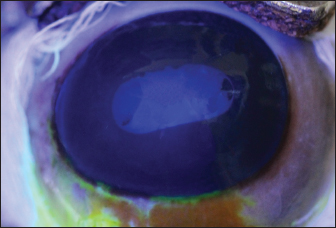
Fig. 5. Specular photomicrographs of the corneal endothelium of a bovine obtained using a contact specular microscope. The mean ECD of the central area was 1,277 cells/mm2. Regarding the morphology, mainly cells with six (74.3%), five (14.7%), and seven sides (10%) were found, along with four-sided and eight-sided cells (Table 2). There was no significant difference in the morphological parameters of the endothelial cells when comparing the right eye to the left eye. We also found no statistically significant difference in ECD (p=0.491) when comparing both eyes (Table 3). DiscussionCorneal endothelial parameters have already been determined in some species, including humans (Abib and Barreto, 2001), alligators (Pigatto et al., 2004), ostriches (Pigatto et al., 2009), chinchillas (Bercht et al., 2015), chickens (Albuquerque et al., 2015), rabbits (Brambatti et al., 2017), dogs (Pigatto et al., 2006; Kobashigawa et al., 2015; Hünning et al., 2018), sheep (Brandao et al., 2006; Coyo et al., 2016) goats (Coyo et al., 2018a, 2018b), marmosets (Morita and Shimomura, 1996), horses (Andrew et al., 2001; Faganello et al., 2016), owls (Coyo et al., 2018a, 2018b), llamas and alpacas (Andrew et al., 2001), Magellan penguins (Pigatto et al., 2005), and pigs (Tamayo-Arango et al., 2009). However, there are few ophthalmological studies related to endothelial parameters in bovine species (Tofflemire et al., 2015). Several methods have been tested to evaluate the proliferative capacity of the corneal endothelium (Katz et al., 1994; Maurizi et al., 2020; Pei et al., 2021; Maurizi et al., 2022). Faye and collaborators reviewed different aspects related to the cultivation of endothelial cells in vitro (Faye et al., 2021). The proliferative capacity of corneal endothelial cells differs between species. While in larger mammals corneal endothelial cells are arrested in a quiescent state, rabbit corneal endothelial cells retain a high proliferative capacity and can repopulate the endothelium upon injury (Català et al., 2023). Due to the differences in corneal endothelial parameters among species, it is important to know the parameters of each species. Only after obtaining these reference values, it is possible to recognize pathological changes related to this layer of the cornea and to perform surgical procedures with greater safety. In the present study, eyes from slaughterhouse discards were evaluated. This methodology was feasible and allowed for scientific research without the need for euthanasia of experimental models for this purpose. Previous studies performed within 6 hours post-mortem have shown that endothelial integrity was maintained (Andrew et al., 2001; Pigatto et al., 2008, Bercht et al., 2015; Faganello et al., 2016). Although the present study was conducted on eyes obtained after enucleation, as no endothelial alterations occurred due to preparation for analysis, the results obtained can be used as a reference and compared with future data obtained from live animals. Unlike optical and scanning electron microscopy, no cellular retraction occurs during cornea preparation with the specular microscope. The specular microscope has been used to analyze both live animal corneas and enucleated eyes (Andrew et al., 2001; Pigatto et al., 2006; Pigatto et al., 2008; Albuquerque et al., 2015; Bercht et al., 2015). The cornea of bovines has characteristics that have been referenced in the literature, where the corneal thickness is 1,015 ± 104 µm, the horizontal corneal diameter is 29.8 ± 1.3 mm, and the vertical diameter is 23.9 ± 1.5 mm. The bovine corneal endothelium is an organized arrangement of polygonal cells of uniform size, of which 67.1% ± 2.7% demonstrated hexagonal morphology. Lwigale (1999), studying bovine corneas, found endothelial cell nuclei with a lobulated morphological pattern, arranged in a regular manner (Doughty et al., 1995; Lwigale, 1999). Specular microscopy is an excellent method for the evaluation of corneal endothelium cells in humans and animals (Price and Chang, 1981; Klais et al., 2003; Pigatto et al., 2006; Coyo et al., 2016; Jirsova et al. , 2017). This non-invasive methodology can reliably be employed in the evaluation of corneal endothelium cells. The normal and pathological structure of the endothelium can be analyzed, and parameters can be quantified and established for clinical or experimental conditions (Abib, 2000; Benetz and Lass, 2018; Chaurasia and Vanathi, 2021). This allows the evaluation of endothelial diseases, healing processes, and aging as well as corneal transplants and the study of intracameral drug toxicity, aiding in the diagnosis and treatment of corneal disorders and improving surgical techniques in different species (Collin and Collin, 1998; Pigatto et al., 2006; Albuquerque et al., 2015; Coyo et al., 2016; Benetz and Lass, 2018; Chaurasia and Vanathi, 2021). Table 1. Comparison of endothelial parameters found in different species.
