| Review Article | ||
Open Vet. J.. 2023; 13(10): 1228-1238 Open Veterinary Journal, (2023), Vol. 13(10): 1228–1238 Review Article Is camel’s urine friend or enemy? Review of its role in human health or diseasesMohamed Tharwat1, Tariq I. Almundarij1,*, Madeh Sadan1,2, Faten Khorshid3,4,5 and Ayman Swelum6,71Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia 2Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt 3Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia 4PMF Natural Products Company, Al-Suez, Egypt 5Yousef Abdul Latif Jameel Scientific Chair of Prophetic Medicine Application, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia 6Department of Animal Production, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia 7Department of Theriogenology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Tariq I. Almundarij. Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia. Email: tmndrj [at] qu.edu.sa Submitted: 17/06/2023 Accepted: 03/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
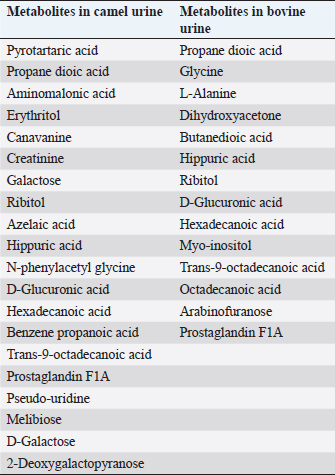
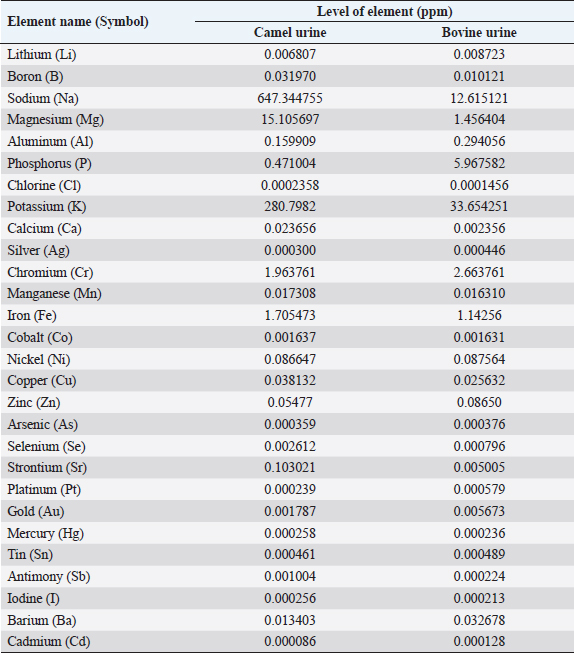
AbstractCamels play an important role in the pastoral mode of life by fulfilling basic demands of livelihood. Various pathologies, such as tuberculosis, hemorrhoids, ascites, increased size of the abdomen, gas colic, anemia, and abdominal tumors, were treated with animal urine, including camels, horses, donkeys, sheep, goats, elephants, and buffalo. Thirty different compounds were analyzed in camel urine by gas chromatography and mass spectrometry. For inductively coupled plasma mass spectrometry analysis, 28 important elements were analyzed in the urine of both camel and bovine. It was found that the inorganic elements are almost similar, except sodium, potassium, iron, zinc, and magnesium are higher in levels in camel urine, while chromium is high in bovine urine. Camel urine also contains different nanoparticles, crystals, and nano-rods with varying shapes and sizes, which offer potent selective cytotoxic activity against several lines of cancer cells. It is believed that the camel’s urine has a therapeutic effect for a wide range of diseases such as chill, fever, or even tumors; therefore, it has been consumed in the Arabian Peninsula for a long time. Usually, patients take it directly or by mixing a few drops with camel milk. Camel urine is also used for therapeutic purposes, most widely in Asia, Africa, the United States, the United Kingdom, and other European countries. The religious aspect of using camel urine in treatment comes from the fact that there has been convincing evidence that the Prophet Mohammad (PBUH) suggested the use of camel urine to treat his companions who were suffering from abdominal pains at that time. The camel’s urine has anti-diabetic, anti-cancer, antibacterial, antiviral, and antifungal properties. It also has hepato-protective and cardiovascular effects. Keywords: Antibacterial, Anti-cancer, Disease, Pathology, Pathophysiology. IntroductionCamels are working animals suited to the desert habitat and are vital in transporting passengers and cargo; they play an important role in the pastoral mode of life by fulfilling basic demands of livelihood. For a long time, camel has been domesticated and provides food such as milk and meat; also, camels provide fiber and felt from hair for textiles. One-humped dromedary camel (Camelus dromedarius) consists about 94% of the world’s camel population; this species is the most famous species of three species of camel, whereas the two-humped Bactrian camel (Camelus bactrianus) forms about 6%. The total camel population was estimated to be 35 million head (FAO, 2013). In the Arab world, the total dromedary population is higher than 1.5 million camels, of which about 53% are living in Saudi Arabia (Abdallah and Faye, 2012). The natural habitats of the dromedary camel and Bactrian camels are hot deserts and cold deserts, respectively (Kula and Tegegne, 2016; Mihic et al., 2016). The wild Bactrian camel is a separate species and is now critically endangered (Chuluunbat et al., 2014; Burger et al., 2019). Camel constitutes cultural, literary, heritage, and civilizational legacies in the Gulf region, besides being used for milk and meat production as well as for transportation. These camels gained their importance in Saudi Arabia because they can live and reproduce under severe harsh climatic conditions of heat and drought, which are not suitable for the survival of other domestic animals (Muyldermans, 2013). Camel’s urine seems like a thick syrup, and camel feces are so dry (Davidson and Jane, 2006). During the past decade, our research group has focused extensively on examination, biopsy, and kidney diseases in dromedary camels (Tharwat et al., 2012a and 2012b, 2017, 2018a and 2018b; Tharwat and Al-Sobayil, 2016; Tharwat, 2020, 2021, 2023a, b, 2024; Almundarij and Tharwat, 2023). The immune system of camels is advanced and differs from other mammals. The antibody molecules of camels consist of two heavy long and two light short chains. In addition, the two heavy chains were discovered in 1993, and they were the main cause of the small size and robust nature of camel antibodies (Koenig, 2007). Camel’s milk is one of the essential food materials of desert tribes. Bedouin can feed on camel milk alone for a long time, nearly 1 month; therefore, camel milk is considered a meal itself (Bulliet, 1975). Cheese could not be manufactured from camel’s milk because rennet cannot coagulate the milk proteins to allow the collection of cruds. The curdling process could be activated by the addition of calcium phosphate and vegetable rennet, but cheese with low levels of cholesterol was produced. Butter can be obtained from camel milk after the milk is soured first, churned, and a clarifying agent is then used (Seifu, 2022). In the Netherlands, they served as warm, fresh camel milk, and camel milk ice cream. Camel meat is mainly eaten in indifferent Arabian and African countries such as the Kingdom of Saudi Arabia, Egypt, Sudan, Libya, Somalia, Djibouti, and other desert lands that use alternative forms of protein (Mukasa-Mugerwa, 1981. Camel blood is consumable in northern Kenya and acts as a source of iron, vitamin D, salts, and minerals when it is drunk with milk (Mukasa-Mugerwa, 1981). Since ancient times, camel milk and urine have been used as medicines in certain parts of Africa and Asia, and recently, scientists have had great interest in exploring the supposed antibacterial and therapeutic effects of camel