| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 360–369 Original Research The impact of dietary probiotic supplementation on welfare and growth performance of Nile tilapia (Oreochromis niloticus)Mohamed Y. I. Youssef, Al-Sadik Y. Saleem, Fayza A. Ahmed*, Enas N. Said, Shereen El. Abdel-Hamid and Heba S. A. GharibDepartment of Behaviour and Management of Animal, Poultry and Aquatic, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Fayza A. Ahmed. Department of Behaviour and Management of Animal, Poultry and Aquatic, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: fayyad568 [at] gmail.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
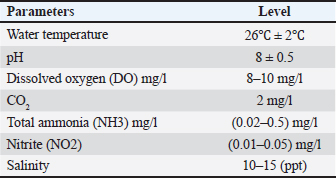
ABSTRACTBackground: The usage of commercial probiotic products as alternatives to traditional antibiotics in fish culture is initiated to be a potential factor for Nile tilapia fish’s welfare and growth. Aim: The purpose of the current study is to show the influence of commercial probiotics (Bacillus amyloliquefaciens) dietary supplementation at different levels on Nile tilapia welfare and growth. Methods: Apparently healthy fingerlings of Nile Tilapia with a total number of 120 with an average initial weight (26.2 ± 0.3 g) were distributed into four groups (each group had 30 fingerlings). The first group (G1) was given a basal diet without additional probiotics, while other groups [second group (G2), the third group (G3), and the fourth group (G4)] were given basal diets supplemented with different levels of commercial probiotics (1 g, 2 g, and 3 g of probiotics per kilogram of diet), respectively (15 fish in each sub group as replicate), in eight glass aquaria (30 × 40 × 100 cm) for 2 months as an experimental period. Results: The results revealed that the probiotic-treated groups especially G4 (3 g probiotics/kg diet) showed a marked increase in the following behavioral patterns such as feeding and swimming behaviors, while G2 (1 g probiotic/kg diet) had an increase in the foraging behavior compared with G1 control group. While surfacing, body care, and aggressive behaviors with all patterns were the highest in the control group (G1) than all probiotics-treated groups. The crossing test showed that fish rose in the probiotic-treated groups (G3 and G4) were more active and could achieve the highest growth rates. While water quality was better in G4 (3 g probiotic /kg diet) than in other groups. Moreover, G4 (3 g probiotic/kg diet) showed a marked increase in all serum biochemical parameters than the control group (G1). Conclusion: The current study proved that the best level of commercial probiotics (B. amyloliquefaciens) was (3 g probiotic/kg diet) for achieving optimal Nile tilapia fingerlings’ growth performance under these experimental conditions. Finally, this work confirms the significance of the addition of probiotics as a feed additive to enhance both growth performance and immunity response, improve water quality, and achieve the welfare of Nile tilapia fingerlings. Keywords: Probiotics, Behavioural patterns, Crossing test, Growth rate, Welfare. IntroductionFisheries are the most important farmed aquaculture in the world. Aquaculture had a potential requirement of fish products which avails a potential supply of the most important proteins in addition to essential micronutrients for healthy nutrition and the best life in the world as it is considered as the most rapidly growing food industry and it is known as a necessary solution to the global nutritional depletion and deficiencies. Nowadays, aquaculture can overrun capture fisheries regarding fish productivity (Subasinghe et al., 2009). Fish culture is a crucial and subservient supply of highly nutritious, easily digestible animal protein, and meets the needs of humans in terms of nutrition. Tilapia is regarded as the best and most sophisticated fish due to its resistance to environmental changes including high amounts of nitrite and ammonia, low levels of dissolved oxygen, and wide pH variations (Ardjosoediro and Ramnarine, 2002). Today, the average global intake of tilapia, which is the star of fish culture and popularly known as “aquatic chicken,” has increased (Fitzsimmons, 2005). The most popular species, tilapia, constitutes more than 65% of all aquaculture productivity (Dickson et al., 2016). One of the most prominent freshwater fish in Egypt and around the globe is Nile tilapia which is characterized by quick growth performance, excellent adaptability to poor water quality, low feed conversion ratio (FCR), and high disease resistance. All these criteria make Nile tilapia a convenient cultured fish (El-Sayed, 2019). Probiotics are thought to be safe substitutes for antibiotics, with numerous positive influences on the aquaculture sector (Banerjee and Ray, 2017). Today, in aquaculture, probiotics have been used as a basic and friendly supplement to enhance fish health. The selection of probiotics is based on their ability to colonize, combat pathogens, increase the activity of microflora, and produce beneficial substances including vitamins, fatty acids, and digestive enzymes. (Vine et al., 2006). Commercial probiotics can be applied as water or feed supplements to minimize challenging circumstances, boost immune function, and subsequently lessen the adverse consequences of various stresses (Taoka et al., 2006). Probiotics are regarded as “live bacterial feed supplement that enhance the health and wellbeing of the host” (Gatesoupe, 1999). The modern trend that has been reported is to isolate, test, and utilize probiotics from fish that will be observed to be more efficient in growth rate, health, and immunity of farmed fishes (Ridha and Azad, 2016). Different studies confirmed that the usage of probiotics in fish culture boosts survival rate (SR) and growth, enhances feeding behavior, improves the immune response (Huerta-R´abago et al., 2019), and increases productivity (Hasan and Banerjee, 2020). Thus the primary aim of the current work was to investigate the influence of probiotic products on behavior, growth performance, welfare, water quality, and