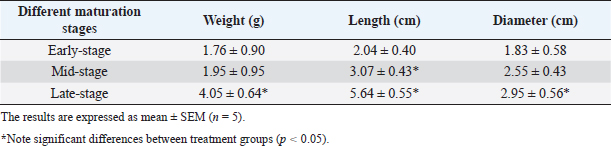| Research Article | ||
Open Vet. J.. 2023; 13(12): 1562-1569 Open Veterinary Journal, (2023), Vol. 13(12): 1562–1569 Original Research Phytochemical compounds, antiradical capacity, and in vitro inhibitory effect against fish pathogenic bacteria of okra fruits (Abelmoschus esculentus L.) at different maturity stagesSalma Guebebia1*, Abdalla A. Mohamed2, Cristóbal Espinosa-Ruiz3, Maria Ángeles Esteban3, Lazhar Zourgui4 and Mehrez Romdhane11Laboratory of Energy, Water, Environment and Process (LR18ES35), National School of Engineers of Gabes (ENIG), University of Gabes, Gabes, Tunisia 2Biomedical Research Team, Department of Medical Nutrition, Faculty of Medical Technology, University of Zawia, Zawia, Libya 3Immunobiology for Aquaculture Group, Department of Cell Biology and Histology, Faculty of Biology, Campus Regional de Excelencia Internacional “Campus Mare Nostrum,” University of Murcia, Murcia, Spain 4Research Unit of Active Biomolecules Valorisation, Department of Biological Engineering, Higher Institute of Applied Biology of Medenine (ISBAM), University of Gabes, Gabes, Tunisia *Corresponding Author: Salma Guebebia. Laboratory of Energy, Water, Environment and Process (LR18ES35), National School of Engineers of Gabes (ENIG), University of Gabes, Gabes, Tunisia. Email: salmaguebebia1992 [at] gmail.com Submitted: 09/07/2023 Accepted: 20/11/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
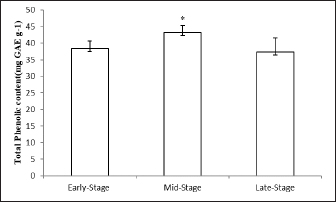
AbstractBackground: Abelmoschus esculentus L., okra, has been known as a healthy plant and classically employed in food and folk medicine for several human and animal diseases. Aim: The in vitro antioxidant and antibacterial capacities, in addition to the phytochemical compounds of the okra fruit extracts gathered at three maturity stages, were the objectives of this study. Methods: This study examined the modifications in total phenolic content (TPC), total flavonoid content (TFC), and antioxidant and antibacterial capacities of three okra fruit hydroalcoholic extracts during three comestible maturity stages. The different maturity stages of okra pods were demonstrated as early-stage, mid-stage, and late-stage maturation. Results: The mid-stage of okra fruit maturity had the highest TPC (43.27 ± 2.029 mg GAE g−1), TFC (29.96 ± 0.19 mg RE g−1), and antioxidant capacity (75.64% ± 0.79%). Moreover, at mid-stage maturity, okra fruit extracts exhibited a major antibacterial effect against Vibrio anguillarum. The phenolic content was significantly increased at the mid-stage maturity, while the flavonoid level and the antioxidant activity were greatly decreased at the end of fruit maturity. Conclusion: The results confirmed that A. esculentus L. fruits at mid-stage maturity are an excellent source of biomolecules with high antiradical and bactericidal activities, which could be used as functional foods and as an option for chemical compounds for fish farming to prevent and treat numerous marine animal diseases. Keywords: Okra fruits, Phenolic compounds, Antioxidant capacity, Antibacterial activity, Maturity stage. IntroductionSince immemorial times vegetables have played a vital role in traditional diets. In addition, vegetables are functional foods used as nutrition and herbal medicines, thanks to their abundant bioactive medicinal molecules and chemical properties (Poobalan et al., 2019). A noticeable amount of work has been carried out on the richness of vegetable extracts in phenol and flavonoid contents with a growing interest in the food industry and the medical field (Mohammed et al., 2016). Then, natural antioxidants are ubiquitous in fruits and vegetables known as medicinal plants. Currently, the demand for biological antioxidants has received more and more attention and has been studied, due to their low toxicity than synthetic antioxidants which explains the increased attention to plants with therapeutic potential in recent years (Umme et al., 2023). Besides, many studies have confirmed that plant species are traditionally used as medicinal plants due to their antibacterial and antifungal agents (Erfan and Marouf, 2019). Abelmoschus esculentus L. also called okra, lady’s finger, and gumbo included in the Malvaceae family (Dhruve et al., 2015). The plant is an essential vegetable culture widely progressed in Africa, Asia, Southern Europe, and America, mostly in tropical, sub-tropical, and warm temperate regions (Xia et al., 2015). Okra is a multifunctional plant thanks to its different uses of pods, leaves, flowers, and seeds (Habtamu et al., 2014). Furthermore, medicinally several diseases are treated using this plant. Abelmoschus esculentus L. and its constituents have many bioactivities such as cardioprotective, renal, neuroprotective, anti-fatigue, anti-cancer, antimicrobial, and hypoglycemic activities (Solomon et al., 2016). Furthermore, okra fruit is a green-colored capsule containing numerous