products. According to previous clinical and laboratory research, camel milk is efficient on its own or when mixed with urine in the treatment of various disorders, including bacterial infections, parasitic manifestation, cancer, diabetes mellitus (DM), autism, and viral hepatitis. In addition, antiplatelet and fibrinolytic actions of camel milk and urine on the cardiovascular system were reported as prospect benefits (Khorshid, 2009; Abdel Gader and Alhaider, 2016; Swelum et al., 2021). Camel urine has been used in Iraq, Jordan, and the Arabian Peninsula for medicinal purposes for centuries as a part of medicine. Bachtiar Nasir, an Islamic scholar, advocated for the consumption of camel urine; he claimed the mixing of camel urine and milk for medicinal benefits. Whereas Abu Yusuf, a student of jurist Abu Hanifah, said that there is no harm in using camel urine for medical treatment (Williams, 1994). The safety profile of camel urine and the fraction extracted from it (PMF) (a fraction extracted from camel urine) has been investigated in animal studies (Khorshid, 2008) and in phase I in studies on healthy volunteers (Khorshid et al., 2010) for its anticancer activity. A novel curative target and successful substitutional anticancer/antiviral agent are predictable (Khorshid et al., 2005; Khorshid and Moshref, 2006). Concurrently, this promising drug (PMF) has gained many international and local awards, and it also has been granted a patent from the European patent office, No. 09162954.3 from EPO on 07/01/2015; from GCC Patent Office, No. (GC0002755) 12/30/2013 and from the US patent office, No. US 10.624,927 B2 at 21/04/2020. PMF was prepared in the manufactory of (PMF Natural Products Company) from natural product PM 701 (powder camel urine), which is a yellowish, odorless powder insoluble in water. PMF was extracted by method according to Prof. Khorshid as previously described (Khorshid et al., 2009; Mahboub et al., 2015). In addition, camel urine contains anti-cancerous agents (Al-Yousef et al., 2012). Therapeutic purposes of camel urine are used in Asia and Africa, since these locations are the largest camel habitats (Kashim et al., 2019). Previous research recorded the treatment of many diseases using camel urine, including liver cirrhosis, skin, and hair problems (Christy, 1994). In addition, it inhibits the growth of tumor cells and shrinks cancer, as well as inhibition of secondary metastases, in vitro and in vivo, in humans and animals (Lillie et al., 1993; Miller et al., 1993; Khorshid et al., 2009; Raouf et al., 2009; El-Shahawy et al., 2010). The camel urine is used for therapeutic purposes most widely in Asia and Africa, since these locations are the largest camel habitats. The religious aspect of using camel urine stems from the fact that there has been convincing evidence that the Prophet Mohammad (PBUH) suggested the use of camel urine to treat his companions who were suffering from abdominal pains at that time (Kashim et al., 2019). This review was written to shed light on the therapeutic, anticancer, antiviral, and antimicrobial characteristics of the camel’s urine in human health or diseases. Camel’s urine properties and compositionUrine is a purified sterile product of blood filtration; it is produced in mammals by the kidneys, medically referred to as plasma ultra-filtrate. Its nature is sterile, transparent, and slightly yellowish in color, and it contains water-soluble, easily excreted components, including urea, creatinine, organic acids, amino acids, ammonia, and inorganic salts. The chemical constituents of urine in camels were first studied early in the last century; it contains creatine, creatinine, urea, chlorides, hippuric acid, and total nitrogen ammonia. Interestingly, slight traces of urea and ammonia were found (Read, 1925). The toxicity and bad smell of camel’s urine are attributed to these traces of urea and ammonia. It contains mineral salts 10 times higher than human urine (Khorshid et al., 2011b, 2015; Al-Yousef et al., 2012). More details about camel’s urine composition using liquid chromatography-mass spectrometry have been recently studied. Antakly (2012) reported many metabolites in camel urine, including major constituents such as Benzoic acid, urea, creatinine, phenylacetate, citric acid, and hippuric acid. The concentration of these substances was similar to the amounts found in elephant and rat urine; the benzoate salt was greatest in camel urine. Phenol, salicylic acid, p-cresol, cinnamic acid, azelaic acid, and benzoic acid as major bioactive acids in camel urine were determined using gas chromatography-mass spectrometry (GC-MS) (Khorshid et al., 2009; Raouf et al., 2009; El-Shahawy et al., 2010; Khedr and Khorshid, 2016). The density of the urine in old camels (5–10 years old) is 1.01 to 1.07, the urea is 18 to 36 mg/dl, and keratin is 0.2 to 0.5 mg/l, while the pH value is weak to strong alkaline (Amer and Al-Hendi, 1996). Microscopic examination of the urine sample revealed the presence of calcium oxalate, phosphorus, and ammonium urate, as well as some epithelial and granular cells (Amer and Al-Hendi, 1996; Khorshid, 2008). According to the results of neutron activation analysis, camel milk, and urine contain considerable levels of sodium, zinc, and potassium; therefore, the patients with electrolyte imbalance due to diarrhea could be neutralized using camel milk and urine (Al-Attas, 2009; El-Shahawy et al., 2010). The results of GC-MS and inductively coupled plasma mass spectrometry (ICP-MS) analysis showed the presence of derivatives of fatty acid, amino acid, benzene propanoic acid, sugars, prostaglandins, and canavanine in the urinary metabolites of camels as reported by (Ahmed et al., 2015). The percentage of metabolites in camel urine is higher than those excreted in the urine of cattle, sheep, and goats. Thirty different compounds were analyzed in urine by GC-MS (Ahamad et al., 2017) (Table 1). ICP-MS analysis of both bovine and camel urine revealed 28 important elements. The inorganic elements are almost the same except sodium, potassium, iron, and magnesium, which are high in camel urine, and chromium, which is high in bovine urine (Ahamad et al., 2017) (Table 2). The cytotoxic activity of camel urine against several lines of cancer cells could be attributed to the presence of various nanoparticles, crystals, and nano-rods with varying shapes and sizes (Khorshid et al., 2011a; Alebie et al., 2017). Table 1. Metabolites were detected in camel and bovine urine by GC-MS (Ahamad et al., 2017).