Table 2. Estimated mean ECD (cells/mm2) and polygonal cells in the central region of bovine eyes using contact specular microscopy (n=20 eyes).
In the present study, contact specular microscopy was employed to analyze and determine the parameters of the corneal endothelium in bovines. One limitation of specular microscopy is the difficulty in obtaining images of oedematous areas of the cornea (Doughty, 2006; Mccarey et al., 2008). In this experiment, using a specular microscope, the corneal endothelium could be analyzed and photographed in all enucleated eyes because only healthy eyes were included and examined. Various methods are employed to quantify endothelial parameters, among which the fixed and variable frame method, the center-to-center method, the corner method, and the comparison method are the most common ones. Regardless of the software used, the accuracy of the evaluation depends on the quality and sharpness of the obtained specular photo (Chaurasia and Vanathi, 2021). In this study, the central endothelial density (CED) was determined using the center marking method in the equipment software. The number of delimited cells varies among studies, and the minimum recommended cell count for maximum accuracy of the analyzed area is 30 cells (Binder et al., 1979; Laing et al., 1979). In the current study, 30 endothelial cells were selected and analyzed from each evaluated cornea. Endothelial analysis mainly includes the analysis of parameters such as cell density (cells/mm2), cell area, polymegathism (cellular variation coefficient) and pleomorphism (hexagonality index) (Abib, 2000; Chaurasia and Vanathi, 2021). In this study, the ECD and endothelial cell morphology were determined, and only parameters of the central area were analyzed. Although the peripheral regions were not analyzed in this study, previous studies found no significant difference in endothelial parameters when comparing peripheral regions with the central region (Mccarey et al., 2008; Bercht et al., 2015; Faganello et al., 2016; Brambatti et al., 2017; Hünning et al., 2018). Based on this, the data obtained in the central region can typically be extrapolated to the peripheral regions. Table 3. Comparative table of endothelial parameters in right and left eyes.
Only one study was found where corneal endothelial analysis of bovines corneal was performed using specular microscopy (Bahn et al., 1986). In this study, the authors evaluated the central corneal density of adult and juvenile bovines and found an average endothelial density of 2,500 cells/mm2. However, the number of endothelial cells per bovine cornea in this study was estimated by multiplying the area of the endothelial surface by the CED, determined by specular microscopy. This measurement of endothelial density is probably more accurate for spherical corneas (dogs, cats, and rabbits) and produces significant sampling errors (about 23%) when applied to the elliptical bovine cornea, overestimating the endothelial density of this species (Bahn et al., 1986). Mean ECD of 2,669 cells/mm2 for llamas and 2,275 cells/mm2 for alpacas were obtained using a non-contact specular microscope (Andrew et al., 1999). In chinchillas, ECD calculated through specular microscopy in groups of different ages was between 2,124 and 3,423 cells/mm2. These previous studies already carried out demonstrate that there is a difference in endothelial density according to the studied species. In the present study, the mean ECD of adult bovines was 1,277 cells/mm2. In the current study, as all the animals were of the same age, the effects of aging were not analyzed. The effect of age on