| Case Report | ||
Open Vet. J.. 2023; 13(11): 1471-1477 Open Veterinary Journal, (2023), Vol. 13(11): 1471-1477 Case Report Management of uroperitoneum through combination of conservative and surgical treatments in two coltsChiara Montano1, Giulia Forni2, Aliai Lanci2, Jole Mariella2, Chiara Del Prete1, Mariaelena de Chiara1,*, Maria Pia Pasolini1 and Riccardo Rinnovati21Department of Veterinary Medicine and Animal Production, University of Naples “Federico II,” Naples, Italy 2Department of Veterinary Medical Sciences, University of Bologna, Bologna, Italy *Corresponding Author: Mariaelena de Chiara. Department of Veterinary Medicine and Animal Production, University of Naples “Federico II,” Naples, Italy Email: mariaelena.dechiara [at] unina.it Submitted: 04/08/2023 Accepted: 13/10/2023 Published: 30/11/2023 © 2023 Open Veterinary Journal
AbstractBackground: Ruptures of the urinary bladder and urachus are the most frequent cause of uroperitoneum in foals. Surgical correction is often the first treatment choice, however, nonsurgical methods, such as urine removal via urinary catheters and abdominal drains, have been successfully performed in foals. Case Description: Two foals were referred to the Equine Perinatology Unit for suspicion of uroperitoneum. The diagnosis was confirmed by hematobiochemical and ultrasound examinations, thus cystorrhaphy and cystoplasty were attempted. Surgeons found a lesion in the dorsocranial margin of the bladder (Case 1) and a tear in the pelvic urethra (Case 2); in the first case, the defect was routinely repaired, while the last lesion was impossible to repair due to its localization. A urinary catheter was left in place in both cases. Uroperitoneum recurred 72 hours after the surgery in both foals: a second surgical correction was not recommended due to the localization of the tears and conservative treatment, with the placement of a 32F chest tube in the most ventral part of the abdomen, was preferred. Abdominal drains were removed 5–7 days after surgery, while urinary catheters were left in place for up to 7–8 days. Colts’ conditions improved during hospitalizations. Two months after bladder surgery, Case 1 was euthanized due to multiple adhesions between the small intestine and the abdominal wall. Case 2 was still alive one year postoperatively. Conclusion: Although it cannot be considered the first choice for the treatment of uroperitoneum in the foal, nonsurgical treatment was successful in both cases in the short-term follow-up. However, the prognosis should be cautious due to the risk of long-term complications. Conservative management may be used to manage bladder/urethral tears that cannot be solved by surgery. Keywords: Abdominal drainage, Cystorrhaphy, Cystoplasty, Foal, Urinary catheter. IntroductionUroperitoneum is defined as urine collection into the peritoneal space secondary to a congenital or acquired leakage from any portion of the urinary tract (Axton et al., 2015; Bernick et al. 2021). In foals, this most commonly results from tearing of the cranial aspect of the dorsal bladder wall (Schott and Woodie, 2019a; DeNotta, 2022). Ruptures of the urachus during parturition or secondary to an umbilical abscess are also common causes (Richardson and Kohn, 1983; Hardy, 1998; Hyman, 2001). Uroperitoneum is a medical emergency and surgery should be performed only when the foal has been adequately stabilized (Hardy, 1998; Kablack et al., 2000; Dunkel et al., 2005). Untreated uroabdomen can lead to hyperkalemia, azotemia, hemoconcentration, hypovolemia, septic peritonitis, and death (Higuchi et al ., 2002; Jenei, 2012). Surgical correction is the first choice to repair a bladder rupture (Rodgerson et al., 1999; Bryant and Gaughan, 2005; Schott and Woodie, 2019a), even if complications, including recurrence, incontinence, infection, urine scalding, and fistulas, are common (Hardy, 1998; Ford et al., 2022). Nonsurgical methods of treating uroperitoneum, such as urine removal via urinary catheters and abdominal drains, have been successfully performed in foals as well. However, data about short- and long-term follow-up are still limited (Kritchevsky et al., 1984; Lavoie and Harnagel, 1988; Hendrickson and Lee, 2012; Ford et al ., 2022). The follow-up of bladder/urethral rupture in two colts treated through the combination of surgical and conservative techniques is reported in the present study. Cases DetailsCase 1History and clinical findings A 21-day-old Standardbred Horse colt weighing 87 kg was admitted at the Equine Clinical Service of the