Therapeutic effects of camel’s urineCamel urine is a “marvelous” medication used in Prophetic Medicine, since the pre-Islamic period when camel milk, as well as urine, were used as oral medication for various health disorders. In many countries, drinking camel urine is a common practice, and it is considered a part of alternative medicine called urotherapy or urine therapy; it is mostly worthy and widely used as alternative medicine in India and China (Khorshid, 2009; Al-Abdalall, 2010; Alhaidar et al., 2011; Gole and Hamido, 2020). Although camel urine seems unpleasant, it has been consumed widely for a long time in the Arabian Peninsula as well as in the United States, United Kingdom, and other European countries. Its use is considered an available option to manage different health disorders such as febrile conditions, colds, and tumors. People usually take it directly or occasionally mix small drops with camel milk (Abdel Gader and Alhaider, 2016). Without informing their doctors, most patients use these products concurrently with their conventional medicines, depending on the fact that the use of natural products in disease treatment has increased significantly. Therefore, understanding patients’ perceptions is essential to assist them in selecting proper natural therapeutics and protect them from any injury due to their improper knowledge about these medications (Alkhamees and Alsanad, 2017). Table 2. Inorganic constituents in freeze-dried camel urine estimated by ICP-MS (Ahamad et al., 2017).
The urine of animals, including goats, sheep, buffalo, elephants, horses, camels, and donkeys, has been used for the treatment of various health problems, such as tuberculosis, hemorrhoids, leprosy, dropsy, abdominal enlargement, flatulence, colic, anemia, and abdominal tumors have been treated with (Al-Abdalall, 2010). The use of llama urine has been reported in the Asian countries of Mongolia and China. Some studies have also reported the use of cattle urine in India and Tibet (Christy, 1994). Camel urine has been used as a prototype model of urotherapy since Avicenna (980–1037 AD) (Abdel Gader and Alhaider, 2016). Medicinal properties of both camel’s milk and urine could be attributed to the (Alhaider et al., 2012, 2013). According to (Hamers-Casterman et al., 1993), camel antibodies consist of two heavy chains, characterized by a smaller size than about one-tenth of human antibodies. Therefore, they have the ability to be transmitted through the milk of the lactating camel and also crossing of the blood–brain barrier or to be filtered in the urine, which is contributed to their small size. In addition, it can cross the intestine into the general circulation of individuals who consume camel’s milk and/or urine (Hamers-Casterman et al., 1993). The single antigen-binding domains of heavy-chain antibodies (Nano bodies) could be used in the development of biosensors, as well as in the diagnosis and treatment of cancer (Muyldermans et al., 2009). Anti-diabetic actionDM is a metabolic disorder caused by insulin deficiency, or resistance of tissues to its effect, resulting in disturbances of carbohydrate, lipid, and protein metabolism, leading to an increase of blood glucose level; it also affects human health and in progress causing general weakness and alteration in the metabolism of fats, protein, and carbohydrates (Ha et al., 2016). The incidence of diabetes has increased in recent years mostly due to the increased rate of prevalence of obesity in individuals, as well as to changes in lifestyle (Wu et al., 2018). The number of diabetes patients may increase to over 640 million by the year 2040 (Lau et al., 2019). In rats with experimentally induced DM with alloxan, it was found that the onset of diabetic complications could be delayed in diabetic rats treated with camel milk or urine (Labbo et al., 2020). In the later study, the serum activities of aspartate aminotransferase and alanine aminotransferase, and the serum concentration of total protein were significantly reduced compared to non-treated rats. In addition, in treated rats with induced DM, decreases in serum of triglycerides and low-density lipoprotein cholesterol and increases in high-density lipoprotein cholesterol were detected also compared to non-treated diabetic rats (Labbo et al., 2020). Anti-cancer propertiesCancer is a major disease all over the world. Each year, thousands of new patients are recorded with high mortality rates (Stewart and Wild, 2014). Diagnosis and treatment of cancer are great challenges in medical practice; there are no available medical modalities that can selectively destroy tumor cells without causing any injury to normal tissues or the functions of important organs. The medicinal claim of camel urine has been put under scrutiny, and with promising results in recent years, it is reported that camel urine can destroy many neoplastic cells at an acceptable dose (Khorshid et al., 2011b; Alghamdi and Khorshid, 2012; Al-Yousef et al., 2012). Functional food plays an important and useful role in health improvement, and it is considered a novel model for controlling health disorders such as tumors (Kontou et al., 2011). Despite being a waste product, camel urine is shown to be effective in treating numerous cancers in humans (Alhaider et al., 2011). Several researches were carried out on the safety camel’s urine profile. According to the results of this research, camel’s urine is safe for humans, and it has no hepatotoxic or nephrotoxic effects under all experimental conditions (Khorshid, 2008; Khorshid et al., 2010, 2015). In Saudi Arabia, a series of in vitro experiments were carried out by a group of researchers; the results of these experiments demonstrate that lyophilized camel urine could suppress cancer cells development (Khorshid et al., 2005; Khorshid and Moshref, 2006; Mushref, 2006). The camel urine and its fraction PMF inhibited malignant cell growth, including brain carcinoma (glioma) (U251), colon carcinoma (HCT116), hepatocellular carcinoma (HEPG2), lung cancer, and leukemia (Khorshid et al., 2009, 2011b). The anticancer action of camel’s urine could be attributed to direct cell cytotoxicity or via an antiangiogenic mode of action through restriction of the blood supply to the tumor cells (Alghamdi and Khorshid, 2012) or combines pH–triggered (Raouf et al., 2009). Later, the observations of Prof Khorshid were proved by conducting a number of experiments using camel urine (3 patents), camel urine, and the fraction extracted from it (PMF) contains in its structures trace elements such as copper and zinc. Many studies mentioned the role of Zn in