serum biochemical parameters on fingerlings of Nile tilapia. Materials and MethodsThe current study was conducted at the Faculty of Veterinary Medicine, Zagazig University, Egypt. It was carried out between the beginning of June (2022) and the end of July (2022). Fish handling and water hygieneFish handlingA particular fish farm in the Governorate of Ismailia provided 120 Nile Tilapia fingerlings, with an average initial weight of (26.2 ± 0.3 g). These fingerlings appeared to be in good health. Fingerlings were moved as soon as they arrived at a cement pond at the Fish Research Unit, Faculty of Veterinary Medicine, Zagazig University, Egypt for a 2-week adaptation period to the water temperature to reduce stress. Before the beginning of the study, fingerlings were kept in eight 90 l aquaria for a 14-day acclimatization period. To start the study, the fingerlings of Nile Tilapia were separated into four groups (Fifteen fish per subgroup). The first group (G1) control group was given a basal diet without additional probiotics, the second group (G2) was given a basal diet supplemented with 1 g probiotics/kg diet, the third group (G3) (2 g probiotics/kg diet), and the fourth group (G4) (3 g probiotics/kg diet). Daily records of mortality and morbidity were kept. Aquaria and aquarium water hygieneA fully prepared eight glass aquaria (two aquaria for each group) measuring (30 × 40 × 100 cm) were used. Electrical aerators and filters were used to provide consistent aeration for each aquarium while also serving as a supply of dissolved oxygen and all organic waste materials were removed. A thermostat (controlled heater) was used to regulate water temperature thermostatically and to keep the aquarium water at an optimum temperature required for fingerlings of Nile Tilapia. A water thermometer for measuring the water temperature daily and a separate nylon hand net were used for each aquarium to make fish transport and handling easier and to prevent the spread of infection. Throughout the experimental period, about 25 % of the aquarium water was changed every day and completely changed weekly by dechlorinated water from the water storage tank for keeping fish at the following aquarium water parameters according to Gregory and Grandin (2013) (Table 1). Table 1. The aquarium’s environmental parameters favorable for Nile tilapia growth.
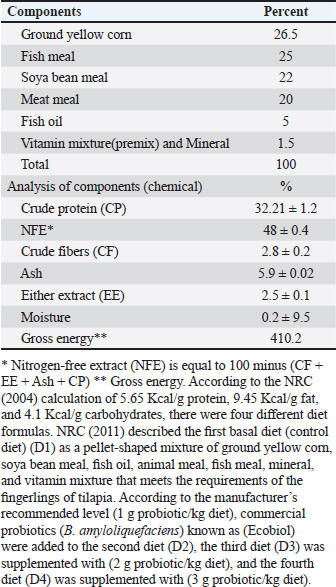
MedicationUsing sodium chloride (1 g/1 l) as protection against any fish disease 2 times weekly after altering the water of all aquaria, as well as potassium permanganate (2 mg/1 l) and Oxytetracycline (50 mg/1 kg b.wt.) for treating fish from the columnaris disease that impacts many freshwater fish throughout stressful situations (Abdel-Fattah et al., 2020). Feeding systemProbioticsIn this study, we utilized commercial probiotics (Bacillus amyloliquefaciens), which were purchased from Attaka Industrial Zone-Suez Gulf, Egypt, commercially known as Ecobiol, Norel Animal Production. Preparation of the basal dietThe basal diet components were utilized according to Yousry et al. (2019) as mentioned in Table 2. Feeding routineFeeding frequency was 3 times per day at 8:00 AM, 12:00 PM, and 4:00 PM, and fingerlings were manually fed 6 days per week. Each daily ration is separated into three parts at the three previously mentioned times. Feeding exactly as much as they can consume in 5 minutes, the daily food intake was kept constant at 3% of the fish’s total weight during the period of study and it was estimated every 2 weeks by weighing all fish of all aquaria (Ahmed, 2020). Table 2. Components of basal diet applied in the study.
Live fish performanceThe following physical performance was noted during the study according to Said et al. (2020). Average daily gain (ADG) per day. This statistic was calculated using the following formula:ADG is equal to (W2-W1)/T, where T is the study’s duration in days. Specific growth rate (SGR). It was calculated using the formula shown below:SGR is equal to [(log (W2) − log (W1))/T] × 100. Body weight gain (WG)/g. The body WG during two consecutive weeks was estimated separately using the formula: BWG (WG/g) is equal to W2 − W1. W1 and W2 are the weights of individual fish in two separate weeks. Feed conversion ratio (FCR). FCR is calculated as follows: [feed intake (g)/WG (g)] × 100. Survival rate (SR). To calculate the SR, multiply the number of fish that survived by the total number of fish. Mortality rate. The mortality rate is equal to the Number of dead fish/duration of the study. Morbidity rate. The morbidity rate is equal to the Number of sick fish/duration of the study. Observation and data collectionThe behavioral observations were noted as follows: each group was monitored twice daily for 15 minutes each period (7.5 minutes for each aquarium) (45 seconds for each fish identified), 4 days a week, at a regularly scheduled time. Intervals via 8 hours per week throughout the 8 weeks of the study for all groups (Abdel-Fattah et al., 2020). The Nile Tilapia fingerlings were identified by short plastic bands of various colors that were attached to the dorsal fins of 10 fingerlings using thin wire to provide easy observation of 10 fingerlings during the study period (Ahmed, 2020). Behavioral observation intervals time were 2 hours per day in periods of 8:00 AM till 4:00 PM throughout the study weeks by utilizing a focal sample technique, multipurpose counter, notebook to note the behavioral patterns, video camera, and stop watch (Said et al., 2020). All presented behaviors were observed according to El-Saadony et al. (2021) as the following: Ingestive behaviorFeeding behaviorFeeding behavior is the act of feed consumption during the feeding period and involves several behavioral responses related to eating, including modes of feeding, the frequency of feeding, feeding habits, and food preferences. Mean frequency of feeding behavior was recorded/8 hours. Mean time (second) of feeding behavior was recorded/8 hours. Foraging behaviorForaging behavior means the searching of fish for food and the usage of food resources.
