| Case Report | ||
Open Vet. J.. 2023; 13(11): 1465-1470 Open Veterinary Journal, (2023), Vol. 13(11): 1465–1470 Case Report Successful treatment of acute respiratory failure following hypertensive crisis in a dog with presumed pheochromocytoma or paragangliomaJun Tamura*, Shino Yoshida, Noriyuki Nagata, Genya Shimbo and Norihiko OyamaVeterinary Teaching Hospital, Faculty of Veterinary Medicine, Hokkaido University, Sapporo, Japan *Corresponding Author: Jun Tamura. Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Hokkaido University, Sapporo, Japan. Email: j-tamura [at] vetmed.hokudai.ac.jp Submitted: 01/08/2023 Accepted: 04/10/2023 Published: 30/11/2023 © 2023 Open Veterinary Journal
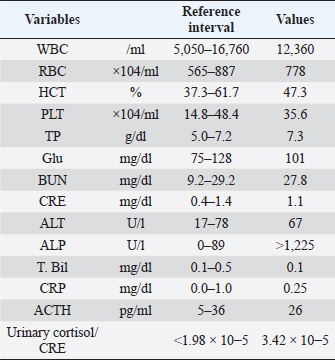
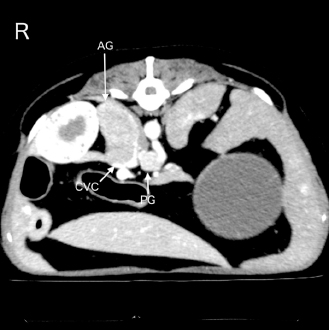
AbstractBackground: Acute respiratory failure has been reported as one of the manifestations of hypertensive crisis in pheochromocytoma in human medicine. In dogs, no reports have been described as acute respiratory failure following hypertensive crisis. Here, we report the clinical presentation, course, and treatment of acute respiratory failure following the hypertensive crisis in a dog with presumed pheochromocytoma or paraganglioma. Case Description: A 12-year-old neutered male toy poodle was referred for the diagnostic evaluation of a right adrenal gland mass. The dog suddenly exhibited severe dyspnea with abnormal hypertension (systolic blood pressure >200 mmHg) 15 minutes after recovery from the anesthesia for the computed tomography (CT) examination. Pulmonary CT and ultrasonography findings suggested acute onset of severe pulmonary edema. Pulmonary edema was treated with mechanical ventilation (pressure-support ventilation with continuous positive airway pressure) and negative fluid balance after the administration of furosemide. Weaning from mechanical ventilation was successful 24 hours after the onset of respiratory failure. Finally, the dog was discharged 3 days after weaning from ventilation without complications. Conclusion: This report outlines a case of acute respiratory failure following a hypertensive crisis requiring mechanical ventilatory management in a dog. The onset and progression of pulmonary edema were extremely rapid. However, improvement in pulmonary edema was also rapid. Hemodynamic stability, in addition to prompt diagnosis and aggressive therapeutic intervention, including mechanical ventilation, may have contributed to the good prognosis of pulmonary edema following hypertensive crisis in a dog, which we attribute to a catecholamine storm. Keywords: Acute respiratory failure, Catecholamine storm, Hypertensive crisis, Mechanical ventilation. IntroductionRespiratory failure occurs due to a disease or injury worsening the lungs’ ability to deliver oxygen or remove carbon dioxide (Summers et al., 2022). Symptoms of acute respiratory failure occur suddenly and can be life threatening (Summers et al., 2022). It requires immediate medication or oxygen supplementation. If oxygenation or ventilation impairment is severe, long-term mechanical ventilation is required. The outcomes of mechanical ventilation for canine respiratory diseases appear to be heavily determined by the underlying disease (Drobatz et al., 1995; Hopper et al., 2007; Edwards et al., 2014; Cagle et al, 2022; Herrería-Bustillo et al., 2022; Nemi et al., 2023). In humans, acute pulmonary edema is one of the manifestations of hypertensive crisis in pheochromocytoma (Ng et al., 2023). It can be either cardiogenic or noncardiogenic (Ng et al., 2023). In dogs, a previous review article described pulmonary edema as a complication of hypertensive crisis in pheochromocytoma (Galac and Korpershoek, 2017). However, the onset and treatment of acute respiratory failure following hypertensive crisis in dogs have not been reported previously. This case describes the clinical presentation, course, and successful treatment of acute