vital body processes, such as regulating the immune system and cell metabolism. Zn also works as an antioxidant for free radicals that damage the cells by blocking the action of activated oxygen atoms (El-Shahawy et al., 2010; Ali et al., 2011). In the murine sponge implant model, the camel milk and urine suppressed the inflammatory angiogenesis (Alhaider et al., 2014a and 2014b). Moreover, further studies reported that the anticancer properties of camel urine are through considerable suppression of the expression of the gene encoding carcinogen-activating enzyme Cyp1a1 at the mRNA level in cancerous liver cells (Alhaider et al., 2011). Furthermore, camel’s milk has some apoptotic anticancer properties (Korashy et al., 2012). Prof Khorshid team showed that camel urine and its fraction PMF contain many organic and inorganic molecules such as calcium oxalate, cystine, tyrosine, uric acid crystals, ammonium urate, and calcium phosphate. In addition to glycine, alanine, and arginine, the previous studies mentioned that the receptor tyrosine-specific protein kinases are an important cell-surface growth-factor receptor, whereas ligand-controlled tyrosine-kinase activity. Therefore, the functions in normal cells were regulated with a vital role in oncogenesis. Tyrosine kinases play an important role in the modulation of growth factor signaling. Glycine and cyctine are made the antioxidant in the body of glutathione; lacking molecules caused the accumulation of reactive oxygen species and affected the immune system. Thus, camel urine and its active fraction PMF have strong selectivity by the presence of tyrosine, which targets only cancer cells, and the presence of glycine and cyctine are enhanced the immune system to fight the cancer cells (El-Shahawy et al, 2010; Ahmed et al., 2015). Although, the anticancer actions of the exact constituents of camel’s milk or urine are unknown, some research findings demonstrated that it could be attributed to the iron-binding, multifunctional protein lactoferrin (Kanwar et al., 2015). Chemotherapy is used as traditional therapy for breast cancer; it suppresses the onset of cancer and the progressive character of its cells, but it has severe side effects such as recrudesce and pain (EBCTCG, 2005). In order to be considered an applicable option for cancer treatment, it has to not only destroy or suppress the growth of the neoplastic cells, but also it needs to stop the metastasis of cancer. Metastasis means the migration of cancer cells to other organs or secondary sites in the body. 90% of mortalities in patients suffering from cancer occur due to metastasis rather than the primary tumor (Deryugina and Quigley, 2006). The anticancer activity of camel urine (PM701) on breast cancer cells was studied by (Khorshid, 2011); the fractions PMF and PMFK that were isolated from PM 701 were proved to have cytotoxic activity against breast cancer, which significantly inhibited the proliferation of MCF7 cells and induced apoptosis by direct effect on the nuclei. The effects of camel urine were proven to inhibit the metastatic ability and growth potential of neoplastic cells in vitro and in vivo. The results also revealed the cytotoxic effect of camel urine against 4T1 cells with dose-dependent. In addition, the antimetastatic effect of camel urine has the ability to inhibit the metastatic process of the 4T1 cells. In vivo treating mice inoculated with 4T1 cells with different doses of camel urine indicated that the tumor had reduced in size in all treated groups as compared to the control group (Romli et al., 2017). The killing effect of camel urine on 4T1 cells could be attributed to DNA fragmentation and regulation of inflammation-related genes (Romli et al., 2017). Hepato-protective effectsIn a study on the effects of camel urine therapy and its hepato-protective effects against carbon tetrachloride (CCl4) induced liver toxicity in rats, it was found that rats given CCL4 with camel urine had a significant improvement in liver cells histopathology and liver enzymes compared to rats given only CCL4 (Khorshid et al., 2016; Mahmoud et al., 2019). In the later study, it was concluded that camel urine is characterized by anti-oxidative, anti-free radical, and scavenging activities of its volatile metabolites and inorganic essential elements. Therefore, it reverses liver dysfunction (Al-Attas et al., 2015; Mahmoud et al., 2019). Antibacterial and antifungal effects of camel’s urineSimilarities have been found between the effects of camel urine on cancer cells and bacterial cells. Rabbit liver tissue infected with Escherichia coli was treated using camel urine in concentrations of up to 100%, and no histopathological effects were reported (Khalifa et al., 2005). Furthermore, other researchers stated that camel urine has strong antimicrobial activity against various pathogenic microorganisms including bacteria (e.g., Staphylococcus aureus and E. coli) and fungi (e.g., Aspergillus niger, Aspergillus flavus, and Candida albicans) (Shoeib and Ba-Hatheq, 2007; Al-Abdalall, 2010; Al-Bashan, 2011; Mostafa and Dwedar, 2016). The antimicrobial properties of camel urine and its fraction PMF were determined against the Gram-positive and Gram–negative bacteria and dermatophytic fungi. The antimicrobial activity of PMF is higher than that of camel urine itself. Its inhibition zone is up to 32 mm with MIC <100 μg/ml, compared with low antimicrobial activity (< 10 mm inhibition zone) with MIC of 100–150 μg/ml of the camel urine (El-Shahawy et al., 2010; Ali et al., 2011). Camel urine has antibacterial properties in vitro, against multidrug-resistant bacteria, as well as strong antifungal activity against C. albicans and Nonalbicans Candida (Mostafa and Dwedar, 2016). In accordance with these findings, the antibiotic and suppressive effects of camel urine against various fungi, such as C. albicans (yeast), A. niger, and F. oxysporum, even after boiling to 100°C, were demonstrated by AL-Awadi and AL-Jedabi (2000), in addition to a suppression activity on the dry weights of yeast and the fungi (Al-Awadi and Al-Jedabi, 2000). Camel’s urine has a high antifungal effect against mycoses-causing dermatophytes, with high performance better than the synthetic drug fluconazole and almost similar to ketoconazole (Kabbashi and Omer, 2016). Shoeib and Ba-Hatheq (2008) evaluated the action of camel’s urine on the morphological properties of some human pathogens. They found that the chemical and organic constituents of urine suppressed the development of bacteria and fungi. Furthermore, treatment with fresh camel’s urine had bactericidal properties against