Surfacing behaviorSurfacing behavior refers to the frequency with which fish periodically rise to the water’s surface to breathe.
Swimming behaviorSwimming behavior means the movement of fish in the surface, middle, and bottom of an aquarium, whether it be quickly or slowly, and without engaging in any behavioral activity.
Body care behaviourBody shakingBody shaking means that a fish shakes its entire body laterally (2) or (3) times in rapid succession.
Scratching (chafing)It is the act of rubbing any part of the fish’s body against an object, regarding (Neto and Giaquinto, 2020).
Aggressive behaviorIt is the act of initiating an attack and refers to fighting. The following Aggressive patterns were defined and noted according to Brandão et al. (2018) and Barreto et al. (2011).
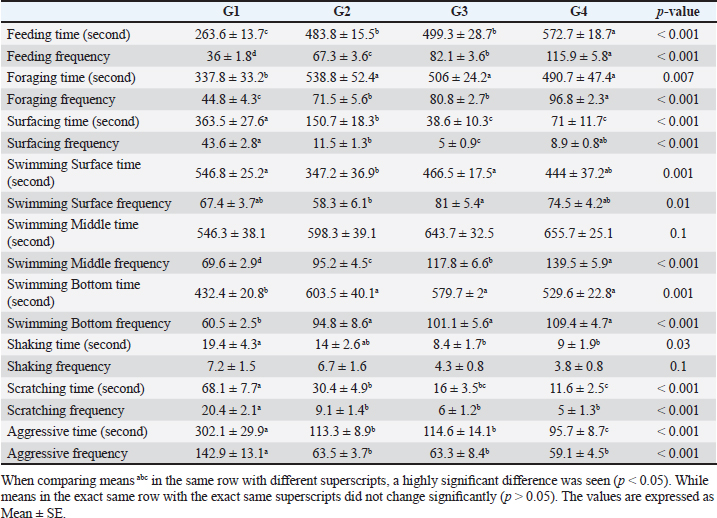
Number of midline crossing testCalculated by counting the number of midline crossings from fish via a 5-minute period in each aquarium by separating the total length of the aquarium by a midline externally according to the calculations and protocol of Scott et al. (2003). Environmental data collectionFor determination of aquarium water parameters: Collection of water sample. Clean sample flasks each of about 1-l volume were equipped with a stopper and were rinsed several times with sampled water. Water samples were taken from below the water surface and the flasks were labeled with the time of collection, date, and locality.For chemical analysis and measuring water parameters, water samples were utilized in the location for measuring immediately dissolved oxygen, PH, ammonia, nitrite, and nitrate (APHA, 1998). Measurements of water quality parameters. Water samples were gathered from aquaria for:(1) Determination of pH using a pH meter (pH - 330). (2) Determination of water temperature by using a thermometer. (3) Determination of ammonia, nitrite, and nitrate by using Aquamerk (Merck kGaA. 64271 Darmstadt, Germany). (4) Determination of the dissolved oxygen by using an oxygen meter (Oxi - 330). (5) Dechlorination of tap water by storage for 24–48 hour. Serum biochemical analysis. The caudal vertebral vein is where the blood samples were taken according to Feldman et al. (2000).At a wavelength of 550 nm, serum albumin was estimated colorimetrically, and serum globulin concentration was determined mathematically (El-Kady et al., 2022). Serum total proteins were recorded at the wavelength of 540 nm, while the method outlined by Demers and Bayne (1997) was used to determine the lysozyme activity of sera. Data handling and statistical analysis. Using the SPSS version 21 Statistical Analysis System package (SPSS, 2012), all study data were gathered, organized, summarized, and then analyzed.1. A one-way analysis of variance (ANOVA) test was performed to examine behavioral variables and variations in fish body weight among groups. After significant results, Tukey’s honesty significant test was used. 2. Crossing test was conducted for distinct groups over the course of successive study weeks using a mixed model ANOVA test. At 8 weeks into the study, an interaction plot was utilized to compare means across multiple groups. All results were expressed as Mean ± SD except results of behavioral parameters expressed as mean ± SE. Statistics were considered significant if p-value < 0.05. ResultsResults in Table 3 revealed that there was a highly significant impact of probiotic dietary supplementation (p < 0.05) on mean time (second) and frequency of feeding and foraging behavior of probiotic-treated groups during 8 weeks of the study compared to G1. As the highest and optimum feeding behavior (time and frequency), and foraging behavior frequency were observed in G4 (572.7 ± 18.7 seconds), (115.9 ± 5.8 bout), (96.8 ± 2.3 bout), respectively, but G2 showed the highest values of foraging behavior time (538.8 ± 52.4 seconds), respectively. The data presented in Table 3 showed that probiotic dietary supplementation significantly affected surfacing behavior time and frequency, where G1 (363.5 ± 27.6 seconds) and (43.6 ± 2.8 bout) showed the highest values of surfacing time and frequency respectively. While G3 showed the lowest values of surfacing time and frequency. Probiotic dietary supplementation significantly influenced swimming behavior (surface, middle, and bottom) time and frequency among groups, where probiotic-treated groups especially G4 showed the highest means of middle swimming behavior time and frequency (655.7 ± 25.1 seconds) and (139.5 ± 5.9 bout), respectively. Concerning the body care behavior of Nile tilapia, as maintenance behavior showed that the control group had the highest body shaking behavior and scratching behavior time and frequency (19.4 ± 4.3 seconds), (7.2 ± 1.5 bout), (68.1 ± 7.7 seconds), (20.4 ± 2.1 bout), respectively, than probiotic-treated groups. Regarding, the aggression patterns time and frequency as revealed G1 demonstrated the greatest values of time and frequency (302.1 ± 29.9 seconds) and (142.9 ± 13.1 bout), respectively, of all aggression patterns than probiotic-treated groups. Table 3. The effect of probiotics on the behavior of four groups throughout 8 weeks of study.