respiratory failure following a hypertensive crisis, possibly due to a catecholamine storm induced by a presumed pheochromocytoma or paraganglioma in a dog. Case DetailsA 12-year-old neutered male toy poodle, weighing 8.6 kg with a body condition score of 7 out of 9, was referred to the Internal Medicine Service at a veterinary teaching hospital for diagnostic evaluation of a right adrenal gland mass and cystic disease of the left kidney. The abnormalities were incidentally discovered during health screening at the referring hospital. The dog had been previously diagnosed with myxomatous mitral valve disease (MMVD) and biliary sludge. Oral administration of pimobendane, isosorbide dinitrate, theophylline, and ursodeoxycholic acid was initiated. Upon initial examination, the dog appeared healthy. The dog showed a normal heart rate (138 beats/minute) and blood pressure (systolic, mean, and diastolic blood pressures were 141, 115, and 103 mmHg, respectively) measured using an oscillometric device (Vet20; Sun Tech Medical Inc., Morrisville, NC). In addition, a grade Ⅲ/Ⅵ systolic murmur was identified. As the patient was panting, the respiratory rate (RR) could not be obtained. The complete blood count and biochemistry panel are shown in Table 1. Thoracic radiography revealed no abnormality. Transthoracic echocardiography revealed mitral regurgitation (peak velocity, 5.4 m/s) without left atrial dilatation (ratio of left atrial to aortic root diameter, 1.27). The mitral valve E-wave velocity was 0.9–1.0 m/s. Abdominal ultrasonography revealed an enlarged right adrenal gland (maximum width, 27 mm) and hyperechoic biliary sludge. It was difficult to determine vascular invasion of the enlarged adrenal gland. The urinary free normetanephrine to creatinine ratio measured by liquid chromatography tandem mass spectrometry at the Hokkaido University One Health Research Center in Sapporo, Japan, was high (259.05), suggesting pheochromocytoma (Sasaki et al., 2021). The plasma adrenocorticotropic hormone concentration and urinary cortisol creatinine ratio, measured by Fujifilm Corporation, Tokyo, Japan, are shown in Table 1. The dog was scheduled to undergo contrast-enhanced computed tomography (CT) with 300 mg iodine/ml iohexol (Omnipaque 300 injection; Daiichi Sankyo Co., Ltd., Tokyo, Japan) at 2 ml/kg for surgical planning of adrenalectomy 7 days after the initial examination at our hospital. A 22-gauge, 31-mm intravenous (IV) catheter (Supercath5; Medikit Co., Ltd., Tokyo, Japan) was placed in the cephalic vein, and general anesthesia was induced with IV administration of butorphanol (Vetorphal; Meiji Animal Health Co., Ltd., Tokyo, Japan) and propofol (PropoFlo; Zoetis Japan, Tokyo, Japan). After intubation, anesthesia was maintained using oxygen and isoflurane (Isoflu; Zoetis Japan, Tokyo, Japan). The electrocardiogram, pulse rate, RR, end-tidal carbon dioxide concentration, indirect mean arterial pressure (MAP), and percutaneous oxygen saturation (SpO2) were monitored using a multiparameter monitor (BSM-5192; Nihon Kohden Corporation, Tokyo, Japan). During the CT scan (Aquilion PRIME; Canon Medical Systems Corporation, Tochigi, Japan), intermittent positive pressure ventilation was maintained. The patient’s pulse rate ranged from 58 to 79 beats/minute, and MAP ranged from 68 to 79 mmHg during anesthesia. The time from the induction of anesthesia to extubation was 24 minutes, and recovery from anesthesia seemed to be very smooth. The dog was moved to the ward after spontaneous head lift was observed. CT scans showed a right adrenal gland mass invading the caudal vena cava (Fig. 1). In addition, the mass around the celiac and cranial mesenteric arteries was consistent with a previous report of paraganglioma (Gombert et al., 2022) (Fig. 1). The CT images of the lungs appeared normal, except for some bullae (Fig. 2A and B). Table 1. Laboratory findings on initial examination day.
