| Research Article | ||
Open Vet. J.. 2023; 13(10): 1308-1317 Open Veterinary Journal, (2023), Vol. 13(10): 1308–1317 Original Research Clinical signs associated with prostatic disorders in canines: Retrospective study in Uruguay (2011–2019)Guillermo Cazzuli1*, Gonzalo Suárez1, Stefania Busconi1, Juan Pablo Damián2, and Paula Pessina11Departamento de Clínicas y Hospital Veterinario, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay 2Departamento de Biociencias Veterinarias, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay *Corresponding Author: Guillermo Cazzuli. Departamento de Clínicas y Hospital Veterinario, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay. Email: guillecazzu [at] hotmail.com Submitted: 20/07/2023 Accepted: 18/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
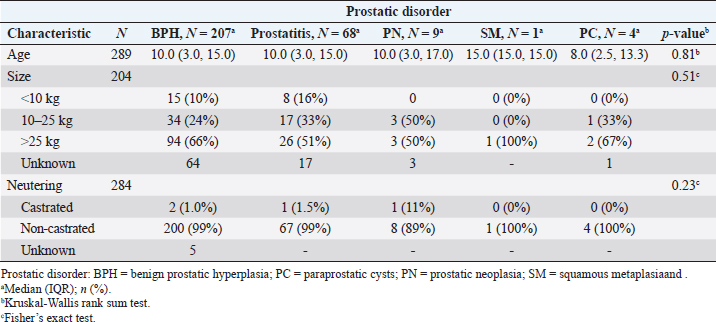
AbstractBackground: Prostate disease represents about 0.7% of diseases in canines. The main diagnosed pathology is benign prostatic hyperplasia (BPH). However, the reports that study the association of a certain clinical sign with a specific prostate disease are scarce. Aim: The main objective of this study was to evaluate the clinical relevance of the most commonly observed clinical signs associated with the different prostatic disorders in canines admitted to the hospital of the Facultad de Veterinaria–Universidad de la República between 2011 and 2019. Methods: This retrospective study included 7,729 male canines treated at the hospital de la Facultad de Veterinaria–Universidad de la República (Montevideo, Uruguay) between 2011 and 2019. 289 canines with a presumptive/definitive diagnosis of prostate diseases were selected, recording the presence/absence of associated clinical signs. Results were reported in terms of odds ratios (ORs) using logistic regression (p < 0.05). Results: The five most frequently reported clinical signs were tenesmus (34%), anorexia (32%), lethargy (27%), prostatomegaly or pain during rectal examination (25%), and abdominal pain from palpation (22%). Diarrhea (3.39 vs. 0.33 OR), anorexia (2.07 vs. 0.39 OR), weight loss (2.27 vs. 0.27 OR), hematuria (3.25 vs. 0.44 OR), and urinary incontinence (2.96 vs. 0.33 OR) indicated a highest predictive value (p < 0.05) with prostatitis versus BPH, respectively. Being weight loss, the clinical sign is more frequently associated with neoplasia (20.2 OR, p=0.002). Conclusion: This study shows that there are clinical signs with a higher degree of association for certain canine prostatic disorders than others. Keywords: Canine, Clinical signs, Odds ratio, Prostatic diseases. IntroductionThe prostate is the only accessory sex gland of the canine reproductive system (Christensen, 2018). In non-castrated male canines, the weight and size of the gland gradually increase with age, and for this reason, the disorder manifests itself in canines older than 6 years (Barsanti and Finco, 1986; Smith, 2008). Testosterone has a key role in the development and predisposition of prostate diseases (Cunto et al., 2019). A fact that highlights the role of testosterone in canine prostatic disorders is that the sharp drop in this hormone in the first week post-castration is accompanied by a significant decrease in the volume and prostatic dimensions assessed by ultrasound (Cazzuli et al., 2022). Although information on prostatic disorders is abundant (Smith, 2008; Christensen, 2018; Cunto et al., 2019; Cunto et al., 2022), there is not much information from population studies on the prevalence of these disorders in canines (Krawiec and Heflin, 1992; Polisca et al., 2016). In canines, a retrospective study conducted by Polisca et al. (2016) in France, showed that benign prostatic hyperplasia (BPH) was the most common prostatic disorder (50%), followed by prostatitis (38.5%). Other prostatic disorders, such as abscesses, neoplasia, cysts, and squamous metaplasia (SM), are usually present, although less frequently (Barsanti and Finco, 1986; Polisca et al., 2016; Cunto et al., 2019). Although biopsy for histopathology is necessary for the definitive diagnosis of these pathologies (Palmieri et al., 2022), it is not always used because it is a very invasive technique (Smith, 2008). In this sense, the combination of fine needle aspiration cytology and B-mode ultrasonography has been reported to have high diagnostic value in prostatic disorders (Rodak et al., 2018). The presumptive diagnosis of prostatic disorders can be reached based on the anamnesis, clinical signs, physical examination, ultrasound findings, and laboratory findings such as serum biochemistry, complete blood count, biomarkers, and semen evaluation (Ruetten et al., 2021; Cunto et al., 2022). Canine prostatic disorders can share many clinical signs, including tenesmus, flattened stools, hematuria, and blood dripping from the urethra (Ruetten et al., 2021), which makes