Veterinary Teaching Hospital (Department of Veterinary Medical Sciences, University of Bologna, Italy), presenting with hyperthermia (39.3℃) and abdominal distention of 48 hours duration. The referring veterinarian treated the animal with ceftiofur (2 mg/kg, IV) for 2 days, amikacin (20 mg/kg, IV) for 1 day, and flunixin meglumine (1.1 mg/kg, IV) for 1 day before admission. On initial physical examination, the colt was depressed, hyperthermic (39.3℃), with elevated respiratory (96 bpm) and heart rate (160 bpm). Mucous membranes were cyanotic and capillary refill time was increased (5″). Mean arterial pressure, measured using a sphygmomanometer (Dynamap PRO 300) with a cuff placed over the coccygeal artery, was 80 mmHg (normal range 82–108 mmHg) (Franco et al., 1986). Hematic concentrations of glucose and lactate were measured using a rapid method (Glucose and β-ketone sensor; Lactate Scout Analyzer, respectively) and resulted, respectively, 7.88 mmol/l (6.11–7.83 mmol/l) and 1.7 mmol/l (0.9–1.65 mmol/l) (Lumsden et al., 1980). Serum IgG concentration was normal (1,545 mg/dl) (>800 mg/dl) (Liepman et al., 2015). Jugular venous blood was collected for hematological and biochemical assessments. Treatment Antimicrobial therapy was started by using ampicillin (70 mg/kg, IV, q.6h) and amikacin (50 mg/kg, IV, q.24h). Fluid therapy included 3 l of isotonic NaCl administered as a bolus and 5% dextrose solution at 5 ml/kg/hour. The foal also received flunixin meglumine (1.1 mg kg, IV, q.12h) and supplemental oxygen (5 l/minute for 3 hours and 3l/minute for 1 hour). Transabdominal ultrasound examination did not show significant findings but, when a 14G French Foley catheter was inserted through the urethra, 2 l of urine were drained. Blood biochemistry showed hyponatremia (130 mmol/l) (136–140 mmol/l), hypochloremia (92.1 mmol/l) (93–100 m/l), and hyperkalemia (6.4 mmol/l) (3.5–4.2 mmol/l) (Lumsden et al., 1980). The clinical situation was suggestive of rupture of the urinary bladder; thus, surgery was recommended. The anesthesia protocol included butorphanol (0.05 mg/kg, IV) and xylazine (0.5 mg/kg, IV) for premedication, then propofol (0.8 mg/kg, IV) and ketamine (1.5 mg/kg, IV) for general anesthesia and isoflurane (1.1%) in 100% oxygen for maintenance. The foal was positioned in dorsal recumbency, and the ventral abdomen was clipped and aseptically prepared. A fusiform incision was made around the external umbilicus. The umbilicus and the residual urachus were dissected from the abdominal wall, and the remnant of the umbilical vein was ligated and transected.
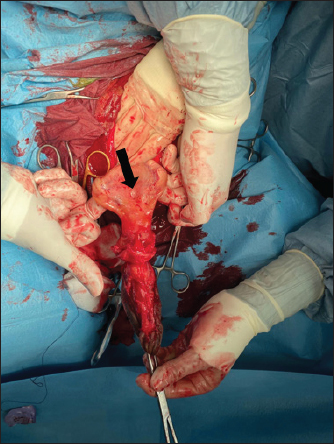
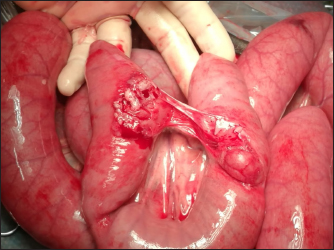
Fig. 1. Bladder rupture in the dorsocranial portion of the urinary bladder in Case 1. Black arrow: bladder tear. The bladder was exposed by traction on the urachus and a 2-cm tear in the dorsocranial margin was revealed (Fig. 1). The tear was closed with a 2–0 glycolide dioxanone monofilament (BiosynTM) using a two-layer inverting suture pattern. Umbilical arteries’ remnants were ligated at the level of the urachal resection and a transverse incision was made across the apex of the bladder to remove the urachus and bladder apex. The incision was closed using a two-layer inverting suture pattern with a 2–0 glycolide dioxanone monofilament (BiosynTM). The bladder closure was tested for leaks by carefully retrograde introduction of 200 ml of sterile saline solution. The Foley urinary catheter was left in place and the abdominal cavity was lavaged with 5 l of warm sterile saline solution. Linea alba was closed with 1 glycole/lactide copolymer absorbable suture (PolysorbTM) with a simple interrupted pattern; the subcutaneous tissue and skin were closed in routine fashion with 0 glycole/lactide copolymer absorbable suture (PolysorbTM) and 0 monofilament nylon (MonosofTM), respectively. After the surgery, the foal’s vital parameters remained within physiological limits and the animal continued to urinate through the urinary catheter. Preoperative therapy was continued. Three days after the surgery, the colt appeared depressed, with a distended abdomen. Ultrasonography of the abdomen showed free fluid in the peritoneal cavity and the cystoscopy showed another tear in the dorsal wall of the bladder, further caudad to the original repair. No signs of suture failure were highlighted. Due to the localization of the tear and for financial reasons, surgical correction was excluded and conservative treatment was preferred. A closed drainage system consisted of a 32F chest tube (REDAX S.r.l., Poggio Rusco (MN), Italy), attached to a Christmas tree connector (Vygon Italia S.r.l, Padova, Italy) closed by a perforable cap (Farmac-Zabban S.p.A, Calderara di Reno (BO), Italy), was placed into the peritoneal cavity at the most ventral aspect of the abdomen, 5 cm to the left midline. The catheter was fixed to the abdominal wall by a 0 nonabsorbable monofilament suture (DafilonTM), with a Chinese finger trap suture. The abdomen was lavaged with 2 l of sterile saline solution twice daily for 2 days. Poor return and normal ultrasound images were noted on day 2, so the abdominal drain was removed. On day 8 after surgery, the urinary catheter was removed, and the colt started to urinate normally. A cystoscopy was repeated on day 10, in which the closure of the tear was confirmed. Blood gas analysis was monitored on alternating days for 4 days and by day 2, all values were within normal limits. Antibiotic therapy was suspended on day 17. The colt was discharged 29 days following admission. Complications after hospital discharge and long-term outcome Two months after hospitalization, the foal was referred to the hospital with signs of pain, abdominal distention, sweating, and the presence of gastric reflux from the nostrils. The patient was depressed, tachycardic (80 ppm), and tachypneic (60 bpm), with congestive mucous membranes and increased capillary refill time (4″). Ultrasound examination showed an immotile, distended, thickened small intestine, without free fluid in the abdomen. Exploratory laparotomy under general anesthesia was performed and revealed multiple adhesions between the abdominal wall and the small intestine, areas as well as necrotic patches in the small intestine, (Fig. 2). According to the owner, due to a guarded prognosis, euthanasia was performed. Case 2History and clinical findings A 2-day-old Quarter Horse foal weighing 46 kg was presented to the Equine Clinical Service with depression, tenesmus, frequent urination, preputial edema, and umbilical hernia. The foal’s delivery had been assisted by the owners, due to the absence of contractions by the mare. The colt received adequate colostrum after birth and passed meconium. Physical examination showed depression, absence of suckling reflex, abdominal distention, opisthotonus, and lingual ptosis; the colt was hyperthermic (39.4℃), tachypneic (56 bpm), tachycardic (112 bpm). The hematic concentration of glucose was elevated (12.88 mmol/l) (6.11–7.83 mmol/l) (Lumsden et al., 1980); lactate level at the admission was elevated (19.4 mmol/l) (11–2.3 mmol/l) (Castagnetti et al., 2010), but decreased during hospitalization. Serum IgG concentration was normal (1,865 mg/dl) (>800 mg/dl) (Liepman et al., 2015).
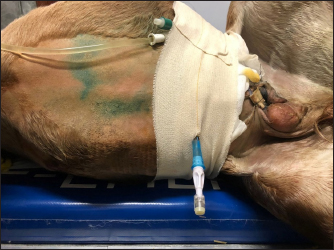
Fig. 2. Intraoperative images of Case 1: adhesions between segments of small intestine. Hematologic, serum biochemistry, and arterial gas analysis were performed, and abnormalities included severe hyponatremia (113 mmol/l) (136–140 mmol/l), hypochloremia (73.3 mmol/l) (93–100 m/l), and elevation of blood urea nitrogen (26.3 mmol/l) (3.1–4.1 mmol/l), and creatinine (499.8 μmol/l) (97–123 μmol/l) (Lumsden et al., 1980). Potassium concentration was normal. Ultrasound examination was performed and revealed free anechoic abdominal fluid and segments of the small intestine suspended in the fluid in the proximity of the umbilicus, suggesting a diagnosis of uroperitoneum. Treatment Therapy was started with amikacin (30 mg/kg, IV, q.24h), ampicillin (50 mg/kg, IV, q.6h), flunixin meglumine (1.1 mg/kg, IV, q.12h), and sucralfate (20 mg/kg, PO, q.12h). Intravenous fluid therapy included isotonic NaCl at 80 ml/kg/hour, 10% DMSO solution at 120 ml/kg/hour, and 10% glucose solution at 120 ml/kg/hour. After urinary catheterization, general anesthesia and surgery were performed as described in Case 1. Surgeons found a tear in the pelvic urethra and based on the difficulty in accessing the defect, it was decided to maintain in place the urinary catheter, to allow the tear to heal by second intention. After the surgery, the colt appeared depressed. Preoperative treatment was continued. On day 2 after the surgery, abdominal ultrasonography showed anechoic fluid in the peritoneal cavity; a peritoneal drain was placed as in the previous case (Fig. 3) and the abdomen was lavaged with 1 l of sterile saline solution once daily for 5 days. On day 7 after the surgery, the abdominal drain was removed due to a failure to obtain fluid, and on day 9, the urinary catheter was removed, as well.
