| Research Article | ||
Open Vet. J.. 2023; 13(10): 1268-1276 Open Veterinary Journal, (2023), Vol. 13(10): 1268–1276 Original Research Possible role of pomegranate fruit in reversing renal damage in rats exposed to PhenylhydrazineNabil Abbas Soliman1,*, Sherif Wajih Mansour2, Mohamed Ahmed Ammar3, Noura Ahmed Hassan4 and Rehab Hamed Abdallah Mohamed51Department of Zoology, Faculty of Science, Zagazig University, Sharkia, Egypt 2Department of Physiology, Faculty of medicine, Zagazig University, Sharkia, Egypt 3Department of Zoology, Faculty of Science, Zawia University, Al Zawia City, Libya 4Department of Pharmacology, Faculty of Pharmacy, Zagazig University, Sharkia, Egypt 5Department of Pharmacognosy, Faculty of Pharmacy, Zagazig University, Sharkia, Egypt *Corresponding Author: Nabil Abbas Soliman. Department of Zoology, Faculty of Science, Zagazig University, Sharkia, Egypt. Email: nabilsoliman54 [at] yahoo.com Submitted: 07/07/2023 Accepted: 10/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
AbstractBackground: Pomegranate granatum (molasses and peels) and its constituents showed protective effects against natural toxins such as phenylhydrazine (PHZ) as well as chemical toxicants such as arsenic, diazinon, and carbon tetrachloride. Aim: The current study aimed to assess the effect of pomegranate molasses (PM), white peel extract, and red peel extract on nephrotoxicity induced by PHZ. Methods: 80 male rats were divided into eight equal groups; a control group, PM pure group, white peel pomegranate pure group, red peel pomegranate pure group, PHZ group, PM + PHZ group, white peel pomegranate + PHZ group and red peel pomegranate + PHZ group. Kidney function, inflammation markers, antioxidant activities, and renal tissue histopathology were investigated. Results: The results revealed that PHZ group showed a significant increase in lactate Dehydrogenase (LDH), malondialdehyde (MDA), creatinine, uric acid, BUNBUN, C - reactive protein (CRP), tumor necrosis factor, thiobarbituric acid reactive substances (TBARSs), and total antioxidant capacity (TAC) with a significant decrease of catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) as compared with a control group. Other pomegranate-treated and PHZ co-treated groups with pomegranate showed a significant decrease of LDH, MDA, creatinine, uric acid, BUN, tumor necrosis factor, TBARSs, and TAC with a significant increase of CAT, GPx, and SOD as compared with PHZ group. Conclusion: Collectively, our data suggest that red, white peels, and molasses have anti-toxic and anti-inflammatory effects on renal function and tissues. Keywords: Peel pomegranate, Molasses, Phenylhydrazine, Thiobarbituric acid reactive substances, Total antioxidant capacity. IntroductionPlant extracts may be a significant replacement for synthetic antioxidants in the oil sector. Pomegranate peel is regarded as a cheap, plentiful, and enhanced source of functional components as well as a sustainable source for the extraction of polyphenolic and flavonoid chemicals with excellent antioxidant ability (El-Hadary and Taha, 2020). Methanol and aqueous extracts of pomegranate peels (Punica granatum L.) from two Egyptian varieties (Wardey and Manfalouty) were screened for phenolic compounds and antioxidant activities. Amounts of phenolic compounds in methanol peel extracts were higher than those in water extracts. The relative contents of phenolic compounds displayed variability as Manfalouty contained a higher percentage of phenolic compounds for protocatchoic acid, P-cumaric acid, chlorogenic acid, catechin, epicatechin, and ellagic acid. However, Wardey peel contained a higher percentage of phenolic compounds for vanillic acid, caffeic acid, and ferulic acid. Antioxidant activities of peel extracts by the β-carotene-linoleate model system showed that inhibition values of methanol in both peel extracts exhibited higher values than water extracts (Zaki et al., 2015). It was reported that pomegranate molasses (PM) was used as a condiment in Turkish cuisine and believed to have significant effects on arteriosclerosis, cholesterol levels, and cancer prevention due to the antioxidant potential of pomegranate fruit itself. In addition, the antioxidant activity of PM depends on several factors, such as cultivar and climatic conditions during fruit maturation and ripening and the part of the fruit used (Akpinar-Bayizit et al., 2016). The high antioxidant and nephropreventive effect of the pomegranate peel extract was attributed to its high phenolic content (Ahmed and Ali, 2010). PHZ intoxication causes oxidative damage to erythrocytes, resulting in hemolytic anemia with the involvement of the spleen and liver. It damages the cell membrane, producing gradual hematological alterations, inflammatory mediators, and increased red cell apoptosis. PHZ was reported to cause lipid peroxidation in the liver, kidney, and spleen of mice. Moreover, PHZ intoxication was