the etiological diagnosis based solely on clinical signs difficult (Christensen, 2018; Cunto et al., 2019). Polisca et al. (2016) observed that in animals with prostatitis/prostatic abscesses and neoplasia, urinary, systemic, and gastrointestinal signs occurred more frequently compared to animals with BPH. However, to the best of our knowledge, no studies have determined the degree of association (odd ratio) of clinical signs in relation to each of the canine prostatic disorders. We hypothesize that the clinical signs are associated with the diagnosis of each prostatic disorder, evidencing a lower probability of observing clinical signs such as tenesmus, anorexia, hematuria, urinary incontinence, lethargy, pain on abdominal, and/or rectal palpation in BPH than in prostatitis. Therefore, the objective of this study was to evaluate the clinical relevance of the main signs associated with the different prostatic disorders in canines who were admitted to the hospital of the Facultad de Veterinaria–Universidad de la República (FVET–UdelaR) between 2011 and 2019. As a secondary objective, we set out to describe the prevalence of each prostatic disorder, age, body size, and reproductive status (neutered vs. non-neutered) in the canine population with prostatic disease studied during the same period. Materials and MethodsMedical recordsThis is a retrospective study. All records (clinical records) of canines admitted at the Hospital of the Facultad de Veterinaria–Universidad de la República (Montevideo, Uruguay) between 2011 and 2019 were reviewed. Inclusion criteriaFirst, all the medical records that matched the following inclusion criteria were selected: species (canine), sex (male), presumptive or definitive diagnosis of prostatic disorder. The presumptive diagnosis of a prostatic disorder was reached when the patient had an anamnesis, clinical signs, and clinical examination consistent with said prostatic disorder (Smith, 2008; Lévy et al., 2014; DiBartola and Westropp, 2019), laboratory findings (e.g., blood biochemistry, urinalysis, urine culture, and prostate cytology) consistent with the suspected disorder (Zinkl, 2008; Teske, 2009; Lacreta et al., 2012) and B-mode ultrasonography of the prostate to assess echostructure of the parenchyma and dimensions compatible with a disorder (Mattoon and Davidson, 2020). No patient had a prostatic biopsy. A definitive diagnosis of a prostatic disorder was considered when the patient had a cytological report by fine needle aspiration added to a B-mode ultrasound image compatible with said prostatic disorder (Rodak et al., 2018). Only the cases of SM and prostatic neoplasia (PN) were considered definitive diagnoses based on anamnesis, clinical signs, laboratory findings, ultrasound images, and compatible cytology. The remaining diagnoses were presumptive. All the canines included in this study received treatment for the diagnosed prostatic disorder, confirming clinical improvement in all cases. From these records, other specific descriptive data were recorded, including age, breed, size, reproductive status (castrated/non-castrated), the reason for consultation, anamnestic data provided by the owner, clinical signs, complementary tests performed, and description of the ultrasound study. In this study, the inclusion of the breed variable was disregarded since 87% of the medical records belonged to mongrel animals, or the breed was not determined. Classification of physiological variablesAge was reported in years. The size was grouped into three categories: <10 kg (small size), ≥10, and ≤25 kg (median size); >25 kg (large size), according to what was reported by Ruel et al. (1998). Description of the reported clinical signsThe clinical signs recorded in patients with a presumptive or definitive diagnosis of prostatic disorder were grouped based on Polisca et al. (2016) but with modifications, in three categories: digestive signs (tenesmus, flat stools, constipation, vomiting, diarrhea, hematochezia, dyschezia, anorexia, and weight loss); urinary signs (hematuria, blood dripping from the urethra, pollakiuria, stranguria, dysuria, anuria, urinary incontinence, and polyuria/polydipsia); and “other signs” (presence or absence of hindlimb weakness, difficulty with locomotion, perineal hernia, abdominal pain from palpation, prostatomegaly or pain during rectal examination, and lethargy). Classification of the different prostatic disordersThe following five prostatic disorders were considered: BPH, with and without the presence of cysts according to the ultrasound report; prostatitis, SM; PN; and paraprostatic cysts (PCs). These prostatic disorders were defined according to anamnesis, clinical signs, physical examination, laboratory findings, and B-mode ultrasonography compatible with each disorder, already described in the inclusion criteria. B-mode ultrasound evaluations