endothelial density has already been studied by means of specular microscopy in horses (Andrew et al., 2001), lhamas/alpacas (Andrew et al., 2001), dogs (Pigatto et al., 2006, 2008), cats (Franzen et al., 2010), sheep (Coyo et al., 2016), among others. ECD decreased with age (Coyo et al., 2018a, 2018b). There was no significant difference in the central ECD between the right and left eyes. In most vertebrates the shape of the normal corneal endothelial cells was mainly hexagonal, pentagonal, and tetragonal cells (Yee et al., 1987; Collin and Collin, 1998). In this study, the predominant morphological pattern was hexagonal (74.3%). There was no significant difference between in the endothelial morphological findings for the right and left eyes, which is in agreement with previous findings (Albuquerque et al., 2015; Bercht et al., 2015; Brambatti et al., 2017; Coyo et al., 2018a, 2018b). In this study, only corneas from male bovines were analyzed, and sex was not a variable considered in this research. Previous studies have shown that in healthy corneas, there is no difference in endothelial parameters between male or female animals (Tuft and Coster, 1990; Tamayo-Arango et al., 2009; Franzen et al., 2010; Brambatti et al., 2017). Experimental studies have evaluated the properties of bovine endothelial cells, demonstrating their proliferative capacity in vitro. Researchers suggest that the culture model of endothelial cells preserves the ability to pump fluids, where the endothelial cell pumping rate was quantified for the first time, constituting an experimental model that could contribute to the development of corneal transplant methods in the future (Narula et al., 1992; Huang et al., 2010). One study reported the in vivo heterologous transplantation in cat corneas, in which the endothelial layer was removed and replaced with endothelial cells from the cornea of bovines previously preserved in culture medium (Gospodarowicz et al., 1979). Cellular integrity was analyzed using the red alizarin dye, which demonstrated the high capacity for reorganization and formation of a monolayer of the bovine endothelial graft in the cat cornea within 8 days, maintaining corneal transparency for months after the experiment. The results showed that bovine corneal endothelial cells maintained in culture remain functional, presenting an alternative to xenotransplantation (Gospodarowicz et al., 1979). In another study, the corneal endothelium of cattle served as an experimental model and was successfully replicated ex vivo by supplementation with growth factors, temperature control, and human plasma culture medium. The supplemented culture medium ensured the maintenance of corneal transparency and hexagonal morphology. Therefore, considering the limited capacity of corneal endothelium regeneration, this study provides a basis for elucidating bovine corneal parameters in the future. Thus, the corneal endothelium of cattle, if kept in an adequate substrate, can serve as an alternative for xenotransplantation (Chou et al., 2014). Using contact specular microscopy, it was possible to analyze and measure the corneal endothelial parameters of bovines, providing a reference for the analysis of bovine corneal endothelium. AcknowledgmentsThe authors would like to thank Angus slaughterhouse LTDA for supplying eyes used in this research. We also would like to thank “Coordination for the Improvement of Higher Education Personnel” (CAPES) which provided the fellowship for three of the authors (M.G.A., M.P.S., and A.F.S.). Conflict of interestThe authors declare that they have no conflicts of interest. Author contributionsMGA: conceptualization, methodology, investigation, writing, review and editing; NPM: methodology, investigation, review and editing; LSC: methodology, investigation; RSR: methodology, investigation; MPS: methodology, investigation; AFS: methodology, investigation; JATP: conceptualization, supervision, writing, funding acquisition. FundingThis research received no specific grant. Data availabilityThe data that support the findings of this study are available from the corresponding author upon request. ReferencesAbib, F.C. 2000. Corneal specular microscopy: manual and atlas, 1st ed. Rio de Janeiro, Brazil: Revinter, p: 140. Abib, F.C. and Barreto, J.R. 2001. Behavior of corneal endothelial density over a lifetime. J. Cataract Refract. Surg. 27(10), 1574–1578. Albuquerque, L., Pigatto, J.A.T. and Pacicco, L.V.R. 2015. Analysis of the corneal endothelium in eyes of chickens using contact specular microscopy. Semina: Cienc. Agrar. 36(2), 4199–4206. Andrew, S.E., Ramsey, D.T., Hauptman, J.G. and Brooks, D.E. 2001. Density of corneal endothelial cells and corneal thickness in eye of euthanized horses. Am. J. Vet. Res. 62(4), 479–482. Andrew, S.E., Samuelson, D.A., Lewis, P.A. and Kubilis, P.S. 1999. Comparison of optisol-GS and neomycin-polymyxin B-gramicidin ophthalmic solution for corneal storage in the dog. Vet. Ophthalmol. 2(3), 155–161. Bahn, C.F., Glassman, R.M., MacCallum, D.K., Lillie, J.H., Meyer, R.F., Robinson, B.J. and Rich, N.M. 1986. Postnatal development of corneal endothelium. Invest. Ophthalmol. Vis. Sci. 27(1), 44–51. Benetz, B.A. and Lass, J.H. 2018. Specular microscopy. Cornea 37, S7–S8. Bercht, B.S., Albuquerque, L., Araújo, A.C.P. and Pigatto, J.A.T. 2015. Specular microscopy to determine corneal endothelial cell morphology and morphometry in chinchillas (Chinchilla lanigera) in vivo. Vet. Ophthalmol. 18(1), 137–142. Binder, P.S., Akers, P. and Zavala, E.Y. 1979. Endothelial cell density determined by specular microscopy and scanning electron microscopy. Ophthalmology 86(10):1831–1847. Brambatti, G., Albuquerque, L., Vargas, E.V.B., Neumann, C.F. and Pigatto, J.A.T. 2017. Corneal endothelial cell density and morphology in rabbits eyes using contact specular microscopy. Cienc. Rural. 47(12), 1–5. Brandao, C.V.S. Ranzani, J.J.T., Rodrigues, G.N., Marinho, L.F.L.P., Peixoto, T.P., Cremonini, D.N., Lima, L.S.A., Chiurciu, J.L.V. and Teixeira, C.R. 2006. Thickness and density of corneal endothelial cells in ovines. Arch. Vet. Sci. 11(1), 16–18. Català, P., Vivensang, F., Beek , D.V., Adriaens, M.E., Dickman, M.M., LaPointe, V.L.S. and Kutmon, M. 2023. Elucidating the corneal endothelial cell proliferation capacity through an interspecies transcriptome comparison. Adv. Biol. 7(12), 2300065. Chaurasia, S. and Vanathi, M. 2021. Specular microscopy in clinical practice. Indian J. Ophthalmol. 69(3), 517–524. Chou, M., Burnouf, T. and Wang, T. 2014. Ex vivo expansion of bovine corneal endothelial cells in xeno-free medium supplemented with platelet releasate. PLoS One 9(6), e99145. Collin, S.P. and Collin, H.B. 1998. A comparative study of the corneal endothelium in vertebrates. Clin. Exp. Optom. 81(6), 245–254. Coyo, N., Peña, M.T., Costa, D., Ríos, J., Lacerda, R. and Leiva, M. 2016. Effects of age and breed on corneal thickness, density, and morphology of corneal endothelial cells in enucleated sheep eyes. Vet. Ophthalmol. 19(5), 367–372. Coyo, N., Leiva, M., Costa, D., Ríos, J. and Peña, M.T. 2018a. Corneal thickness, endothelial cell density, and morphological and morphometric features of corneal endothelial cells in goats. Am. J. Vet. Res. 79(10), 1087–1092. Coyo, N., Leiva, M., Costa, D., Molina, R., Nicolás, O., Ríos, J. and Peña, M.T. 2018b. Endothelial cell density and characterization of corneal endothelial cells in the Tawny Owl (Strix aluco) using specular microscopy. Vet. Ophthalmol. 