Fig. 3. Abdominal drain in Case 2. The colt’s conditions improved during hospitalization, and no abnormalities were found in clinical parameters following day 5 after surgery. Hematology and serum biochemistry were monitored twice weekly until discharge, and by day 8, all values were within normal limits. The foal was discharged 17 days following admission. Complications after hospital discharge and long-term outcome The colt showed no complications after discharge and was healthy at the phone follow-up 1-year post after the surgery. DiscussionThese case reports describe the treatment of uroperitoneum in two colts, combining surgical repair and conservative management. Uroperitoneum is a common disease in foals, with an incidence of 0.2%–2.5% (Hackett, 1984) and a higher prevalence in colts than in fillies (Dunkel et al., 2005). The reason for this sex difference has been attributed to the longer male urethra (Hackett, 1984). This condition is most frequently seen in the first week of life, whereby morbidity range can vary from 1 to 60 days after birth (Bernick et al., 2021). Trauma during delivery or afterbirth, focal necrotic cystitis, or congenital defects can lead to uroperitoneum (Schott and Woodie, 2019a). Tears usually occur in the dorsal wall of the bladder, which can be explained by the reduced thickness of this area (Kablack et al., 2000). The uroabdomen can also evolve from a rupture of the urachus, which may be a result of traction of the umbilical cord at the time of delivery (Hardy, 1998) or by umbilical infections (Mendoza et al., 2010). In the cases reported here, the rupture of the bladder was assumed to be the result of an external trauma (Case 1) or excessive traction of the umbilical cord during delivery (Case 2). In this last case, the umbilical cord has probably produced excessive stretching and trauma of the pelvic urethra, producing the rupture. Cystorrhaphy and cystoplasty are often the first choice to repair a bladder rupture (Richardson and Kohn, 1983; Adams et al., 1988; Schott and Woodie, 2019a); however, surgery and general anesthesia are associated with high mortality, particularly in metabolically compromised patients (Peitzmeier et al., 2016). Moreover, accessing tears in the ventral part of the bladder is often impossible (Schott and Woodie, 2019a). Conservative management of bladder lacerations through correction of metabolic imbalances and drain of urine using indwelling urinary catheters and abdominal drains in adult horses is advocated by some practitioners, with successful outcomes (Peitzmeier et al., 2016; Gosling et al., 2021). Abdominal drainage and urinary catheterization were considered alternative techniques to treat uroperitoneum in critically ill foals (Lavoie and Harnagel, 1988). Medical treatment associated with urine drainage is also performed to stabilize compromised foals before surgical therapy of the uroperitoneum (Kritchevsky et al., 1984; Lavoie and Harnagel, 1988; Butter, 2008). The advantages of surgical technique over conservative management include a short convalescence and antibiotic therapy, as well as the absence of complications (cystitis, peritonitis) (Schott and Woodie, 2019a). On the other hand, conservative handling of bladder ruptures avoids complications associated with recumbent surgical repair (Peitzmeier et al., 2016). At first, conservative management appeared as a possible alternative to a second surgery in both treated foals. After stabilization of the colts, a cystorraphy (Case 1) and cystoplasty (Cases 1 and 2) were performed under general anesthesia. Surgeons performed a double-layer suture without debridement of the defect, as described in the literature (Schott and Woodie, 2019a; Fubini and Delco, 2022). Suture material and pattern and the decision to debride the bladder tear margins have been associated with the development of complications (Bryant and Gaughan, 2005; Schott and Woodie, 2019a; Ford et al., 2022; Fubini and Delco, 2022). Although the recurrence of uroperitoneum appears to be more common in cases where no debridement is performed, Ford et al. (2022) have not highlighted a clear relationship