| Review Article | ||
Open Vet. J.. 2023; 13(9): 1056-1070 Open Veterinary Journal, (2023), Vol. 13(9): 1056–1070 Review Article Advancing the frontiers: Revolutionary control and prevention paradigms against Nipah virusFredmoore L. Orosco1,2*1Virology and Vaccine Institute of the Philippines Program, Industrial Technology Development Institute, Department of Science and Technology, Taguig City, Philippines 2S&T Fellows Program, Department of Science and Technology, Taguig City, Philippines *Corresponding Author: Fredmoore L. Orosco. Virology and Vaccine Institute of the Philippines Program, Industrial Technology Development Institute, Department of Science and Technology, Taguig City, Philippines. Email: orosco.fredmoore [at] gmail.com Submitted: 21/05/2023 Accepted: 22/08/2023 Published: 30/09/2023 © 2023 Open Veterinary Journal
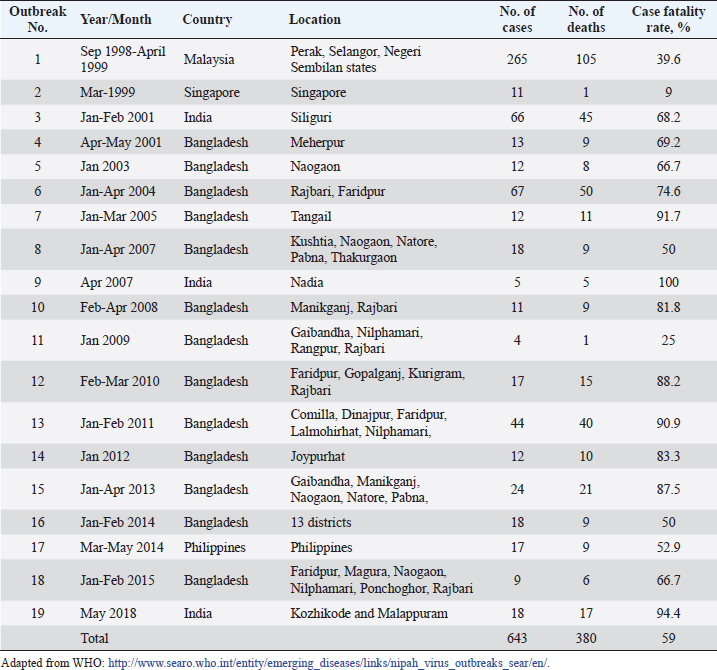
AbstractNipah Virus (NiV) is a highly virulent pathogen that poses a significant threat to human and animal populations. This review provides a comprehensive overview of the latest control and prevention strategies against NiV, focusing on vaccine development, antiviral drug discovery, early diagnosis, surveillance, and high-level biosecurity measures. Advancements in vaccine research, including live-attenuated vaccines, virus-like particles, and mRNA-based vaccines, hold promise for preventing NiV infections. In addition, antiviral drugs, such as remdesivir, ribavirin, and favipiravir, have the potential to inhibit NiV replication. Early diagnosis through molecular and serological assays, immunohistochemistry, and real-time reverse transcription polymerase chain reaction plays a crucial role in timely detection. Surveillance efforts encompassing cluster-based and case-based systems enhance outbreak identification and provide valuable insights into transmission dynamics. Furthermore, the implementation of high-level biosecurity measures in agriculture, livestock practices, and healthcare settings is essential to minimize transmission risks. Collaboration among researchers, public health agencies, and policymakers is pivotal in refining and implementing these strategies to effectively control and prevent NiV outbreaks and safeguard public health on a global scale. Keywords: Antivirals, Control, Nipah virus, Prevention, Vaccines. IntroductionNipah virus (NiV) is a zoonotic paramyxovirus transmitted by bats, which causes lethal encephalitis in humans and has been reported in several countries, including Singapore (Paton et al., 1999), Malaysia (Goh et al., 2000), Bangladesh (Rahman and Chakraborty, 2012), India (Chadha et al., 2006), and the Philippines (Ching et al., 2015). NiV is categorized under the order Mononegavirales, which includes other deadly viruses, such as Hendra, Ebola, and Marburg (Amarasinghe et al., 2019). The first NiV outbreak was identified in 1999 when pig farmers and exporters in Malaysia and Singapore were affected, leading to the collapse of the billion-dollar pig export industry (Doucleff and Greenhalgh, 2017). Initially, control measures focused on the Japanese encephalitis (JE) outbreak, and the spread of NiV could not be detected until the isolation of NiV from the cerebrospinal fluid of a victim after 2 months (Looi and Chua, 2007). The virus was named after the village of Kampung Sungai Nipah, where it was first discovered (Kulkarni et al., 2013). Subsequent NiV outbreaks occurred in India and Bangladesh in 2001 and 2007 (Mahedi et al., 2023), and eight outbreaks from 2001 to 2012 (Ambat et al., 2019). The most recent outbreak was reported in Kozhikode district, Kerala, India, on May 19, 2018 (Table 1) (Thomas et al., 2019). NiV and Hendra virus (HeV) are closely related and share 80% genome sequence identity (Ang et al., 2018). These viruses have been categorized within the genus Henipavirus, alongside the Cedar virus (Marsh et al., 2012), Ghanaian bat virus (Drexler et al., 2009), and Mojiang virus (Wu et al., 2014). Fruit bats of the genus Pteropus have been identified as natural reservoirs for NiV. This pathogen poses a significant threat to both humans and animals, including pigs (Orosco, 2023), with human-to-human and animal-to-human transmission occurring via infected bats and pigs (Thomas et al., 2019). Notably, NiV has an alarming case fatality rate of up to 100% (Table 1). Owing to its high fatality rate and the absence of effective therapeutics or vaccines, the World Health Organization has designated NiV as a biosafety level 4 (BSL4) pathogen (Freiberg et al., 2010). Consequently, urgent measures are required to effectively prevent and control NiV infection and safeguard vulnerable populations. In light of the persistent threat posed by NiV to human and animal health, this review paper seeks to comprehensively address the multifaceted challenge of developing effective control and prevention strategies. The paper aims to explore the current state of knowledge in areas including vaccine development, antiviral drug discovery, early diagnosis, surveillance, and high-level biosecurity measures. By delving into these interconnected aspects, this review endeavors to offer a synthesized understanding of the current landscape and prospects in combatting NiV infections. Table 1. Case fatality rates of NiV infection in humans.