reported to increase serum urea concentration and cause histopathological alterations in the kidneys of Wistar rats. In addition, oxidative stress-induced hemodynamic disturbance and vascular dysfunction, as well as derangement in electrolytes, were reported to occur following PHZ intoxication. Decreased glomerular filtration rate, albuminuria, and proteinuria were also reported to occur following hemolysis (Amama et al., 2022). Therefore, the objective of this study was to study the protective effect of pomegranate fruit in reversing renal damage in rats exposed to phenylhydrazine (PHZ), through evaluating LDH, MDA, creatinine, uric acid, blood urea nitrogen (BUN), C - reactive protein (CRP), tumor necrosis factor, thiobarbituric acid reactive substances (TBARSs), and total antioxidant capacity (TAC) and histopathological examination of kidney. Materials and MethodsPlant materialPomegranates were obtained from an indigenous market and peeled off, and then the seeds were squeezed to obtain the juice. Nine liters of pomegranate fresh juice were filtered to remove seeds and then subjected to lyophilization Freez dryer (Model SB4, England Chemlab, and England) to give 770 gram of PM (Hasan et al., 2016; Riaz and Khan, 2016). Pomegranate white peel (fresh mesocarp) was cut into small pieces and air dried for a few days to give 235 gm powder. The powdered drug was extracted with 80% aqueous ethyl alc (4 × 8 L), the solvent was removed under reduced pressure to give 155 gm viscous residue, and 27 gm of viscous residue was added to 200 ml distilled water (Hasan et al., 2016). Pomegranate red peel (leathery mesocarp) was cut into small pieces, and air dried for a few days to give 202gm powder. The powdered drug was extracted with 80% aqueous ethyl alc (4 × 8 L), the solvent was removed under reduced pressure to give 155 gm viscous residue, and then 17 gm of viscous residue was added to 200 ml distilled water (Hasan et al., 2016). Experimental animalsThe study was performed on 80 male W/A rats 6–8 weeks old, weighing 250–275 g (Zagazig University, Zagazig, Egypt). Each four rats was housed in clear polypropylene cages and provided free access to purified water and standard rodent pellets. Constant animal housing conditions were applied, constituting alternating 12 hours of light and dark, a temperature of 22°C ± 3°C, relative humidity of 50%–60%, and adequate ventilation. Experimental protocolsAfter acclimatization, animals were randomly divided into eight groups (ten each); the first group (control) received corn, maize, and barley for two weeks. The second group (molasses pomegranate pure group) was orally given 0.25 ml/kg body weight for two weeks. The third group (white peel pomegranate pure group) was orally given 0.28 ml/kg body weight for two weeks. The fourth group (red peel pomegranate pure group) was given orally at 0.28 ml/kg body weight for two weeks. The fifth group (PHZ group) was intraperitoneally injected with 0.75 ml/ kg body weight (PHZ and sodium chloride) for 3 days. The sixth group (molasses pomegranate + penylhydrazine group) was orally given 0.25 ml/kg body weight for two weeks, followed by intraperitoneal injection of 0.75 ml/ kg body weight (PHZ and sodium chloride) for 3 days with continued giving molasses pomegranate extract (PE). The seventh group (white peel pomegranate + phenylhdrazine group) was orally given 0.28 ml/kg body weight for two weeks, followed by intraperitoneal injection of 0.75 ml/kg body weight (PHZ and sodium chloride) for 3 days with the continued giving of white peel PE. The eighth group (red peel pomegranate + penylhydrazine group) was orally given 0.28 ml/kg body weight for two weeks, followed by intraperitoneal injection of 0.75 ml/ kg body weight (PHZ and sodium chloride) for 3 days with the continued giving of red peel PE. At the end of the experiment, rats were fasted overnight and sacrificed, and blood samples were drawn from the orbital sinus of the eyes; the blood was allowed to clot at room temperature for 20–30 minutes and then centrifuged at 3,000 rpm for 15 minutes. The sera were kept at 200c until used for analysis. The kidneys were removed and fixed in 10% neutral buffered formalin for histopathological examinations. High Performance Liquid Chromatography (HPLC) analysis of pomegranateAgilent 1260 infinity HPLC Series (Agilent, USA), equipped with Quaternary pump, a Zorbax Eclipse plus C18 column 100 × 4.6 mm i.d., (Agilent technologies, USA), operated at 25ºC.The separation is achieved using a ternary linear elution gradient with (A) HPLC grade water % 0.2 H3PO4 (vv), (B) methanol, and (C) acetonitrile. The injected volume was 20 µL. Detection: VWD detector set at 284 nm (Noratiqah et al., 2018). Evaluation of oxidative stress biomarkersA) Lactate dehydrogenase (LDH) was carried out by the LDH enzymatic assay kit, which measures the concentration of LDH using a direct, plate-based, colorimetric reaction. B) Malondialdehyde (MDA) Serum MDA measurement was carried out by using a commercial kit obtained from Diagnostic Systems Laboratories Inc. TBARSs (Colorimetric Assay kit) (Dawn-Linsley et al. (2005). Evaluation of antioxidant biomarkers and TACThe activities of superoxide dismutase (SOD) and catalase (CAT) and the level of reduced glutathione (GSH) were estimated according to methods described previously (Aebi, 1984); Nishikimi et al., 1972; Ursini et al., 1985). TAC was determined using kit reagents & the ZellX TAC assay (Trachootham et al., 2008). Biochemical analysisCreatinine, uric acid, and BUN were measured. Serum creatinine concentration was measured by Jaffe’s method (Colorimetric—kinetic) (Bowers and Wong, 1980). Serum level of Uric acid was measured by (Enzymatic Colorimetric method) (White-Stevens, 1982; Young, 2000). The BUN colorimetric procedure is a modification of the Berthelot reaction. Urea is converted to ammonium using urease (Tabacco et al., 1979). Determination of Inflammation markers and oxidation status
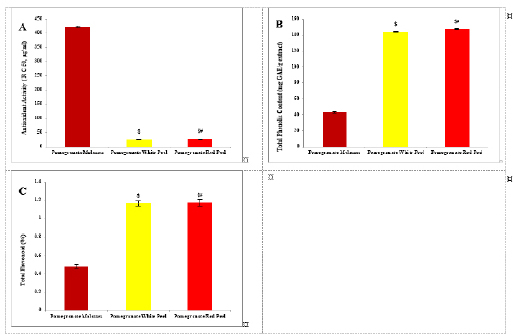
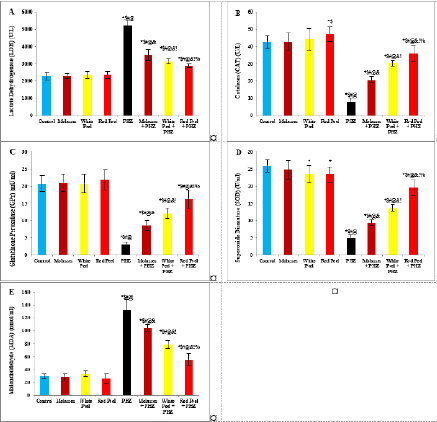
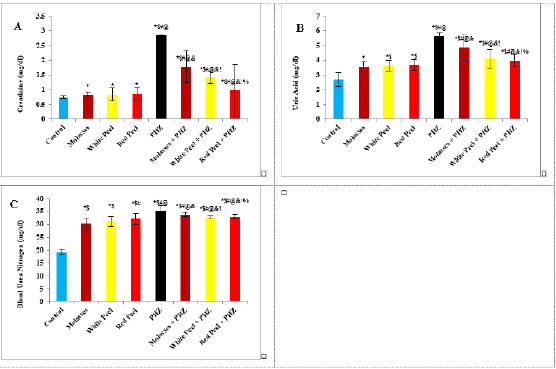
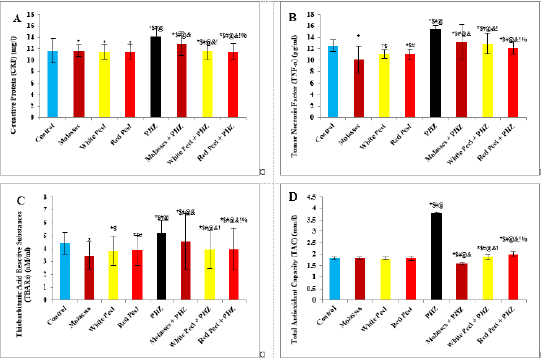
Histopathological examinationKidney specimens that were fixed in 10% neutral buffered formalin were embedded in paraffin blocks. Paraffin-embedded tissue sections (5 µm thick) were cut, de-waxed in xylene, hydrated using graded ethanol, and stained with hematoxylin and eosin (H&E) dyes for histopathological examination. The slides were examined by light microscopy (Bancroft and Gamble, 2008). Statistical analysisSPSS Statistics is a software package used for interactive or batched statistical analysis produced by SPSS Inc. It was acquired by IBM in 2009. The current versions (2015) is named IBM SPSS Statistics. Analysis of variance (ANOVA) is a collection of statistical models and their associated procedures (such as “variation” among and between groups) used to analyze the differences among group means. ANOVA was developed by the statistician and evolutionary biologist Ronald Fisher. F-test: An F-test is any statistical test in which the test statistic has an F-distribution under the null hypothesis. Post hoc analysis: In a scientific study, post hoc analysis (from Latin post hoc, “after this”) consists of analyses that were not specified before seeing the data (Ling and Roberts, 1975). Ethical approvalThe animal study was reviewed and approved by the experimental design and animal handling procedures as indicated by the guidelines of the Ethical Committee for Animal Handling at Zagazig University (ZU-IACUC/2/F/26/2022). ResultsHPLC analysis of pomegranateHPLC characterization of pomegranate revealed lower values of total phenolic content and total flavonoid (42.7 ± 0.8; 0.48 ± 0.02 mg GAE/g extract), respectively, in PM. But, total phenolic content and total flavonoid are higher (144.1 ± 0.1; 147.2 ± 0.2; 1.16 ± 0.03; 1.17 ± 0.04 mg GAE/g extract), respectively, in white peel pomegranate and red peel pomegranate (Fig. 1). Oxidative stress biomarkers (MDA, LDH)Both MDA, LDH (132.5 ± 16.83 nmol/ml; 5234 ± 267.8 U/L), respectively, showed a significant increase in the PHZ group as compared with the control group (29.32 ± 3.825 nmol/ml, 2272 ± 245.4 U/L), respectively, while in pomegranate treated and PHZ co-treated groups with pomegranate, they showed a significant decrease (Fig. 2). Antioxidant biomarkersEnzymatic activities of SOD and CAT, as well as GPx level, showed a significant decrease in PHZ group (4.927 ± 0.919 U/ml; 7.985 ± 2.355 U/L; 3.105 ± 0.841 mU/ml), respectively, as compared with control (26.03 ± 1.777 U/ml; 42.67 ± 3.494 U/L; 20.74 ± 2.317 mU/ml), respectively. On the other hand, other pomegranate-treated and PHZ co-treated groups with pomegranate showed a significant increase in CAT, GPx, and SOD levels (Fig. 2). Biochemical findingsThe results revealed a marked reduction in renal function, as characterized by significant increases in creatinine. Uric acid and BUN levels in the PHZ group (2.84 ± 0.02 mg/dl; 5.65 ± 0.23 mg/dl; 35.14 ± 2.01 mg/dl), respectively, as compared with control (0.74 ± 0.05 mg/dl; 2.70 ± 0.46 mg/dl; 19.25 ± 1.09 mg/dl), respectively. On the other hand, pomegranate-treated and PHZ co-treated groups with pomegranate showed a significant decrease in creatinine. Uric acid and BUN levels (Fig. 3). Inflammation markers and oxidation statusIt was observed that CRP, tumor necrosis factor, TBARSs, and TAC showed a significant increase in the PHZ group (14.17 ± 1.86 mg/l; 15.35 ± 0.76 pg/ml; 5.21 ± 0.99 nM/ml; 3.78 ± 0.04 mm/l; 3.78 ± 0.04 mm/l), respectively, as compared with control (11.68 ± 2.13 mg/l; 12.54 ± 0.94 pg/ml; 4.41 ± 0.84 nM/ml; 1.83 ± 0.04 mm/l; 1.83 ± 0.04 mm/l), respectively. On the other hand, other pomegranate-treated and PHZ co-treated groups with pomegranate showed a significant decrease of that CRP, tumor necrosis factor, TBARS, and TAC (Fig. 4).