22(2):177–182. De Sanctis, U., Machetta, F., Razzano, L., Dalmasso, P. and Grignolo, F.M. 2006. Corneal endothelium evaluation with 2 noncontact specular microscopes and their semiautomated methods of analysis. Cornea 25(5), 501–506. Doughty, M.J., Petrou, S. and Macmillan, H. 1995. Anatomy and morphology of the cornea of bovine eyes from a slaughterhouse. Can. J. Zool. 73, 2159–2165. Doughty, M. J. 2006. Subjective vs. objective analysis of the corneal endothelial cells in the rabbit cornea by scanning electron microscopy - a comparison of two different methods of corneal fixation. Vet Ophthalmol. 9(2), 127–135. Faganello, C.S., Silva, V.R.M., Andrade, M.C.C., Carissimi, A.S. and Pigatto, J.A.T. 2016. Morphology of endothelial cells from different regions of the equine cornea. Cienc. Rural. 46(12), 2223–2228. Faye, P.A., Poumeaud, F., Chazelas, P., Duchesne, M., Rassat, M., Merissi, F., Lia, A.S., Sturtz, F., Robert, P.Y., Favreau, F. and Banayoun, Y. 2021. Focus on cell therapy to treat corneal endothelial diseases. Exp. Eye Res. 204, 10846. Franzen, A.A., Pigatto, J.A.T., Abib, F.C., Albuquerque, L. and Laus, J.L. 2010. Use of specular microscopy to determine corneal endothelial cell morphology and morphometry in enucleated cat eyes. Vet. Ophthalmol. 13(4), 222–226. Gospodarowicz, D., Greenburg, G. and Alvarado, J. 1979. Transplantation of cultured bovine corneal endothelial cells to species with nonregenerative endothelium: the cat as an experimental model. Arch. Ophthalmol. 97(11), 2163–2169. Huang, L., Harkenrider, M., Thompson, M., Zeng, P., Tanaka, H., Gilley, D., Ingram, D.A., Bonanno, J.A. and Yoder, M.C. 2010. A hierarchy of endothelial colony-forming cell activity displayed by bovine corneal endothelial cells. Invest. Ophthalmol. Vis. Sci. 51(8), 3943–3949. Hünning, P.S., Andrade, M.C.C., Carissimi, A. and Pigatto, J.A.T. 2018. Morphology of endothelial cells from different regions of the cornea of dogs. Cienc. Rural. 48(10), e20180596. Jirsova, K., Clover, J., Stoeger, C.G. and Gilles, T. 2017. Various Approaches to the microscopic assessment of the cornea, visualization and image analysis of the corneal endothelium. In Light and specular microscopy of the cornea. Eds., Jirsova, K. Cham, Switzerland: Springer International Publishing, vol. 1, pp: 59–71. Joyce, N.C. 2003. Proliferative capacity of corneal endothelium. Prog. Retin. Eye Res. 22(3), 359–389. Katz, A., Fish, A.J., Pe’er, J., Frucht-Pery, J., Ron, V. and Vlodavsky, I. 1994. Entactin/Nidogen: synthesis by bovine corneal endothelial cells and distribution in the human cornea. Invest. Ophthalmol. Vis. Sci. 35(2), 495–502. Klais, C.M.C., Bühren, J. and Kohnen, T. 2003. Comparison of endothelial cell count using confocal and contact specular microscopy. Ophthalmologica 217(2), 99–103. Kobashigawa, K.K., Lima, T.B., Padua, I.R.M., Sobrinho, A.A.F.B., Marinho, F.A., Ortêncio, K.P. and Laus, J.L. 2015. Ophthalmic parameters in adult Shih Tzu dogs. Cienc. Rural. 45, 1280–1285. Laing, R.A., Sandstrom, M.M. and Leibowitz, H.M. 1979. Clinical specular microscopy. I. Optical principles. Arch. Ophthalmol. 