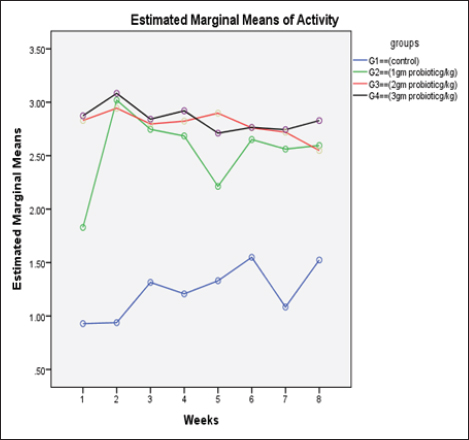
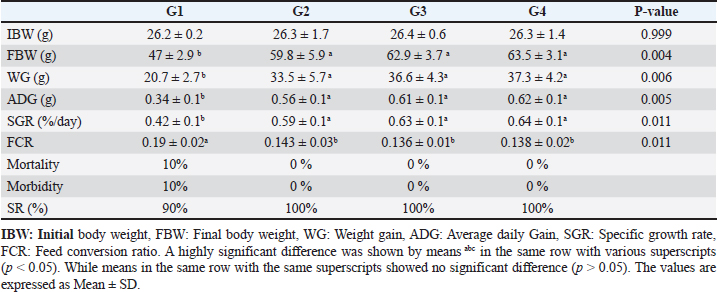
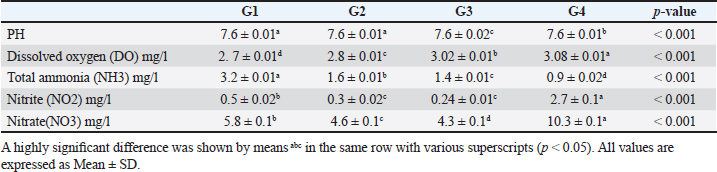
Data from Figure 1 indicated that probiotic dietary supplementation had a significant effect on the frequency of the midline crossing test which was the greatest in G4 (3.1 ± 0.5 bout) and the lowest in G1 (0.9 ± 0.5 bout), respectively. The presented results in Table 4 showed that the growth performance was significantly affected by various probiotic levels during the study weeks, as final body weight (FBW) was the highest in G4 (63.5 ± 3 g) and the lowest in G1 (47 ± 2.3 g). Increasing commercial probiotic (B. amyloliquefaciens) levels in the study diets up to 3 g probiotic/kg diet significantly improved WG, ADG, and SGR, whereas the highest commercial probiotic (B. amyloliquefaciens) level had the greatest values (37.3 ± 4.2 g), (0.62 ± 0.1 g), and (0.64 ± 0.1 g), respectively, compared to G1. The highest FCR values were found in the probiotic-treated groups. Furthermore, the data in Table 4 revealed the impact of various probiotic levels on mortality and morbidity among groups. Whereas probiotics-treated groups showed no mortality and morbidity while G1 (control group) showed mortality (10%), and morbidity (10%). Results presented in Table 5 revealed the influence of different probiotic levels on water quality. G4 had the highest dissolved oxygen level (3.08 ± 0.01 mg/l), but G2 had the highest water pH levels (7.6 ± 0.01), while total ammonia, nitrite, and nitrate were the lowest levels as recorded in G4 (0.9 ± 0.02 mg/l), (2.7 ± 0.1 mg/l), and (10.3 ± 0.1 mg/l), respectively, in comparison to G1.
Fig. 1. The effect of probiotics on the crossing test of four groups throughout 8 weeks of study. (G1): control group; (G2): 1 g probiotic/kg diet; (G3): 2 g probiotic/kg diet; (G4): 3 g probiotic/kg diet. Table 4. The effect of probiotics on growth rate, mortality, and morbidity of Nile tilapia.