consisted of abdominal and pelvic examinations to locate the bladder and, subsequently, to identify the prostate. Images were captured to determine prostate volume by the ellipse formula: length × width × height × 0.523 (Ruel et al., 1998; Cazzuli et al., 2022). In the sagittal position, the length was measured as the maximum diameter of the gland along the urethral axis and the height as the maximum diameter perpendicular to the length axis. In the transverse position, the width was measured as the maximum diameter from the prostate measured from right to left (Mattoon and Davidson, 2020). Prostatic disorders that occurred infrequently (≤5%) were excluded from further analysis. Statistical analysisThe prevalence (%) of prostatic diseases in the study population was defined by the number of canines (n) that experienced a prostatic disorder in relation to the total number of canines admitted in the hospital of the FVET–UdelaR consultation during the researched period. The prevalence (%) of different prostatic disorders was calculated as the number of canines experiencing a diagnosed prostatic disorder (BPH, prostatitis, SM, PN, and PC) relative to the total number of canines that were recorded with prostatic diseases. Statistical differences in age, size, and neutering by prostatic disorder were calculated using the Kruskal-Wallis rank sum test or Fisher’s exact test. The associations of clinical signs with each prostatic disorder (BPH, prostatitis, and PN) were examined using multiple logistic regression analysis (explanatory variables: digestive, urinary, or other signs). The results were reported in terms of frequency (%), odds ratios (ORs), 95% confidence interval (CI), and p-value (<0.05 was considered significant). All analyses were performed in R (Version 4.2.2, 2022) and Rstudio (version 2022.12.0 Build 353) (Rstudio Team, 2022). Ethical approvalNot needed for this study. ResultsPopulation dataOf the total medical records ( n=7729) in 9 years of care at the hospital of the FVET–UdelaR, the recorded prostatic disease prevalence was 3.7% (n=289). The prevalence of different prostatic disorder was greater for BPH 71.6% (n=207), followed by prostatitis 23.5% (n=68), PN 3.1% (n=9), PC 1.3% (n=4), and SM 0.3% (n=1) (Chi-Square 1,796, df 5, p < 0.01). In turn, 66% of the BPH cases had cystic structures present. The average age of presentation of the prostatic disease was 10 years (ranging from 3 to 15 years), with 62% (n=126) corresponding to animals of large size, 27% (n=55) to animals of medium size and 11% (n=23) to animals of small size. 99% of canines (n=280) were linked to a non-castrated reproductive status. Table 1 shows that prostatic disorders had no association with the variables age (Kruskal-Wallis, p=0.81), size (Fisher’s exact test, p=0.51), and castrated or non-castrated status (Fisher’s exact test, p=0.23). Table 2 indicates the frequency of clinical signs within each explanatory variable (digestive, urinary, or other signs) for all canines registered with prostatic disease without considering the diagnosed prostatic disorder (n=289). In the set of explanatory variables, the most relevant clinical signs (>20%) in order of frequency of presentation were tenesmus, anorexia, lethargy, prostatomegaly, abdominal pain from palpation, and perineal hernia. Prostatic disorder and clinical signs observedOf the 289 canines evaluated, there were 28 individuals with a diagnosis of prostatic disease but with no clinical signs. Of those with clinical signs (n=261), digestive (48%, 45%, 52%), urinary (18%, 19%, and 6%), and other signs (35%, 36%, and 42%) were more prevalent for BPH, prostatitis and PN, respectively. Table 1. Prostatic disorders (BPH, prostatitis, PN, SM, and PC) in canines from the retrospective study (2011–2019) according to age, size (<10 kg; ≥10 and ≤25 kg; and >25 kg), and neutering (castrated/non-castrated).
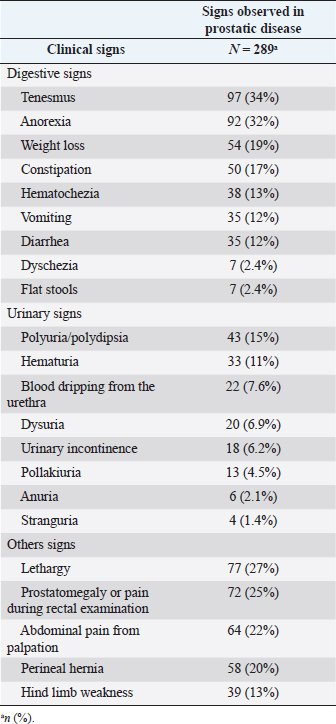
Table 2. Frequency of clinical signs (%) by explanatory variable (digestive, urinary, and others) in canines with prostatic disease in the retrospective study (2011–2019).
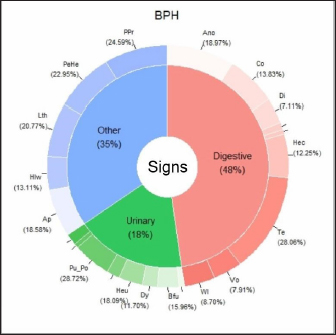
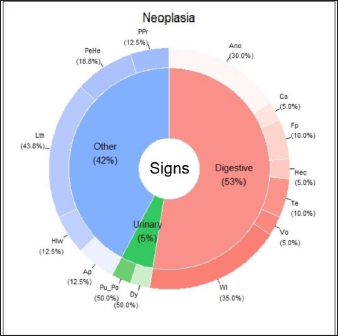
The main clinical signs of BPH, prostatitis, and PN are shown in Figures 1,–3. These three figures demonstrate a wide range of clinical signs associated with the three main prostatic disorders. Digestive signs were the most frequent for BPH (Fig. 1). Of the digestive signs, the most frequent clinical signs in BPH were tenesmus, followed by anorexia, constipation, and hematochezia. The “other signs” category was also frequent, with prostatomegaly, pain during rectal examination, perineal hernia, and lethargy being the clinical signs that occurred most frequently. Of the urinary signs, polyuria/polydipsia was the most frequent, followed by hematuria and blood dripping from the urethra.