between debridement procedure, uroperitoneum recurrence, or survival rate. Fubini and Delco (2022) suggested that debridement is not necessary if the bladder is closed with an inverted suture. In an experimental ovine model, performing a double-layer suture in cystotomy leads to a higher leaking pressure than a single layer (Duffy et al., 2019). Conversely, a single-layer suture pattern for cystotomy closure in 144 dogs and cats has proven to be a safe and effective procedure with minimal complications (37%) (Thieman-Mankin et al., 2012). Albeit in calves, there is some experimental evidence that a single appositional layer may be sufficient to ensure bladder closure (Bouré et al., 2005), in foals, there is no information regarding the best security of the double-layer suture than the single-layer. In our experience, the bladder suture was effective in repairing the defect in Case 1, as the endoscopy did not show any sign of suture failure; in Case 2, access to the urethral defect was not possible. A few days after the surgery, a recurrence of the uroperitoneum was diagnosed by ultrasonography (Cases 1 and 2) and endoscopy (Case 1), because of the presence of a further bladder tear that was not highlighted during surgery (Case 1) and a tear in the pelvic urethra (Case 2). A conservative management has been undertaken to solve those recurrences, since a second surgery was not considered, due to economic reasons, localization of the defect (Case 2) and to avoid all possible complications associated with another general anesthesia and surgery. Urinary catheters were placed in those patients to reduce the amount of urine accumulating in the abdomen and the tension on bladder walls (Osborne et al., 1996; Walesby et al., 2002). Catheters were placed with a sterile technique and were connected to a urine bag, anchored to the patient’s body, creating a close system that prevented the development of pneumoperitoneum, peritonitis, or ascending urinary tract infections (Schott and Woodie, 2019a). Catheters were replaced every 2–3 days and patients were treated with antibiotics, to prevent ascending infections, as reported in several studies (Peitzmeier et al., 2016). Furthermore, in Case 2, the urinary catheter was left in place to prevent mucosal damage by urine and facilitate healing by second intention on the tear (Castagnetti et al., 2010; Schott and Woodie, 2019b). Abdominal drains were introduced in both colts to remove urine from the abdomen, decreasing chemical irritation in the peritoneum (Genetzky and Hagemoser, 1985) and assisting correction of electrolyte imbalance (Schott and Woodie, 2019a). Abdominal drains were successfully placed in both awake and sedated patients. Drains have been used as ingress and egress portals for peritoneal lavage, with sterile saline solution. Reuss et al. (2006) performed abdominal lavage with a solution of 1,5% dextrose in a balanced electrolyte fluid, which creates a hypertonic dialysate that allows diffusion of urea, creatinine, and other elevated electrolytes from blood to peritoneal fluid, which is then drained. Short-term complications of prolonged drainage were not experienced in these cases, and on days 2 and 7 in Cases 1 and 2, respectively, drains were removed due to poor return. The time for the equine bladder to heal by second intention is still unknown (Peitzmeier et al., 2016); the urinary catheter, in this case report, was maintained for 8 and 9 days (Cases 1 and 2, respectively). Controlled cystoscopy was performed only in Case 1, and on day 10, showed a complete repair of the tear in the bladder wall. Further studies are necessary to determine the healing times of the bladder wall, allowing to reduce the time urinary catheter is left in situ and, consequently, the use of antibiotics. Some authors prefer to not leave the urinary catheter in place, but in our experience, leaving it in situ for a few days allows a better postoperative outcome. The most common complication of urinary surgery is a recurrence of uroperitoneum due to dehiscence of the suture or failure to completely suture the tear (Hardy, 1998), incontinence, infection, urine