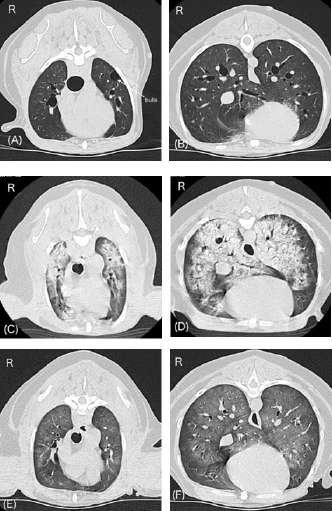
Approximately 15 minutes after being moved to the ward, the dog exhibited dyspnea and nose bleeding. The oral mucous membrane was pale, systolic blood pressure was >200 mmHg, and SpO2 ranged from 70% to 80%. The pulse rate was not recorded. A catecholamine crisis was suspected, and IV acepromazine (PromAce injectable; Boehringer Ingelheim Animal Health, Ridgefield, CT) at 0.01 mg/kg was administered for vasodilation and sedation. In addition, oxygen supply via a mask or flow-by was initiated after upper airway suctioning of blood and secretions. Following these treatments, the patient’s blood pressure decreased. Systolic and mean blood pressures were 140 and 100 mmHg, respectively. However, SpO2 remained at approximately 90% despite oxygen therapy, and respiratory distress was evident. Lung ultrasonography (LOGIQ e Premium; GE HealthCare, Tokyo, Japan) revealed a shred sign suggestive of lung consolidation with aeration in the left caudodorsal and perihilar lung regions. Multiple B lines suggestive of a wet lung were also observed in the left lung. The awake CT images taken 90 minutes after anesthetic recovery showed severe pulmonary consolidation with air bronchograms throughout all lung fields (Fig. 2C and D), and a decision was made to initiate ventilatory support under stable hemodynamics. Blood sampling for measurement of plasma catecholamine levels could not be performed given that our top priority was to stabilize a critical hypoxemia following the hypertensive crisis.
Fig. 1. An abdominal CT image of the present case is shown. A right adrenal gland mass with invasion of the caudal vena cava is observed. In addition, a mass around the celiac and cranial mesenteric arteries, suggestive of a paraganglioma, is also detected. AG, adrenal gland mass; CVC, caudal vena cava; and PG, the mass suspecting a paraganglioma. The dog was anesthetized with IV propofol to effect with 0.2 mg/kg of IV butorphanol. After intubation, anesthesia was maintained with 1.5% isoflurane in oxygen (1 l/minute) delivered via a circular rebreathing system and an anesthetic machine with an out-of-circuit vaporizer (FO-20A; Acoma Medical Industry Co., Ltd., Tokyo, Japan). Spontaneous breathing was maintained, and an end-expiratory positive pressure of 5 cm H2O, adjusted by a manual semi-closed adjustable pressure-limiting valve, was applied for ventilatory support. In addition, 1 mg/kg IV of furosemide (Nichi-Iko Pharmaceutical Co., Ltd., Toyama, Japan) was administered to reduce pulmonary capillary osmotic pressure. Mild improvement was observed in pulmonary CT images 3 hours after the initiation of ventilatory support. During treatment, the pulse rate and MAP ranged from 108 to 140 beats/minute and 81 to 92 mmHg, respectively. The RR varied between 43 and 48 breaths/minute, and the end-tidal carbon dioxide concentration was between 32 and 35 mmHg. SpO2 was maintained between 96% and 100%. The partial pressures of arterial oxygen (PaO2) and carbon dioxide (PaCO2) analyzed using a blood gas analyzer (GEM-Premier 5000; IL Japan Co., Ltd., Tokyo, Japan) after the third CT scan, were 152 and 46 mmHg, respectively. The ratio of the PaO2 to the fraction of inspired oxygen (P/F ratio) was 152, and a diagnosis was made of severe oxygenation failure due to acute respiratory failure. After informed consent was obtained, a decision was made to implement more aggressive ventilatory management in the intensive care unit (ICU).