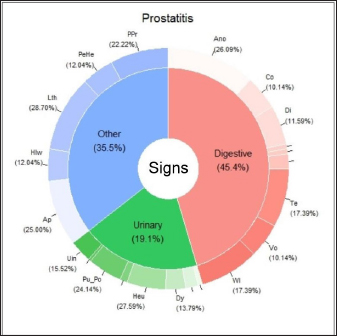
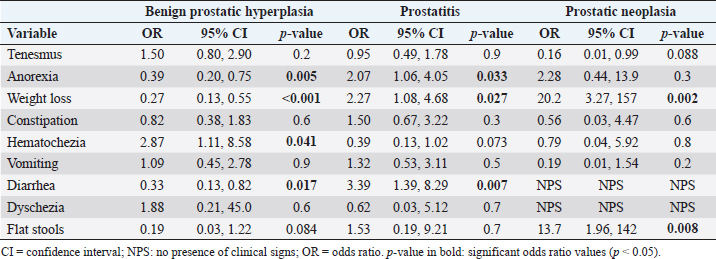
Fig. 1. Percentage of each sign (digestive, urinary, and others) affected and associated clinical signs for BPH. Clinical signs: Ano: anorexia; Co: constipation; Di: diarrhea; Hec: hematochezia; Te: tenesmus; Vo: vomiting; Wl: weight loss Bfu: blood dripping from the urethra; Dy: dysuria; Heu: hematuria; Pu_Po: polyuria/polydipsia; Ap: abdominal pain from palpation; Hlw: hind limb weakness; Lth: lethargy; PeHe: perineal hernia; and PPr: prostatomegaly or pain when rectal examination. The line without signs (-) indicates clinical signs that are not included because they appear in less than 5%. In the case of prostatitis (Fig. 2), the frequency of signs categories was similar (digestive signs, other signs, and urinary signs, respectively), but there were variations in the prevalence within each category. Of the digestive signs, anorexia became the most frequent, followed by tenesmus and weight loss. Lethargy and abdominal pain from palpation were the most frequently seen in the category of “other signs,” followed by prostatomegaly or pain during the rectal examination. In the case of PN (Fig. 3), weight loss and anorexia were the most frequent digestive signs observed. Nearly half of the “other signs” category consisted of lethargy, followed in similar proportions by perineal hernia, abdominal pain from palpation, hind limb weakness, and prostatomegaly or pain during rectal examination. Urinary signs constituted a lower percentage, with only polyuria/polydipsia and dysuria signs observed. Main associated clinical signsThe ORs for prostatic disorders adjusted for clinical associations such as digestive, urinary, and “other signs” are presented in Tables 3–5, respectively. After adjusting for associated digestive signs (Table 3), we noticed that the OR for anorexia (p=0.033), weight loss (p=0.027), and diarrhea (p=0.007) were very high for prostatitis. The OR for hematochezia (p=0.041) increased only for BPH. In the case of PN, the main clinical signs were strongly associated with flat stools (p=0.008) and weight loss (p=0.002). Patients with PN did not present clinical signs of diarrhea and dysquezia.
Fig. 2. Percentage of each sign (digestive, urinary, and others) affected and associated clinical signs for prostatitis. Clinical signs: Ano: anorexia; Co: constipation; Di: diarrhea; Te: tenesmus; Vo: vomiting; Wl: weight loss; Dy: dysuria; Heu: hematuria; Pu_Po: polyuria/polydipsia; Uin: Urinary incontinence; Ap: abdominal pain from palpation; Hlw: hind limb weakness; Lth: lethargy; PeHe: perineal hernia; and PPr: prostatomegaly or pain during rectal examination. The line without signs (-) indicates clinical signs that are not included because they appear in less than 5%.
Fig. 3. Percentage of each sign (digestive, urinary, and other) affected and associated clinical signs for the PN. clinical signs: Ano: anorexia; Co: constipation; Fp: flat stools; Hec: hematochezia; Te: tenesmus; Vo: vomiting; Wl: weight loss; Dy: dysuria; Pu_Po: polyuria/polydipsia; Ap: abdominal pain from palpation; Hlw: hind limb weakness; Lth: lethargy; PeHe: perineal hernia; and PPr: prostatomegaly or pain when rectal examination. Table 3. OR, CI (95% CI), and significance (p-value) of digestive signs in BPH, prostatitis, and PN.
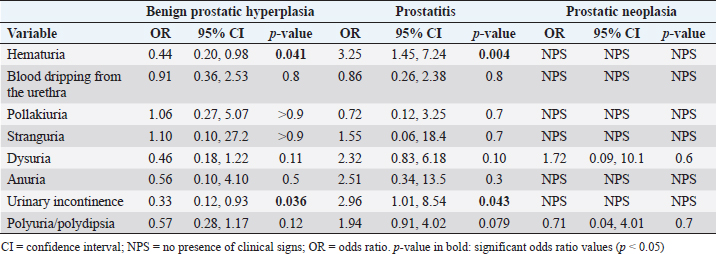
A similar pattern can be described for urinary signs (Table 4), where the OR of hematuria (p=0.004) and urinary incontinence (p=0.043) were strongly associated with prostatitis. In the rest of the prostatic disorders, no tendency towards any particular sign was displayed. Patients with PN did not present urinary signs such as hematuria, blood dripping from the urethra, pollakiuria, stranguria, anuria, or urinary incontinence. Table 4. OR, CI (95% CI), and significance (p-value) of urinary signs in BPH, prostatitis, and PN.
Table 5. OR, CI (95% CI), and significance (p-value) of other signs in BPH, prostatitis, and PN.