Molecular biology of Nipah virusNipah virus, a member of the Paramyxoviridae family, shares its viral family with notable human pathogens, including HeV, measles virus, mumps virus, respiratory syncytial virus, and human parainfluenza virus (Amarasinghe et al., 2019). These paramyxoviruses possess a single-stranded, nonsegmented, negative-sense RNA genome, which is entirely enclosed by envelope proteins consisting of a cell receptor-binding protein known as the glycoprotein (G) specific to henipaviruses, as well as hemagglutinin (H) or hemagglutinin/neuraminidase (HN), along with a distinct fusion (F) protein (Fig. 1). In-depth analyses of various gene regions of the Nipah virus compared to other paramyxoviruses have affirmed that NiV, alongside HeV, constitutes a unique cluster within the Paramyxoviridae family, ultimately designated as the Henipavirus genus (Chua et al., 2000). NiV-G and NiV-F proteins exhibit a close physical association, and viral fusion is triggered by conformational changes that occur subsequent to receptor binding (Guillaume et al., 2006). Among these proteins, residue E533 of the NiV-G protein plays a crucial role in receptor binding, and shares structural and functional similarities with residue R533 of the measles virus attachment hemagglutinin (Sawatsky et al., 2016). In contrast, the NiV-F protein undergoes glycosylation at multiple sites, resulting in reduced fusion efficiency compared to mutated F proteins. Interestingly, unlike other paramyxoviruses, the N-glycans present in NiV may shield proteins from neutralizing antibodies (Aguilar et al., 2006). While the NiV-G and NiV-F proteins are vital for host cell binding, fusion, and entry, their involvement in viral budding is relatively minor compared to the essential role played by the viral matrix protein M. The M protein appears to be integral to the viral organization and budding processes (Patch et al., 2007). Notably, the NiV genome encompasses six genes that encode structural proteins of the viral envelope (F, G, and M), as well as the nucleocapsid protein (N), polymerase (L), and phosphoprotein (P). Within the P gene, in addition to encoding phosphoprotein P, crucial proteins, such as C, V, and W, contribute significantly to the pathogenicity of NiV (Fig. 1) (Uchida et al., 2018; Hauser et al., 2021). The genomes of NiV-Bangladesh (NiV-B) and NiV-Malaysia (NiV-M) exhibit a substantial homology of 91.8%. However, a noteworthy difference between the two genomes lies in their length, with the NiV-B genome being six nucleotides longer than that of NiV-M. Interestingly, these additional nucleotides are specifically located in the 5’ nontranslated region of the F protein gene (Harcourt et al., 2004). Furthermore, while minimal variations were observed among the open reading frames of the two strains, the V gene displayed variability (Patch et al., 2007). The V gene encodes a protein (V) that has been implicated in multiple mechanisms aimed at suppressing the host immune response during NiV infection. Nevertheless, the precise implications and significance of these variations within the V gene among different NiV strains remain to be fully elucidated (Uchida et al., 2018). Transmission of Nipah virusBat-to-bat transmission Pteropus spp. bats, commonly known as flying foxes, serve as natural reservoir hosts for both the Hendra and Nipah viruses. These bats have a broad geographic distribution, and evidence of Henipavirus infection has been detected in bats from various regions, including Southeast Asia, Africa, and South and Central America (Enchéry and Horvat, 2017). However, the impact of henipavirus infection on naturally infected bats remains poorly understood. Experimental studies involving Pteropus spp. bats have revealed that they do not exhibit signs of disease and display only limited pathological changes upon infection (Halpin et al., 2011).
Fig. 1. NiV structure and genome organization. During infection, henipaviruses are primarily shed through urine, although viruses have also been detected in throat and rectal swabs (Edson et al., 2015). Vertical transmission of HeV has been observed in experimentally infected pregnant bats (Williamson et al., 2000). However, no HeV RNA has been detected in fetal tissues of naturally infected bats (Goldspink et al., 2015). Furthermore, contact transmission of HeV from P. poliocephalus bats to naive animals has not been observed in previous studies, likely due to the absence of virus shedding from the inoculated bats (Williamson et al., 2000). In contrast, the transmission of NiV among bats is believed to occur through direct contact, facilitated by the high population density within roosts and the use of urine during grooming (Middleton et al., 2007; Agarwal and Singh, 2020). Horse-to-horse transmission In horses, natural infection with henipaviruses manifests as depression and fever, progressing to neurological and respiratory manifestations, with a notable presence of copious frothy nasal discharge observed in the advanced stages of the disease (Selvey et al., 1995). Horses affected by henipaviruses experience a high case fatality rate, estimated to be approximately 90% (Field, 2016). Experimental studies involving infected horses have demonstrated the detection of viral RNA in urine and oral and nasal swabs, with nasal swabs exhibiting the highest viral shedding levels that persist for an extended duration (Marsh et al., 2011). Notably, environmental swabs collected from stalls housing naturally infected horses also yielded detectable viral RNA, indicating a potential for environmental contamination (Field et al., 2010; Chowdhury et al., 2022). Viral shedding can occur before the onset of clinical signs in experimentally infected horses, suggesting that asymptomatic horses have the potential to contribute to the spread of infection (Marsh et al., 2011). However, in the context of housing experimentally infected and naive horses in adjacent stalls, no transmission of the virus to naive animals has been observed (Williamson et al., 1998). It is important to note that this lack of transmission could potentially be attributed to the absence of the copious frothy nasal discharge that has been proposed to play a crucial role in both inter-horse transmission and zoonotic transmission (Marsh et al., 2011; Alaoui et al., 2021). Collectively, the available data from both natural and experimental infections in horses strongly suggest that the primary route of HeV transmission between horses occurs through close contact with infected horses during the end stage of the disease when viral shedding is at its peak. In addition, the extensive handling of horses by humans without adequate measures to prevent cross-contamination could contribute to the spread of henipaviruses among horses (Field et al., 2010; Shams et al., 2023). Pig-to-pig transmission Naturally infected pigs exhibit acute febrile illness characterized by respiratory signs, including nasal discharge, barking cough, labored breathing, and neurological manifestations (Mohd Nor et al., 2000). The severity of NiV disease in pigs is influenced by their age. In experimental studies involving pigs inoculated with NiV, a spectrum of disease signs was observed, ranging from subclinical to clinical, with respiratory and neurological symptoms. Viral shedding was detected in the nasal and throat secretions of experimentally infected pigs, with the highest viral load observed in the nasal samples. Viral shedding has been observed in both clinically and clinically infected pigs (Weingartl et al., 2005; Rashid et al., 2022). Transmission of the virus from experimentally infected pigs to naive pen-mates has been observed several days after the onset of viral shedding in inoculated animals (Middleton et al., 2002). Consistent with epidemiological data from the