Fig. 2. Changes in lung CT images over time. A and B, images with breath hold at 10 cm H2O under general anesthesia before a hypertensive crisis. C and D, awake images 75 minutes after the onset of respiratory failure following a hypertensive crisis. E and F, images under spontaneous breathing 24 hours after onset of respiratory failure. Panels A, C, and E, cranial level; panels B, D, and F, caudal level. A and B show normal canine lungs except for the presence of bulla. C and D reveal acute onset of severe pulmonary consolidation with air bronchograms throughout all lung fields compared with A and B. E and F reveal dramatic improvement of pulmonary consolidation compared with C and D. The patient was connected to an intensive care ventilator (SERVO-air; Fukuda Denshi, Tokyo, Japan), and isoflurane anesthesia was discontinued. General anesthesia was maintained with propofol (Propofol 1% IV injectable; Pfizer Japan Inc., Tokyo, Japan) and fentanyl (Terumo Corporation, Tokyo, Japan) with constant-rate infusion (0.3 mg/kg/minute and 0.1 μg/kg/minute, respectively). An additional IV bolus of propofol, fentanyl, or midazolam (Maruishi Pharmaceutical. Co., Ltd., Osaka, Japan) was administered to maintain the patient’s ventilatory synchrony. Pressure-support ventilation with continuous positive airway pressure (CPAP) was initiated and maintained overnight. The initial ventilatory settings were: support pressure, 8 cm H2O (ΔPsupport); CPAP, 5 cm H2O; time for pressure to rise to the target pressure, 0.15 seconds; expiration trigger sensitivity, 30%; and flow trigger, 1.0 l/minute. During ventilation, ΔPsupport ranged from 7 to 10 cm H2O, and the tidal volumes were between 8 and 14 ml/kg. The fraction of inspired oxygen (FiO2) had gradually decreased to 30% by the next morning, while SpO2 was maintained above 95%. In addition to ventilatory management, 0.01 mg/kg of IV acepromazine was administered every 4 hours for additional sedation and prophylaxis of hypertension. Fluid therapy, consisting of a hypotonic crystalloid solution (Soldem3; Terumo Corporation, Tokyo, Japan) at a rate of 1 ml/kg/hour was maintained. The total volume, including other drugs was calculated as 2–3 ml/kg/hour. Three bolus doses of IV furosemide (0.5 or 1 mg/kg) were used to maintain a negative fluid balance. Cefazoline (LTL Pharma Co., Ltd., Tokyo, Japan; 20 mg/kg IV) was administered every 8 hours as a prophylactic antibiotic. During ventilation, the patient’s hemodynamics remained stable, and the spontaneous RR gradually decreased to 20–30 breaths/minute. On the morning after 14 hours of ICU ventilation, PaO2 and PaCO2 were 93 and 33 mmHg, respectively. The P/F ratio was calculated to be 310. The ventilatory settings were: ΔPsupport, 7 cm H2O; CPAP, 5 cm H2O; and FiO2, 0.3. No cardiorespiratory instability was observed. In addition, only a few B lines were observed on lung ultrasonography. Improvement in the pulmonary CT images was also noted (Fig. 2E and F). Therefore, an attempt to wean the patient from the ventilator was initiated 15 hours after starting ICU ventilation (24 hours after the onset of respiratory failure). To allow weaning from the ventilator, constant-rate infusions were discontinued. Ten minutes later, the patient was no longer accepting the endotracheal tube, at which time the patient was extubated. Blood sputum adhesion was observed at the tip of the extubated tube. The patient was able to breathe effectively with some respiratory effort, and SpO2 was 96% under nasal oxygen supply at 0.5 l/minute. PaO2 and PaCO2 were 77 and 27 mmHg, respectively. The body weight after weaning was 170 g less than that before the onset of respiratory failure. Cefazoline and enrofloxacin (Baytril 2.5% injection for dogs and cats; Elanco Japan, Tokyo, Japan) were administered as prophylactic antibiotics during hospitalization. In addition, oral