scalding, and fistulas (Steward and Rubio-Martinez, 2021). Bladder atony, renal failure, intestinal adhesions, peritonitis, and pleural effusion are less frequently reported sequelae (Ford et al., 2022). Mortality is higher in the immediate perioperative period, especially for hyperkalemia and respiratory distress (Bernick et al., 2021). Conversely, conservative treatment may leave raw defects in the bladder, which may predispose to adhesion between abdominal organs (Peitzmeier et al., 2016). Development of abdominal adhesions is possible but not as frequent as in intestinal surgery (Hardy, 1998); however, in Case 1, the foal was referred a second time to the hospital two months after bladder surgery for colic syndrome. In the explorative laparotomy, it was seen that the colt had developed serious adhesions between the small intestine, the bladder, and the abdominal wall, a probable consequence of viscera manipulation, the presence of the suture, or urine irritation. The outcome of the two clinical cases may be related to various aspects. Both bladder ruptures had a traumatic etiology, although the foals’ age and dynamics of the trauma were different. Moreover, referral time may have had an important effect on the outcome, as the foal that survived was referred to the hospital shortly after the onset of symptoms. Early intervention can reduce exposure of the abdominal organs and tissues to urine and, therefore, reduce postoperative complications. Studies about short and long-term follow-up of both surgical and conservative treatments of uroperitoneum in foals are still limited, so further research is needed to clarify which factors have an influence on the prognosis. ConclusionIn the present study, conservative management consisted of continuous peritoneal fluid and urine leakage with an indwelling Foley urinary catheter and abdominal drainage. Even if the short-term prognosis was favorable, caution in the long term is necessary due to the risk of adhesions. AcknowledgmentsThe authors would like to thank all the veterinarians and students at the Equine Clinical Service (University of Bologna) for their help in taking care of the patients. Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. FundingNot applicable. Author contributionsConceptualization: Ricardo Rinnovati, Chiara Montano, Giulia Formi. Methodology: Ricardo Rinnovati, Jole Mariella, Alia Lanci, Maria Pia Pasolini, Chiara Del Prete. Validation: Maria Pia Pasolini, Ricardo Rinnovati. Formal analysis: Aliai Lanci, Jole Mariella, Chiara Del Prete. Investigation: Ricardo Rinnovati, Giulia Forni, Chiara Montano. Resources: Ricardo Rinnovati, Jole Mariella, Alia Lanci, Maria Pia Pasolini. Data curation: Chiara Montano, Giulia Forni, Mariaelena de Chiara. Writing -original draft preparation: Chiara Montano. Writing-review and editing: Giulia Forni, Mariaelena de Chiara, Maria Pia Pasolini, Ricardo Rinnovati. Supervision: Jole Mariella, Alia Lanci, Maria Pia Pasolini, Ricardo Rinnovati. Data availabilityData about this study are available from the corresponding author upon reasonable request. ReferencesAdams, R., Koterba, A.M., Cudd, T.C. and Baker, W.A. 1988. Exploratory celiotomy for suspected urinary tract disruption in neonatal foals: a review of 18 cases. Equine. Vet. J. 20(1), 13–17. Axton, J.E., Russell, C.M. and Wilkins, P.A. 2015. Neonatology. In Equine emergency and critical care medicine. Eds., Southwood, L.L., Wilkins, P.A., CRC Press, Boca Raton, FL, pp: 511–554. Bernick, A., Nieth, J. and Wehrend, A. 2021. Uroperitoneum in neonatal foals-a review of the literature. Tierarztl. Prax. Ausg. G. Grosstiere/Nutztiere. 49(1), 41–50. Bouré, L.P., Kerr, C.L., Pearce, S.G., Runciman, R.J., Lansdowne, J.L. and Caswell, J.L. 2005. Comparison of two laparoscopic suture patterns for repair of experimentally ruptured urinary bladders in normal neonatal calves. Vet. Surg. 34(1), 47–54. Bryant, J.E. and Gaughan, E.J. 2005. Abdominal surgery in neonatal foals. Vet. Clin. North. Am. Equine. Pract. 