Fig. 1. Analysis of pomegranate by HPLC. (A) Antioxidant activity ( R C 50, μg/ml). (B) Total phenolic content (mg GAE/g extract). (C) Total flavonoid (%). Results are expressed as mean ± SEM (n=3 for all groups). $ p < 0.05 when compared to the corresponding PM values, # p < 0.05 when compared to pomegranate white peel values using one-way ANOVA followed by Tukey’s post-hoc test.
Fig. 2. Effect of oral administration of molasses extract, white peel pomegranate extract, and red peel pomegranate extract to control or PHZ rats both at 25 mg/kg/day for 2 weeks on (A) Lactate Dehydrogenase (LDH) (U/L). (B) CAT (U/L). (C) Glutathione Peroxidase (GPx) (mU/ml). (D) SOD (U/ml). (E) MDA (nmol/ml). Results are expressed as mean ± SEM (n=8 for all groups). * p < 0.05 when compared to the corresponding control values, $ p < 0.05 when compared to the corresponding molasses values, # p < 0.05 when compared to white peel values, [at] p < 0.05 when compared to red peel values, & p < 0.05 when compared to PHZ values, ! p < 0.05 when compared to molasses+PHZ values, % p < 0.05 when compared to white peel+PHZ values; using one-way ANOVA followed by Tukey’s post-hoc test.
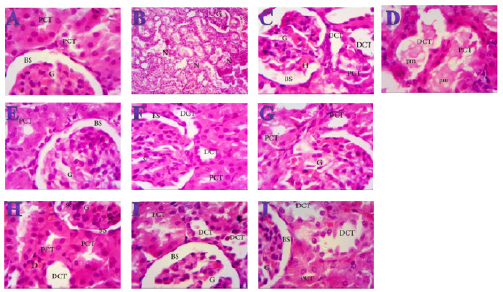
Fig. 3. Effect of oral administration of molasses extract, white peel pomegranate extract, and red peel pomegranate extract to control or PHZ rats both at 25 mg/kg/day for 2 weeks on (A) Creatinine (mg/dl). (B) Uric acid (mg/dl). (C) BUN (mg/dl). Results are expressed as mean ± SEM (n=8 for all groups). * p < 0.05 when compared to the corresponding control values, $ p < 0.05 when compared to the corresponding molasses values, # p < 0.05 when compared to white peel values, [at] p < 0.05 when compared to red peel values, and p < 0.05 when compared to PHZ values, ! p < 0.05 when compared to molasses+PHZ values, %p < 0.05 when compared to white peel+PHZ values; using one-way ANOVA followed by Tukey’s post-hoc test. Histopathological observationsHistopathological changes in renal tissues in the different groups treated are shown in Figure 5. PHZ exposed group showed disorganization and spread of necrosis throughout the renal parenchyma with severely damaged glomeruli, damaged and lobulated glomerulus (G) with intra-glomerular hemorrhage and widened Bowman’s space, damaged and necrotic proximal and distal convoluted tubules (DCT) furthermore, highly destructed proximal and DCT with hyaline proteinaceous material. Pomegranate pure-treated groups showed nearly normal renal parenchyma containing G and proximal convoluted tubules (PCT) with good profile and regular Bowman’s space except for some sort of thickening in the parietal layer of Bowman’s capsule. PHZ co-treated groups with permanganate showed good renal parenchyma; G, PCT, and DCT, but the G still contained sclerosis together with damaged renal nuclei. DiscussionPomegranate has extensively been used in folk medicine for several purposes. Recently, studies have shown that pomegranate has several pharmacological activities such as antimicrobial (Akpinar-Bayizit et al., 2016), antioxidant, anti-inflammatory, and anticarcinogenic effects (Ahmed and Ali, 2010). Pomegranate–derived products have shown beneficial effects on the treatment and prevention of various diseases such as cancer, cardiovascular disease, neurological disorders diabetes, and other diseases. The kidneys are easily susceptible to damage from drugs because of larger perfusion and accumulation of excreted compounds that occur in renal tubular cells during absorption and secretion. In the current study, it was observed that PHZ induced a reduction in renal function, which improved in pomegranate co-treated groups. Phenols and flavonoids, which have antioxidant activities have been shown to be very important plant constituents. The plant phenolics are commonly present in fruits, vegetables, leaves, nuts, seeds, barks, roots, and other plant parts. Pomegranate is an important source of vitamin C. The antioxidant and free radical scavenging activities of pomegranate phenolic compounds and vitamin C have been reported. It appears that PE has affected the glomeruli in the cortex more than other parts, so the flavonoids - containing compounds might cause vasodilation and increase blood flow in the kidney (Kumar et al., 2011). In addition, it was reported that polyphenolic compounds could elevate nitric oxides (NOs) that increase blood flow. This increased blood flow can also influence glomeruli and increase the size of the glomeruli. This could be a main factor in increasing the weight, volume density, and volume of the cortex, the volume density of glomeruli, and the total volume of glomeruli (Mansouri et al., 2016).