treatment with pimobendane (VETMEDIN chewable tablet; Boehringer Ingelheim Animal Health, Tokyo, Japan) was resumed. Oxygen therapy was continued for 24 hours after extubation. Finally, the dog was discharged 3 days after weaning from ventilation. The owner refused additional treatment for the intra-abdominal lesions. The dog survived without any respiratory or neurological complications for 14 months after weaning from mechanical ventilation. DiscussionTo the best of our knowledge, this is the first clinical case report describing the successful recovery and discharge of a dog with acute respiratory failure following hypertensive crisis. There is a high possibility that this hypertensive crisis was associated with a catecholamine storm due to a preexisting pheochromocytoma or paraganglioma. In pheochromocytoma, a perianesthetic catecholamine storm may be induced by many factors, including stress, changes in intra-abdominal pressure, hypoventilation, drugs causing histamine release (e.g., morphine and meperidine), and drugs affecting the sympathetic nervous system (e.g., ketamine, ephedrine, and metoclopramide) (Fischer, 2022). In the present dog, overweight and cystic disease of the left kidney may have aggravated the changes in intra-abdominal pressure occurring during positional changes. There are pros and cons (Bessell-Browne and O’Malley, 2007), IV administration of iodine contrast agents may also induce catecholamine storm in patients with pheochromocytoma. The triggers of the hypertensive crisis in the present dog may have been stress, changes in intra-abdominal pressure, or the iodine-based contrast agent administered for the contrast-enhanced CT scan. Hypertensive crisis is a major complication of pheochromocytoma and paraganglioma (Galac and Korpershoek, 2017; Ng et al., 2023). In humans, acute pulmonary edema is a manifestation of hypertensive crisis in pheochromocytoma and can be either cardiogenic or noncardiogenic (Galac and Korpershoek, 2017; Ng et al., 2023). Cardiogenic pulmonary edema is more common and may be due to catecholamine-induced type of cardiomyopathy, including dilated cardiomyopathy, hypertrophic cardiomyopathy, or Takotubo cardiomyopathy in humans (Kumar et al., 2021; Ng et al., 2023). However, cardiovascular abnormalities were not detected in the present case, except for mild-to-moderate MMVD, and no evidence of elevated left atrial pressure leading to cardiogenic pulmonary edema was obtained on preanesthetic examination. During mechanical ventilation, no findings suggestive of these cardiomyopathies were observed by subjective echocardiography, and the hemodynamics remained stable without cardiovascular medication. On the other hand, noncardiogenic pulmonary edema is rare in humans (Ng et al., 2023). The cause of noncardiogenic pulmonary edema in cases of pheochromocytomas is similar to that of neurogenic pulmonary edema. The pathogenesis of neurogenic pulmonary edema is also associated with catecholamine storms (Davison et al., 2012; Drobatz, 2019; Unger and Martin, 2023). In this pathology, high catecholamine concentration can cause a sudden increase in pulmonary capillary pressure due to massive vasoconstriction and alter pulmonary capillary permeability (Davison et al., 2012; Drobatz, 2019; Unger and Martin, 2023). Unfortunately, we did not measure plasma catecholamine levels during the hypertensive crisis and, therefore, lack direct proof of a catecholamine storm in the present case. The observation of a high urinary-free normetanephrine to creatinine ratio on the initial examination day suggests that catecholamine storms were occurring randomly in this patient. The common radiographic findings of neurogenic pulmonary edema in dogs are bilateral, symmetric, multifocal, mixed alveolar to interstitial lung patterns, focused on the caudal lung lobe or distributed throughout