97(9), 1714–1719. Lwigale, P.Y. 1999. Nuclear morphologies of bovine corneal cells as visualized by confocal microscopy. Cel. Tis. Org. 165(2), 104–112. MacCallum, D.K., Bahn, F.C., Lillie, J.H., Meyer, R.F. and Martonyi, C.L. 1983. Evidence for corneal endothelial cell hypertrophy during postnatal growth of the cat cornea. Invest. Ophthalmol. Vis. Sci. 24(2), 247–250. Maurizi, E., Schiroli, D., Zini, R., Limongelli, A., Mistò, R., Macaluso, C. and Pellegrini, G. 2020. A fine-tuned β-catenin regulation during proliferation of corneal endothelial cells revealed using proteomics analysis. Scient. Rep. 10(13841). Maurizi, E., Merra, A., Schiroli, D., Ghezzi, B., Macaluso, C. and Pellegrini, G. 2022. Fluctuations in corneal endothelial LAP2 expression levels correlate with passage dependent declines in their cell proliferative activity. Int. J. Mol. Sci. 23(10), 5859. Mccarey, B.E., Edelhauser, H.F. and Lynn, M.J. 2008. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices and new intraocular drugs and solutions. Cornea 27(1), 1–16. Miller, W.W. 2001. Evaluation and management of corneal ulcerations: a systematic approach. Clin. Tech. Small Anim. Pract. 16(1), 51–57. Mimura, T., Yamagami, S., Amano, S. 2013. Corneal endothelial regeneration and tissue engineering. Prog. Retin. Eye Res. 35, 1–17. Morita, H. and Shimomura, K. 1996. Specular microscopy of the corneal endothelial cells in common marmosets. J. Vet. Med. Sci. 58(3), 277–279. Narula, P., Xu, M., Kuang, K.Y., Akiyama, R. and Fischbarg, J. 1992. Fluid transport across cultured bovine corneal endothelial cell monolayers. Am. J. Physiol. 262(1), C98–C103. Nishida, T., Saika, S. and Morishige, N. 2022. Cornea and sclera: anatomy and physiology. In Cornea. Eds., Mannis, M.J. Holland: E.J. Elsevier, vol. 5, pp: 33–54. Pei, W., Chen, J., Wu, W., Wei W., Yu, Y. and Feng, Y. 2021. Comparison of the rabbit and human corneal endothelial proteomes regarding proliferative capacity. Exp. Eye Res. 209, 108629. Pigatto, J.A.T., Abib, F.C., Pereira, G.T., Barros, P.S.M., Freire, C.D. and Laus, J.L. 2006. Density of corneal endothelial cells in eyes of dogs using specular microscopy. Braz. J. Vet. Res. Anim. Sci. 43(4), 476–480. Pigatto, J.A.T., Andrade, M.C., Laus, J.L., Santos, J.M., Brooks, D.E., Guedes, P.M. and Barros, P.S.M. 2004. Morphometric analysis of the corneal endothelium of Yacare caiman (Caiman yacare) using scanning electron microscopy. Vet. Ophthalmol. 7(3), 205–208. Pigatto, J.A.T., Cerva, C., Freire, C.D., Abib, F.C., Bellini, L.P., Barros, P.S.M. and Laus, J.L. 2008. Morphological analysis of the corneal endothelium in eyes of dogs using specular microscopy. Pesq. Vet. Bras. 28(9), 427–430. Pigatto, J.A.T., Franzen, A.A., Pereira, F.Q., Almeida, A.C.V.R., Laus, J.L., Santos, J.M., Guedes, P.M. and Barros, P.S.M. 2009. Scanning electron microscopy of the corneal endothelium of ostrich. Cienc. Rural. 39(3), 926–929. Pigatto, J.A.T., Laus, J.L., Santos, J.M, Cerva, C., Cunha, L.S., Ruoppolo, V. and Barros, P.S.M. 2005. Corneal endothelium of the Magellanic penguin (Spheniscus magellanicus) by scanning electron microscopy. J. Zoo Wild Life Med. 36(4), 702–705. Price, N.C. and Cheng, H. 1981. Contact and noncontact specular microscopy. Br. J. Ophthalmol. 65(8), 568–574. Spinozzi, D., Miron, A., Bruinsma, M., Dapena, I., Kocaba, V., Jager, M.J., Melles, G.R.J., Dhubhghaill, S.N. and Oellerich, S. 2021. New developments in corneal endothelial cell replacement. Acta Ophthalmol. 99(7), 712–729. Tamayo-Arango, L.J., Baraldi-Artoni, S.M., Laus, J.L., Vicenti, F.A.M., Pigatto, J.A.T. and Abib, F.C. 2009. Ultrastructural morphology and morphometry of the normal corneal endothelium of adult crossbred pig. Cienc. Rural. 39(1), 117–122. Taylor, M.J. and Hunt, C.J. 1981. Dual staining of corneal endothelium with trypan blue and alizarin red S: importance of PH for the dye—lake reaction. Br. J. Ophthalmol. 