P. aeruginosa and E. coli, in addition to causing loss of the bacterial plasmids. On the other hand, the bacterial cells did not lose their resistance against various antibiotics. In addition, stored camel urine did not have an effect on the viability of bacterial cells when compared to the control treatment. These studies demonstrated that continuous exposure of the bacterial cells to camel urine leads to no disintegration (bacteriolysis after death) (Höltje, 1998) due to loss of plasmids from the bacterial cell (Al-Abdalall, 2010). Antibacterial and antifungal effects of camel urine could be attributed to the presence of a high concentration of salt and its high alkalinity in addition to the presence of natural bioactive components gained from the plants consumed by the camels, along with some resident bacteria and excreted antimicrobial agents. The camel urine is alkaline, unlike other animal urine; it contains high concentrations of magnesium, potassium, and albuminous protein and low levels of uric acid, sodium, and creatine (Kamalu et al., 2004; Mostafa and Dwedar, 2016). Antiviral effects of camel’s urineCamel’s milk gives much better results than mare milk when treating chronic hepatitis (Sharmanov et al., 1981). In addition, bilharzial liver disease could be effectively treated using camel urine, and it was found that the patients recovered at a better rate and had considerable changes in their cirrhotic livers (Ohag et al., 1998). The camel urine has potential antiviral activities; in-vitro PMF (a fraction extracted from camel urine) treatment of Middle East Respiratory Syndrome Corona (MERS-CoV) and Influenza A (H1N1) viral infections showed a significant reduction in the titers of the two viruses across time, in addition to the absence of a role of camel urine in MERS-CoV transmission to humans (Al Attas et al., 2019). There was no evidence of shedding of MERS-CoV RNA in urine; even camels had evidence of an acute MERS-CoV infection at the time of urine sampling (Adney et al., 2014; Ali et al., 2017; Farag et al., 2019). Cardiovascular activityThere are powerful cardiovascular activities exhibited by both camel urine and milk; Lactating camel urine exhibits a strong repression reaction on human platelet aggregation. Strong platelet-blocking properties of camel urine have been determined, and they are similar to the effects of commonly used antiplatelet drugs, such as clopidogrel and aspirin. Furthermore, the strongest antioxidants, such as uric acid and creatinine, are found in camel urine (Al-Harbi et al., 1996; Alhaider et al., 2011; Alyahya et al., 2016; Abdel Gader and Alhaider, 2016). It has not yet been assured whether the function of camel urine and that of aspirin are similar or not or if it works with the prostaglandin pathway (Agrawal et al., 2004; Alhaider et al., 2011; Malik et al., 2012). Camel’s milk also has a strong thrombolytic effect because it decreases the plasma fibrinogen level of diabetic rats (Korish et al., 2015). The claimed anticancer activity of camel’s milk and urine could be attributed to the previously detected antiplatelet action, since the suppression of fibrin formation and coagulation prevent the expansion and development of metastatic tumor cells (Abdel Gader and Alhaider, 2016). Camel urine can also be used as a bronchodilator because it possesses anticholinergic characteristics, as was reported by another Iranian research (Zibayi et al., 2015). ConclusionCamel’s urine is different in composition from bovines. It contains 30 different compounds and 28 important elements. The inorganic elements in camel, including sodium, potassium, iron, zinc, and magnesium, are high in camel urine compared to bovine urine. Camel urine also contains different nanoparticles, crystals, and nano-rods with varying shapes and sizes, which offer potent selective cytotoxic activity against several lines of cancer cells. It was proved that camel’s urine has anti-diabetic, anti-cancer, antibacterial, antiviral, and antifungal properties. It also has hepato-protective and cardiovascular effects. More research and long-term follow-up clinical studies are warranted to clarify the exact pharmacological effects of camel urine on several human diseases and the recommended doses and treatment duration. Further studies are needed to compare the physical and chemical properties and composition of urine in camels of different physiological statuses under different environmental, nutritional, and management conditions. In addition, further studies are needed to ensure that all camel urines have therapeutic and antimicrobial characteristics or if the therapeutic and antimicrobial characteristics are related only to the urine of some camel with specific physiological and nutritional status. Additional studies are also warranted to evaluate the effect of camel urine on the immunity and health condition of apparently healthy humans. AcknowledgmentsResearchers would like to thank the Deanship of Scientific Research, Qassim University, for funding the publication of this project. Conflict of interestThe authors declare that there is no conflict of interest. FundingPublication of this research was funded by the Deanship of Scientific Research, Qassim University, Saudi Arabia. Author contributionsMT and MS concept and design the proposal. MS, TA, and MT collect the required data. MS, TA, AS, FKH, and MT analyzed the collected data and wrote the manuscript. All authors revised and approved the final manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript and no additional data sources are required. ReferencesAbdallah, H. and Faye, B. 2012. Phenotypic classification of Saudi Arabian camel (Camelus dromedarius) by their body measurements. Emir. J. Food. Agric. 24, 272–280. Abdel Gader, A.M. and Alhaider, A.A. 2016. The unique medicinal properties of camel products: a review of the scientific evidence. J. Taibah. Univ. Med. Sci. 11, 98–103. Adney, D.R., van Doremalen, N., Brown, V.R., Bushmaker, T., Scott, D. and de Wit, E. 2014. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerging. Infect. Dis. 20, 1999–2005 Agrawal, R., Kochar, D., Sahani, M., Tuteja, F. and Ghorui, S. 2004. Hypoglycemic activity of camel milk in streptozotocin induced diabetic rats. Int. J. Diab. Dev. Countries. 