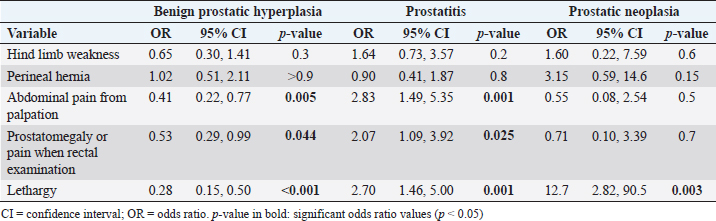
Finally, regarding “other signs” category (Table 5), we observed that the OR was almost three times higher for abdominal pain from palpation (p=0.001) and lethargy (p=0.001), and two times higher for prostatomegaly or pain during rectal examination (p=0.025) for prostatitis. In the case of PN, only lethargy (p=0.003) seemed to be relevant and was strongly associated with this prostatic disorder. DiscussionThis study is the first to show that there are clinical signs with a greater degree of association for certain prostatic disorders in canines. Although several clinical signs are common and overlap in different prostatic disorders, signs such as diarrhea, anorexia, weight loss, hematuria, and urinary incontinence have been shown to have a higher ORs for prostatitis than for animals with BPH. However, hematochezia had a higher predictive value for BPH, not being a significant sign in cases of prostatitis. On the other hand, weight loss and lethargy were clinical signs strongly associated with PN. Thus, this study contributes from another perspective to the one provided by Polisca et al. (2016), who compared the frequency of clinical signs observed in prostatic disorders. Furthermore, unlike the report by Polisca et al. (2016), our study shows that even within each group of signs (digestive, urinary, or systemic), some of them are more associated with a prostatic disorder than others. For example, within digestive signs, diarrhea was largely associated with prostatitis, while hematochezia was more strongly associated with BPH. Therefore, our study shows that there are specific signs (and not necessarily a group of clinical signs) that are greatly associated with certain prostatic disorders in canines. In addition, this 9-year retrospective study on prostatic disorders in canines conducted in Latin America (Uruguay), expands the information available from other epidemiological studies conducted in other regions of the world (Krawiec and Heflin, 1992; Polisca et al., 2016). Briefly, in our country, BPH was the canine prostatic disorder most frequently diagnosed, followed by prostatitis and, less frequently, PN, PCs, and SM. Besides, prostatic diseases occurred in large, non-castrated canines, with a mean age of 10 years, which agrees with what was reported in the bibliography (Smith, 2008; Foster, 2012; Polisca et al., 2016; Cunto et al., 2019). Our main objective was to evaluate the clinical relevance of the main clinical signs associated with the different prostatic disorders in canines who were admitted to the hospital of the FVET–UdelaR between 2011 and 2019. Based on the results of this study, we were able to confirm our hypothesis. From the analysis of the clinical signs and their association with the different canine prostatic disorders, we were able to observe that our results could contribute to the differentiation of prostatic disorders. Thus, urinary signs (hematuria and urinary incontinence) were strongly associated with prostatitis. Animals with suspected prostatic diseases and hematuria were three times more likely to present with prostatitis than any other prostatic disease. In turn, the OR for urinary incontinence was almost three times greater for prostatitis than for any other prostatic disorder. It is possible that urinary signs are more frequently observed in prostatitis because it often shares infection with the bladder and urethra (Lévy et al., 2014). In addition, if prostatitis becomes chronic, recurrent clinical signs of cystitis and urinary tract inflammation may be the only signs observed in the patient (Barsanti and Finco, 1986). Within the digestive signs, tenesmus was one of the most frequently observed clinical signs in prostatic diseases, not being associated with any particular prostatic disorder. This is to be expected due to the anatomical position of the prostate, located ventrally to the colon (Kutzler and Yeager, 2005; Smith, 2008), and because all prostatic disorders present with prostatomegaly causing the colonic lumen to decrease in size due to pressure from the diseased prostate gland (Christensen, 2018). The effort to defecate could cause small lacerations at the level of the anal sphincter and cause the appearance of hematochezia. This could explain why, in our study, hematochezia could only be associated with BPH, being almost three times more likely to be seen in canines with BPH suspected of prostatic disorder. Although anorexia was associated with both BPH and prostatitis, the association was much stronger for the latter. The OR that a canine with anorexia and suspected of having prostatic diseases had prostatitis was two times higher than that of having any other prostatic disorder. Anorexia could be explained by lethargy, another sign also associated with the main prostatic disorders (BPH, prostatitis, and neoplasia), but more strongly associated with prostatitis. Another possible explanation for anorexia in animals with prostatitis could be the strong pain caused by the gland’s inflammation, described in this work as abdominal pain from palpation, making it almost three times more likely that a patient with pain and suspicion of prostatic diseases had prostatitis. All clinical signs of prostatitis found coincided with what had previously been reported in other studies (Barsanti and Finco, 1986; Smith, 2008; Lévy et al., 2014). Weight loss and lethargy were clinical signs