65(12), 815–819. Tofflemire, K.L., Whitley, E.M., Gould, S.A., Dewell, R.D., Allbaugh, R.A., Ben-Shlomo, G., O’Connor, A.M. and Whitley, R.D. 2015. Schirmer tear test I and rebound tonometry findings in healthy calves. Vet. Ophthalmol. 18(2), 147–151. Tuft, S.J. and Coster, D.J. 1990. The corneal endothelium. Eye 4, 389–424. Wen, Q., Diecke, F.P., Iserovich, P., Kuang, K., Sparrow, J. and Fischbarg, J. 2001. Immunocytochemical localization of aquaporin-1 in bovine corneal endothelial cells and keratocytes. Exp. Biol. Med. 226(5), 463–467. Yee, R.W., Edelhauser, H.F. and Stern, M.E. 1987. Specular microscopy of vertebrate corneal endothelium: a comparative study. Exp. Eye Res. 44(5), 703–714. Zavala, J., Jaime, G.R.L., Barrientos, C.A.R. and Valdez-Garcia, J. 2013. Corneal endothelium: developmental strategies for regeneration. Eye 27(5), 579–588. | ||
| How to Cite this Article |
| Pubmed Style Azevedo MG, Méndez NP, Cargnin LS, Rocha RS, Seibel MP, Silva AFD, Pigatto JAT. Specular microscopy of the corneal endothelial cells of bovines: An ex vivo study. Open Vet. J.. 2023; 13(12): 1554-1561. doi:10.5455/OVJ.2023.v13.i12.5 Web Style Azevedo MG, Méndez NP, Cargnin LS, Rocha RS, Seibel MP, Silva AFD, Pigatto JAT. Specular microscopy of the corneal endothelial cells of bovines: An ex vivo study. https://www.openveterinaryjournal.com/?mno=153541 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i12.5 AMA (American Medical Association) Style Azevedo MG, Méndez NP, Cargnin LS, Rocha RS, Seibel MP, Silva AFD, Pigatto JAT. Specular microscopy of the corneal endothelial cells of bovines: An ex vivo study. Open Vet. J.. 2023; 13(12): 1554-1561. doi:10.5455/OVJ.2023.v13.i12.5 Vancouver/ICMJE Style Azevedo MG, Méndez NP, Cargnin LS, Rocha RS, Seibel MP, Silva AFD, Pigatto JAT. Specular microscopy of the corneal endothelial cells of bovines: An ex vivo study. Open Vet. J.. (2023), [cited January 25, 2026]; 13(12): 1554-1561. doi:10.5455/OVJ.2023.v13.i12.5 Harvard Style Azevedo, M. G., Méndez, . N. P., Cargnin, . L. S., Rocha, . R. S., Seibel, . M. P., Silva, . A. F. D. & Pigatto, . J. A. T. (2023) Specular microscopy of the corneal endothelial cells of bovines: An ex vivo study. Open Vet. J., 13 (12), 1554-1561. doi:10.5455/OVJ.2023.v13.i12.5 Turabian Style Azevedo, Mariane Gallicchio, Natália Pons Méndez, Luísa Soares Cargnin, Rafaella Silva Rocha, Maiara Poersch Seibel, Alessandra Fernandez Da Silva, and João Antonio Tadeu Pigatto. 2023. Specular microscopy of the corneal endothelial cells of bovines: An ex vivo study. Open Veterinary Journal, 13 (12), 1554-1561. doi:10.5455/OVJ.2023.v13.i12.5 Chicago Style Azevedo, Mariane Gallicchio, Natália Pons Méndez, Luísa Soares Cargnin, Rafaella Silva Rocha, Maiara Poersch Seibel, Alessandra Fernandez Da Silva, and João Antonio Tadeu Pigatto. "Specular microscopy of the corneal endothelial cells of bovines: An ex vivo study." Open Veterinary Journal 13 (2023), 1554-1561. doi:10.5455/OVJ.2023.v13.i12.5 MLA (The Modern Language Association) Style Azevedo, Mariane Gallicchio, Natália Pons Méndez, Luísa Soares Cargnin, Rafaella Silva Rocha, Maiara Poersch Seibel, Alessandra Fernandez Da Silva, and João Antonio Tadeu Pigatto. "Specular microscopy of the corneal endothelial cells of bovines: An ex vivo study." Open Veterinary Journal 13.12 (2023), 1554-1561. Print. doi:10.5455/OVJ.2023.v13.i12.5 APA (American Psychological Association) Style Azevedo, M. G., Méndez, . N. P., Cargnin, . L. S., Rocha, . R. S., Seibel, . M. P., Silva, . A. F. D. & Pigatto, . J. A. T. (2023) Specular microscopy of the corneal endothelial cells of bovines: An ex vivo study. Open Veterinary Journal, 13 (12), 1554-1561. doi:10.5455/OVJ.2023.v13.i12.5 |