24, 47–49. Ahamad, S.R., Alhaider, A., Raish, M. and Shakeel, F. 2017. Metabolomic and elemental analysis of camel and bovine urine by GC–MS and ICP–MS. Saudi. J. Biol. Sci. 24, 23–29. Ahmed, G.A.R., Faten, A.K., Alaa, K., Salem, M. and Numan, A.S. 2015. The effect of PMF camel urine nanoparticles on A549 cells: the mechanism of action and drug delivery. Life. Sci. J. 12, 63–75. Al-Abdalall, A.H.A. 2010. The inhibitory effect of camel’s urine on mycotoxins and fungal growth. Afr. J. Agric. Res. 5, 1331–1337. Al-Attas, A. 2009. Determination of essential elements in milk and urine of camel and in Nigella sativa seeds. Arab. J. Nucl. Sci. Appl. 42, 59–67. Al-Attas, S.G., Khorshid, F.K., Elsourojy, Y.A., Noor, S.O. and Tawfik, N. In-vitro evaluation of cytotoxicity, antiviral and virostatic activity of PMF derived from camel urine. International Conference on Biology and Biomedical Engineering. In Proceedings of 2015 the International Conference on Energy, Environment and Material Science, 2015, pp 72–76. Al Attas, S.A., Khorshid, F.A., Kao, M. and Bahieldin, A. 2019. Antiviral activity of extracted fraction from camel urine against corona and influenza A (H1N1) viruses. Appl. Ecol. Environ. Res. 17, 11023–11031. Al-Awadi, A. and Al-Jedabi, A. 2000. Antimicrobial agents in camel’s urine. JUAB 9, 265–281. Al-Bashan, M.M. 2011. In vitro assessment of the antimicrobial activity and biochemical properties of camel’s urine against some human pathogenic microbes. Middle. East. J. Sci. Res. 7, 947–958. Alebie, G., Yohannes, S. and Worku, A. 2017. Therapeutic applications of camel’s milk and urine against cancer: current development efforts and future perspectives. J. Cancer. Sci. Ther. 9, 5. Alghamdi, Z. and Khorshid, F. 2012. Cytotoxicity of the urine of different camel breeds on the proliferation of lung cancer cells, A549. J. Nat. Sci. Res. 2, 9–16. Alhaider, A.A., Abdel Gader, A.G.M., Almeshaal, N. and Saraswati, S. 2014a. Camel milk inhibits inflammatory angiogenesis via downregulation of proangiogenic and proinflammatory cytokines in mice. APMIS 122, 599–607. Alhaider, A.A., Abdel Gader, A.G.M., Almeshal, N. and Saraswati, S. 2014b. Camel urine inhibits inflammatory angiogenesis in murine sponge implant angiogenesis model. Biomed. Aging. Pathol. 4, 9–16. Alhaidar, A., Abdel Gader, A.G.M. and Mousa, S.A. 2011. The antiplatelet activity of camel urine. J. Altern. Complement. Med. 17, 803–808. Alhaider, A.A., Bayoumy, N., Argo, E., Gader, A.G. and Stead, D.A. 2012. Survey of the camel urinary proteome by shotgun proteomics using a multiple database search strategy. Proteomics 12, 3403–3406. Alhaider, A.A., El-Gendy, M.A., Korashy, H.M. and El-Kadi, A.O. 2011. Camel urine inhibits the cytochrome P450 1a1 gene expression through an AhR-dependent mechanism in Hepa 1c1c7 cell line. J. Ethnopharmacol. 133, 184–190. Alhaider, A., Murray, K., Abdelgader, A., Kiemele, L., Hansen, K., Shan, B., Ma, B., Hunsucker, S. and Duncan, M. 2013. Identification of the peptides & proteins in the milk of the one humped camel (Camelus dromedarius) by mass spectrometry. J. Mass. Spectros. 48, 77. Al-Harbi, M., Qureshi, S., Ahmed, M., Raza, M., Baig, M. and Shah, A. 1996. Effect of camel urine on the cytological and biochemical changes induced by cyclophosphamide in mice. J. Ethnopharmacol. 52, 129–137. Ali, A., Khorshid, F., Aboarky, H. and Osman, A.M. 2011. Tumor lung cancer model for assessing anti-neoplastic effect of PMF in rodents: histopathological study. Trends. Appl. Sci. Res. 6, 1214. Ali, M.A., Shehata, M.M., Gomaa, M.R., Kandeil, A., El-Shesheny, R. and Kayed, A.S. 2017. Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg. Microbes. Infect. 6(1), e1. Alkhamees, O.A. and Alsanad, S.M. 2017. A review of the therapeutic characteristics of camel urine. Afr. J. Tradit. Complement. Altern. Med. 14, 120–126. Almundarij, T. and Tharwat, M. 2023. Impact of intestinal and urinary tracts obstruction on oxidative stress biomarkers in dromedary camels. Int. J. Vet. Sci. 12(3), 422–427. Alyahya, A.M., Gader, A.G.M.A. and Alhaider, A.A. 2016. Characterization of inhibitory activity of camel urine on human platelet function. J. Taibah. Univ. Med. Sci. 11, 26–31. Al-Yousef, N., Gaafar, A., Al-Otaibi, B., Al-Jammaz, I., Al-Hussein, K. and Aboussekhra, A. 2012. Camel urine components display anti-cancer properties in vitro. J. Ethnopharmacol. 143, 819–825. Amer, H. and Al-Hendi, A. 1996. Physical, biochemical and microscopically analysis of camel urine. J. Camel. Pract. Res. 3, 17–21. Antakly, T. 2012. Bioactive compounds in camel urine and milk. WO Patents WO2012019295A1. Bulliet, R.W. 1975. The camel and the wheel. New York, NY: Columbia University Press, pp: 23, 25, 28, 35–36, 38–40. Burger, P.A., Ciani, E. and Faye, B. 2019. Old world camels in a modern world—a balancing act between conservation and genetic improvement. Anim. Genet. 50, 598–612. Christy, M.M. 1994. Your Own Perfect Medicine, Self-Healing Press, Mesa, Arizona, USA. Chuluunbat, B., Charruau, P., Silbermayr, K., Khorloojav, T. and Burger, P.A. 2014. Genetic diversity and population structure of Mongolian domestic bactrian camels (Camelus bactrianus) . Anim. Genet. 45, 550–558. Davidson, A. and Jane, T. 2006. The oxford companion to food. Oxford, UK: Oxford University Press. Deryugina, E.I. and Quigley, J.P. 2006. Matrix metalloproteinases and tumor metastasis. Cancer. Metastasis. Rev. 25, 9–34. (EBCTCG. 2005. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365, 1687–1717. El-Shahawy, A., El-Sawi, N., Backer, W.S., Wadiah, S.B., Khorshid, F.A. and Geweely, N.S. 2010. Spectral analysis, molecular orbital calculations and antimicrobial activity of PMF-G fraction extracted from PM-701. Int. J. Pharm. Bio. Sci. 1, 1–19. FAO. 2013. Statistical year book. Rome, Italy: Food and Agriculture Organization of the United Nations. Farag, E.A., Haagmans, B.L., Al-Romaihi, H.E., Mohran, K., Haroun, M., El-Sayed, A.M., Koopmans, M., AlHajri, M. and Reusken, C.B.E.M. 2019. Failure to detect MERS-CoV RNA in urine of naturally infected dromedary camels. Zoonoses. Public. Health. 66, 437–438. Gole, F.A. and Hamido, A.J. 2020. Review on health benefits of camel urine: therapeutics effects and potential impact on public health around east hararghe district. Int. J. Pure. Appl. Biosci. 2, 183–191. Ha, W.T., Jeong, H.Y., Lee, S.Y. and Song, H. 2016. Effects of the insulin-like growth factor pathway on the regulation of mammary gland development. Dev. Reprod. 20, 179–185. Hamers-Casterman, C., Atarhouch, T., Muyldermans, S., Robinson, G., Hammers, C., Songa, E.B., Bendahman, N. and Hammers, R. 1993. Naturally occurring antibodies devoid of light chains. Nature 363, 446–448. Höltje, J.V. 1998. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 62, 181–203. Kabbashi, M.A. and Omer, F.A. 2016. In vitro antifungal activity of camel’s urine against dermatophytes. Am. J. Res. Comm. 4, 183–191. Kamalu, T., Okpe, G. and Williams, A. 2004. Mineral contents of extracellular fluids in camel and cattle in the North East Sahel region of Nigeria. Niger. Vet. J. 24, 13–20. Kanwar, J.R., Roy, K., Patel, Y., Zhou, S.F., Singh, M.R., Singh, D., Nasir, M., Sehgal, R., Sehgal, A. and Singh, R.S. 2015. Multifunctional iron bound lactoferrin and nanomedicinal approaches to enhance its bioactive functions. Molecules 20, 9703–9731. Kashim, M.A.M., Mohamad, M.N., Sukor, A.S.A., Adnan, N.I.M., Safiai, M.H. and Jamsari, E.A. 2019. Animal urine therapy according to Islamic and scientific perspectives. Int. J. Civ. Eng.10, 2280–2286. Khalifa, S., Al-Elyani, R. and Al-Alwani, A. 2005. Histological, cytological and histochemical studies on the effect of camel’s urine on liver of rabbits infected by Escherichia coli. SJBS 2, 66–80. Khedr, A. and Khorshid, F. 2016. Characterization and determination of major bioactive acids in camel urine using gas chromatography mass spectrometry. Indian. J. Pharm. Sci. 78, 680–687. Khorshid, F.A. 2008. Preclinical evaluation of PM 701 in experimental animals. Int. J. Pharmacol. 4, 443–451. Khorshid, F.A., 2009. Potential anticancer natural product against human lung cancer cells. Trends. Med. Res. 4, 8–15. Khorshid, F. 2011. The cytotoxic effect of PM 701 and its fractions on cell proliferation of breast cancer cells, McF7. Am. J. Drug. Discov. Dev. 1, 200–208. Khorshid, F., Al-Attas, S., Elsourojy, Y., Noor, S., Tawfik, N. and Alshehri, R. 2016. Evaluation of anticancer, antiviral and hepatic protective activities of PMF derived from camel urine. Int. J. Drug. Dev. Res. 1, 10–22. Khorshid, F.A. and Moshref, S.S. 2006. In vitro anticancer agent, I—tissue culture study of human lung cancer cells A549 II—tissue culture study of mice leukemia cells L1210. Int. J. Cancer. Res. 2, 330–344. Khorshid, F.A., Moshref, S.S. and Heffny, N. 2005. An ideal selective anticancer agent in vitro, I- tissue culture study of human lung cancer cells A549. Med. Sci. 12(1), 3–18. Khorshid, F.A., Osman, A.A. and Abdulsattar, E. 2009. Cytotoxicity of bioactive fractions from PM 701. Elec. J. Env. Agricult. Food. Chem. 8, 1091–1098. Khorshid, F.A., Rabah, S.O., Abuaraki, H.A., Ali, A., Noor, S.O. and Alkabkaby, H. 2015. Safety of oral administration of PMF a fraction derived from camel urine: acute study on mice. Int. J. Emerg. Technol. Adv. Eng. 5, 365–370. Khorshid, F.A., Rahimaldeen, S.A. and Alameri, J.S. 2011b. Apoptosis study on the effect of PMF on different cancer cells. Int. J. Biol. Chem. 5, 150–155. Khorshid, F., Raouf, G.A., El-Hamidy S.M., Al-Amri, G.S., Alotaibi, N.A. and Kumosani, T.A. 2011a. PMF cesium & rubidium nanoparticles induce apoptosis in A549 cells. Life. Sci. J. 8, 534–542. Khorshid, F.A., Shazly, H., Al-Jefery, A. and Osman, A.A. 2010. Dose escalation phase I study in healthy volunteers to evaluate the safety of a natural product PM 701. Int. J. Pharm. Toxic. 5, 91–97. Koenig, R. 2007. Veterinary medicine. ‘Camelized’ antibodies make waves. Science 318(5855), 1373. Kontou, N., Psaltopoulou, T., Panagiotakos, D., Dimopoulos, M.A. and Linos, A. 2011. The mediterranean diet in cancer prevention: a review. J. Med. Food. 14, 1065–1078. Korashy, H.M., Maayah, Z.H., Abdallah, A.R., El-Kadi, A.O. and Alhaider, A.A. 2012. Camel milk triggers apoptotic signaling pathways in human hepatoma HepG2 and breast cancer MCF7 cell lines through transcriptional mechanism. J. Biomed. Biotechnol. 2012, 593195. Korish, A.A., Gader, A. and Alhaidar, A.A. 2015. The effects of camel milk on platelet function and coagulation parameters in streptozotocin diabetic rats. Intern. J. Diary. Technol. 68, 7. Kula, J.T. and Tegegne, D. 2016. Chemical composition and medicinal values of camel milk. Int. J. Res. Stud. Biosci. 4, 13–25. Labbo, A.M., Sheshe, S.M., Bello, H.J., Shehu, Z. and Bello, Z.H. 2020. Biochemical and histopathological studies on the efficacy of camel milk and urine against alloxan induced diabetic rats. Asian. J. Biochem. Genet. Mol. Biol. 3, 37–45. Lau, L.H., Lew, J., Borschmann, K., Thijs, V. and Ekinci, E.I. 2019. Prevalence of diabetes and its effects on stroke outcomes: a meta-analysis and literature review. J. Diabetes. Investig. 10, 780–792. Lillie, J.W., O’keefe, M., Valinski, H., Hamlin, H.A., Varban, M.L. and Kaddurah-Daouk, R. 1993. Cyclocreatine (1-carboxymethyl-2-iminoimidazolidine) inhibits growth of a broad spectrum of cancer cells derived from solid tumors. Cancer. Res. 53, 3172–3178. Mahboub, A., Faten, A.K. and Abdul-Hamid, ME. 2015. The cytotoxic effect of small and large molecules of PMF fraction extracted from camel urine on cancer cells. Br. J. Med. Med. Res. 6, 4. Mahmoud, H.S., Elsaed, W.M. and Gabr, S.A. 2019. Camel urotherapy and hepatoprotective effects against carbon tetrachloride-induced liver toxicity. Int. J. Pharmacol. 15, 696–705. Malik, A., Al-Senaidy, A., Skrzypczak-Jankun, E. and Jankun, J. 2012. A study of the anti-diabetic agents of camel milk. Int. J. Mol. Med. 30, 585–592. Mihic, T., Rainkie, D., Wilby, K.J. and Pawluk, S.A. 2016. The therapeutic effects of camel milk: a systematic review of animal and human trials. J. Evid. Based. Complementary. Altern. Med. 21, 110–126. Miller, E.E., Evans, A.E. and Cohn, M. 1993. Inhibition of rate of tumor growth by creatine and cyclocreatine. Proc. Natl. Acad. Sci. 90, 3304–3308. Mostafa, M.S. and Dwedar, R.A. 2016. Antimicrobial activity of camel’s urine and its effect on multidrug resistant clinical bacterial and fungal isolates. Br. J. Pharm. Res. 13(4), 1–6. Mukasa-Mugerwa, E. 1981. The camel (Camelus dromedarius): a bibliographical review. International Livestock Centre for Africa Monograph 5. Addis Ababa, Ethiopia: International Livestock Centre for Africa, pp: 1, 3, 20–21, 65, 67–68. Mushref, F.K.S. 2006. In vitro anticancer agent I-tissue culture study of human lung cancer cells A549 II-tissue culture study of mice leukemia cells L1210. Int. J. Cancer. Res. 2, 330–344. Muyldermans, S. 2013. Nano bodies: natural single domain antibodies. Annu. Rev. Biochem. 82, 775–797. Muyldermans, S., Baral, T., Retamozzo, V.C., De-Baetselier, P., De-Genst, E., Kinne, J., Leonhardt, H., MAGEZ, S., Nguyen, V. and Revets, H. 2009. Camelid immunoglobulins and nanobody technology. Vet. Immunol. Immunopathol. 128, 178–183. Ohag, H., Mohamed, A., Saeed, O.K., Elawad, A., Elsayed, S., Elhussein, S. and Daffalla, G. 1998. Clinical trials for treatment of ascites with camel urine. J. Arab. Board. Med. Spec. 7, 25–29. Raouf, G.A., Khorshid, F.A. and Kumosani, T. 2009. FT-IR spectroscopy as a tool for identification of apoptosis-induced structural changes in A549 cells dry samples treated with PM 701. Int. J. Nano. Biomater. 