observed with high frequency and were highly associated with PN, although to a lesser extent, it was also associated with prostatitis. In this sense, canines suspected of having a prostate disease that presents weight loss are 20 times more likely to be suffering from PN versus prostatitis, and 12 times more likely that the canine has PN versus prostatitis if you have lethargy. These results could be expected due to the systemic effects produced by the PN and how advanced it usually is at the time of diagnosis. PN often presents regional and/or distant metastases at the time of consultation, causing a lot of pain and multi-organ failure (Teske, 2009; Lévy et al., 2014). In summary, prostatitis and PN were the disorders to which some signs could be more strongly associated. Prostatitis was characterized by a greater number of clinical signs related to inflammation and pain, and a greater systemic repercussion in the animal. PN presented an increase in clinical signs that were related to digestive and other processes linked to general systemic repercussions to the detriment of urinary signs. The information obtained in this study may be useful for the veterinarian, contributing to the presumptive diagnosis of canine prostatic disease based on the association of clinical signs that patients present, once a prostatic disorder is presumed based on the support of the B-mode ultrasonographic study. This will allow the veterinarian to prioritize the choice of the best complementary diagnostic method that will contribute to obtaining a definitive diagnosis. Our secondary objective was to describe the prevalence of prostatic disorders, age, body size, and reproductive status of the canine population under study. This study was carried out with canines that attended the veterinary clinic with a prostatic disorder and clinical signs compatible with it. Only a small proportion of the canines in this study were asymptomatic, which were considered to have some prostatic disorder based on B-mode ultrasonography imaging consistent with an enlarged prostate and echostructure consistent with prostatic disorder (Cunto et al., 2019; Mattoon and Davidson, 2020) and is reported in the results. Some canines are known to have BPH and are asymptomatic (Christensen, 2018; Cunto et al., 2022) until prostatic enlargement is sufficient to produce clinical signs (Smith, 2008). For this reason, we must be cautious since it is likely that patients with asymptomatic prostatic disorders are older, since dogs rarely come to the FVET–UdelaR hospital for prostate control without clinical signs compatible with prostatic disorder. The population of canines with prostate disease in our study was mostly represented by medium-sized and large-size canines, non-castrated and with a mean age that far exceeds that reported in the bibliography (Polisca et al., 2016; Cunto et al., 2022). The prostatic disorder that occurred most frequently was BPH, followed by prostatitis, which coincides with what has already been reported internationally (Johnston et al., 2001; Smith, 2008; Foster, 2012; Polisca et al., 2016; Cunto et al., 2019). In our work, BPH represented 71.6% of the prostatic disorders studied, similar to the 63% mentioned by Foster (2012), but higher than the 45.9% reported by Polisca et al. (2016). The difference in the proportion of presentation of BPH of our work with those of the bibliography could be related to the way of diagnosis of prostatic disorders. In many veterinary care centers around the world, diagnoses of prostatic disorder are presumptive and based on anamnesis, clinical signs, and ultrasonographic studies (Zambelli et al., 2012). This situation is similar to what happens in the hospital of the FVET–UdelaR. In a study with similar characteristics to ours, Polisca et al. (2016) reported similar difficulties, where more than 50% of the cases lacked a definitive diagnosis. The second most frequent prostatic disorder after BPH was prostatitis, with 23.5%, coinciding with what has been reported internationally (Johnston et al., 2001; Smith, 2008; Foster, 2012; Polisca et al., 2016; Cunto et al., 2019). It is known that BPH is the underlying cause of some prostatic disorders, such as prostatitis, caused by bacterial contamination of the fluid from cystic structures (Hecht, 2008; DiBartola and Westropp, 2019). As we mentioned above, since most of the cases included in this study, as in many other international reports, do not have a definitive diagnosis, we could be overestimating the occurrence of BPH in the hospital of FVET–UdelaR. Part of the population with BPH could be patients with prostatitis with similar clinical signs and with subtle ultrasound changes that did not allow differentiation between the two disorders. In this same sense, chronic prostatitis can be asymptomatic and/or present nonspecific ultrasound changes (Hecht, 2008; Cunto et al., 2019; Palmieri et al., 2022). For all these reasons, it is relevant to reach a definitive diagnosis, which will allow establishing an adequate treatment and avoiding recurrences. Although the definitive diagnosis can be reached by means of a biopsy for histopathology (Palmieri et al., 2022), it is not usually recommended (Cunto et al., 2022). Another less invasive technique that can be used is ultrasound-guided fine needle aspiration. High diagnostic value of prostatic disorders has been reported when fine needle cytology is combined with