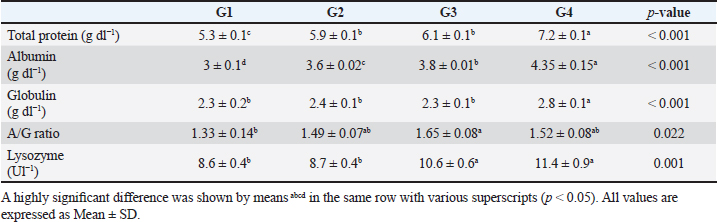
The data presented in Table 6 showed the impact of probiotic dietary supplementation (p ≤ 0.05) on serum biochemical parameters where probiotics-treated groups especially G4 had the optimal values of total proteins (7.2 ± 0.1 g dl−1), serum albumin (4.4 ± 0.2 g dl−1), serum globulin (2.8 ± 0.1 g dl−1), and lysozyme activity (11.4 ± 0.9 Ul−1), respectively. DiscussionProbiotics have been described as appropriate substitutes for traditional antibiotics. Thus, probiotic dietary supplementation can be a nutritional strategy to enhance growth performance and improve behavioral patterns of Nile tilapia fish. Probiotic dietary supplementation affected well on the ingestive behavior of Nile tilapia, this influence may be due to the impact of probiotics on digestive processes by increasing the population of helpful microorganisms, microbial enzyme activity, and intestinal microbial balance, which in turn improves food digestibility and absorption as well as diet palatability and feed utilization (Sakr, 2003). Therefore, it is concluded that supplementation of commercial probiotics (B. amyloliquefaciens) with the level of 3 g/kg diet to Nile tilapia’s diet can improve the fish welfare. These outcomes appear to roughly reflect those attained by Soltan and El-Laithy (2008) who discovered that fish fed the probiotic-supplemented diet exhibited the greatest and optimal values of feeding behavior among all treatments. It was found that probiotic dietary supplementation reduced the surfacing behavior and these findings coincided with the data reported by Noga (1996) who highlighted that fish surface (come to the water surface) more frequently as a result of low dissolved oxygen levels in the aquarium, thus this behavior serves as a good indicator of the dissolved oxygen content of the aquarium’s water. These findings may be due to the reasons found by Diab et al. (2002) who hypothesized that Biogen® as a probiotic product might improve fish body cell metabolism, increase feed utilization efficiency, and balance the secretion of several secretary glands. In addition, it enhanced immunological responses and promoted the vitality of cells by supplying oxygen to the entire body leading to reduced surfacing behavior in all treated probiotics groups especially G3 (2 g probiotic/kg diet). Thus, it can be inferred that supplementing the food of Nile tilapia with commercial probiotics (B. amyloliquefaciens) at a level of 3 g per kilogram of diet will enhance its welfare. Besides, probiotic dietary supplementation significantly influenced swimming behavior (surface, middle, and bottom), these findings concurred with the data cited by Martins et al. (2012) who documented how swimming behavior was modified by water quality indicators. For instance, hypoxia (low level of dissolved oxygen), as seen in G1 (the control group), might cause a lowering in swimming activity and speed. Similarly, Diab et al. (2002) found that supplemented fish with probiotics in the diet resulted in promoting the vitality of fish leading to markedly increasing swimming behavior as seen in G4 (3 g probiotic/kg diet). Table 5. The effect of probiotics on water hygiene.
Table 6. The effect of probiotics on serum biochemical parameters.
Concerning the body care behavior of Nile tilapia, maintenance behavior is influenced well by probiotic dietary supplementation. These results support the findings of El-Kady et al. (2022) who estimated that adding probiotics to farmed aquatic species could enhance the quality of water, lower ammonia, and pollution levels, and lessen the need for bioremediation leading to achieving fish welfare. These findings were reinforced by Noga (1996) who discovered that rubbing a fish’s body against the sides and bottom of the tank is one of the clinical indications of fish ammonia poisoning. Therefore, the main causes of these findings may be related to poor management and hygiene (increasing ammonia emission resulting from high amounts of food residues due to reducing fish appetite). Regarding, the aggression patterns, they were decreased in the probiotic-treated groups. These results disagree with the study of Soltan and El-Laithy (2008) who discovered no appreciable difference between fish fed the various probiotic-supplemented diets and the control diet in terms of aggressive behavior. This contrast between studies may be referred to different management techniques. Probiotic dietary supplementation had a significant effect on the frequency of midline crossing tests. These findings seem to be roughly parallel to those reported by Abd El-Maksoud et al. (2020) who discovered an increase in the vitality of fish-fed probiotic-supplemented diets. These results may be due to the positive relationship between the crossing test and swimming behavior. These findings also concurred with the study of Jahangiri and Esteban (2018) who established that microbial balance in the environment is improved by adding probiotics to culture water or feed, which in turn improves the general health of the farmed aquatic species leads to increasing the fish activity. Thus, it can be concluded that supplementing the food of Nile tilapia with probiotics at a level of 3 g per kg of diet will improve its welfare. The growth performance was significantly affected by various probiotic levels during the study period. Increasing the commercial probiotic (B. amyloliquefaciens) level in the study diets up to 3 g probiotic/kg diet significantly improved WG, ADG, and SGR. Whereas the highest commercial probiotic (B. amyloliquefaciens) level (3 g probiotic/kg diet) had the greatest values