all lung fields (Bouyssou et al., 2017). The CT and lung ultrasonography images of the present dog were consistent with previous reports. We surmise that the main pathogenesis of acute respiratory failure in the present dog was a noncardiogenic pulmonary edema, such as a neurogenic pulmonary edema induced by a catecholamine storm. The involvement of MMVD may have exacerbated the pulmonary edema. A productive cough associated with pink, frothy nasal discharge or sputum, commonly seen in severe cardiogenic pulmonary edema (Oyama, 2019), was not observed in the present dog. In addition, endotracheal tube suctioning during mechanical ventilation was not required. A small amount of blood sputum was observed at the tip of the extubated tube, but this may have originated from aspiration of nasal bleeding following hypertensive crisis. Initially, it was also suspected that the acute dyspnea was caused by upper airway obstruction following nasal bleeding consequent to the hypertensive crisis. However, despite prompt efforts to secure the upper airway through sedation, suction, and mouth opening, we observed no improvement in respiratory status, and no obstruction of the airway was observed at the time of CT imaging. In the present case, the onset and progression of pulmonary edema were extremely rapid. Severe oxygenation failure was detected based on the P/F ratio before initiation of ICU ventilation. Pulmonary edema in the present dog was treated with mechanical ventilation and negative fluid balance (minus 170 g based on body weight measurements) after the administration of furosemide. In this dog, pressure-support ventilation with CPAP was adopted to reduce spontaneous respiratory effort and improve oxygenation. The response to treatment was good, and the improvement in pulmonary edema was dynamic. The dog was extubated after mechanical ventilation for 24 hours. Similar to the present case, rapid onset and recovery of neurogenic pulmonary edema have been reported in dogs (Drobatz et al., 1995; Drobatz, 2019; Herrería-Bustillo et al., 2022; Nemi et al., 2023; Unger and Martin, 2023). A high mortality rate was observed in dogs treated with mechanical ventilation for neurogenic pulmonary edema (Drobatz et al., 1995; Nemi et al., 2023). The unfavorable outcomes are attributed to the severity of the underlying disease rather than to the complications of pulmonary edema or mechanical ventilation (Drobatz et al., 1995; Drobatz, 2019; Unger and Martin, 2023). In the present case, no additional hypertensive crisis was observed during mechanical ventilation. To control hypertensive crisis in pheochromocytoma, short-acting injectable alpha-blockers, such as phentolamine, are commonly administered. On the other hand, long-acting oral alpha-blockers, such as phenoxybenzamine, are commonly used for preoperative stabilization in pheochromocytoma (Herrera et al., 2008). Acepromazine is a long-acting injectable major tranquilizer with simultaneous alpha 1 blockade. The main reason for using acepromazine during ICU ventilation was our expectation of obtaining hemodynamic stabilization in the present dog, similar to preoperative alpha-blocker therapy in pheochromocytoma. Simultaneously, acepromazine likely contributed to sedation during mechanical ventilation. We believe that hemodynamic stability was key to the successful treatment of this dog. This case illustrates the successful weaning and hospital discharge of a dog with acute respiratory failure following a hypertensive crisis requiring mechanical ventilatory management for 24 hours. A catecholamine storm associated with presumed pheochromocytoma or paraganglioma may have caused pulmonary edema in the present dog. The onset and progression of the pulmonary edema were extremely rapid. However, improvement in pulmonary edema was also rapid. In addition to prompt diagnosis