ultrasound evaluation of the prostate (Rodak et al., 2018). On the other hand, the culture of prostatic fluid or urine, obtained by ultrasound-guided aseptic puncture, could be a useful, less expensive, and less invasive tool to differentiate BPH from prostatitis (Lévy et al., 2014; Das et al., 2017). Some authors mention that in some cases, ultrasound-guided fine-needle aspiration is contraindicated, such as in the presence of prostatic abscess (Smith, 2008) and PN (Christensen, 2018), due to the possibility of seeding bacteria with the needle and neoplastic cells, respectively. In the countries where it is available, the serum determination of canine prostatic specific esterase (CPSE) could be a useful and less invasive tool as a complement to the diagnosis of prostatic disorders. Significantly elevated serum CPSE concentrations have been found in BPH canines compared to normal dogs (Wolf et al., 2012; Holst et al., 2017; Pinheiro et al., 2017; Dearakhshandeh et al., 2020). Therefore, CPSE is a valuable biomarker, and its use has been suggested in the diagnosis of dogs with clinical signs of BPH, follow-up treatment of prostatic diseases, and routine check-ups in geriatric patients (Holst et al., 2017; Pinheiro et al., 2017). In this sense, the correct diagnosis of prostate disease is a challenge. Submit canines older than 6 or 7 years to prostatic B-mode ultrasonography, as suggested by Polisca et al. (2016); adding the serum determination of CPSE could serve as screening to detect asymptomatic canines with prostatic disorders early. In conclusion, this study shows that there are clinical signs with a higher degree of association for certain canine prostatic disorders than others, as evidenced by the higher predictive value presented by diarrhea, anorexia, weight loss, hematuria, and urinary incontinence for prostatitis compared with BPH. In addition, this 9-year retrospective study on prostatic disorders in canines conducted in Latin America (Uruguay) expands and complements the information available from other epidemiological studies conducted in other regions of the world. AcknowledgmentsTo the administrative staff of the hospital of the FVET–UdelaR, especially to Francisco, for his great help in the search and selection of clinical records. To Emiliano D’Anatro for his collaboration in reviewing the clinical records. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsDr. Guillermo Cazzuli: research design, acquisition, analysis, and interpretation of data, drafting the paper. Dr. Gonzalo Suárez: analysis and interpretation of data, critical review of the manuscript. Dra. Stefania Busconi: acquisition of data. Dr. Juan Pablo Damian: analysis and interpretation of data, drafting the paper, critical review of the manuscript. Dra. Paula Pessina: research design, interpretation of data, critical review of the manuscript. FundingThis research received no specific grant. Data availabilityThe data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. ReferencesBarsanti, J. and Finco, D.R. 1986. Canine prostatic disease. Vet. Clin. North Am. 16, 587–599. Cazzuli, G., Damián, J.P., Molina, E. and Pessina P. 2022. Post-castration prostatic involution: a morphometric and endocrine study of healthy canines and those with benign prostatic hyperplasia. Reprod. Domest. Anim. 57(2), 157–164. Cunto, M., Mariani, E., Anicito Guido, E., Ballotta, G. and Zambelli, D. 2019. Clinical approach to prostatic diseases in the dog. Reprod. Dom. Anim. 54, 815–822. Cunto, M., Ballotta, G. and Zambelli, D. 2022. Benign prostatic hyperplasia in the dog. Anim. Reprod. Sci. 247, 107096. Christensen, B.W. 2018. Canine prostate disease. Vet. Clin. Small Anim. 48, 701–719. Das, M.R., Patra, R.C., Das, R.K., Rath, P.K., Mishra, B.P. 2017. Hemato-biochemical alterations and urinalysis in dogs suffering from benign prostatic hyperplasia. Vet. World 10, 331–335. Dearakhshandeh, N., Mogheiseh, A., Nazifi, S., Khafi, M.S.A., Hasiri, M.A., Golchin-Rad, K. 2020. Treatment of experimentally induced benign prostatic hiperplasia with Tadalafil and castration in dogs. Theriogenology 142, 236–245. DiBartola, S.P. and Westropp, J.L. 2019. Urinary tract disorder. In Small animal internal medicine, 6th ed. Eds., Nelson, R.W and Couto, C.G. St. Louis, MO: Mosby Elsevier, pp: 649–739. Foster, R.A. 2012. Common lesions in the male reproductive tract of cats and dogs. Vet. Clin. North Am. Small Anim. Pract. 42, 527–545. Hecht, S. 2008. Male reproductive tract. In Atlas of small animal ultrasonography. Eds., Penninck, D. and d’Anjou, M.A. Ames, IA: Iowa State University Press, pp: 685–769. Holst, B.S., Holmroos, E., Friling, L., Hanas, S., Langborg, L.M., Franko, M.A. and Hansson, K. 2017. The association between the serum concentration of canine prostate specific esterase (CPSE) and the size of the canine prostate. Theriogenology 93, 33–39. Johnston, S.D., Root Krustritz, M.V. and Olson, P.N.S. 2001. Canine and feline theriogenology. Philadelphia, PA: Saunders. Krawiec, D.R. and Heflin, D. 1992. Study of prostatic disease in dogs: 177 cases (1981–1986). J. Am. Vet. Med. Assoc. 