21(2), 511–535. Butter, A. 2008. Medical and surgical management of uroperitoneum in a foal. Can. Vet. J. 49(4), 401–403. Castagnetti, C., Pirrone, J., Mariella, J. and Mari, G. 2010. Venous blood lactate evaluation in equine neonatal intensive care. Theriogenology 73(3), 343–357. DeNotta, S.L. 2022. Urinary tract disorders of foals. Vet. Clin. North. Am. Equine. Pract. 38(1), 47–56. Duffy, D.J., Kindra, C.G. and Moore, G.E. 2019. Comparison of initial leak pressures after single- and double-layer cystotomy closure with barbed and nonbarbed monofilament suture material in an ex vivo ovine model. Vet. Surg. 48(3), 424–430. Dunkel, B., Palmer, J.E., Olson, K., Boston, R.C. and Wilkins, P.A. 2005. Uroperitoneum in 32 foals: influence of intravenous fluid therapy, infection, and sepsis. J. Vet. Intern. Med. 19(6), 889–893. Ford, M.G., Nelson, B.B., Ford, T.S., Souza, C., Easley, J.T. and Hackett, E.S. 2022. Complications and comorbidities in foals undergoing surgical repair for uroperitoneum. J. Equine. Vet. Sci. 110, 103852. Franco, R.M., Ousey, J.C., Cash, R.S.G., Rossdale, P.D. and Silver, M. 1986. Study of arterial blood pressure in newborn foals using an electronic sphygmomanometer. Equine. Vet. J. 18(6), 475–478. Fubini, S.L. and Delco, M. 2022 Surgery of the equine urinary tract. Vet. Clin. North. Am. Equine. Pract. 38(1), 141–153. Genetzky, R.M. and Hagemoser, W.A. 1985. Physical and clinical pathological findings associated with experimentally induced rupture of the equine urinary bladder. Can. Vet. J. 26(12), 391. Gosling, L., Anderson, J. and Rendle, D. 2021. Conservative management of iatrogenic bladder rupture and uroperitoneum in a gelding with urolithiasis. Equine. Vet. Educ. 33(3), e53–e57. Hackett, R.P. 1984. Rupture of the urinary bladder in neonatal foals. Compend. Contin. Educ. Pract. Vet. 6(8), S488. Hardy, J. 1998. Uroabdomen in foals. Equine. Vet. Educ. 10, 21–25. Hendrickson, D.A. and Lee, M. 2012. Repair of the ruptured equine bladder. In Advances in equine laparoscopy. Ed., Ragle, C.A. MI: John Wiley and Sons, Inc, Ames, IA, pp: 221–228. Higuchi, T., Nanao, Y. and Senba, H. 2002. Repair of urinary bladder rupture through a urethrotomy and urethral sphincterotomy in four postpartum mares. Vet. Surg. 31(4), 344–348. Hyman, S.S. 2001. Uroperitoneum in the equine neonate. In Recent advances in equine neonatal care. Ed., Wilkins, P.A. Ithaca, NY:International Veterinary Information Service. Jenei, T.M. 2012. Bladder rupture in the mature horse: diagnostic techniques. Equine. Vet. Educ. 24, 517–519. Kablack, K.A., Embertson, R.M., Bernard, W.V., Bramlage, L.R., Hance, S., Reimer, J.M. and Barton, M.H. 2000. Uroperitoneum in the hospitalised equine neonate: retrospective study of 31 cases, 1988–1997. Equine. Vet. J. 32(6), 505–508. Kritchevsky, J.E., Stevens, D.L., Christopher, J. and Cook, W.O. 1984. Peritoneal dialysis for presurgical management of ruptured bladder in a foal. JAVMA 185(1), 81–82. Lavoie, J.P. and Harnagel, S.H. 1988. Nonsurgical management of ruptured urinary bladder in critically ill foal. JAVMA 192(22), 1577–1580. Liepman, R.S., Dembek, K.A., Slovis, N.M., Reed, S.M. and Toribio, R.E. 2015. Validation of IgG cut-off values and their association with survival in neonatal foals. Equine. Vet. J. 47(5), 526–530. Lumsden, J.H., Rowe, R. and Mullen, K. 1980. Hematology and biochemistry reference values for the light horse. Can. J. Comp. Med. 44(1), 32–42. Mendoza, F.J., Lopez, M., Diez, E., Perez-Ecija, A. and Estepa, J.C. 2010. Uroperitoneum secondary to rupture of the urachus associated with Clostridium spp. Infection in a foal: a case report. Vet. Med. 55(8), 399–404. Osborne, C.A., Sanderson, S.L., Lulich, J.P., Johnston, G.R. and Polzin, D.J. 1996. Medical management of iatrogenic rents in the wall of the feline urinary bladder. Vet. Clin. North. Am. Small. Anim. Pract. 26(3), 551–562. Peitzmeier, M.D., Mc Nally, T.P., Slone, D.E. and Lynch, T.M. 2016. Conservative management of cystorrhexis in four adult horses. Equine. Vet. Educ. 28(11), 631–635. Reuss, S.M., Franklin, R.P., Peloso, J.G. and Gallatin, L.L. How to perform continuous dialysis in an adult horse. In AAEP Annual Convention, American Association of Equine Practitioners, San Antonio, TX, 2006. Richardson, D.W. and Kohn, C.W. 1983. Uroperitoneum in the foal. J. Am. Vet. Med. Assoc. 