NiV outbreak in Malaysia and transmission studies in experimentally infected animals, it has been established that the transmission of NiV among pigs in close proximity is rapid and efficient. The primary mode of transmission is likely direct contact with nasal secretions, and both clinically and subclinically infected pigs have the potential to transmit the virus (Mohd Nor et al., 2000). Human-to-human transmission In humans, NiV infection results in an acute febrile illness accompanied by respiratory symptoms (Table 2). The virus is shed in the urine and respiratory secretions, including those from the mouth and nose, during the acute phase of the disease (Chua et al., 2001). The clinical course often involves progression to acute respiratory distress syndrome and severe neurological disease, which can have long-term consequences (Sejvar et al., 2007) and relapses (Tan et al., 2002). Human-to-human transmission has played a significant role in the spread of NiV in India, Bangladesh, and the Philippines. Notably, during the 2001 outbreak in India, 75% of cases were acquired through human-to-human transmission (Chadha et al., 2006). Patients with respiratory symptoms and coughing are more likely to transmit the virus, and fatal cases were more prone to transmission compared to nonfatal cases (Luby et al., 2009, pp. 2001–2007). Transmission of NiV from deceased patients to individuals who come in contact with the body after death has also been reported (Halpin et al., 2011; Sazzad et al., 2013). Although nosocomial transmission of NiV is rare in Malaysia (Mounts et al., 2001) and Bangladesh (Gurley et al., 2007b), it is the primary source of cases in Siliguri, India, where 25 out of 66 NiV infections occur among hospital staff (Chadha et al., 2006). Despite the potential for transmission through aerosols or respiratory droplets, given the respiratory tract infection caused by the virus, epidemiological studies have consistently shown that close contact is necessary for human-to-human transmission of NiV (Gurley et al., 2007a; Homaira et al., 2010). In Bangladesh, the contact transmission of NiV is facilitated by healthcare and cultural practices that involve family members caring for hospitalized patients and the significance of physical contact with sick relatives and friends (Blum et al., 2009). Although there is a risk of transmission through aerosols or respiratory droplets, epidemiological evidence underscores the importance of close contact for the spread of NiV (Gurley et al., 2007a; Homaira et al., 2010). Bat-to-pig-to-human transmission In Malaysia, human cases of NiV infection are primarily attributed to the transmission of the virus from infected pigs, which serve as intermediate amplifying hosts (Looi and Chua, 2007). The spillover of the virus from bats to pigs is believed to occur through the consumption of partially eaten fruits contaminated by bats carrying NiV (Luby, 2013). The transmission of the virus from pigs to humans is facilitated through direct contact with infected pigs, whereas human-to-human transmission can occur through direct contact with aerosols or fomites (Looi and Chua, 2007). Abattoir workers, who come into contact with excretions and secretions such as urine, saliva, pharyngeal, and respiratory secretions of infected pigs, as well as raw pig meat and other contaminated products, are particularly at risk (Paton et al., 1999). In addition, because infected pigs experience severe respiratory distress, the aerosol spread of NiV to humans is considered an important route of respiratory transmission (Mohd Nor et al., 2000). The importation of infected pigs from Malaysia to Singapore has resulted in the spread of infection among pig farmers living in close proximity to pig sites (Paton et al., 1999). Prevention and control strategiesNiV vaccines Various vaccines have been extensively explored (Table 3) in light of the expanding understanding of the molecular biology of NiV (Fig. 2). Initial investigations demonstrated the generation of neutralizing antibodies and protection against fatal infections in mice and hamsters upon vaccination with vaccinia virus recombinants expressing either NiV-G or F proteins (Guillaume et al., 2004). Recent vaccine studies have focused on the G and F proteins (Hauser et al., 2021). Notably, a subunit vaccine incorporating the G glycoprotein of HeV, which shares 83% amino acid identity with the NiV G protein, displayed promising efficacy in safeguarding ferrets against NiV infection following exposure to lethal doses of the virus (Pallister et al., 2013). Several vector-based vaccines are currently being developed. The ChAdOx1 NiV-B vaccine employs a replication-deficient simian adenovirus vector that encodes glycoprotein G of NiV-B (Doremalen et al., 2019). Female Golden Syrian hamsters were immunized with this vaccine at different intervals before being challenged with NiV-B, with some hamsters receiving a booster dose of the vaccine before the challenge. Following a single vaccine dose, all animals exhibited virus-neutralizing antibodies. Throughout the study, all vaccinated animals survived without viral RNA detection in oropharyngeal swabs or during necropsy (Doremalen et al., 2019). Recombinant vesicular stomatitis virus (rVSV) has emerged as a widely employed vector for vaccine delivery and has been used to develop a NiV vaccine that expresses the NiV G glycoprotein in conjunction with an incompatible F protein (Prescott et al., 2015). To assess its efficacy, three African green monkeys were intratracheally challenged with NiV-M three weeks after vaccination (Orosco, 2024). Remarkably, all three vaccinated monkeys survived the duration of the study, without exhibiting any clinical disease or detecting the presence of the virus. In contrast, two of the three control monkeys experienced symptoms, such as increased work of breathing and lethargy, and NiV RNA was detected in their oropharyngeal swabs and blood samples. However, both control monkeys eventually recovered and survived until the end of the study period (Prescott et al., 2015). Table 2. Laboratory and radiological diagnosis of Nipah virus disease.
Table 3. Different vaccine prototypes are under development for NiV infection.
To address the seemingly heightened pathogenicity of NiV-B compared to NiV-M, a vaccine specifically targeting NiV-B was constructed utilizing rVSV that expressed either the G or F protein. Following vaccination, all animals developed NiV-B-neutralizing antibodies within three weeks. Upon subsequent challenge with NiV-B, the mice demonstrated remarkable survival without any evidence of infection (Mire et al., 2019). An examination was conducted to explore the potential of a live attenuated rabies virus-based vaccine as a preventive measure against NiV in wildlife. This approach capitalizes on robust humoral immune responses elicited by vaccines based on the rabies virus. The inclusion of the NiV-B glycoprotein G within the rabies virus vector resulted in seroconversion in mice subjected to the vaccination regimen (Keshwara et al., 2019). The authors proposed the development of a live-attenuated rabies-NiV hybrid as a promising vaccine candidate for wildlife, offering the potential for dual protection against both rabies and NiV (Keshwara et al., 2019). Virus-like particles (VLPs) offer a promising approach to vaccine development as they elicit robust immune responses by presenting both native F and G glycoproteins without the potential risks associated with using complete virions. A study conducted on Syrian golden hamsters demonstrated the protective efficacy and induction of neutralizing antibody titers by a vaccine based on NiV VLPs (Walpita et al., 2017). In addition, an mRNA vaccine encoding the soluble subunit of the HeV glycoprotein is currently in the developmental stage and exhibits partial protection against NiV infection in Syrian hamsters (Lo et al., 2020). Further preclinical and clinical investigations are necessary to validate the effectiveness of these vaccine constructs. Anti-NiV therapeutics Several antiviral agents have been investigated for their potential in treating NiV infection, although only a limited number have been evaluated in animal model studies (Table 4). Notable examples include ribavirin, remdesivir, and favipiravir. Ribavirin was one of the first antiviral drugs used against NiV. During the outbreak in Malaysia between 1998 and 1999, ribavirin treatment resulted in a 36% reduction in the mortality rate of 140 NiV-infected patients (Goh et al., 2000). However, in vitro evaluation using a combination of ribavirin and chloroquine in hamsters did not yield a significant reduction in mortality (Freiberg et al., 2010). The efficacy of ribavirin was further examined during the 2018 NiV outbreak in Kerala, where oral ribavirin was administered to six patients, but only two survived (Banerjee et al., 2019).