seeds. Abelmoschus esculentus L. fruit is slightly curved measuring up to about 5–10 cm long and 1.5–3 cm in diameter (Lengsfeld et al., 2004). Moreover, thanks to their wealth in secondary metabolites, okra fruit extract has multiple bioactivities. Thus, A. esculentus L. fruit extract contains mostly phenolic compounds, flavonoids, and tannins (Saha et al., 2011). Various studies have confirmed that okra fruit phenolic compounds deliberated the principal responsible for its biological effects, such as antioxidant, anti-diabetic, and antibacterial properties (Jiang et al., 2017; Zhang et al., 2018). Besides, cultivars, growing conditions, and fruit sizes can swing okra fruit phenolic content and their bioactivities. Particularly, the quality of the harvested okra fruit is linked to their size proportional to time (Petropoulos et al., 2018). However, huge variation in level and chemical composition has been noted between the harvest stage of okra pods (Piloo and Kabir, 2011). Besides, once okra fruit reaches a length of 3–5 cm, it is collected in the Mediterranean countries contrary to other markets of the world where the harvested okra fruit size is longer (7–12 cm or larger) (Petropoulos et al., 2018). Whereas, A. esculentus L. fruit is the most consumed part of the plant. Hence, for the reason of studying the phenolic compounds in okra fruits, this work aims to evaluate A. esculentus L. fruits at three marketable sizes to reveal ripe-stage extract with high levels of phenolic and flavonoid compounds as well as to compare their antiradical and antibacterial activities from okra fruit produced in Gabes, Tunisia. Materials and MethodsPlant material and sample preparationAbelmoschus esculentus L. fresh fruits were harvested separately at three maturity stages based on the fruits’ morphology (size) from July to September 2020 from a local farm (Gabes, Tunisia). The three maturity stages were consumables. The samples were established as early-stage maturity, mid-stage maturity, and late-stage maturity. Afterward, to get rid of the foreign materials, samples were washed extensively with distilled water. After that, they were sliced into portions of 5 mm. As well, until their masses stabilized, they were parched at 40°C for 6 days. Then, they were powdered and sieved before being sifted to a smooth powder. Finally, plant material samples were stored at 4°C in the dark until their analysis. Besides, ethanol (70%) (PanReac AppliChem) was used for the preparation of extracts as a safe solvent for both food and natural medicinal purposes. Then, ethanol/water has been used to extract strongly phenolic and antioxidant compounds from natural substances (Sultana et al., 2009). Extracts were prepared by microwave extraction. Briefly, 1 g of each dried powdered okra fruit, was mixed with 25 ml of ethanol (70%) solution. Samples of microwave-assisted extractions were carried at 100 W for 2 minutes. After that, all mixed samples were filtered using a 100 µm pore size nylon net filter before their centrifugation at 8,000 g for 10 minutes. Then, the supernatants are collected, filtered, and stored at 4°C until utilized. In conformity with Elik et al. (2017) with some modifications, the solid–liquid extraction was provided. Determination of okra fruit weight, length, and diameterThe harvested fruit was transported in a closed plastic sack to the laboratory. After that, the length and diameter (size) of the fruit were measured. In addition, fruit weight was resolved using an electronic balance. Determination of phenolic contentsAs a principal quantitative analysis, the total phenolic content (TPC) of okra fruit extracts was determined using the Folin–Ciocalteu’s approach following the procedure described previously by Singleton and Lamuela-Raventos (2012) with modifications. Briefly, 0.4 ml of fruit extract was reacted with 2 ml of Folin–Ciocalteu reagent (LOBA Chemie). Then, 1.6 ml of Na2CO3 (Chemi-Pharma) solution (7%) was added to each sample with simple agitation. Following that, the absorbance was measured using a UV-visible spectrophotometer (T60 UV-visible spectrophotometer) after 45 minutes in the dark conditions at room temperature. All measurements were performed at a wavelength=765 nm. The data were expressed in milligrams of gallic acid per gram (mg GAE g−1) of dry sample. Determination of flavonoid contentsThe total flavonoid content (TFC) was measured as per the colorimetric method of Bahroun et al. (1996) with minor modifications. Besides, 1 ml of each okra fruit aliquot was mixed with 1 ml of an aluminum solution (AlCl3 10%) (LOBA Chemie). After an incubation of 30 minutes, the measurement of absorbance at 430 nm was determined. The results were expressed as milligrams of rutin equivalent per gram of dry extract (mg RE g−1). Determination of total antioxidant activity using the DPPH methodThe total antioxidant activity was determined using the DPPH method. The ability to scavenge DPPH