compared to G1. The highest FCR values were recorded in the probiotic-treated groups and that might be due to the increased feed intake, and reduced amount of feed required for fish growth by effectively using all nutrients in the aquarium, and subsequently could lead to decreasing production cost. These findings coincided with the study of El-Dakar et al. (2004) who discovered that adding probiotics to fish’s diet enhanced several growth-related parameters, including initial body weight, FBW, WG, and SGR. These results also agree with the data recorded by Soltan et al. (2016) who observed that all Biogen® levels (1–4 g/kg) could give the best growth performance indices (IBW, FBW, WG, and SGR) compared to the control diet. These results might be attributed to the effect of probiotics, which prevent prospective pathogens from colonizing the gastrointestinal tract through antibiosis, competition for nutrients and space, and changes in microbial metabolism (Hoshino et al., 1997). Besides, probiotics aid in forming vitamins such as biotin and vitamin B12 and detoxifying the potentially toxic chemicals in feeds (Spanggaard et al., 2001). However, El-Kady et al. (2022) estimated that increasing probiotic levels caused a decrease in the feed intake leading to a reduction in the growth rate. Probiotics-treated groups showed no mortality compared with the control group. These results agree with those established by El-Okaby (2015) who observed that introducing Micro Pan AQUA® to the Sparus aurata fingerlings’ rearing water improved their growth performance, consumption of feed, and SR in comparison to the control group. This agreement might be attributed to the similarity in the management program. The influence of different probiotic levels on water quality was clear. As probiotic-treated groups had the highest values of dissolved oxygen level and pH levels. While total ammonia, nitrite, and nitrate were the lowest levels as recorded in G4 in comparison to the control group. These findings concurred with the study of Lakshmi et al. (2015) who stated that the level of dissolved oxygen was greater in probiotic-treated groups than in the control group. This result may be attributed to the greater microbial load in the control group while the other water quality parameters such as total ammonia, nitrite, and nitrate levels were found to be markedly lower than in the control group. Similarly, Wang et al. (2017) stated that probiotics were employed as water additives and had been shown to have a beneficial effect on the microbe populations of aquatic species and the environment. As a result, the immune system is strengthened and water quality is improved. All these reasons made the level of commercial probiotic 3 g probiotic/kg diet is the best for improving Nile Tilapia’s welfare. Probiotic dietary supplementation had a marked effect on serum biochemical parameters where probiotic-treated groups especially G4 had the optimal values of total proteins, serum albumin, serum globulin, and lysozyme activity, but G2 (1 g probiotic/kg diet had the highest A/G ratio in comparison to the control group. These current findings corroborated Reda and Selim’s (2015) observation that Nile tilapia serum total protein, albumin, and globulin levels were increased significantly after probiotic (B. amyloliquefaciens) supplementation. These findings also agreed with those of El-Kady et al. (2022) who observed that probiotic-treated groups had significantly improved serum biochemistry and immunological parameters (lysozyme and phagocytic activity). These findings contrasted with those of Wang et al. (2008) who found that Nile tilapia exposed to Enterococcus faecium ZJ4 at 1 × 107 CFU ml−1 in aquarium water every 4 days for 40 days had an unremarkable rise in serum total protein, serum albumin, globulin, A/G, and lysozyme activity. This disagreement may be attributed to different cultural environments. ConclusionWhen small amounts of commercial probiotics (3 g probiotic/kg diet) are applied to Nile tilapia-raising ponds together with a decrease in water exchange, the growth rate, FCR, and fish body WG are all improved. This study therefore supports the significance of commercial probiotics (B. amyloliquefaciens) as a feed additive to enhance fish growth performance, water quality, and behavior, and increase economic return and profit. Probiotics are therefore recommended as a crucial ingredient in the aquaculture industries for good growth performance and welfare of Nile tilapia. AcknowledgmentsWe appreciate our colleagues at the Faculty of Veterinary Medicine, Zagazig University, Egypt, Department of Behaviour and Management of Animal, Poultry, and Aquatic for contributing to the study’s materials. In addition, we appreciate the helpful suggestions provided by the anonymous referees for this manuscript. List of AbbreviationsConflict of interestThe authors declare that they have no conflict of interest. FundingNot applicable. Data availabilityAll data will be available upon a reasonable request. Authors’ contributionFAA conducted all experimental work and drafted the manuscript, and MY and AYS supervised the work and planned the study design. ENS, SA, and HG collected the data, shared it in the laboratory work, and drafted the manuscript. All authors agreed to the final version of this manuscript. ReferencesAbd El-Maksoud, A.M., Aboul-Fotoh, G.E., Allam, S.M., Abou Zied, R.M., Abdel hamid, F.M., Elshopakey, G. E and Aziza, A.E. 2020. Ameliorative effects of dietary Chlorella vulgaris and β-glucan against diazinon-induced toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 96, 