200(8), 1119–1122. Kutzler, M.A. and Yeager, A. 2005. Prostatic disease. In Textbook of veterinary internal medicine, 6th ed. Eds., Ettinger, S.J. and Feldman, E.C. St. Louis, MO: Elsevier Saunders, pp: 1809–1819. Lacreta, J., de Castro, M.B., Sobreira, L.D.R. and Canola, J.C. 2012. Aspectos ultrassonográficos e citopatológicos das prostatopatias em 52 cães. Biotemas 25(1), 137–149. Lévy, X., Nizanski, W., von Heimendahl, A. and Mimouni, P. 2014. Diagnosis of common prostatic conditions in dogs: an update. Reprod. Domest. Anim. 49(Suppl 2), 50–57. Mattoon, J.S. and Davidson, A. 2020. Prostate and testes. In Small animal diagnostic ultrasound, 4th ed. Eds., Mattoon, J.S., Sellon, R.K. and Berry, C.R. St. Louis, MO: Elsevier, pp: 635–664. Palmieri, C., Fonseca-Alves, C.E. and Laufer-Amorim, R. 2022. A review on canine and feline prostate pathology. Front. Vet. Sci. 9, 881232. Pinheiro, D., Machado, J., Viegas, C., Baptista, C., Bastos, E., Magalhaes, J., Pires, M.A., Cardoso, L. and Martins-Bessa, A. 2017. Evaluation of biomarker canine-prostate specific arginine esterase (CPSE) for the diagnosis of benign prostatic hyperplasia. BMC Vet. Res. 13, 76. Polisca, A., Troisi, A., Fontaine, E., Menchetti, L. and Fontbonne, L. 2016. A retrospective study of canine prostatic diseases from 2002 to 2009 at the Alfort Veterinary College in France. Theriogenology 85, 835–840. Rodak, O., Dzimira, S., Podolak, A., Plociennik, M. and Nizanski, W. 2018. Accuracy of ultrasonography and fine-needle aspiration cytology in the diagnosis of prostate diseases in dogs. Reprod. Dom. Anim. 53(Suppl 3), 79–84. RStudio Team. 2022. RStudio: integrated development environment for R. R Core Team R: a language and environment for statistical computing. Vienna, Austria: R Core Team. Ruel, Y., Barthez, P.Y., Mailles, A. and Begonm D. 1998. Ultrasonographic evaluation of the prostate in healthy intact dogs. Vet. Radiol. Ultrasound 39, 212–216. Ruetten, H., Wehber, M., Murphy, M., Cole, C., Sandhu, S., Oakes, S., Bjorling, D., Waller K.III, Viviano, K. and Vezina, C. 2021. A retrospective review of canine benign prostatic hyperplasia with and without prostatitis. Clin. Theriogenol. 13(4), 360. Smith, J. 2008. Canine prostatic disease: a review of anatomy, pathology, diagnosis, and treatment. Theriogenology 70, 375–383. Teske, E. Urogenital cytology: part I–prostatic diseases. In Proceedings of the 34th World Small Animal Veterinary Congress, San Pablo, 2009, p 5. Wolf, K., Kayacelebi, H., Urhausen, C., Piechotta, M., Mischke, R. and Kramer, S. 2012. Testicular steroids, prolactin, relaxin and prostate gland markers in peripheralblood and seminal plasma of normal dogs and dogs with prostatic hyperplasia. Reprod. Domest. Anim. 47(Suppl 6), 243–246. Zambelli, D., Cunto, M. and Gentilini, F. 2012. Validation of a model to develop a symptom index for benign prostatic hyperplasia in dogs. Reprod. Domest. Anim. 47, 229–231. Zinkl, J. 2008. The male reproductive tract: prostate, testes and semen. In Diagnostic cytology and hematology of the dog and cat, 3th ed. Eds., Cowell, R.L., Tyler, R.D., Meinkoth, J.H. and DeNicola, D.B. St. Louis, MO: Mosby Elsevier, pp: 369–377. | ||
| How to Cite this Article |
| Pubmed Style Cazzuli G, Suárez G, Busconi S, Damián JP, Pessina P. Clinical signs associated with prostatic disorders in canines: Retrospective study in Uruguay (2011-2019). Open Vet. J.. 2023; 13(10): 1308-1317. doi:10.5455/OVJ.2023.v13.i10.10 Web Style Cazzuli G, Suárez G, Busconi S, Damián JP, Pessina P. Clinical signs associated with prostatic disorders in canines: Retrospective study in Uruguay (2011-2019). https://www.openveterinaryjournal.com/?mno=161823 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i10.10 AMA (American Medical Association) Style Cazzuli G, Suárez G, Busconi S, Damián JP, Pessina P. Clinical signs associated with prostatic disorders in canines: Retrospective study in Uruguay (2011-2019). Open Vet. J.. 2023; 13(10): 1308-1317. doi:10.5455/OVJ.2023.v13.i10.10 Vancouver/ICMJE Style Cazzuli G, Suárez G, Busconi S, Damián JP, Pessina P. Clinical signs associated with prostatic disorders in canines: Retrospective study in Uruguay (2011-2019). Open Vet. J.. (2023), [cited January 25, 2026]; 13(10): 1308-1317. doi:10.5455/OVJ.2023.v13.i10.10 Harvard Style Cazzuli, G., Suárez, . G., Busconi, . S., Damián, . J. P. & Pessina, . P. (2023) Clinical signs associated with prostatic disorders in canines: Retrospective study in Uruguay (2011-2019). Open Vet. J., 13 (10), 1308-1317. doi:10.5455/OVJ.2023.v13.i10.10 Turabian Style Cazzuli, Guillermo, Gonzalo Suárez, Stefania Busconi, Juan Pablo Damián, and Paula Pessina. 2023. Clinical signs associated with prostatic disorders in canines: Retrospective study in Uruguay (2011-2019). Open Veterinary Journal, 13 (10), 1308-1317. doi:10.5455/OVJ.2023.v13.i10.10 Chicago Style Cazzuli, Guillermo, Gonzalo Suárez, Stefania Busconi, Juan Pablo Damián, and Paula Pessina. "Clinical signs associated with prostatic disorders in canines: Retrospective study in Uruguay (2011-2019)." Open Veterinary Journal 13 (2023), 1308-1317. doi:10.5455/OVJ.2023.v13.i10.10 MLA (The Modern Language Association) Style Cazzuli, Guillermo, Gonzalo Suárez, Stefania Busconi, Juan Pablo Damián, and Paula Pessina. "Clinical signs associated with prostatic disorders in canines: Retrospective study in Uruguay (2011-2019)." Open Veterinary Journal 13.10 (2023), 1308-1317. Print. doi:10.5455/OVJ.2023.v13.i10.10 APA (American Psychological Association) Style Cazzuli, G., Suárez, . G., Busconi, . S., Damián, . J. P. & Pessina, . P. (2023) Clinical signs associated with prostatic disorders in canines: Retrospective study in Uruguay (2011-2019). Open Veterinary Journal, 13 (10), 1308-1317. doi:10.5455/OVJ.2023.v13.i10.10 |