Fig. 2. Transmission routes of NiV. (A) In Malaysia, bat-bitten fruits contaminated with NiV-M were consumed by pigs, and workers handling the pigs were infected with NiV-M. (B) In Bangladesh, bat saliva- and excreta-contaminated palm sap consumption led to NiV-B infection in humans and was spread further via nosocomial mode. Infected bats shed the virus in their urine, excreta, and saliva. (C) In India, the possibility of direct bat-to-human transmission has been reported in Kerala state, but this was not supported by adequate evidence. Nosocomial spread of NiV-B has been reported in two different states—Kerala and West Bengal. Considering the limited therapeutic options available for NiV, recent efforts have focused on evaluating the potential of alternative antiviral drugs. Among these, remdesivir, an adenosine nucleoside antiviral drug, has shown promise in preclinical studies in African green monkeys. Notably, when African green monkeys were treated with remdesivir, only two out of four animals developed mild respiratory symptoms, whereas all untreated animals exhibited severe respiratory symptoms, highlighting the potential efficacy of remdesivir against NiV infection (Lo et al., 2019). It is worth mentioning that remdesivir has gained significant recognition in the field of viral therapeutics, as it has received approval from the United States Food and Drug Administration for the treatment of SARS-CoV-2 infection. Moreover, numerous countries, including Bangladesh, Singapore, Taiwan, India, Japan, and Australia, have authorized its emergency use, further attesting to its therapeutic potential (Beigel et al., 2020). During the NiV outbreak in Singapore in 1999, a combination therapy consisting of acyclovir (Zovirax) and ceftriaxone was administered to nine abattoir workers, resulting in a remarkable survival rate of eight out of nine individuals (Paton et al., 1999). However, it is worth noting that no in vitro data are available regarding the effectiveness of acyclovir against NiV. In contrast, favipiravir, marketed as Avigan, has demonstrated inhibitory effects on NiV replication in in vitro studies (Srinivasan and Rao, 2021). Moreover, in a hamster model study, favipiravir exhibited the highest antiviral activity against NiV infection compared with other compounds (Dawes et al., 2018). Another potential therapeutic candidate, rintatolimid (Ampligen), an immunomodulator, has shown efficacy in inhibiting NiV replication and in providing protection against viral challenges in hamsters through the induction of IFN-α and IFN-β (Georges-Courbot et al., 2006). Nonetheless, it is imperative to highlight the need for further comprehensive investigations to establish solid evidence regarding the efficacy of antiviral drugs suitable for the treatment of NiV infections in humans. Currently, available in vitro and in vivo studies are limited. Continued research efforts are warranted to identify and develop effective treatment options against NiV infections (Liew et al., 2022). Early diagnosis The early and accurate diagnosis of NiV infection plays a pivotal role in mitigating the significant fatality rate associated with this disease. To achieve this objective, a comprehensive diagnostic approach encompasses the collection of diverse specimens from both infected individuals and animals. In human patients, various samples such as nasal swabs, throat swabs, urine, blood, and cerebrospinal fluid (CSF) are obtained for diagnostic purposes (Mazzola and Kelly-Cirino, 2019). Similarly, deceased animals provide valuable diagnostic specimens, such as lungs, spleen, and kidneys, which aid in the identification and isolation of NiV (Daniels et al., 2001). Diagnostic procedures for NiV are conducted in highly controlled and specialized facilities, specifically in enhanced Biosafety Level 3 (BSL3+) or Biosafety Level 4 (BSL4) laboratories. A range of diagnostic tests is available for the detection of NiV, including molecular and serological assays, immunohistochemistry, histopathology, virus isolation, and neutralization techniques (Mazzola and Kelly-Cirino, 2019). Notably, Vero cells are employed for the cultivation of NiV, with observable cytopathic effects typically manifesting within a three-day timeframe (Daniels et al., 2001). Among the various diagnostic methods available for NiV detection, polymerase chain reaction (PCR) is the most preferred and highly sensitive. This molecular approach utilizes reverse-transcription PCR (RT-PCR) and nested PCR, targeting conserved segments of the viral genome, namely N, M, and P (Mazzola and Kelly-Cirino, 2019). In particular, real-time RT-PCR, despite its relatively high cost, is widely used because of its exceptional sensitivity, enabling accurate and efficient NiV detection and diagnosis (Wang and Daniels, 2012). The utilization of PCR-based methods, particularly real-time RT-PCR, has revolutionized the field of NiV diagnostics by providing rapid and highly sensitive results. In addition to traditional diagnostic approaches, next-generation sequencing (NGS) has emerged as a valuable method for accurate identification of NiV strains. Although NGS offers substantial benefits in terms of strain characterization, its utilization in routine diagnosis is limited owing to cost considerations (Mazzola and Kelly-Cirino, 2019). Alternatively, immunohistochemistry is a safe and reliable method for NiV detection, particularly when using formalin-fixed tissue samples (Wang and Daniels, 2012). The enzyme-linked immunosorbent assay (ELISA) plays a crucial role in the detection of NiV antigens and antibodies in serum samples. ELISA is an effective and widely employed technique that is often followed by confirmatory tests such as serum neutralization or PCR to enhance diagnostic accuracy (Daniels et al., 2001). In addition, virus isolation and neutralization methods are utilized for NiV diagnosis; however, their implementation is restricted to high-containment BSL4 facilities because of the infectious nature of the virus (Wang and Daniels, 2012; Mazzola and Kelly-Cirino, 2019). NiV surveillance The NiV outbreak in 2018 was a significant occurrence characterized by a high case fatality rate, whereas the subsequent outbreak can be considered comparatively minor in scale. The primary cases in both outbreaks likely resulted from inadvertent exposure to NiV-infected bats or the consumption of food contaminated by bat secretions (Bruno et al., 2023). However, in the 2018 outbreak, it is plausible that the initial spillover event was further propagated through person-to-person transmission within hospital settings, as subsequent cases were predominantly from close family or hospital contacts. These two incidents can be regarded as isolated and localized