182(3), 267–271. Rodgerson, D.H., Spirito, M.A., Thorpe, P.E. and Hanson, R.R. 1999. Standing surgical repair of cystorrhexis in two mares. Vet. Surg. 28(2), 113–116. Schott, H.C. and Woodie, J.B. 2019a. Bladder. In Equine surgery. Eds., Auer, J.A. and Stick, J.A. MI: W.B. Saunders, St. Louis, MO, pp: 1129–1145. Schott, H.C. and Woodie, J.B. 2019b. Urethra. In Equine surgery. Eds., Auer, J.A. and Stick, J.A. MI: W.B. Saunders, St. Louis, MO, pp: 1145–1155. Steward, S.K.T. and Rubio-Martinez, L.M. 2021. Complications of urinary surgery. In Complications in equine surgery. Eds., Rubio-Martinez, L.M. and Hendrickson, D.A. Wiley & sons, Inc, Hoboken, NY, pp: 571–582. Thieman-Mankin, K.M., Ellison, G.W., Jeyapaul, C.J. and Glotfelty-Ortiz, C.S. 2012. Comparison of short-term complication rates between dogs and cats undergoing appositional single-layer or inverting double-layer cystotomy closure: 144 cases (1993–2010). J. Am. Vet. Med. Assoc. 240(1), 65–68. Walesby, H.A., Ragle, C.A. and Booth, L.C. 2002. Laparoscopic repair of ruptured urinary bladder in a stallion. J. Am. Vet. Med. Assoc. 221(12), 1737–1741. | ||
| How to Cite this Article |
| Pubmed Style Montano C, Forni G, Lanci A, Mariella J, Del-prete C, De-chiara M, Pasolini MP, Rinnovati R. Management of uroperitoneum through combination of conservative and surgical treatments in two colts. Open Vet. J.. 2023; 13(11): 1471-1477. doi:10.5455/OVJ.2023.v13.i11.11 Web Style Montano C, Forni G, Lanci A, Mariella J, Del-prete C, De-chiara M, Pasolini MP, Rinnovati R. Management of uroperitoneum through combination of conservative and surgical treatments in two colts. https://www.openveterinaryjournal.com/?mno=162871 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i11.11 AMA (American Medical Association) Style Montano C, Forni G, Lanci A, Mariella J, Del-prete C, De-chiara M, Pasolini MP, Rinnovati R. Management of uroperitoneum through combination of conservative and surgical treatments in two colts. Open Vet. J.. 2023; 13(11): 1471-1477. doi:10.5455/OVJ.2023.v13.i11.11 Vancouver/ICMJE Style Montano C, Forni G, Lanci A, Mariella J, Del-prete C, De-chiara M, Pasolini MP, Rinnovati R. Management of uroperitoneum through combination of conservative and surgical treatments in two colts. Open Vet. J.. (2023), [cited January 25, 2026]; 13(11): 1471-1477. doi:10.5455/OVJ.2023.v13.i11.11 Harvard Style Montano, C., Forni, . G., Lanci, . A., Mariella, . J., Del-prete, . C., De-chiara, . M., Pasolini, . M. P. & Rinnovati, . R. (2023) Management of uroperitoneum through combination of conservative and surgical treatments in two colts. Open Vet. J., 13 (11), 1471-1477. doi:10.5455/OVJ.2023.v13.i11.11 Turabian Style Montano, Chiara, Giulia Forni, Aliai Lanci, Jole Mariella, Chiara Del-prete, Mariaelena De-chiara, Maria Pia Pasolini, and Riccardo Rinnovati. 2023. Management of uroperitoneum through combination of conservative and surgical treatments in two colts. Open Veterinary Journal, 13 (11), 1471-1477. doi:10.5455/OVJ.2023.v13.i11.11 Chicago Style Montano, Chiara, Giulia Forni, Aliai Lanci, Jole Mariella, Chiara Del-prete, Mariaelena De-chiara, Maria Pia Pasolini, and Riccardo Rinnovati. "Management of uroperitoneum through combination of conservative and surgical treatments in two colts." Open Veterinary Journal 13 (2023), 1471-1477. doi:10.5455/OVJ.2023.v13.i11.11 MLA (The Modern Language Association) Style Montano, Chiara, Giulia Forni, Aliai Lanci, Jole Mariella, Chiara Del-prete, Mariaelena De-chiara, Maria Pia Pasolini, and Riccardo Rinnovati. "Management of uroperitoneum through combination of conservative and surgical treatments in two colts." Open Veterinary Journal 13.11 (2023), 1471-1477. Print. doi:10.5455/OVJ.2023.v13.i11.11 APA (American Psychological Association) Style Montano, C., Forni, . G., Lanci, . A., Mariella, . J., Del-prete, . C., De-chiara, . M., Pasolini, . M. P. & Rinnovati, . R. (2023) Management of uroperitoneum through combination of conservative and surgical treatments in two colts. Open Veterinary Journal, 13 (11), 1471-1477. doi:10.5455/OVJ.2023.v13.i11.11 |