213–22. Abdel-Fattah, F.A., Ahmed, F.A., Saleem, A.S.Y., Mohammed, H.H., Youssef, M.Y. and Said, E.N. 2020. Effect of the different stocking density on behavior, performance and welfare of the Nile tilapia (Oreochromis niloticus). Egypt. J .Aquat. Biol. Fish. 24(5), 539–560. Ahmed, F.A. 2020. Behaviour and performance of tilapia nilotica in relation to management. M. Vet. Sci. thesis, Fac.Vet. Med. Zagazig University, Zagazig, Egypt. APHA (American Public Health Association), 1998. Standard methods for the examination of water and waste, 20th ed. Washington, DC: APHA, WEF and AWWA, vol. 11, pp: 1193. Ardjosoediro, I. and Ramnarine, I.W. 2002. The influence of turbidity on growth, feed conversion and survivorship of the Jamaica red tilapia strain. Aquaculture 212(1–4), 159–165. Banerjee, G. and Ray, A.K. 2017. The advancement of probiotics research and its application in fish farming industries. Res. J. Vet. Sci. 115, 66–77. Barreto, R.E., Carvalho, G.G.A. and Volpato, G.L. 2011.The aggressive behaviour of Nile tilapia introduced into novel environments with variation in enrichment. Zoology 114(1), 53–57. Brandão, M.L., Colognesi, G., Bolognesi, M.C., Costa-Ferreira, R.S., Carvalho, T.B. and Gonçalves-de-Freitas, E. 2018. Water temperature affects aggressive interactions in a Neotropical cichlid fish. Neotrop. Ichthyol. 16(1), 170081. Demers, N.E. and Bayne, C.J. 1997. The immediate effect of stress on hormones and plasma lysozyme in rainbow trout. Dev. Comp. Immunol. 21, 363–373. Diab, A.S., El-Nagar, G.O. and Abd El-Hady, Y.M. 2002. Evaluation of Nigella sativa L. (Black seed (baraka), Allium sativum (garlic) & Biogen® as a feed additives on growth performance and immunostimulants of Oreochromis niloticus fingerlings. SCVMJ. 5(2), 745–753. Dickson, M., Nasr-Allah, A., Kenawy, D. and Kruijssen, F. 2016. Increasing fish farm profitability through aquaculture best management practice training in Egypt. Aquaculture 465, 172–178. El-Dakar, A.Y., Hassanien G.D.I., Gad, S.S. and Sakr, S.E. 2004. Use of medical and aromatic plants in fish diets: 2. Effect of dried basil leaves on performance of hybrid tilapia Oreochromis niloticus × O. aureus, Fingerlings.3 rd Inter. Conf. on Anim. Production and Health in semi-Arid Areas, Suez Canal University, pp: 265–277. El-Kady, A.A., Magouz, F.I., Mahmoud, S.A. and Abdel-Rahim, M.M. 2022. The effects of some commercial probiotics as water additive on water quality, fish performance, blood biochemical parameters, expression of growth and immune-related genes, and histology of Nile tilapia (Oreochromis niloticus). Aquaculture 546, 737249. El-Okaby, M.A.S. 2015. Improving the productive performance of farmed marine fish. PH.D. Thesis, Faculty of Agriculture (Saba-Basha). Alexandria University, Egypt. El-Saadony, M.T., Alkhatib, F.M., Alzahrani, S.O., Shafi, M.E., Abdel-Hamid, S.E., Taha, T.F. and Ahmed, N.H. 2021. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 28(8), 4592–4604. El-Sayed, A.F.M. 2019. Tilapia culture. Cambridge, MA: Academic Press. Feldman, B.F., Zink, J.G. and Jain, N.C. 2000. Schalm’s veterinary hematology, 5th ed. New York, NY: Lippincott Williams and Wilkins. Fitzsimmons, K. 2005.Tilapia culture. In American Fisheries Society Symposium (46): 563–590. Gatesoupe, F.J. 1999.The use of probiotics in aquaculture. Aquaculture 180, 147–165. Gregory, N. and Grandin, T. 2013. Animal welfare and meat production. Wallingford, Oxfordshire, UK; Cambridge, MA: CABI. Hasan, K.N. and Banerjee, G. 2020. Recent studies on probiotics as beneficial mediator in aquaculture: a review. J. Basic Appl. Zool. 81(1), 1–16. Hoshino T., Ishizaki K., Sakamoto T., Kumeta H.,Yumoto I., Matsuyama H and Ohgiya S. 1997. Isolation of a Pseudomonas species from fish intestine produces a protease active at low temperature. Lett. Appl. Microbiol. 25(1), 70–72. Huerta-R´abago, J.A., Martínez-Porchas, M., Miranda-Baeza, A., Nieves-Soto, M., Rivas- Vega, M.E. and Martínez-C´ordova, L.R. 2019. Addition of commercial probiotic in a biofloc shrimp farm of Litopenaeus vannamei during the nursery pHase: effect on bacterial diversity using massive sequencing 16S rRNA. Aquaculture 502, 391–399. Jahangiri, L. and Esteban, M.´A. 2018. Administration of probiotics in the water in finfish aquaculture systems: a review. Fishes 3(3), 33. Lakshmi, B., Viswanath, B. and Gopal, D.S. 2015. Influence of the isolated probiotic bacteria on the water quality parameters of shrimp pond and their effect on growth and survival of the shrimp. Int. J. Sci. Eng. Res. 6(2), 15–22. Martins, C.I.M., Galhardo, L., Noble, C., Damsgård, B., Spedicato, M.T., Zupa, W. and Kristiansen, T. 2012. Behavioural indicators of welfare in farmed fish. Fish. Physiol. Biochem. 38, 17–41. Neto, J.F. and Giaquinto, P.C. 2020. Environmental enrichment techniques and tryptopHan supplementation used to improve the quality of life and animal welfare of Nile tilapia. Aquac. Rep. 17, 100354. Noga, E.J. 1996. Fish diseases diagnosis and treatment, 1st edition. St. Louis, MO: Mosby electronic publishing. NRC (National Research Council). 2011. Nutritional Requirements of Fish and Shrimp. National Washington, DC: Academy Press. NRC (National Research Council). 