radicals was performed using the method illustrated previously by Velazquez et al. (2003). Briefly, each extract (0.75 ml) was combined with 1.5 ml of DPPH solution in methanol 90% (20 mg l−1). After that, the mixture was incubated for 15 minutes at room temperature and the absorbance was measured at 517 nm using a spectrophotometer. In this analysis, we have zeroed the spectrophotometer with methanol. On the other hand, we have considered DPPH radical absorbance without extract as a control. All tests were performed in triplicate. The antioxidant activity was expressed as a percentage inhibition using the following formula: Inhibition (%)=[(AB − AS) / AB] × 100 where AS is the sample (tested extract solution) absorbance and AB is the blank absorbance. Evaluation of bactericidal activityPathogens and growth conditions To evaluate the bactericidal effect of A. esculentus L. fruit extract at three ripening stages, pathogenic bacteria for fish (Vibrio anguillarum) were adopted. The sterilized tryptic soy agar (Difco Laboratories) was used to cultivate the bacteria. Thus, single colonies were first inoculated in tryptic soy broth 30 g l−1 (TSB, Difco Laboratories). Then, they were enhanced with NaCl until reaching a final concentration of 1% (w/v), for 24 hours at 25°C with continuous agitation. Considerably proliferating bacteria were washed and resuspended in sterile PBS at 108 cfu ml−1. The in vitro bactericidal activity of the okra extracts was estimated using the method described by Stevens et al. (1991) with minor modifications. Antibacterial assay In this bactericidal assay, aliquots of 20 µl the bacterial suspensions were conducted in each well of a flat-bottomed plate (96-well plate). Then, they were incubated (5 hours, 25°C) with 20 µl of each tested extract (0.5 mg ml−1). After that, instead of the extracts, PBS solution was inserted into some wells as a positive control. While, to evaluate the growth of bacteria without treatment (0% activity), the control sample was prepared only with the bacterial solution and TSB medium and to ensure the sterility of the analysis, the blank contains only 200 µl of culture medium. After the incubation time, 25 µl of MTT (1 mg ml−1), giving a purple color, was added to each well. After that, the plates were re-intubated (10 minutes, 25°C) to promote the formation of formazan. Finally, they were centrifuged (2,000 × g, 10 minutes). After the removal of the supernatant, each precipitate was dissolved in 200 µl of DMSO. The absorbance of the dissolved formazan was then measured at 570 nm. The bacterial capacity was expressed as a percentage of viable bacteria, measured by the difference between the absorbance of the existing bacteria in test samples and the absorbance of bacteria from positive controls (100%). Statistical analysisAll analyses were realized in triplicates and the experimental data were reported as means ± SEM. One-factor analysis of variance analyses using Xlstat software were used to evaluate the statistical differences among the treatments. The significance level was 95% in all cases (p < 0.05) and was assessed using the Tukey test. Ethical approvalNot needed for this study. ResultsWeight, length, and diameter of okra fruits at different maturation stagesThe changes in physical properties [fruit weight (g), length (cm), and diameter (cm)] of A. esculentus L. fruits at different maturity stages are indicated in Table 1. Furthermore, during maturation, the weight, length, and diameter of fresh okra fruits increased considerably. In addition, the maximum level was achieved at the end of maturation. Extracts phenolic compound contentsThe main phenolic compounds present in ethanolic A. esculentus L. fruit extracts are represented in Figure 1. In the current research, the phenolic contents were present in important quantities in all maturation stages of okra extracts. Although, the mid-maturation stage of okra fruit extract showed significantly higher phenolic levels (43.27 ± 2.029 mg GAE g−1) than the fruits harvested early and the late harvested ones (37.38 ± 4.22 mg GAE g−1), respectively. Extracts flavonoids contentsIn this study, the results showed that the flavonoid contents were present in important quantities in the different maturation stages of A. esculentus L. fruit extracts (Fig. 2). As regards, Figure 2 revealed the variations of TFC in okra fruit extracts belonging to their stage of maturity. Briefly, the TFC of okra fruits increased significantly from 25.08 ± 0.93 g RE g−1 (mid-stage maturity) to 29.96 ± 0.19 g RE g−1 (late-stage maturity). Table 1. Weight, length, and diameter of okra fruits at different stages of maturity of A. esculentus L. fruits.
Fig. 1. Effect of different maturity stages on TPC of ethanolic okra fruit extracts. The results are representative of at least three independent experiments and are expressed as mean ± SEM (n=5). (*): Significant differences between treatment groups (p < 0.05).