Fig. 4. Effect of oral administration of molasses extract, white peel pomegranate extract, and red peel pomegranate extract to control or PHZ rats both at 25 mg/kg/day for 2 weeks on (A) CRP (mg/l). (B) Tumor necrosis factor (TNF-a) (pg/ml). (C) Thiobarbituric acid reactive substances (TBARs) (nM/ml). (D) TAC (mm/l). Results are expressed as mean ± SEM (n=8 for all groups). * p < 0.05 when compared to the corresponding control values, $ p < 0.05 when compared to the corresponding molasses values, # p < 0.05 when compared to white peel values, [at] p < 0.05 when compared to red peel values, & p < 0.05 when compared to PHZ values! p < 0.05 when compared to molasses+PHZ values, %p < 0.05 when compared to white peel+PHZ values; using one-way ANOVA followed by Tukey’s post-hoc test. Flavonoids, polyphenols, pectin, and ascorbic acid form 1.5% of the weight of pomegranate juice (PJ). The soluble polyphenol content is between 0.2% and 1% depending on variety, and includes mainly anthocyanins (Ignarro et al., 2006; Darwish et al., 2009). The total content of anthocyanins was found to be higher in pomegranate than in any other fruit juice tested for antioxidant activity. Anthocyanins are glycosides of anthocyanidin and are the most abundant water-soluble plant pigments responsible for the red, blue and purple colors of fruits. They are potent antioxidants, and their activity is directly related to their unique chemical structures (Rice-Evans et al., 1996). Concerning oxidative stress biomarkers, both MDA (secondary product of lipid peroxidation) and LDH showed a significant increase in the PHZ group as compared with the control group, while in pomegranate-treated and PHZ co-treated groups with pomegranate, they showed a significant decrease. Similarly, it was reported that PJ caused a significant decrease in MDA in the kidney cortex of rats under oxidative stress. In addition, the pomegranate methanol extract from the peel caused a significant decrease in MDA levels in kidney samples of rats (Fatma, 2014). Furthermore, previous studies demonstrated that PHZ treatment of rats was associated with a marked elevation in serum MDA as well as a significant reduction in the level of TAC. Feeding rats with 5% dried green beet reduced serum MDA levels as well as improving the level of TAC (Elaby and Ali, 2018). In this study, the enzymatic activities of SOD and CAT, as well as GPx levels, showed a significant decrease in the PHZ group as compared with the control group. On the other hand, PHZ co-treated groups with pomegranate showed a significant increase in CAT, GPx, and SOD levels, where PM, pomegranate white peel and pomegranate red peel analysis showed considerable antioxidant activity due to their high phenolic, and flavonoid contents. Polyphenols are considered the major class of phytochemicals in pomegranate fruit. They have an antioxidant activity in vivo and in vitro. The antioxidant activities of dietary polyphenols include reactive species scavenging, enzyme modulation to interfere with cell signaling and oxidative stability. In addition, pomegranate is a major source of soluble polyphenols such as gallic acid, ellagic acid, punicalagin, and quercetin. Furthermore, it was demonstrated that polyphenols possess powerful antioxidant properties, which represent the mechanism most likely responsible for the protective benefits of pomegranate. The antioxidant capacity of pomegranate is shown to be three times higher than that of red wine or green tea infusion. In addition, pomegranate is rich in polyphenolic class antioxidants, including flavonoids such as gallotannins, ellagitannins, ellagic, ferulic, and gallagic acids, anthocyanins, quercetins, and catechins. The polyphenols show important biological activities, including oxidation inhibition, free radical elimination, and reduction of the risks of cardiovascular diseases. Ellagitannins may be responsible for the anti-mutagenic and antioxidant activities of pomegranate (Doostan et al., 2017).