occurrences, indicating containment of outbreaks within specific geographic areas and populations (Singhai et al., 2021). The early warning signs of this outbreak could have been detected through diligent reporting of unusual events, such as the unexpected sudden death of an otherwise healthy young male. Epidemiologically linked cases in subsequent outbreaks could have been prevented through strict adherence to surveillance, infection control, and biomedical waste management practices at all levels of healthcare centers (Singhai et al., 2021). In response to the 2018 outbreak, the Indian National Center for Disease Control established guidelines for the definition of suspected, probable, and confirmed cases of NiV infection to facilitate early detection and control of future outbreaks (Table 5) (Bruno et al., 2023). Through surveillance efforts, the analysis of viral strains and monitoring of viral factors have been instrumental in understanding the Nipah outbreaks. Cluster-based surveillance has been effective in identifying two distinct types of Nipah outbreaks that are characterized by high fatality rates. Similarly, case-based surveillance has helped detect sporadic introductions of NiV in Bangladesh (Naser et al., 2015). In Bangladesh, the establishment of a surveillance system in five hospitals along the NiV belt has contributed to early detection of outbreaks (Dhillon and Banerjee, 2015). During the 1999 outbreak in Malaysia, a comprehensive surveillance study encompassed human health, animal health, and reservoir hosts. Within the human health sector, three categories are covered: disease surveillance, patient surveillance, and high-risk group surveillance (Ambat et al., 2019). These surveillance efforts have been crucial for improving our understanding of NiV dynamics and informing effective prevention and control strategies (Dhillon and Banerjee, 2015). Table 4. Drugs with potential antiviral activity against NiV.
Table 5. Surveillance case definitions for Nipah virus infection as defined by the National Centre for Disease Control, India.
High-level biosecurity Given the absence of specific standardized therapy and the limited availability of large-scale vaccines against NiV, the implementation of biosecurity measures is crucial for preventing disease outbreaks and effectively mitigating the risk of transmission to both humans and animals (Bruno et al., 2023). Particularly in the agricultural and livestock sectors, the adoption of good pig farming practices and the prevention of crop contamination by bats play a fundamental role in averting further outbreaks (Chua et al., 2001). Rigorous enforcement of proper hygiene protocols and thorough handwashing by all personnel involved are of paramount importance. In the event of a suspected outbreak, it is imperative for all personnel to strictly adhere to the use of personal protective equipment (PPE), including masks, gloves, protective goggles, gowns, and boots. Furthermore, meticulous cleaning and disinfection of PPE after use must be performed (Lam and Chua, 2002). Within hospital settings, strict adherence to standard infection control precautions is imperative for all healthcare personnel involved in patient care activities and procedures that generate aerosols. In the event of a NiV infection, additional measures, including droplet, contact, and airborne precautions, should be implemented (Gurley et al., 2007a). Droplet precautions involve the isolation of patients from individual rooms or cohorts with existing roommates. Immediate isolation and implementation of infection control measures are essential when a patient meets the criteria for a suspected Nipah infection (Siegel et al., 2007). Hospitals located in high-risk areas must be adequately prepared to manage Nipah cases through the implementation of hospital screening, admission procedures, triage systems, and regulation of visitor access and movement to minimize potential exposure. Standard precautions must be rigorously observed when handling patients, managing deceased individuals, handling specimens, and carrying out cleaning and waste disposal procedures (Boyce et al., 2002). Thorough hand hygiene is of the utmost importance in preventing the transmission of NiV and should be practiced by healthcare workers before and after every patient contact. Evidence from Bangladesh has highlighted the ability of NiV to survive on surfaces, posing a potential risk of transmission of infection to caregivers (Gurley et al., 2007b). A lack of hand hygiene practices and limited access to water in healthcare settings have been implicated in the infection of healthcare workers during outbreaks (Boyce et al., 2002). The significance of proper hand hygiene cannot be overstated, as it serves as a critical cornerstone for preventing the spread of infection (Siegel et al., 2007). Implementing hand washing with soap and water or utilizing alcohol-based hand rubs is essential for maintaining hand hygiene standards and reducing the risk of NiV transmission (Boyce et al., 2002). ConclusionIn conclusion, control and prevention strategies for NiV represent a critical area of research and development that has gained significant attention in recent years. The multifaceted approach encompassing vaccine development, antiviral drug discovery, early diagnosis, surveillance, and high-level biosecurity measures holds great promise for mitigating the impact of NiV outbreaks and safeguarding public health. Several areas warrant further exploration to enhance our understanding and control of NiV. First, comprehensive clinical trials are needed to assess the safety and efficacy of candidate vaccines and antiviral drugs in humans. Long-term monitoring of vaccine recipients and the development of strategies to optimize vaccine coverage and delivery should also be prioritized. Advancements in diagnostic technologies and accessibility to resource-limited settings are crucial. The development of rapid point-of-care diagnostic tests can facilitate early detection and timely intervention. In addition, the integration of surveillance data from multiple sources, including humans, animals, and the environment, can enhance our ability to detect and respond to potential outbreaks of NiV. Collaborative efforts among researchers, public health agencies, and policymakers are paramount for strengthening NiV control and prevention strategies. Establishing international partnerships and knowledge-sharing platforms can foster an exchange of information, resources, and expertise to help combat NiV infections on a global scale. AcknowledgmentsThe author would like to thank the DOST S&T Fellows Program and the Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development (DOST-PCAARRD) for funding this research project, and the Industrial Technology Development Institute (DOST-ITDI) for hosting this research project. Conflict of interestThe author declares that there are no conflicts of interest. FundingThis research is funded by the Department of Science and Technology - Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development (DOST-PCAARRD). ReferencesAgarwal, P. and Singh, R. 2020. Modelling of transmission dynamics of Nipah virus (Niv): a fractional order approach. Phys. Stat. Mech. Appl. 547, 124243. Aguilar, H.C., Matreyek, K.A., Filone, C.M., Hashimi, S.T., Levroney, E.L., Negrete, O.A., Bertolotti-Ciarlet, A., Choi, D.Y., McHardy, I., Fulcher, J.A., Su, S.V., Wolf, M.C., Kohatsu, L., Baum, L. G. and Lee, B. 2006. N-Glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J. Virol. 