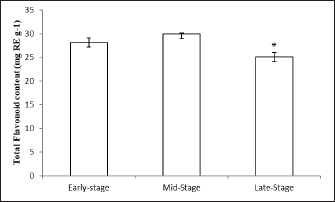
Fig. 2. Effect of different maturity stages on TFC of ethanolic okra fruit extracts. The results are representative of at least three independent experiments and are expressed as mean ± SEM (n=5). (*): Significant differences between treatment groups (p < 0.05). In fact, the TPCs and TFCs of okra fruits were significantly influenced by their ripening process. While the mid-ripe ethanolic extract of A. esculentus L. fruits contained the highest phenolic and flavonoid contents. DPPH radical-scavenging activityIn the present research, the antioxidant activity of okra was determined using the DPPH method. The results are highlighted in Figure 3. As regards, the percent inhibition of fruit extracts was determined. As shown in Figure 3, all ripening stages of okra fruits have a major anti-radical effect. In addition, the extract capacity was dependent on the maturation stage. Furthermore, the antioxidant capacities of okra fruits contribute partially to the health amelioration (Xia et al., 2015). The antioxidant capacities of okra fruit extracts were ranged from 60.7% ± 0.26% to 75.64% ± 0.79%. Wahyuningsih et al. (2021) suggested also that the ethanolic extract of red okra fruits has an important percent inhibition via the DPPH test.
Fig. 3. Effect of different ripening stages on radical scavenging activity of okra fruit. The results are representative of at least three independent experiments and are expressed as mean ± SEM (n=5). (*): Significant differences between treatment groups (p < 0.05).
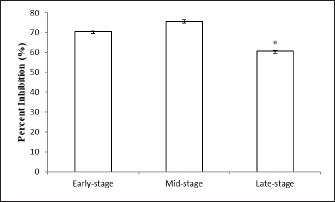
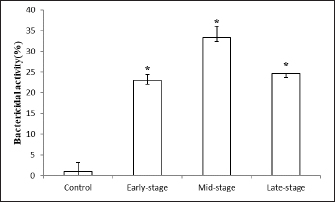
Fig. 4. Bactericidal activity (expressed as a percentage) of okra fruit extract against V. anguillarum depending on different maturity stages. The results are representative of at least three independent experiments and are expressed as mean ± SEM (n =5). (*): Significant differences between treatment groups (p < 0.05). All the antioxidant-tested activities of okra fruits varied depending on their maturity stages. While, the extract showed the highest antioxidant effect at a medium maturity, followed by fruits harvested at the beginning of maturity. While the DPPH radical scavenging activity of okra fruit was significantly lower at the late ripe stage (70.61% ± 0.15%). This result can be connected to the TPC that could be the origin of the antiradical capacity. These data are in correlation with previous results that indicate that several vegetal phenolic compounds are important sources of natural antioxidants (Khomsug et al., 2010). Bactericidal activityThe in vitro evaluation of A. esculentus L. fruits extracts bactericidal effect belonging to three maturity stages was assigned against V. anguillarum, fish pathogenic bacteria (Fig. 4). However, the results demonstrated that the viability of V. anguillarum was influenced by the fruit extract at all the tested stages. Then, as shown in Figure 4, the bactericidal capacity quantitatively varied depending on the maturity stages. Vibrio anguillarum showed a remarkable sensitivity to all okra fruit extracts. Then, the ethanolic extracts at the mid-maturity stage recorded the major inhibitory effect of the bacteria viability. Furthermore, at the three maturity stages the okra fruit extracts have noticeably affected the bacteria growth compared to the control. DiscussionThe length of fresh fruit is considered a major indicator for okra fruit harvest. Generally, the two sizes of okra fruits (small and big) are collected and consumed (Petropoulos et al., 2018). The increase in size and weight indicates an improvement in the quality of okra fruit during maturity (Uchoi et al., 2018). Besides, phenolic elements are considered crucial biomolecules in okra fruits (Hussain et al., 2012). In the same context, Shen et al. (2019) have shown that the main bioactive component in okra fruits is phenolic compounds. Whereas the fruit ripening stage had a marked influence on the secondary metabolite contents extracts. During the ripening of okra, various changes in phenolic level have been established ranging from 37.38 ± 4.22 mg GAE g−1 (late harvested fruits) to 43.27 ± 2.029 mg GAE g−1 (mid-stage maturation of fruits). Similar to our findings, previous research presented a similar level of TPC varied from 33.33 ± 0.02 to 37.38 ± 4.22 mg GAE g−1 (Mohammed et al., 2016). While, previous studies have reported a similar phenolic level in okra fruits (49.72 ± 2.24 mg GAE g−1) (Rohman et al., 2010). Otherwise, another study indicated a lower