Fig. 5. Photomicrographs (H&E) of kidney sections of (A) Control (×1000) showing normal structure with no histopathological changes, normal G and PCTs, Bowmen’s capsule with ordinary regular urinary Bowman space (BS). (B) PHZ (1 mg) (×400) illustrating disorganization and spread of necrosis (N) throughout the renal parenchyma with severely damaged glomeruli (G). (C) PHZ (1 mg) (×1000) illustrating damaged and lobulated G with intra-glomerular hemorrhage (H) and widened Bowman’s space (BS), damaged and necrotic proximal and distal convoluted tubules (PCT and DCT, respectively). (D) PHZ (1 mg) (×1000) showing highly destructed proximal and distal convoluted tubules (PCT and DCT, respectively) with hyaline proteinaceous material. (E) Red peel pomegranate-administered rat (×1000) showing nearly normal renal parenchyma containing G and PCTs with good profile and regular Bowman’s space (BS) except some sort of thickening in the parietal layer of Bowman’s capsule. (F) White peel pomegranate-administered rat (×1000) showing renal elements G, PCT, DCT, and BS with good histological appearance except some sort of lobulation in the G. (G) Molasses pomegranate-administered rat (×1000) showing renal elements; G, PCT, DCT, and BS with good histological appearance except some sort of vacuolization in the G, which contains the characteristic red pigment. (H) Red peel pomegranate (Red Peel + PHZ) (×1000) showing good renal parenchyma; G, PCT, and DCT but the G still containing sclerosis (Sc) together with damaged (D) renal nuclei. (I) White peel pomegranate (White Peel + PHZ) (×1000) showing a low level of repair in the renal elements where the G is still lobulated with widened Bowman’s space, PCT and DCT are still vacuolated and damaged. (J) Molasses pomegranate (Molasses + PHZ) (×1000) showing a nearly normal G, BS, and PCT, but the DCT is still damaged. Pomegranate (P. granatum L.) was found to be high in natural antioxidants (anthocyanins, catechins, quercetin, gallotannins, ellagitannins, ellagic, ferulic, and gallic acid). Ellagitannins (punicalagin and its derivatives) were found out to be the most abundant polyphenols in the peel, and they are responsible for the peel’s potent antioxidant properties (El-Wakf et al., 2018). However, pomegranate’s most potent antioxidant activity is due to its inclusion of several phenolic components (ellagic acid, punicalin, and punicalagin) instead of single pure polyphenols (Doostan et al., 2017). Our results revealed a marked reduction in renal function, as characterized by significant increases in creatinine, uric acid, and BUN levels in the PHZ group as compared with the control group. On the other hand, pomegranate-treated and PHZ co-treated groups with pomegranate showed a significant decrease in creatinine, uric acid, and BUN levels. In the same respect, serum creatinine and urea levels increased significantly in the PHZ group, and feeding 5% dried beet green significantly decreased the levels of serum urea and creatinine. Renal dysfunction and structural damage have been attenuated using beetroot through the reduction of oxidative stress, inflammation, and apoptosis in the kidney (Elaby and Ali, 2018). In addition, the rats that received meatballs containing PM or peel and molasses mix exhibited a significant decline in BUN level compared with the control group. Moreover, prophylaxis with pomegranate peel extract for a week resulted in reducing serum creatinine and urea values in oxidative damage induced by Ferric nitrilotriaacetate (Fe-NTA) in rats. In addition, the administration of PJ and pomegranate peel methanol extract in rats with chronic renal failure significantly decreased serum levels of creatinine, BUN, and uric acid (Fatma, 2014). PM reduced the levels of urea, uric acid, and creatinine. However, ciplastin elevated the levels of kidney functions (Abdel Moneim et al., 2011). Regarding CRP, tumor necrosis factor, TBARSs, and TAC, our results are in agreement with (Fatma, 2014; Elaby and Ali, 2018). It was reported that pomegranate peel methanol extracts lowered thiobarbituric acid reactive substance (TBAR) and significantly decreased serum tumor necrosis factor-α (TNF-α) and CRP accompanied by an increase in NO level. PJ was found to have inhibitory effects on renal tubular cell injury and oxidative stress (Fatma, 2014). Our finding is supported by the study of Cekmen et al. (2013), who concluded that PE has an overall protective effect against GEN-induced nephrotoxicity in rat models. The observed protective effects can be attributed to the antioxidant properties of PE. However, pomegranate is a highly free-radical scavenger agent and offers protection against GEN-induced acute renal failure. Similarly, treatment with PJ) before, during, and after diethylnitrosamine (DEN) and Carbon tetrachloride (CCl4) administration showed a reduction in the levels of kidney caspase-3, DNA fragmentation (DNAF), NO as well as creatinine, urea, and uric acid levels compared with the DEN group. This means that polyphenolic compounds in pomegranate play an important role in quenching the free radicals resulting from the metabolism of DEN, thereby inhibiting lipid peroxidation and protecting membrane lipids from oxidative damage and, in turn, preventing apoptosis (Hamouda et al., 2015). Concerning the histopathological changes, it