80, 4878–4889. Alaoui, A.L., Tilioua, M., Sidi Ammi, M. R. and Agarwal, P. 2021. Dynamical analysis of a Caputo fractional order SIR epidemic model with a general treatment function. In Analysis of infectious disease problems (Covid-19) and their global impact. Eds., Agarwal, P., Nieto, J.J., Ruzhansky, M., Torres, D.F.M. Infosys Science Foundation Series. Singapore: Springer, pp: 17–33. Amarasinghe, G.K., Ayllón, M.A., Bào, Y., Basler, C.F., Bavari, S., Blasdell, K.R., Briese, T., Brown, P.A., Bukreyev, A., Balkema-Buschmann, A., Buchholz, U.J., Chabi-Jesus, C., Chandran, K., Chiapponi, C., Crozier, I., de Swart, R.L., Dietzgen, R.G., Dolnik, O., Drexler, J.F., Dürrwald, R., Dundon, W.G., Duprex, W.P., Dye, J.M., Easton, A.J., Fooks, A.R., Formenty, P.B.H., Fouchier, R.A.M., Freitas-Astúa, J., Griffiths, A., Hewson, R., Horie, M., Hyndman, T.H., Jiāng, D., Kitajima, E.W., Kobinger, G.P., Kondō, H., Kurath, G., Kuzmin, I.V., Lamb, R.A., Lavazza, A., Lee, B., Lelli, D., Leroy, E.M., Lǐ, J., Maes, P., Marzano, S.-Y.L., Moreno, A., Mühlberger, E., Netesov, S.V., Nowotny, N., Nylund, A., Økland, A.L., Palacios, G., Pályi, B., Pawęska, J.T., Payne, S.L., Prosperi, A., Ramos-González, P.L., Rima, B.K., Rota, P., Rubbenstroth, D., Shī, M., Simmonds, P., Smither, S.J., Sozzi, E., Spann, K., Stenglein, M.D., Stone, D.M., Takada, A., Tesh, R.B., Tomonaga, K., Tordo, N., Towner, J.S., van den Hoogen, B., Vasilakis, N., Wahl, V., Walker, P.J., Wang, L.-F., Whitfield, A.E., Williams, J.V., Zerbini, F.M., Zhāng, T., Zhang, Y.-Z. and Kuhn, J.H. 2019. Taxonomy of the order Mononegavirales: update 2019. Arch. Virol. 164, 1967–1980. Ambat, A.S., Zubair, S.M., Prasad, N., Pundir, P., Rajwar, E., Patil, D.S. and Mangad, P. 2019. Nipah virus: a review on epidemiological characteristics and outbreaks to inform public health decision making. J. Infect. Public Health 12, 634–639. Ang, B.S.P., Lim, T.C.C. and Wang, L. 2018. Nipah virus infection. J. Clin. Microbiol. 56, e01875-17. Banerjee, S., Niyas, V.K.M., Soneja, M., Shibeesh, A.P., Basheer, M., Sadanandan, R., Wig, N. and Biswas, A. 2019. First experience of ribavirin postexposure prophylaxis for Nipah virus, tried during the 2018 outbreak in Kerala, India. J. Infect. 78, 491–503. Beigel, J.H., Tomashek, K.M., Dodd, L.E., Mehta, A.K., Zingman, B.S., Kalil, A.C., Hohmann, E., Chu, H.Y., Luetkemeyer, A., Kline, S., Lopez de Castilla, D., Finberg, R.W., Dierberg, K., Tapson, V., Hsieh, L., Patterson, T.F., Paredes, R., Sweeney, D.A., Short, W.R., Touloumi, G., Lye, D.C., Ohmagari, N., Oh, M., Ruiz-Palacios, G.M., Benfield, T., Fätkenheuer, G., Kortepeter, M.G., Atmar, R.L., Creech, C.B., Lundgren, J., Babiker, A.G., Pett, S., Neaton, J.D., Burgess, T.H., Bonnett, T., Green, M., Makowski, M., Osinusi, A., Nayak, S. and Lane, H.C. 2020. Remdesivir for the treatment of Covid-19—Final report. N. Engl. J. Med. 383, 1813–1826. Blum, L.S., Khan, R., Nahar, N. and Breiman, R.F. 2009. In-depth assessment of an outbreak of Nipah encephalitis with person-to-person transmission in Bangladesh: implications for prevention and control strategies. Am. J. Trop. Med. Hyg. 80, 96–102. Boyce, J.M., Pittet, D., Healthcare Infection Control Practices Advisory Committee and HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. 2002. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm. Rep. 51, 1–45, quiz CE1–4. Bruno, L., Nappo, M.A., Ferrari, L., Di Lecce, R., Guarnieri, C., Cantoni, A.M. and Corradi, A. 2023. Nipah virus disease: epidemiological, clinical, diagnostic and legislative aspects of this unpredictable emerging zoonosis. Animals 13, 159. Chadha, M.S., Comer, J.A., Lowe, L., Rota, P.A., Rollin, P.E., Bellini, W.J., Ksiazek, T.G. and Mishra, A. 2006. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg. Infect. Dis. 12, 235–240. Ching, P.K.G., de los Reyes, V.C., Sucaldito, M.N., Tayag, E., Columna-Vingno, A.B., Malbas, F.F., Bolo, G.C., Sejvar, J.J., Eagles, D., Playford, G., Dueger, E., Kaku, Y., Morikawa, S., Kuroda, M., Marsh, G.A., McCullough, S. and Foxwell, A.R. 2015. Outbreak of Henipavirus Infection, Philippines, 2014. Emerg. Infect. Dis. 21, 328–331. Chowdhury, S.M.E.K., Forkan, M., Ahmed, S.F., Agarwal, P., Shawkat Ali, A.B.M. and Muyeen, S.M. 2022. Modeling the SARS-CoV-2 parallel transmission dynamics: asymptomatic and symptomatic pathways. Comput. Biol. Med. 143, 105264. Chua, K.B., Bellini, W.J., Rota, P.A., Harcourt, B.H., Tamin, A., Lam, S.K., Ksiazek, T.G., Rollin, P.E., Zaki, S.R., Shieh, W.-J., Goldsmith, C.S., Gubler, D.J., Roehrig, J.T., Eaton, B., Gould, A.R., Olson, J., Field, H., Daniels, P., Ling, A.E., Peters, C.J. Anderson, L.J. and Mahy, B.W.J. 2000. Nipah virus: a recently emergent deadly paramyxovirus. Science 288, 1432–1435. Chua, K.B., Lam, S.K., Goh, K.J., Hooi, P.S., Ksiazek, T.G., Kamarulzaman, A., Olson, J. and Tan, C.T. 2001. The presence of Nipah virus in respiratory secretions and urine of patients during an outbreak of Nipah virus encephalitis in Malaysia. J. Infect. 42, 40–43. Daniels, P., Ksiazek, T. and Eaton, B.T. 2001. Laboratory diagnosis of Nipah and Hendra virus infections. Microbes Infect. 3, 289–295. Dawes, B.E., Kalveram, B., Ikegami, T., Juelich, T., Smith, J.K., Zhang, L., Park, A., Lee, B., Komeno, T., Furuta, Y. and Freiberg, A.N. 2018. Favipiravir (T-705) protects against Nipah virus infection in the hamster model. Sci. Rep. 8, 7604. Dhillon, J. and Banerjee, A. 2015. Controlling Nipah virus encephalitis in Bangladesh: policy options. J. Public Health Policy 36, 270–282. Doremalen, N. van, Lambe, T., Sebastian, S., Bushmaker, T., Fischer, R., Feldmann, F., Haddock, E., Letko, M., Avanzato, V.A., Rissanen, I., LaCasse, R., Scott, D., Bowden, T.A., Gilbert, S. and Munster, V. 2019. A single-dose ChAdOx1-vectored vaccine provides complete protection against Nipah Bangladesh and Malaysia in Syrian golden hamsters. PLoS Negl. Trop. Dis. 13, e0007462. Doucleff, M. and Greenhalgh, J. 2017. A taste for pork helped a deadly virus jump to humans. Washington, DC: NPR. Drexler, J.F., Corman, V.M., Gloza-Rausch, F., Seebens, A., Annan, A., Ipsen, A., Kruppa, T., Müller, M.A., Kalko, E.K. and Adu-Sarkodie, Y. 2009. Henipavirus RNA in African bats. PloS One 4, e6367. Edson, D., Field, H., McMichael, L., Vidgen, M., Goldspink, L., Broos, A., Melville, D., Kristoffersen, J., de Jong, C., McLaughlin, A., Davis, R., Kung, N., Jordan, D., Kirkland, P. and Smith, C. 2015. Routes of hendra virus excretion in naturally-infected flying-foxes: implications for viral transmission and spillover risk. PLoS ONE 10, e0140670. Enchéry, F. and Horvat, B. 2017. Understanding the interaction between henipaviruses and their natural host, fruit bats: paving the way toward control of highly lethal infection in humans. Int. Rev. Immunol. 36, 108–121. Field, H.E. 2016. Hendra virus ecology and transmission. Curr. Opin. Virol. 16, 120–125. Field, H.E., Schaaf, K., Kung, N., Simon, C., Waltisbuhl, D., Hobert, H., Moore, F., Middleton, D., Crook, A., Smith, G., Daniels, P., Glanville, R. and Lovell, D. 2010. Hendra virus outbreak with novel clinical features, Australia. Emerg. Infect. Dis. 16, 338–340. Freiberg, A.N., Worthy, M.N., Lee, B. and Holbrook, M.R. 2010. Combined chloroquine and ribavirin treatment does not prevent death in a hamster model of Nipah and Hendra virus infection. J. Gen. Virol. 91, 765–772. Georges-Courbot, M.C., Contamin, H., Faure, C., Loth, P., Baize, S., Leyssen, P., Neyts, J. and Deubel, V. 2006. Poly(I)-poly(C12U) but not ribavirin prevents death in a hamster model of Nipah virus infection. Antimicrob. Agents Chemother. 50, 1768–1772. Goh, K.J., Tan, C.T., Chew, N.K., Tan, P.S.K., Kamarulzaman, A., Sarji, S.A., Wong, K.T., Abdullah, B.J.J., Chua, K.B. and Lam, S.K. 2000. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N. Engl. J. Med. 342, 1229–1235. Goldspink, L.K., Edson, D.W., Vidgen, M.E., Bingham, J., Field, H.E. and Smith, C.S. 2015. Natural hendra virus infection in flying-foxes: tissue tropism and risk factors. PloS One 10, e0128835. Guillaume, V., Aslan, H., Ainouze, M., Guerbois, M., Wild, T.F., Buckland, R. and Langedijk, J.P.M. 2006. Evidence of a potential receptor-binding site on the Nipah virus G protein (NiV-G): identification of globular head residues with a role in fusion promotion and their localization on an NiV-G structural model. J. Virol. 