level of okra fruit phenolic content was indicated (13.456 mg GAE g−1) (Zainuddin et al., 2022). The difference in phenols concentration can also relied on the extraction technique and the polarity of the solvent used (Jing et al., 2015). In addition, these variations are often connected with the difference in geographical origin, the extraction technique used and the experimental protocol adopted (Benchikh et al., 2014). In the current study, the effect of ripening stages on flavonoids level was investigated. The medium stage of maturity reported the most important extractive yield of flavonoids (25.08 ± 0.93 g RE g−1). Our findings are in correlation with Shen et al. (2019) who highlighted the TFC variation of okra fruits during fruit maturity. Literature has explained these results by the dilution effect slowing the biosynthesis of new phenolic compounds which increase the size during ripening for fruits (Anand and Aradhya, 2005). Accordingly, with our results, Mohammed et al. (2016) indicated okra fruit flavonoid contents about 29.979 ± 0.036 mg RE g−1. In addition, similar flavonoid contents of okra fruits have been earlier expressed (31.21 ± 8.64 mg RE g−1) (Rohman et al., 2010). Whereas, Shen et al. (2019) noted that okra fruits flavonoid contents were 10 times lower than ours (2.95 ± 0.06 mg RE g−1). Generally, fluctuations in the reported flavonoid values can arise from several factors like nonrepresentative sampling, different cultivars, and different growing and, analytical conditions (Harnly et al., 2006). Besides, such variations in flavonoid contents might be due to a number of causes as well as genotype, maturity stages, environmental and post-harvest conditions techniques (Koh et al., 2009). In addition, previous research suggested, similarly, that small fruit (3–5 cm) had a higher nutritional value (Petropoulos et al., 2018). The antioxidant activity of A. esculentus L. fruits is correlated with the ripening stage. In that sense, our results showed variation in DPPH value among maturity process from 60.7% ± 0.26% to 75.64% ± 0.79%. Our findings are in accordance with Siddique et al. (2022), which highlighted that Pakistan fruit extract has a scavenging percentage of DPPH radicals around 13.487% lower than the Tunisian one. This fluctuation is caused by the existence of biomolecules such as quercetin and catechin which are more soluble in methanol as a solvent (Wahyuningsih et al., 2020). Numerous further studies have highlighted a directly proportional relationship between phenolic, and flavonoid contents and antioxidant effects of vegetable extracts (Abdel-Hameed, 2009; Shen et al., 2019). In addition, the high antioxidant capacity of okra fruit extract could be explained by its high content of phenolic compounds which function as singlet oxygen quenchers, reduction agents, and hydrogen donors (Reine et al., 2018). Otherwise, as already reported by Uchoi et al. (2018) antioxidant activity can increase depending on the maturation stage of fruits (between the beginning of okra maturity to the mid-ripe stage) contradictory to a previous study by Rekha et al. (2012) that revealed the reduction in phenolic contents and then in antioxidant capacity as shown between mid-ripe stage and the late one of okra fruits. Concerning the antibacterial activity, the inhibitory effect of A. esculentus L. fruits extracts against Gram-negative fish pathogenic bacteria (V. anguillarum) was illustrated. Because of its causes of fish diseases and important economic losses in the aquaculture industry, V. anguillarum was selected. Overall, at three maturity stages, A. esculentus L. fruit extracts were significantly active against V. anguillarum viability. This result was previously reported by Shaeroun et al. (2021), who detected the bactericidal effect of ethanolic extract of okra fruit against two Gram-negative bacterial pathogens (Klebsiella and Escherichia coli). The antibacterial capacity of okra fruit could be derived from the bioactive molecules present in okra fruits which show antibacterial capacity (Septianingrum et al., 2018). Furthermore, the major inhibitory effect was estimated at the middle maturity stage of fruits. Recently, this illustration was confirmed by the study of Aldhanhani et al. (2022) which detected that at all maturity stages the jujube fruit extracts showed growth inhibition against several bacteria at different degrees. However, these mentioned data were in accordance with those found by Ghazghazi et al. (2021) showing the antimicrobial potential of Eucalyptus