and aggressive therapeutic intervention with mechanical ventilation, the achievement of hemodynamic stability may have contributed to a good prognosis; in this case of pulmonary edema following a hypertensive crisis in a dog, we attribute to a catecholamine storm induced by a presumed pheochromocytoma or paraganglioma. AcknowledgmentsNot applicable. Conflict of interestThe authors declare that there is no conflict of interest. FundingNo sources of funding for the work presented here. Author contributionsJT and NO performed anesthesia and ventilatory management of this patient. JT wrote the manuscript. Internal medicine veterinarians NN and SY performed the case evaluation and follow-up. GS was responsible for diagnostic imaging. All authors discussed this patient and approved this manuscript. Data availabilityAll data supporting the findings of this case report are available within the manuscript. ReferencesBessell-Browne, R. and O’Malley, M.E. 2007. CT of pheochromocytoma and paraganglioma: risk of adverse events with i.v. administration of nonionic contrast material. AJR. Am. J. Roentgenol. 188, 970–974. Bouyssou, S., Specchi, S., Desquilbet, L. and Pey, P. 2017. Radiographic appearance of presumed noncardiogenic pulmonary edema and correlation with the underlying cause in dogs and cats. Vet. Radiol. Ultrasound. 58, 259–265. Cagle, L.A., Hopper, K. and Epstein, S.E. 2022. Complications associated with long-term positive-pressure ventilation in dogs and cats: 67 cases. J. Vet. Emerg. Crit. Care. (San Antonio). 32, 376–385. Davison, D.L., Terek, M. and Chawla, L.S. 2012. Neurogenic pulmonary edema. Crit. Care. 16, 212. Drobatz, K.J. 2019. Neurogenic pulmonary edema. In Textbook of small animal emergency medicine, 1st ed. Eds., Drobatz, K.J., Hopper, K., Rozanski, E. and Silverstein, D.C. Hoboken, NJ: Willey Blackwell, pp: 247–252. Drobatz, K.J., Saunders, H.M., Pugh, C.R. and Hendricks, J.C. 1995. Noncardiogenic pulmonary edema in dogs and cats: 26 cases (1987-1993). J. Am. Vet. Med. Assoc. 206, 1732–1736. Edwards, T.H., Erickson Coleman, A.E., Brainard, B.M., DeFrancesco, T.C., Hansen, B.D., Keene, B.W. and Koenig, A. 2014. Outcome of positive-pressure ventilation in dogs and cats with congestive heart failure: 16 cases (1992-2012). J. Vet. Emerg. Crit. Care. (San Antonio). 24, 586–593. Fischer, B.L. 2022. Endocrine disease. In Canine and feline anesthetic and co-existing disease, 2nd ed. Eds., Johnson, R.A., Snyder, L.B.C. and Schroeder, C.A. Hoboken, NJ: Willey Blackwell, pp: 299–335. Galac, S. and Korpershoek, E. 2017. Pheochromocytomas and paragangliomas in humans and dogs. Vet. Comp. Oncol. 15, 1158–1170. Gombert, A., Diana, A., Hecht, S., Nicoli, S., Fracassi, F., Mortier, J., Reyes-Gomez, E. and Pey, P. 2022. Imaging features of retroperitoneal extra-adrenal paragangliomas in 10 dogs. Vet. Radiol. Ultrasound. 63, 393–402. Herrera, M.A., Mehl, M.L., Kass, P.H., Pascoe, P.J., Feldman, E.C. and Nelson, R.W. 2008. Predictive factors and the effect of phenoxybenzamine on outcome in dogs undergoing adrenalectomy for pheochromocytoma. J. Vet. Intern. Med. 22, 1333–1339. Herrería-Bustillo, V.J., Adamantos, S., Lamb, C.R., García-Arce, M., Thomas, E., Saiz-Álvarez, M.R., Cook, S. and Cortellini, S. 2022. Retrospective evaluation of negative-pressure pulmonary edema in dogs (2006-2018): 35 cases. J. Vet. Emerg. Crit. Care. (San Antonio). 32, 397–404. Hopper, K., Haskins, S.C., Kass, P.H., Rezende, M.L. and Aldrich, J. 2007. Indications, management, and outcome of long-term positive-pressure ventilation in dogs and cats: 148 cases (1990-2001). J. Am. Vet. Med. Assoc. 230, 64–75. Kumar, A., Pappachan, J.M. and Fernandez, C.J. 2021. Catecholamine-induced cardiomyopathy: an endocrinologist’s perspective. Rev. Cardiovasc. Med. 22, 1215–1228. Nemi, J.R., Hopper, K. and Epstein, S.E. 2023. Retrospective evaluation of noncardiogenic pulmonary edema in dogs and cats (2000-2021): 31 cases. J. Vet. Emerg. Crit. Care. (San Antonio). 