2004. Nutrient requirement of fish. Washington, DC: National Academy Press. Reda, R.M. and Selim, K.M. 2015. Evaluation of Bacillus amyloliquefaciens on the growth performance, intestinal morpHology, hematology and body composition of Nile tilapia, Oreochromis niloticus. Aquac. Int. 23, 203–217. Ridha, M.T. and Azad, I.S. 2016. Effect of autochthonous and commercial probiotic bacteria on growth, persistence, immunity and disease resistance in juvenile and adult Nile tilapia Oreochromis niloticus. Aquac. Res. 47(9), 2757–2767. Said, E.N., Ahmed, F.A.A., Saleem, A.S.Y., Mohammed, H.H., Youssef, M.Y. and Abdel-Fattah, F.A. 2020. Behavioural response, welfare, and performance of Nile tilapia (Oreochromis niloticus) under different water temperatures. Int. J. Fish Aquat. Stud. 8, 1–11. Sakr, S.E. 2003. Studies on the feeding attractants for fish. M. Vet. Sci. thesis, Fac. Environ. Agric. Sci. El-Arish, Suez Canal University, Cairo, Egypt. Scott, G.R., Sloman, K.A., Rouleau, C. and Wood, C.M. 2003. Cadmium disrupts behavioural and pHysiological responses to alarm substance in juvenile rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 206, 1779–1790. Soltan, M. and El- Laithy, S. 2008. Effect of probiotics and some spices as feed additives on the performance and behaviour of the Nile tilapia, Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish. 12(2), 63–80. Soltan, M.A., Fouad, I. M and Elfeky, A. 2016. Growth and feed utilization of Nile tilapia, Oreochromis niloticus fed diets containing probiotic. Glob. Vet. 17(5), 442–450. Spanggaard, B., Huber, I., Nielson, J., Sick, E.B., Pipper, C.B., Martinussen, T., Slierendrecht, W.J. and Gram. I. 2001. The probiotic potential against vibriosis of the indigenous micro-flora of rainbow trout. Environ. Microbiol. 3, 755–765. SPSS version 21 .2012. IBM Corp. Armonk, NY: IBM SPSS Statistics for Windows. Subasinghe, R., Soto, D. and Jia, J. 2009. Global aquaculture and its role in sustainable development. Rev. Aquaculture 1, 2. Taoka, Y., Maeda, H. and Jo, J.Y. 2006. Growth, stress tolerance and non-specific immune response of Japanese flounder Paralichthys olivaceusto probiotics in a closed recirculating system. Fish Sci. 72(2), 310–321. Vine, N.G., Leukes, W.D. and Kaiser, H. 2006. Probiotics in marine larviculture. FEMS Microbiol. Rev. 30(3), 404–427. Wang, M., Liu, G.B., Lu, M.X., Ke, X.L., Liu, Z.G., Gao, F.Y., Cao, J.M., Zhu, H.P., Yi, M.M. and Yu, D.G. 2017. Effect of Bacillus cereus as a water or feed additive on the gut microbiota and immunological parameters of Nile tilapia. Aquac. Res. 48(6), 3163–3173. Wang, Y.B., Tian, Z.Q., Yao, J.T. and Li, W.F. 2008. Effect of probiotics, Enteroccus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture 277(3-4), 203–207. Yousry, H., Tawfeek, M.I., Reda, R. and El-Gafaary, N. 2019. Impact of commercial and isolated Bacillus amyloliquefaciens probiotic bacteria and/or overcrowding on growth performance and immune status of Oreochromis niloticus. J. Prod. Devel. 24(4), 851–868. | ||
| How to Cite this Article |
| Pubmed Style Youssef MY, Saleem AY, Ahmed FA, Said EN, Abdel-hamid SE, Gharib HS. The impact of dietary probiotic supplementation on welfare and growth performance of Nile tilapia (Oreochromis niloticus). Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 360-369. doi:10.5455/OVJ.2024.v14.i1.33 Web Style Youssef MY, Saleem AY, Ahmed FA, Said EN, Abdel-hamid SE, Gharib HS. The impact of dietary probiotic supplementation on welfare and growth performance of Nile tilapia (Oreochromis niloticus). https://www.openveterinaryjournal.com/?mno=179184 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i1.33 AMA (American Medical Association) Style Youssef MY, Saleem AY, Ahmed FA, Said EN, Abdel-hamid SE, Gharib HS. The impact of dietary probiotic supplementation on welfare and growth performance of Nile tilapia (Oreochromis niloticus). Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 360-369. doi:10.5455/OVJ.2024.v14.i1.33 Vancouver/ICMJE Style Youssef MY, Saleem AY, Ahmed FA, Said EN, Abdel-hamid SE, Gharib HS. The impact of dietary probiotic supplementation on welfare and growth performance of Nile tilapia (Oreochromis niloticus). Open Vet. J.. (2024), [cited January 25, 2026]; 14((1) (Zagazig Veterinary Conference)): 360-369. doi:10.5455/OVJ.2024.v14.i1.33 Harvard Style Youssef, M. Y., Saleem, . A. Y., Ahmed, . F. A., Said, . E. N., Abdel-hamid, . S. E. & Gharib, . H. S. (2024) The impact of dietary probiotic supplementation on welfare and growth performance of Nile tilapia (Oreochromis niloticus). Open Vet. J., 14 ((1) (Zagazig Veterinary Conference)), 360-369. doi:10.5455/OVJ.2024.v14.i1.33 Turabian Style Youssef, Mohamed Y.i., Al-sadik Y. Saleem, Fayza A. Ahmed, Enas N. Said, Shereen E. Abdel-hamid, and Heba S.a. Gharib. 2024. The impact of dietary probiotic supplementation on welfare and growth performance of Nile tilapia (Oreochromis niloticus). Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 360-369. doi:10.5455/OVJ.2024.v14.i1.33 Chicago Style Youssef, Mohamed Y.i., Al-sadik Y. Saleem, Fayza A. Ahmed, Enas N. Said, Shereen E. Abdel-hamid, and Heba S.a. Gharib. "The impact of dietary probiotic supplementation on welfare and growth performance of Nile tilapia (Oreochromis niloticus)." Open Veterinary Journal 14 (2024), 360-369. doi:10.5455/OVJ.2024.v14.i1.33 MLA (The Modern Language Association) Style Youssef, Mohamed Y.i., Al-sadik Y. Saleem, Fayza A. Ahmed, Enas N. Said, Shereen E. Abdel-hamid, and Heba S.a. Gharib. "The impact of dietary probiotic supplementation on welfare and growth performance of Nile tilapia (Oreochromis niloticus)." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 360-369. Print. doi:10.5455/OVJ.2024.v14.i1.33 APA (American Psychological Association) Style Youssef, M. Y., Saleem, . A. Y., Ahmed, . F. A., Said, . E. N., Abdel-hamid, . S. E. & Gharib, . H. S. (2024) The impact of dietary probiotic supplementation on welfare and growth performance of Nile tilapia (Oreochromis niloticus). Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 360-369. doi:10.5455/OVJ.2024.v14.i1.33 |