marginata’s fruits was associated with the fruit ripening stage against four species of pathogenic bacteria. The current investigation estimated that mid-stage maturity fruits had the highest antibacterial capacity, followed by late-stage and early-stage maturity fruits respectively. This may be explained by the important phenolic compound content detected in mid-stage and late-stage fruits than that in early-stage maturity of okra fruits. ConclusionTo conclude, in the present research, noticeable fluctuations in A. esculentus L. fruits’ phenolic, flavonoid contents, and antioxidant activities were observed at three ripening stages. The results highlighted the okra fruit as an excellent source of phenolic compounds with a large amount of flavonoids and optimal antioxidant and antibacterial capacities in its mid-stage maturity. Therefore, okra fruits could be used as functional foods for industrial exploitation or for the production of dietary supplements. AcknowledgmentsSalma Guebebia thankfully acknowledges the scholarship assistance from Gabes University (Gabes, Tunisia). Authors contributionSG drafted the manuscript. LZ and AAM revise and edit the manuscripts. SG and MR took part in preparing and critically checking this manuscript. MAE, CER,` and SG performed the methodology. All authors have read and agreed to the published version of the manuscript. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no external funding. Data availabilityData is available upon request from the corresponding author. ReferencesAbdel-Hameed, E.S.S. 2009. Total phenolic contents and free radical scavenging of certain Egyptian Ficus species leaf samples. Food Chem. 114, 1271–1277. Aldhanhani, A.R.H., Ahmed, Z.F.R., Tzortzakis, N. and Singh, Z. 2022. Maturity stage at harvest influences antioxidant phytochemicals and antibacterial activity of jujube fruit (Ziziphus mauritiana Lamk. and Ziziphus spina-christi L.). Ann. Agric. Sci. 67(2), 196–203. Anand, P.K. and Aradhya, S.M. 2015. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chem. 93(2), 319–324. Bahroun, T., Gressier, B., Trotim, F., Brunet, C., Dime, T., Luyvkx, M., Vasseur, J., Cazin, M., Cazin, J.C. and Pinkas, M. 1996. Oxygen species scavenging activity of phenolic extracts from hawthom fresh plant organs and pharmaceutical preparations. Arzneimittelforschung 46, 1086–1089. Benchikh, Y., Louaileche, H., George, B. and Merlin, A. 2014. Changes in bioactive phytochemical content and in vitro antioxidant activity of carob (Ceratoniasiliqua L.) as influenced by fruit ripening. Ind. Crop. Prod. 60, 298–303. Dhruve, J.J., Shukla, Y.M., Shah, R., Patel, J. and Talati, J.G. 2015. Contribution of okra (Abelmoschus esculentus L.) seeds towards the nutritional characterization. World J. Pharm. Sci. 4, 1009–1023. Elik, A., Kocak, D. and Gogus, F. 2017. Optimization of microwave-assisted extraction of phenolics from organic strawberry using response surface methodology. Harran Tarım Gıda Bilimleri Derg. 21(2), 143–154. Erfan, A.M. and Marouf, S. 2019. Cinnamon oil downregulates virulence genes of poultry respiratory bacterial agents and revealed significant bacterial inhibition: an in vitro perspective. Vet. World 12(11), 1707–1715. Ghazghazi, H., Essghaier, B., Jawadi, I., Riahi, L., Ben Salem, R. and Rigane, G. 2021. Effects of fruit maturity stages on GC-FID fatty acid profiles, phenolic contents, and biological activities of Eucalyptus marginata L. J. Food Qual. 2021, 5546969. Habtamu, F., Ratta, N., Haki, G.D., Woldegiorgis, A.Z. and Beyene, F. 2014. Nutritional quality and health benefits of okra (Abelmoschus esculentus): a review. J. Food Sci. Qual. Manag. 33, 87–96. Harnly, J.M., Doherty, R.F., Beecher, G.R., Holden, J.M., Haytowitz, D.B., Bhagwat, S. and Gebhart, S. 2006. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 54, 9966−9977. Hussain, M.S., Fareed, S., Saba Ansari, M., Rahman, A., Ahmad, I.Z. and Saeed, M. 2012. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied. Sci. 4, 10–20. Jiang, N., Liu, C., Li, D., Zhang, Z., Liu, C., Wang, D., Niu, L. and Zhang, M. 2017. Evaluation of freeze drying combined with microwave vacuum drying for functional okra snacks: antioxidant properties, sensory quality, and energy consumption. LWT Food Sci. Technol. 82, 216–226. Jing, L., Ma, H., Fan, P., Gao, R. and Jia, Z. 2015. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complem. Altern. Med. 15, 1–12. Khomsug, P., Thongjaroenbuangam, W., Pakdeenarong, N., Suttajit, M. and Chantiratikul, P. 2010. Antioxidative activities and phenolic content of extracts from okra (Abelmoschus esculentus L.). Res. J. Biol. Sci. 5, 310–313. Koh, E., Wimalasiri, K.M.S., Chassy, A.W. and Mitchell, A.E. 2009. Content of ascorbic acid, quercetin, kaempferol and total phenolics in commercial broccoli. J. Food Comp. Anal. 22, 637–643. Lengsfeld, C., Titgemeyer, F., Faller, G. and Hensel, A. 2004. Glycosylated compounds from okra inhibit adhesion of Helicobacter pylori to human gastric mucosa. J. Agric. Food Chem. 52, 1495–1503. Mohammed, M.I., Bayero, A.S. and Shettima, U.I. 2016. Levels of total phenolic and flavonoids in Abelmoschus esculentus L. from some irrigation areas of kano state-Nigeria. Bayero J. Pure Appl. Sci. 9(2), 121–124. Petropoulos, S., Fernandes, A., Barros, L. and Ferreira, I.C.F.R. 2018. Chemical composition, nutritional value and antioxidant properties of Mediterranean okra genotypes in relation to harvest stage. Food Chem. 242, 466–474. Piloo, N. and Kabir, J. 2011. Effect of age of harvest on fruit quality of okra (Abelmoschus esculentus L.). J. Environ. Res. Develop. 5(3), 615–622. Poobalan, V., Praneetha, S., Arumugam, T., Kumaravadivel, N. and Jeyakumar, P. 2019. Medicinal properties of vegetable crops. Int. J. Chem. Stud. 7(5), 1538–1542. Reine, B.G.S., Houndekon, B.A.P., Marcelline, G., Pascal, A.D.C., Worou, C.N., Hounankpon, Y. and Félicien, A. 2018. Phytochemical composition and antioxydant capacity of Abelmoschus esculentus L. fresh immature fruits. Am. J. Food Sci. Technol. 6(5), 223–227. Rekha, C., Poornima, G., Manasa, M., Abhipsa, V., Pavithra Devi, J., Vijay Kumar, H.T. and Prashith Kekuda, T.R. 2012. Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. Chem. Sci. Trans. 1(2), 303–310. Rohman, A., Riyanto, S., Yuniarti, N., Saputra, W.R., Utami, R. and Mulatsih, W. 2010. Antioxidant activity, total phenolic, and total flavonoid of extracts and fractions of red fruit (Pandanus conoideus Lam). Int. Food Res. J. 17, 97–106. Saha, D., Jain, B. and Jain, V.K. 2011. Phytochemical evaluation and characterization of hypoglycemic activity of various extracts of Abelmoschus esculentus Lin. fruit. Int. J. Pharm. Pharm. Sci. 3(2), 183–185. Septianingrum, N.M., Hapsari, W. and Syariffudin, A. 2018. Identifikasi Kandungan Fitokimia Ekstrak Okra Merah (Abelmoschus esculentus) dan Uji Aktivitas Antibiotik Terhadap Bakteri Escherichia coli. J. Insan. Farm. Indones. 1(2), 170–177. Shaeroun, E., Alshebani, A.M.A., Rashed, A. and Oshkondali, S.T.M. 2021. Phytochemical screening and antibacterial activity of okra extract. S. Asian Res. J. Biol. Appl. Biosci. 3(4), 51–56. Shen, D.D., Li, X., Qin, Y.L., Li, M.T., Han, Q.H., Zhou, J. and Wu, D.T. 2019. Physicochemical properties, phenolic profiles, antioxidant capacities, and inhibitory effects on digestive enzymes of okra (Abelmoschus esculentus) fruit at different maturation stages. J. Food Sci. Technol. 56(3), 1275–1286. Siddique, M.H., Ashraf, A., Hayat, S., Aslam, B., Fakhar-e-Alam, M., Muzammil, S., Atif, M., Shahid, M., Sulman Shafeeq, S. Afzal, M. and Ahmad, S. 2022. Antidiabetic and antioxidant potentials of Abelmoschus esculentus: in vitro combined with molecular docking approach. J. Saudi Chem. Soc. 26, 101418. Singleton, V.I. and Lamuela-Raventos, R.M. 2012. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 15, 152–178. Solomon, S., Muruganatham, N. and Senthamiselvi, M. 2016. Anticancer activity of Abelmoschus esculentus (flower) against human liver cancer. Int. J. Pharmacol. Biol. Sci. 6, 145–157. Stevens, M.G., Kehrli, M.E. and Canning, P.C. 1991. A colorimetric assay for quantitating bovine neutrophil bactericidal activity. Vet. Immunol. Immunopathol. 28, 45–56. Sultana, B., Anwar, F. and Ashraf, M. 2009. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 14, 2167–2180. Uchoi, J., Singh, R.S., Bhargava, R., Krishna, H., Venkatesan, K. and Kumar, A. 2018. Effect of harvesting of fruits at different stage of maturity on nutritional quality of Kinnow under Hot Arid Zone of North western India. Green Farming Int. J. 9(2), 379–382. Umme, A., Ksenia, M., Giovanna, F. and Matteo, S. 2023. Apples and apple by-products: antioxidant properties and food applications. Antioxidants 12(7), 1456. Velazquez, E., Tournier, H.A., Mordujovich de Buschiazzo, P., Saavedra, G. and Schinella, G.R. 2003. Antioxydant activity of Paraguayan plant extracts. Fitoterapia 74, 91–97. Wahyuningsih, S.P.A., Winarni, D., Pramudya, M., Setianingsih, N., Mwendolwa, A.A. and Nindyasari, F. 2021. Antioxidant potential of red okra pods (Abelmoschus esculentus Moench). EPiC