could be suggested that excessive production of reactive oxygen species (ROS) and lipid peroxidation (LPO) play a key role in renal damage induced by phenylhydrazin, and these protective effects of pomegranate could be due to the presence of a variety of biologically active compounds. Antioxidant and free radicals scavenging properties of pomegranate might be partially responsible for its nephroprotective activity. ConclusionThe present results suggest that pomegranate can be an effective agent against the toxic effects of phenylhydrazin both at the biochemical and histological levels and show beneficial effects in phenylhydrazin-induced kidney dysfunction and organ damage. These protective effects could be due to the presence of a variety of biologically active compounds, such as antioxidants, vitamins, minerals, phenols, and flavonoids, but the explanation and mechanism of this protection need further studies. AcknowledgmentsWe thank Professor Abeer EL-Bayoumy of the Department of Physiology at the Faculty of Medicine at Zagazig University for her first help. FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Data availabilityThe data that support the findings of this study are available from the corresponding author upon reasonable request. Authors contributionsAll authors discussed the results, drafted, revised and approved the manuscript. Conflict of interestAll authors declare that there is no conflict of interest. ReferencesAbdel Moneim, A.E., Dkhil, M.A. and Al-Quraishy, S. 2011. Studies on the effect of pomegranate (Punica granatum) juice and peel on liver and kidney in adult male rats. J. Med. Plant. Res. 5(20), 5083–5088. Aebi, H. 1984. Catalase in vitro. Methods Enzymol. 105, 121–126. Ahmed, M.M. and Ali, S.E. 2010. Protective effect of pomegranate peel ethanol extract against ferric nitrilotriacetate induced renal oxidative damage in rats. J. Cell Mol. Biol. 7(2), 35–43. Akpinar-Bayizit, A., Ozcan, T., Yilmaz-Ersan, L. and Yildiz, E. 2016. Evaluation of antioxidant activity of pomegranate Molasses by 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Method. Int. J. Chem. Engin. Appl. 7(1), 71–74. Amama, E.A., Udefa, A.L., Beshel, F.N., Archibong, E.A., Okpa, S., Nwangwa, J.N., Felix, N.I. and Akronyi, G. 2022. Fresh palm oil improves impaired renal function in phenylhydrazine-induced anaemic Wistar rats via its anti-anaemic effect and modulation of expressions of pro-oxidant/antioxidants, inflammatory cytokines and caspase-3 in the kidneys. Physiol. Pharmacol. 26, 299–312. Ashiba, H., Oyamada, C., Hosokawa, K., Ueno, K. and Fujimaki, M. 2020. Sensitive detection of C-reactive protein by one-step method based on a waveguide-mode sensor. Sensors (Basel). 20(11), 3195. Bancroft, J.D. and Gamble, M. 2008. Theory and practice of histological technique, 4th ed. New York, London, San Francisco, Tokyo: Churchil Livingston. Bowers, L.D. and Wong, E.T. 1980. Kinetic serum creatinine assays. II. A critical evaluation and review. Clin. Chem. 26(5), 555–561. Cekmen, M., Otunctemur, A., Ozbek, E., Cakir, S.S., Dursun, M., Polat, E.C., Somay, A. and Ozbay, N. 2013. Pomegranate extract attenuates gentamicin-induced nephrotoxicity in rats by reducing oxidative stress. Renal Failure. 35(2), 268–274. Darwish, M.M., Osman, N.N. and Farag, M.F.S. 2009. Protective effect of pomegranate (P. granatum) juice in rats consuming aspartame on lipid peroxidation and antioxidant status. J. Rad. Res. Appl. Sci. 2(3), 535–548. Dawn-Linsley, M., Ekinci, F.J., Ortiz, D., Rogers, E. and Shea, T.B. 2005. Monitoring thiobarbituric acid-reactive substances (TBARs) as an assay for oxidative damage in neuronal cultures and central nervous system. J. Neurosci. Methods 141(2), 219–222. Doostan, F., Vafafar, R., Zakeri-Milani, P., Pouri, A., Afshar, R.A. and Abbasi, M.M. 2017. Effects of pomegranate (Punica granatum L.) seed and peel methanolic extracts on oxidative stress and lipid profile changes induced by methotrexate in rats. Adv. Pharm. Bull. 7(2), 269–274. Elaby, S.M. and Ali, J.B. 2018. The anti-anemic effect of dried beet green in phenylhydrazine treated rats. Arch. Pharm. Sci. Ain Shams Univ. 2(2), 54–69. El-Hadary, A.E. and Taha, M. 2020. Pomegranate peel methanolic-extract improves the shelf-life of edible-oils under accelerated oxidation conditions. Food Sci. Nutr. 8, 1798–1811. El-Wakf, A.M., El-Habibi, E.S.M., Barakat, N.M., Attia, A.M., Hussein, A.M. and Ali, I.I. 2018. Cardiovascular toxic effects of chlorpyrifos: a possible protective role for pomegranate extracts. J. Clin. Toxicol. 8(1), 1–7. Fatma, M.E. 2014. Protective effect of pomegranate (peel and molasses) incorporated into roasted meat balls on renal failure in rats. J. Food Dairy Sci. Mansoura Univ. 5(6), 435–449. Hamouda, A.F., Shaban, N.Z. and Talaat, I.M. 2015. Effects of some pyrimidine derivatives and pomegranate juice on male rat kidney injuries induced by diethylnitrosamine and carbon tetrachloride. J. Biol. Chem. Res. 201, 215–229. Hasan, S.M.H., Abou-Rawash, A.E.A. and Bekheet, M.S. 2016. Protective role of an aqueous extract of Punica granatum (Pomegranate) peel on lead-induced anemia in rats. Alexandria J. Vet. Sci. 50(1), 99–108. Ignarro, L.J., Byrns, R.E., Sumi, D., De Nigris, F. and Napoli, C. 2006. Pomegranate juice protects nitric oxide against oxidative destruction and enhances the biological actions of nitric oxide. Nitric. Oxide.15(2), 93–102. Kumar, S., Prahalathan, P. and Raja, B. 2011. Antihypertensive and antioxidant potential of vanillic acid, a phenolic compound in L-NAME-induced hypertensive rats: a dose-dependence study. Redox. Rep. 16(5), 208–215. Lefèvre, G., Beljean-Leymarie, M., Beyerle, F., Bonnefont-Rousselot, D., Cristol, J.P., Thérond, P. and Torreilles, J. 1998. Evaluation of lipid peroxidation by measuring thiobarbituric acid reactive substances. Ann. Biol. Clin. (Paris). 