33, 354–359. Ng, D.Z., Than Yu, K.P. and Rajkanna, J. 2023. Acute pulmonary edema as a cardiovascular manifestation of pheochromocytoma. Cureus 15, e33675. Oyama, M.A. 2019. Cardiogenic pulmonary edema. In Textbook of small animal emergency medicine, 1st ed. Eds., Drobatz, K.J., Hopper, K., Rozanski, E. and Silverstein, D.C. Hoboken, NJ: Willey Blackwell, pp: 242–246. Sasaki, N., Ikenaka, Y., Inoue, Y., Ichise, T., Nagata, N., Ishizuka, M., Nakayama, S.M., Nakamura, K. and Takiguchi, M. 2021. Urinary free metanephrines measurement in dogs with adrenal gland diseases using a new simple liquid chromatography tandem mass spectrometry method. J. Vet. Med. Sci. 83, 648–655. Summers, C., Todd, R.S., Vercruysse, G.A. and Moore, F.A. 2022. Acute respiratory failure. Perioper. Med. 2022, 576–586. Unger, K. and Martin, L.G. 2023. Noncardiogenic pulmonary edema in small animals. J. Vet. Emerg. Crit. Care. (San Antonio). 33, 156–172. | ||
| How to Cite this Article |
| Pubmed Style Tamura J, Yoshida S, Nagata N, Shimbo G, Oyama N. Successful treatment of acute respiratory failure following hypertensive crisis in a dog with presumed pheochromocytoma or paraganglioma. Open Vet. J.. 2023; 13(11): 1465-1470. doi:10.5455/OVJ.2023.v13.i11.10 Web Style Tamura J, Yoshida S, Nagata N, Shimbo G, Oyama N. Successful treatment of acute respiratory failure following hypertensive crisis in a dog with presumed pheochromocytoma or paraganglioma. https://www.openveterinaryjournal.com/?mno=162925 [Access: January 24, 2026]. doi:10.5455/OVJ.2023.v13.i11.10 AMA (American Medical Association) Style Tamura J, Yoshida S, Nagata N, Shimbo G, Oyama N. Successful treatment of acute respiratory failure following hypertensive crisis in a dog with presumed pheochromocytoma or paraganglioma. Open Vet. J.. 2023; 13(11): 1465-1470. doi:10.5455/OVJ.2023.v13.i11.10 Vancouver/ICMJE Style Tamura J, Yoshida S, Nagata N, Shimbo G, Oyama N. Successful treatment of acute respiratory failure following hypertensive crisis in a dog with presumed pheochromocytoma or paraganglioma. Open Vet. J.. (2023), [cited January 24, 2026]; 13(11): 1465-1470. doi:10.5455/OVJ.2023.v13.i11.10 Harvard Style Tamura, J., Yoshida, . S., Nagata, . N., Shimbo, . G. & Oyama, . N. (2023) Successful treatment of acute respiratory failure following hypertensive crisis in a dog with presumed pheochromocytoma or paraganglioma. Open Vet. J., 13 (11), 1465-1470. doi:10.5455/OVJ.2023.v13.i11.10 Turabian Style Tamura, Jun, Shino Yoshida, Noriyuki Nagata, Genya Shimbo, and Norihiko Oyama. 2023. Successful treatment of acute respiratory failure following hypertensive crisis in a dog with presumed pheochromocytoma or paraganglioma. Open Veterinary Journal, 13 (11), 1465-1470. doi:10.5455/OVJ.2023.v13.i11.10 Chicago Style Tamura, Jun, Shino Yoshida, Noriyuki Nagata, Genya Shimbo, and Norihiko Oyama. "Successful treatment of acute respiratory failure following hypertensive crisis in a dog with presumed pheochromocytoma or paraganglioma." Open Veterinary Journal 13 (2023), 1465-1470. doi:10.5455/OVJ.2023.v13.i11.10 MLA (The Modern Language Association) Style Tamura, Jun, Shino Yoshida, Noriyuki Nagata, Genya Shimbo, and Norihiko Oyama. "Successful treatment of acute respiratory failure following hypertensive crisis in a dog with presumed pheochromocytoma or paraganglioma." Open Veterinary Journal 13.11 (2023), 1465-1470. Print. doi:10.5455/OVJ.2023.v13.i11.10 APA (American Psychological Association) Style Tamura, J., Yoshida, . S., Nagata, . N., Shimbo, . G. & Oyama, . N. (2023) Successful treatment of acute respiratory failure following hypertensive crisis in a dog with presumed pheochromocytoma or paraganglioma. Open Veterinary Journal, 13 (11), 1465-1470. doi:10.5455/OVJ.2023.v13.i11.10 |