2, 396–408. Read, B.E. 1925. Chemical constituents of camel’s urine. J. Biol. Chem. 64, 615–617. Rehan, S. and Qureshi, A. 2006. Microscopic evaluation of the heart, kidneys and adrenal glands of one-humped camel calves (Camelus dromedarius) using semi-automated image analysis system. J. Camel. Pract. Res. 13, 123. Rehan, S. and Qureshi, A.S. 2007. Morphometric analysis of heart, kidneys and adrenal glands in dromedary camel calves. Camel. Pract. Res. 14, 27. Romli, F., Abu, V., Khorshid, F.A., Najmuddin, S.U.F.S., Keong, Y.S., Mohamad, N.E., Hamid, M., Alitheen, N.B. and Abd Rahman, N.A.M.N. 2017. The growth inhibitory potential and antimetastatic effect of camel urine on breast cancer cells in vitro and in vivo. Integr. Cancer. Ther. 16, 540–555. Seifu, E. 2022. Recent advances on camel milk: Nutritional and health benefits and processing implications—A review. AIMS Agri. Food 7, 777–804. Sharmanov, T., Zhangabylov, A. and Zhaksylykova, R. 1981. Mechanism of the therapeutic action of whole mare’s and camel’s milk in chronic hepatitis. Vopr. Pitan. 1, 17–23. Shoeib, A.A. and Ba-Hatheq, A. 2007. Effect of camel´s urine on pathogenic P. aeruginosa and E. coli isolates, towards its maintains to their antibiotic(s) resistance and the presence of plasmid(s). Saudi. J. Bio. Sci. 14, 177–184. Shoeib, A. and Ba-Hatheq, A. 2008. Electromicroscopic study of camel urine effect on the morphology of some human pathogenic bacteria. Comparison with the antibiotic cefuroxime. Saudi. J. Biolo. Sci. 15, 119–125. Stewart, B. and Wild, C. 2014. World cancer report. Lyon, France: International Agency for Research on Cancer. Swelum, A.A., El-Saadony, M.T., Abdo, M., Ombarak, R.A., Hussein, E.O.S., Suliman, G., Alhimaidi, A.R., Ammari, A.A., Ba-Awadh, H., Taha, A.E., El-Tarabily, K.A. and Abd El-Hack, M.E. 2021. Nutritional, antimicrobial and medicinal properties of camel’s milk: a review. Saudi. J. Biolo. Sci. 28(5), 3126–3136. Tharwat, M. 2021. Obstructive urolithiasis in dromedary camels: clinical, ultrasonographic and postmortem findings. J. Camel. Pract. Res. 28, 85–93. Tharwat, M. 2023a. Advanced biomarkers and its usage in Arabian camel medicine—a review. J. App. Anim. Res. 51, 350–357. Tharwat, M. 2023b. Changes in acid-base balance, blood gases and hemato-biochemical parameters in Arabian camels with different urinary tract disorders. Int. J. Vet. Sci. 12, 724–729. Tharwat, M. 2024. Fundamentals of diagnostic ultrasound in dromedary camel medicine. Int. J. Vet. Sci. 13, 1–6. Tharwat, M. 2020. Ultrasonography of the kidneys in healthy and diseased camels (Camelus dromedaries). Vet. Med. Int. 2020, 7814927. Tharwat, M., Al-Sobayil, F. 2016. Ultrasonographic findings in camels (Camelus dromedarius) with different urinary affections. J. Camel. Pract. Res. 23, 301–308. Tharwat, M., Al-Sobayil, F., Ali, A. and Buczinski, S. 2012a. Ultrasonography of the liver and kidneys of healthy camels (Camelus dromedarius). Can. Vet. J. 53, 1273–1278. Tharwat, M., Al-Sobayil, F., Ali, A., Derar, D. and Khodeir, M. 2017. Renal cell carcinoma in a female aranian camel: clinical, hematobiochemical, ultrasonographic and pathologic findings. J. Camel. Pract. Res. 24, 61–66. Tharwat, M., Al-Sobayil, F. and Buczinski, S. 2012b. Ultrasound-guided hepatic and renal biopsy in camels (Camelus dromedarius): technique development and assessment of the safety. Small. Rumin. Res. 103, 211–219. Tharwat, M., Sadan, M., El-Shafaey, E., Al-Hawas, A. and Saeed, E.M.A. 2018a. Unilateral nephrectomy in a female dromedary camel with pyelonephritis caused by Staphylococcus lugdunensis. Pak. Vet. J. 38, 116–118. Tharwat, M., Sadan, M., El-Shafaey, E., El-hassan, S.E. and Al-hawas, A. 2018b. Bilateral renal abscessation and chronic active pyelonephritis in a male camel (Camelus dromedarius) caused by Escherichia coli. J. Vet. Med. Sci. 80, 778–783. Williams, J.A. 1994. The word of Islam. Austin, TX: University of Texas Press. Wu, C., Tsai, Y., Lin, J., Fu, S. and Lai, J. 2018. Chinese herbal products and the reduction of risk of breast cancer among females with type 2 diabetes in Taiwan: a case-control study. Medicine (Baltimore) 97, 31. Zibayi, S., Hosseini, S.M. and Anooshiravani, M. 2015. Anti-allergic and anti-cancer properties of camel milk and urine. Avicenna. J. Phytomed. 5, 20–21. | ||
| How to Cite this Article |
| Pubmed Style Tharwat M, Almundarij TI, Sadan M, Khorshid F, Swelum A. Is camel’s urine friend or enemy? Review of its role in human health or diseases. Open Vet. J.. 2023; 13(10): 1228-1238. doi:10.5455/OVJ.2023.v13.i10.1 Web Style Tharwat M, Almundarij TI, Sadan M, Khorshid F, Swelum A. Is camel’s urine friend or enemy? Review of its role in human health or diseases. https://www.openveterinaryjournal.com/?mno=157867 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i10.1 AMA (American Medical Association) Style Tharwat M, Almundarij TI, Sadan M, Khorshid F, Swelum A. Is camel’s urine friend or enemy? Review of its role in human health or diseases. Open Vet. J.. 2023; 13(10): 1228-1238. doi:10.5455/OVJ.2023.v13.i10.1 Vancouver/ICMJE Style Tharwat M, Almundarij TI, Sadan M, Khorshid F, Swelum A. Is camel’s urine friend or enemy? Review of its role in human health or diseases. Open Vet. J.. (2023), [cited January 25, 2026]; 13(10): 1228-1238. doi:10.5455/OVJ.2023.v13.i10.1 Harvard Style Tharwat, M., Almundarij, . T. I., Sadan, . M., Khorshid, . F. & Swelum, . A. (2023) Is camel’s urine friend or enemy? Review of its role in human health or diseases. Open Vet. J., 13 (10), 1228-1238. doi:10.5455/OVJ.2023.v13.i10.1 Turabian Style Tharwat, Mohamed, Tariq I Almundarij, Madeh Sadan, Faten Khorshid, and Ayman Swelum. 2023. Is camel’s urine friend or enemy? Review of its role in human health or diseases. Open Veterinary Journal, 13 (10), 1228-1238. doi:10.5455/OVJ.2023.v13.i10.1 Chicago Style Tharwat, Mohamed, Tariq I Almundarij, Madeh Sadan, Faten Khorshid, and Ayman Swelum. "Is camel’s urine friend or enemy? Review of its role in human health or diseases." Open Veterinary Journal 13 (2023), 1228-1238. doi:10.5455/OVJ.2023.v13.i10.1 MLA (The Modern Language Association) Style Tharwat, Mohamed, Tariq I Almundarij, Madeh Sadan, Faten Khorshid, and Ayman Swelum. "Is camel’s urine friend or enemy? Review of its role in human health or diseases." Open Veterinary Journal 13.10 (2023), 1228-1238. Print. doi:10.5455/OVJ.2023.v13.i10.1 APA (American Psychological Association) Style Tharwat, M., Almundarij, . T. I., Sadan, . M., Khorshid, . F. & Swelum, . A. (2023) Is camel’s urine friend or enemy? Review of its role in human health or diseases. Open Veterinary Journal, 13 (10), 1228-1238. doi:10.5455/OVJ.2023.v13.i10.1 |