56(3), 305–319. Ling, R.F. and Roberts, H.V. 1975. IDA: An Approach to Interactive Data Analysis in Teaching. J. Business 48(3), 411–451. Mansouri, E., Basgen, J. and Saremy, S. 2016. The effects of pomegranate extract on normal adult rat kidney: a stereological study. Vet. Res. Forum. 7(1), 1–6. Nishikimi, M., Roa, N.A. and Yogi, K. 1972. Measurement of superoxide dismutase. Blochem. Biophys. Res. Common. 46, 849–854. Noratiqah, J.M., Norhaslinda, R., Amin, B.A. and Khalili, R.M.A. 2018. Quantitative and optimization of phenolic acid extracted from pomegranate by high performance liquid chromatography (HPLC). Pharm. J. 10(5), 969-972. Quintero, D., Ancel, T., Cassie, G., Ceron, R. and Darwish, A. 2013. Workload optimized systems: tuning POWER7 for analytics. Riaz, A. and Khan, R.A. 2016. Anticoagulant, antiplatelet and antianemic effects of Punica granatum (pomegranate) juice in rabbits. Blood Coagul. Fibrinolysis 27(3), 287–293. Rice-Evans, C.A., Miller, N.J. and Paganga, G. 1996. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Comparative study, Free Radic. Biol. Med. 20(7), 933–956. Ruddle, N.H. 1992. Tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta). Curr. Opin. Immunol. 4(3), 327–332. Tabacco, A., Meiattini, F., Moda, E. and Tarli, P. 1979. Simplified enzymic/colorimetric serum urea nitrogen determination. Clin. Chem. 25(2), 336–337. Trachootham, D., Lu, W., Ogasawara, M.A., Nilsa, R.-D.V. and Huang, P. 2008. Redox regulation of cell survival. Antioxid. Redox. Signal. 10(8), 1343–1374. Ursini, F., Maiorino, M., and Gregolin, C. 1985. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta. 839(1), 62–70. White-Stevens, R.H. 1982. Interference by ascorbic acid in test systems involving peroxidase. I. Reversible indicators and the effects of copper, iron, and mercury. Clin. Chem. 28(4), 578–588. Young, D.S. 1995. Effects of drugs on clinical laboratory tests, 4th Ed., Washington, DC: AACC Press. Young, D.S. 2000. Effects of drugs on clinical laboratory tests, 5th ed. Washington, DC: AACC Press. Zaki, S.A., Abdelatif, S.H., Abdelmohsen, N.R. and Ismail, F.A. 2015. Phenolic compounds and antioxidant activities of pomegranate peels. Int. J. Food Engin. 1(2), 73–76. | ||
| How to Cite this Article |
| Pubmed Style Soliman NA, Mansour SW, Ammar MA, Hassan NA, Mohamed RH. Possible role of pomegranate fruit in reversing renal damage in rats exposed to Phenylhydrazine. Open Vet. J.. 2023; 13(10): 1268-1276. doi:10.5455/OVJ.2023.v13.i10.5 Web Style Soliman NA, Mansour SW, Ammar MA, Hassan NA, Mohamed RH. Possible role of pomegranate fruit in reversing renal damage in rats exposed to Phenylhydrazine. https://www.openveterinaryjournal.com/?mno=159167 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i10.5 AMA (American Medical Association) Style Soliman NA, Mansour SW, Ammar MA, Hassan NA, Mohamed RH. Possible role of pomegranate fruit in reversing renal damage in rats exposed to Phenylhydrazine. Open Vet. J.. 2023; 13(10): 1268-1276. doi:10.5455/OVJ.2023.v13.i10.5 Vancouver/ICMJE Style Soliman NA, Mansour SW, Ammar MA, Hassan NA, Mohamed RH. Possible role of pomegranate fruit in reversing renal damage in rats exposed to Phenylhydrazine. Open Vet. J.. (2023), [cited January 25, 2026]; 13(10): 1268-1276. doi:10.5455/OVJ.2023.v13.i10.5 Harvard Style Soliman, N. A., Mansour, . S. W., Ammar, . M. A., Hassan, . N. A. & Mohamed, . R. H. (2023) Possible role of pomegranate fruit in reversing renal damage in rats exposed to Phenylhydrazine. Open Vet. J., 13 (10), 1268-1276. doi:10.5455/OVJ.2023.v13.i10.5 Turabian Style Soliman, Nabil Abbas, Sherif Wajih Mansour, Mohamed Ahmed Ammar, Noura Ahmed Hassan, and Rehab Hamed Mohamed. 2023. Possible role of pomegranate fruit in reversing renal damage in rats exposed to Phenylhydrazine. Open Veterinary Journal, 13 (10), 1268-1276. doi:10.5455/OVJ.2023.v13.i10.5 Chicago Style Soliman, Nabil Abbas, Sherif Wajih Mansour, Mohamed Ahmed Ammar, Noura Ahmed Hassan, and Rehab Hamed Mohamed. "Possible role of pomegranate fruit in reversing renal damage in rats exposed to Phenylhydrazine." Open Veterinary Journal 13 (2023), 1268-1276. doi:10.5455/OVJ.2023.v13.i10.5 MLA (The Modern Language Association) Style Soliman, Nabil Abbas, Sherif Wajih Mansour, Mohamed Ahmed Ammar, Noura Ahmed Hassan, and Rehab Hamed Mohamed. "Possible role of pomegranate fruit in reversing renal damage in rats exposed to Phenylhydrazine." Open Veterinary Journal 13.10 (2023), 1268-1276. Print. doi:10.5455/OVJ.2023.v13.i10.5 APA (American Psychological Association) Style Soliman, N. A., Mansour, . S. W., Ammar, . M. A., Hassan, . N. A. & Mohamed, . R. H. (2023) Possible role of pomegranate fruit in reversing renal damage in rats exposed to Phenylhydrazine. Open Veterinary Journal, 13 (10), 1268-1276. doi:10.5455/OVJ.2023.v13.i10.5 |