Series Biol. Sci. 1, 158–163. Wahyuningsih, S.P.A., Savira, N.I.I., Anggraini, D.W., Winarni, D., Suhargo, L., Kusuma, B.W.A., Nindtasari, F., Setianingsih, N. and Mwendolwa, A.A. 2020. Antioxidant and nephroprotective effects of okra pods extract (Abelmoschus esculentus L.) against lead acetate-induced toxicity in mice. Scientifica 2020, 4237205. Xia, F., Zhong, Y., Li, M., Chang, Q., Liao, Y., Liu, X. and Pan, R. 2015. Antioxidant and anti-fatigue constituents of okra. Nutrients 7(10), 8846–8858. Zainuddin, A., Sabilu, Y., Majid, R., Pratiwi, A. and Jafriati, D. 2022. Analysis of phytochemical compounds, total phenolic content, and antioxidant activity test of ethanol extract of okra (Abelmoschus esculentus L.) from the traditional market of Kendari. J. Hunan Univ. (Nat. Sci.) 49(7), 89–95. Zhang, T., Xiang, J., Zheng, G., Yan, R. and Min, X. 2018. Preliminary characterization and anti-hyperglycemic activity of a pectic polysaccharide from okra (Abelmoschus esculentus (L.) Moench). J. Funct. Food 41, 19–24. | ||
| How to Cite this Article |
| Pubmed Style Guebebia S, Mohamed AA, Espinosa-ruiz C, Esteban M�, Zourgui L, Romdhane M. Phytochemical compounds, antiradical capacity, and in vitro inhibitory effect against fish pathogenic bacteria of okra fruits (Abelmoschus esculentus L.) at different maturity stages. Open Vet. J.. 2023; 13(12): 1562-1569. doi:10.5455/OVJ.2023.v13.i12.6 Web Style Guebebia S, Mohamed AA, Espinosa-ruiz C, Esteban M�, Zourgui L, Romdhane M. Phytochemical compounds, antiradical capacity, and in vitro inhibitory effect against fish pathogenic bacteria of okra fruits (Abelmoschus esculentus L.) at different maturity stages. https://www.openveterinaryjournal.com/?mno=159593 [Access: January 14, 2026]. doi:10.5455/OVJ.2023.v13.i12.6 AMA (American Medical Association) Style Guebebia S, Mohamed AA, Espinosa-ruiz C, Esteban M�, Zourgui L, Romdhane M. Phytochemical compounds, antiradical capacity, and in vitro inhibitory effect against fish pathogenic bacteria of okra fruits (Abelmoschus esculentus L.) at different maturity stages. Open Vet. J.. 2023; 13(12): 1562-1569. doi:10.5455/OVJ.2023.v13.i12.6 Vancouver/ICMJE Style Guebebia S, Mohamed AA, Espinosa-ruiz C, Esteban M�, Zourgui L, Romdhane M. Phytochemical compounds, antiradical capacity, and in vitro inhibitory effect against fish pathogenic bacteria of okra fruits (Abelmoschus esculentus L.) at different maturity stages. Open Vet. J.. (2023), [cited January 14, 2026]; 13(12): 1562-1569. doi:10.5455/OVJ.2023.v13.i12.6 Harvard Style Guebebia, S., Mohamed, . A. A., Espinosa-ruiz, . C., Esteban, . M. �., Zourgui, . L. & Romdhane, . M. (2023) Phytochemical compounds, antiradical capacity, and in vitro inhibitory effect against fish pathogenic bacteria of okra fruits (Abelmoschus esculentus L.) at different maturity stages. Open Vet. J., 13 (12), 1562-1569. doi:10.5455/OVJ.2023.v13.i12.6 Turabian Style Guebebia, Salma, Abdalla A. Mohamed, Cristóbal Espinosa-ruiz, Maria Ángeles Esteban, Lazhar Zourgui, and Mehrez Romdhane. 2023. Phytochemical compounds, antiradical capacity, and in vitro inhibitory effect against fish pathogenic bacteria of okra fruits (Abelmoschus esculentus L.) at different maturity stages. Open Veterinary Journal, 13 (12), 1562-1569. doi:10.5455/OVJ.2023.v13.i12.6 Chicago Style Guebebia, Salma, Abdalla A. Mohamed, Cristóbal Espinosa-ruiz, Maria Ángeles Esteban, Lazhar Zourgui, and Mehrez Romdhane. "Phytochemical compounds, antiradical capacity, and in vitro inhibitory effect against fish pathogenic bacteria of okra fruits (Abelmoschus esculentus L.) at different maturity stages." Open Veterinary Journal 13 (2023), 1562-1569. doi:10.5455/OVJ.2023.v13.i12.6 MLA (The Modern Language Association) Style Guebebia, Salma, Abdalla A. Mohamed, Cristóbal Espinosa-ruiz, Maria Ángeles Esteban, Lazhar Zourgui, and Mehrez Romdhane. "Phytochemical compounds, antiradical capacity, and in vitro inhibitory effect against fish pathogenic bacteria of okra fruits (Abelmoschus esculentus L.) at different maturity stages." Open Veterinary Journal 13.12 (2023), 1562-1569. Print. doi:10.5455/OVJ.2023.v13.i12.6 APA (American Psychological Association) Style Guebebia, S., Mohamed, . A. A., Espinosa-ruiz, . C., Esteban, . M. �., Zourgui, . L. & Romdhane, . M. (2023) Phytochemical compounds, antiradical capacity, and in vitro inhibitory effect against fish pathogenic bacteria of okra fruits (Abelmoschus esculentus L.) at different maturity stages. Open Veterinary Journal, 13 (12), 1562-1569. doi:10.5455/OVJ.2023.v13.i12.6 |