80, 7546–7554. Guillaume, V., Contamin, H., Loth, P., Georges-Courbot, M.-C., Lefeuvre, A., Marianneau, P., Chua, K.B., Lam, S.K., Buckland, R., Deubel, V. and Wild, T.F. 2004. Nipah virus: vaccination and passive protection studies in a hamster model. J. Virol. 78, 834–840. Gurley, E.S., Montgomery, J.M., Hossain, M.J., Bell, M., Azad, A.K., Islam, M.R., Molla, M.A.R., Carroll, D.S., Ksiazek, T.G., Rota, P.A., Lowe, L., Comer, J.A., Rollin, P., Czub, M., Grolla, A., Feldmann, H., Luby, S.P., Woodward, J.L. and Breiman, R.F. 2007a. Person-to-person transmission of Nipah virus in a Bangladeshi Community. Emerg. Infect. Dis. 13, 1031–1037. Gurley, E.S., Montgomery, J.M., Hossain, M.J., Islam, M.R., Molla, M.A.R., Shamsuzzaman, S.M., Akram, K., Zaman, K., Asgari, N., Comer, J.A., Azad, A.K., Rollin, P.E., Ksiazek, T.G. and Breiman, R.F. 2007b. Risk of nosocomial transmission of Nipah virus in a Bangladesh hospital. Infect. Control Hosp. Epidemiol. 28, 740–742. Halpin, K., Hyatt, A.D., Fogarty, R., Middleton, D., Bingham, J., Epstein, J.H., Rahman, S.A., Hughes, T., Smith, C., Field, H.E., Daszak, P. and Henipavirus Ecology Research Group. 2011. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 85, 946–951. Harcourt, B.H., Lowe, L., Tamin, A., Yu, Z., Bankamp, B., Bowden, N., Rollin, P.E., Comer, J.A., Ksiazek, T.G., Hossain, M.J., Gurley, E.S., Breiman, R.F., Bellini, W.J. and Rota, P.A. 2005. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg. Infect. Dis. 11, 1594–1597. Hauser, N., Gushiken, A.C., Narayanan, S., Kottilil, S. and Chua, J.V. 2021. Evolution of Nipah virus infection: past, present, and future considerations. Trop. Med. Infect. Dis. 6, 24. Homaira, N., Rahman, M., Hossain, M.J., Epstein, J.H., Sultana, R., Khan, M.S.U., Podder, G., Nahar, K., Ahmed, B., Gurley, E.S., Daszak, P., Lipkin, W.I., Rollin, P.E., Comer, J.A., Ksiazek, T.G. and Luby, S.P. 2010. Nipah virus outbreak with person-to-person transmission in a district of Bangladesh, 2007. Epidemiol. Infect. 138, 1630–1636. Hotard, A.L., He, B., Nichol, S.T., Spiropoulou, C.F. and Lo, M.K. 2017. 4′-Azidocytidine (R1479) inhibits henipaviruses and other paramyxoviruses with high potency. Antiviral. Res. 144, 147–152. Keshwara, R., Shiels, T., Postnikova, E., Kurup, D., Wirblich, C., Johnson, R.F. and Schnell, M.J. 2019. Rabies-based vaccine induces potent immune responses against Nipah virus. Npj Vaccines 4, 1–10. Kulkarni, D.D., Tosh, C., Venkatesh, G. and Senthil Kumar, D. 2013. Nipah virus infection: current scenario. Indian J. Virol. 24, 398–408. Lam, S.K. and Chua, K.B. 2002. Nipah virus encephalitis outbreak in Malaysia. Clin. Infect. Dis. 34, S48–S51; doi:10.1086/338818. Liew, Y.J.M., Ibrahim, P.A.S., Ong, H.M., Chong, C.N., Tan, C.T., Schee, J.P., Gómez Román, R., Cherian, N.G., Wong, W.F. and Chang, L.-Y. 2022. The immunobiology of Nipah virus. Microorganisms 10, 1162. Lo, M.K., Feldmann, F., Gary, J.M., Jordan, R., Bannister, R., Cronin, J., Patel, N.R., Klena, J.D., Nichol, S.T., Cihlar, T., Zaki, S.R., Feldmann, H., Spiropoulou, C.F. and de Wit, E. 2019. Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge. Sci. Transl. Med. 11, eaau9242. Lo, M.K., Jordan, R., Arvey, A., Sudhamsu, J., Shrivastava-Ranjan, P., Hotard, A.L., Flint, M., McMullan, L.K., Siegel, D., Clarke, M.O., Mackman, R.L., Hui, H.C., Perron, M., Ray, A.S., Cihlar, T., Nichol, S.T. and Spiropoulou, C.F. 2017. GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and Paramyxoviruses. Sci. Rep. 7, 43395. Lo, M.K., Spengler, J.R., Welch, S.R., Harmon, J.R., Coleman-McCray, J.D., Scholte, F.E.M., Shrivastava-Ranjan, P., Montgomery, J.M., Nichol, S.T., Weissman, D. and Spiropoulou, C.F. 2020. Evaluation of a single-dose nucleoside-modified messenger RNA vaccine encoding hendra virus-soluble glycoprotein against lethal Nipah virus challenge in Syrian hamsters. J. Infect. Dis. 221, S493–S498. Looi, L.-M. and Chua, K.-B. 2007. Lessons from the Nipah virus outbreak in Malaysia. Malays. J. Pathol. 29, 63–67. Luby, S.P. 2013. The pandemic potential of Nipah virus. Antiviral Res. 100, 38–43. Luby, S.P., Hossain, M.J., Gurley, E.S., Ahmed, B.N., Banu, S., Khan, S.U., Homaira, N., Rota, P.A., Rollin, P.E., Comer, J.A., Kenah, E., Ksiazek, T.G. and Rahman, M. 2009. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg. Infect. Dis. 15, 1229–1235. Mahedi, M.R.A., Rawat, A., Rabbi, F., Babu, K.S., Tasayco, E.S., Areche, F.O., Pacovilca-Alejo, O.V., Flores, D.D.C., Aguilar, S.V., Orosco, F.L., Syrmos, N., Mudhafar, M., Afrin, S. and Rahman, M.M. 2023. Understanding the global transmission and demographic distribution of Nipah virus (NiV). Res. J. Pharm. Technol. 16, 3588–3594. Marsh, G.A., Haining, J., Hancock, T.J., Robinson, R., Foord, A.J., Barr, J.A., Riddell, S., Heine, H.G., White, J.R., Crameri, G., Field, H.E., Wang, L.-F. and Middleton, D. 2011. Experimental infection of horses with hendra virus/Australia/Horse/2008/Redlands. Emerg. Infect. Dis. 17, 2232–2238. Marsh, G.A., Jong, C. de, Barr, J.A., Tachedjian, M., Smith, C., Middleton, D., Yu, M., Todd, S., Foord, A.J., Haring, V., Payne, J., Robinson, R., Broz, I., Crameri, G., Field, H.E. and Wang, L.-F. 2012. Cedar virus: a novel henipavirus isolated from Australian bats. PLOS Pathog. 8, e1002836. Mazzola, L.T. and Kelly-Cirino, C. 2019. Diagnostics for Nipah virus: a zoonotic pathogen endemic to Southeast Asia. BMJ Glob. Health 4, e001118. Middleton, D.J., Morrissy, C.J., van der Heide, B.M., Russell, G.M., Braun, M.A., Westbury, H.A., Halpin, K. and Daniels, P.W. 2007. Experimental Nipah virus infection in pteropid bats (Pteropus poliocephalus). J. Comp. Pathol. 136, 266–272. Middleton, D.J., Westbury, H.A., Morrissy, C.J., van der Heide, B.M., Russell, G.M., Braun, M.A. and Hyatt, A.D. 2002. Experimental Nipah virus infection in pigs and cats. J. Comp. Pathol. 126, 124–136. Mire, C.E., Geisbert, J.B., Agans, K.N., Versteeg, K.M., Deer, D.J., Satterfield, B.A., Fenton, K.A. and Geisbert, T.W. 2019. Use of single-injection recombinant vesicular stomatitis virus vaccine to protect nonhuman primates against lethal Nipah virus disease. Emerg. Infect. Dis. 25, 1144–1152. Mohd Nor, M.N., Gan, C.H. and Ong, B.L. 2000. Nipah virus infection of pigs in peninsular Malaysia. Rev. Sci. Tech. Int. Off. Epizoot. 19, 160–165. Mounts, A.W., Kaur, H., Parashar, U.D., Ksiazek, T.G., Cannon, D., Arokiasamy, J.T., Anderson, L.J., Lye, M.S. and Nipah Virus Nosocomial Study Group. 2001. A cohort study of health care workers to assess nosocomial transmissibility of Nipah virus, Malaysia, 1999. J. Infect. Dis. 183, 810–813. Naser, A.M., Hossain, M.J., Sazzad, H.M.S., Homaira, N., Gurley, E.S., Podder, G., Afroj, S., Banu, S., Rollin, P.E., Daszak, P., Ahmed, B.-N., Rahman, M. and Luby, S.P. 2015. Integrated cluster- and case-based surveillance for detecting stage III zoonotic pathogens: an example of Nipah virus surveillance in Bangladesh. Epidemiol. Infect. 143, 1922–1930. Orosco, F.L. 2023. Current progress in diagnostics, therapeutics, and vaccines for African swine fever virus. Vet. Integr. Sci. 21, 751–781. Orosco, F.L. 2024. Immune evasion mechanisms of porcine epidemic diarrhea virus: a comprehensive review. Vet. Integr. Sci. 22, 171–192. Pallister, J.A., Klein, R., Arkinstall, R., Haining, J., Long, F., White, J.R., Payne, J., Feng, Y.-R., Wang, L.-F., Broder, C.C. and Middleton, D. 2013. Vaccination of ferrets with a recombinant G glycoprotein subunit vaccine provides protection against Nipah virus disease for over 12 months. Virol. J. 10, 237. Patch, J.R., Crameri, G., Wang, L.-F., Eaton, B.T. and Broder, C.C. 2007. Quantitative analysis of Nipah virus proteins released as virus-like particles reveals central role for the matrix protein. Virol. J. 4, 1. Paton, N.I., Leo, Y.S., Zaki, S.R., Auchus, A.P., Lee, K.E., Ling, A.E., Chew, S.K., Ang, B., Rollin, P.E., Umapathi, T., Sng, I., Lee, C.C., Lim, E. and Ksiazek, T.G. 1999. Outbreak of Nipah-virus infection among abattoir workers in Singapore. Lancet 354, 1253–1256. Pedrera, M., Macchi, F., McLean, R.K., Franceschi, V., Thakur, N., Russo, L., Medfai, L., Todd, S., Tchilian, E.Z., Audonnet, J.C., Chappell, K., Isaacs, A., Watterson, D., Young, P.R., Marsh, G.A., Bailey, D., Graham, S.P. and Donofrio, G. 2020. Bovine herpesvirus-4-vectored delivery of Nipah virus glycoproteins enhances T cell immunogenicity in pigs. Vaccines 8, 115. Prescott, J., DeBuysscher, B.L., Feldmann, F., Gardner, D.J., Haddock, E., Martellaro, C., Scott, D. and Feldmann, H. 2015. Single-dose live-attenuated vesicular stomatitis virus-based vaccine protects African green monkeys from Nipah virus disease. Vaccine 33, 2823–2829. Rahman, M. and Chakraborty, A. 2012. Nipah virus outbreaks in Bangladesh: a deadly infectious disease. WHO South-East Asia J. Public Health 1, 208. Rashid, S., Kubra, K.T., Sultana, S., Agarwal, P. and Osman, M.S. 2022. An approximate analytical view of physical and biological models in the setting of Caputo operator via Elzaki transform decomposition method. J. Comput. Appl. Math. 413, 114378. Sawatsky, B., Bente, D.A., Czub, M. and von Messling, V. 2016. Morbillivirus and henipavirus attachment protein cytoplasmic domains differently affect protein expression, fusion support and particle assembly. J. Gen. Virol. 97, 1066–1076. Sazzad, H.M.S., Hossain, M.J., Gurley, E.S., Ameen, K.M.H., Parveen, S., Islam, M.S., Faruque, L.I., Podder, G., Banu, S.S., Lo, M.K., Rollin, P.E., Rota, P.A., Daszak, P., Rahman, M. and Luby, S.P. 2013. Nipah virus infection outbreak with nosocomial and corpse-to-human transmission, Bangladesh. Emerg. Infect. Dis. 19, 210–217. Sejvar, J.J., Hossain, J., Saha, S.K., Gurley, E.S., Banu, S., Hamadani, J.D., Faiz, M.A., Siddiqui, F.M., Mohammad, Q.D., Mollah, A.H., Uddin, R., Alam, R., Rahman, R., Tan, C.T., Bellini, W., Rota, P., Breiman, R.F. and Luby, S.P. 2007. Long-term neurological and functional outcome in Nipah virus infection. Ann. Neurol. 62, 235–242. Selvey, L.A., Wells, R.M., McCormack, J.G., Ansford, A.J., Murray, K., Rogers, R.J., Lavercombe, P.S., Selleck, P. and Sheridan, J.W. 1995. Infection of humans and horses by a newly described morbillivirus. Med. J. Aust. 162, 642–645. Shams, M., Kausar, N., Samaniego, C., Agarwal, P., Ahmed, S.F. and Momani, S. 2023. On efficient fractional caputo-type simultaneous scheme for finding all roots of polynomial equations with biomedical engineering applications. Fractals 31, 2340075. Siegel, J.D., Rhinehart, E., Jackson, M. and Chiarello, L. 2007. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am. J. Infect. Control 35, S65–S164. Singhai, M., Jain, R., Jain, S., Bala, M., Singh, S. and Goyal, R. Nipah virus disease: recent perspective and one health approach. Ann. Glob. Health 87, 102. Srinivasan, K. and Rao, M. 2021. Understanding the clinical utility of favipiravir (T-705) in coronavirus disease of 2019: a review. Ther. Adv. Infect. Dis. 8, 20499361211063016. Tan, C.T., Goh, K.J., Wong, K.T., Sarji, S.A., Chua, K.B., Chew, N.K., Murugasu, P., Loh, Y.L., Chong, H.T., Tan, K.S., Thayaparan, T., Kumar, S. and Jusoh, M.R. 2002. Relapsed and late-onset Nipah encephalitis. Ann. Neurol. 51, 703–708. Thomas, B., Chandran, P., Lilabi, M.P., George, B., Sivakumar, C.P., Jayadev, V.K., Bindu, V., Rajasi, R.S., Vijayan, B., Mohandas, A. and Hafeez, N. 2019. Nipah virus infection in Kozhikode, Kerala, South India, in 2018: epidemiology of an outbreak of an emerging disease. Indian J. Community Med. 44, 383–387. Uchida, S., Horie, R., Sato, H., Kai, C. and Yoneda, M. 2018. Possible role of the Nipah virus V protein in the regulation of the interferon beta induction by interacting with UBX domain-containing protein1. Sci. Rep. 8, 7682. Walpita, P., Cong, Y., Jahrling, P.B., Rojas, O., Postnikova, E., Yu, S., Johns, L. and Holbrook, M.R. 2017. A VLP-based vaccine provides complete protection against Nipah virus challenge following multiple-dose or single-dose vaccination schedules in a hamster model. Npj Vaccines 2, 1–9. Wang, L.-F. and Daniels, P. 2012. Diagnosis of Henipavirus infection: current capabilities and future directions. In Henipavirus: ecology, molecular virology, and pathogenesis. Eds., Lee, B., Rota, P.A. Current topics in microbiology and immunology. Berlin, Germany: Springer, pp: 179–196. Weingartl, H., Czub, S., Copps, J., Berhane, Y., Middleton, D., Marszal, P., Gren, J., Smith, G., Ganske, S., Manning, L. and Czub, M. 2005. Invasion of the central nervous system in a porcine host by Nipah virus. J. Virol. 79, 7528–7534. Williamson, M.M., Hooper, P.T., Selleck, P.W., Gleeson, L.J., Daniels, P.W., Westbury, H.A. and Murray, P.K. 1998. Transmission studies of Hendra virus (equine morbillivirus) in fruit bats, horses and cats. Aust. Vet. J. 76, 813–818. Williamson, M.M., Hooper, P.T., Selleck, P.W., Westbury, H.A. and Slocombe, R.F. 2000. Experimental hendra virus infectionin pregnant guinea-pigs and fruit Bats (Pteropus poliocephalus). J. Comp. Pathol. 122, 201–207. Wu, Z., Yang, L., Yang, F., Ren, X., Jiang, J., Dong, J., Sun, L., Zhu, Y., Zhou, H. and Jin, Q. 2014. Novel Henipa-like Virus, Mojiang Paramyxovirus, in Rats, China, 2012. Emerg. Infect. Dis. 20, 1064–1066. Yoneda, M., Georges-Courbot, M.C., Ikeda, F., Ishii, M., Nagata, N., Jacquot, F., Raoul, H., Sato, H. and Kai, C. 2013. Recombinant measles virus vaccine expressing the Nipah virus glycoprotein protects against lethal Nipah virus challenge. PLoS One 8, e58414. | ||
| How to Cite this Article |
| Pubmed Style Fredmoore Orosco|. Advancing the frontiers: Revolutionary control and prevention paradigms against Nipah virus. Open Vet. J.. 2023; 13(9): 1056-1070. doi:10.5455/OVJ.2023.v13.i9.1 Web Style Fredmoore Orosco|. Advancing the frontiers: Revolutionary control and prevention paradigms against Nipah virus. https://www.openveterinaryjournal.com/?mno=154077 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i9.1 AMA (American Medical Association) Style Fredmoore Orosco|. Advancing the frontiers: Revolutionary control and prevention paradigms against Nipah virus. Open Vet. J.. 2023; 13(9): 1056-1070. doi:10.5455/OVJ.2023.v13.i9.1 Vancouver/ICMJE Style Fredmoore Orosco|. Advancing the frontiers: Revolutionary control and prevention paradigms against Nipah virus. Open Vet. J.. (2023), [cited January 25, 2026]; 13(9): 1056-1070. doi:10.5455/OVJ.2023.v13.i9.1 Harvard Style Fredmoore Orosco| (2023) Advancing the frontiers: Revolutionary control and prevention paradigms against Nipah virus. Open Vet. J., 13 (9), 1056-1070. doi:10.5455/OVJ.2023.v13.i9.1 Turabian Style Fredmoore Orosco|. 2023. Advancing the frontiers: Revolutionary control and prevention paradigms against Nipah virus. Open Veterinary Journal, 13 (9), 1056-1070. doi:10.5455/OVJ.2023.v13.i9.1 Chicago Style Fredmoore Orosco|. "Advancing the frontiers: Revolutionary control and prevention paradigms against Nipah virus." Open Veterinary Journal 13 (2023), 1056-1070. doi:10.5455/OVJ.2023.v13.i9.1 MLA (The Modern Language Association) Style Fredmoore Orosco|. "Advancing the frontiers: Revolutionary control and prevention paradigms against Nipah virus." Open Veterinary Journal 13.9 (2023), 1056-1070. Print. doi:10.5455/OVJ.2023.v13.i9.1 APA (American Psychological Association) Style Fredmoore Orosco| (2023) Advancing the frontiers: Revolutionary control and prevention paradigms against Nipah virus. Open Veterinary Journal, 13 (9), 1056-1070. doi:10.5455/OVJ.2023.v13.i9.1 |