| Review Article | ||
Open Vet. J.. 2024; 14(5): 1081-1097 Open Veterinary Journal, (2024), Vol. 14(5): 1081–1097 Review Article Brucellosis: Unveiling the complexities of a pervasive zoonotic disease and its global impactsAswin Rafif Khairullah1, Shendy Canadya Kurniawan2, Yulianna Puspitasari3, Suhita Aryaloka4, Otto Sahat Martua Silaen5, Sheila Marty Yanestria6, Agus Widodo7, Ikechukwu Benjamin Moses8, Mustofa Helmi Effendi9*, Daniah Ashri Afnani10, Sancaka Chasyer Ramandinianto11, Abdullah Hasib12 and Katty Hendriana Priscilia Riwu131Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 2Master Program of Animal Sciences, Department of Animal Sciences, Specialisation in Molecule, Cell and Organ Functioning, Wageningen University and Research, Wageningen, The Netherlands 3Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Master Program of Veterinary Agribusiness, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Doctoral Program in Biomedical Science, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia 6Faculty of Veterinary Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia 7Department of Health, Faculty of Vocational Studies, Universitas Airlangga, Surabaya, Indonesia 8Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 9Division of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 10Department of Microbiology and Parasitology, Faculty of Veterinary Medicine, Universitas Pendidikan Mandalika, Mataram, Indonesia 11Lingkar Satwa Animal Care Clinic, Surabaya, Indonesia 12School of Agriculture and Food Sustainability, The University of Queensland, Gatton, Queensland 13Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Pendidikan Mandalika, Mataram, Indonesia *Corresponding Author: Mustofa Helmi Effendi. Division of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: mhelmieffendi [at] gmail.com Submitted: 14/01/2024 Accepted: 08/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
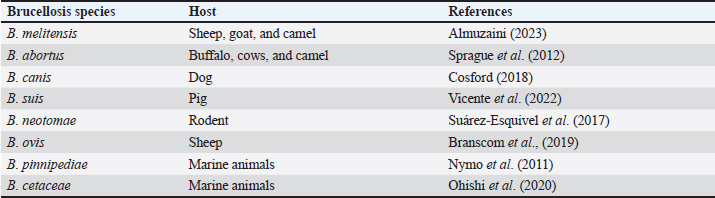
AbstractOne zoonotic infectious animal disease is brucellosis. The bacteria that cause brucellosis belong to the genus Brucella. Numerous animal and human species are affected by brucellosis, with an estimated 500,000 human cases recorded annually worldwide. The occurrence of new areas of infection and the resurgence of infection in already infected areas indicate how dynamically brucellosis is distributed throughout different geographic regions. Bacteria originate from the blood and are found in the reticuloendothelial system, the liver, the spleen, and numerous other locations, including the joints, kidneys, heart, and genital tract. Diagnosis of this disease can be done by bacterial isolation, molecular tests, modified acid-fast stain, rose bengal test (RBT), milk ring test, complement fixation test, enzyme-linked immunosorbent assay, and serum agglutination test. The primary sign of a Brucella abortus infection is infertility, which can result in abortion and the birth of a frail fetus that may go on to infect other animals. In humans, the main symptoms are acute febrile illness, with or without localization signs, and chronic infection. Female cattle have a greater risk of contracting Brucella disease. Human populations at high risk of contracting brucellosis include those who care for cattle, veterinarians, slaughterhouse employees, and butchers. Antibiotic treatment of brucellosis is often unsuccessful due to the intracellular survival of Brucella and its adaptability in macrophages. A “one health” strategy is necessary to control illnesses like brucellosis. Keywords: Brucellosis, Zoonosis, Illness, Brucella abortus, Public health. IntroductionOne zoonotic infectious animal disease is brucellosis. This illness affects financial losses significantly and is global in scope (Moriyón et al., 2023). Numerous animal and human species are affected by the disease, with an estimated 500,000 human cases recorded annually worldwide (Zhou et al., 2020). Most developing nations have an endemic form of this illness, which has a devastating financial impact on the cattle sector, particularly on small-scale farmers (Lokamar et al., 2020). The World Health Organization (WHO) has designated this disease as one of the world’s most important “neglected zoonotic diseases” due to the impact it exerts, especially on low-income nations (Franc et al., 2018). Brucellosis is thought to have existed for a very long time; new evidence from Egyptian skeletons indicates that the disease has been around since at least 750 BC (Bamaiyi, 2016). Brucellosis is also known by other names such as Malta fever, Gibraltar fever, Bang disease, Crimean fever, Mediterranean fever, infectious abortion, undulant fever, stone fever, and intermittent fever (Brangsch et al., 2023). The Gram-negative coccobacilli of the genus Brucella, which infects practically all pets, cattle, and human species, are the cause of this bacterial infection (Khan and Zahoor, 2018). A number of species of Brucella, including Brucella melitensis, Brucella ovis, Brucella pinnipediae, Brucella suis, Brucella neotomae, Brucella cetaceae, Brucella abortus, and Brucella canis, are responsible for this illness (Pfefer et al., 2018). In essence, brucellosis is a sexually transmitted illness that primarily affects the female and male reproductive systems, particularly the uterus during pregnancy (Li et al., 2020). Most Brucella species are stimulated to grow by the allantoic factor. These factors include erythritol, possibly steroid hormones, and other substances (Khurana et al., 2021). In the animal agriculture industry, brucellosis results in significant financial losses as well as issues with public health (Tulu, 2022). This causes economic losses due to reproductive failure through infertility, failure to birth calves, reduced meat and milk production, as well as culling and banning international trade (Singh et al., 2015). Clinical manifestations of brucellosis include orchitis and epididymitis in bulls, and abortion and retained fetal membranes in cows (Megid et al., 2010). Public health is significantly impacted by brucellosis in people, despite the fact that many nations have had success with initiatives to eradicate and control animals (Lai et al., 2021). In people, this illness usually manifests as a fever with a variety of clinical signs and symptoms and an unclear origin (Shi et al., 2021). Patients frequently experience severe localized side effects such neurobrucellosis, endocarditis, or spondylitis (Zhang et al., 2021). Because direct or indirect contact with infected animals or products is the primary cause of brucellosis in people, the focus of prevention should be on removing said contact. A clear route to eliminating animal-borne illness is sometimes out of reach for the financial and human resources of many poor nations (Godfroid, 2017). There are 10 times more unreported cases of clinically manifested brucellosis (Sun et al., 2021). Thus, this is one of the most important issues in public health. All age groups and genders are susceptible to brucellosis, and controlling the disease in humans depends on reducing animal infection through immunization and treatment initiatives (Yuan et al., 2020). The one health approach promotes local, national, and international multidisciplinary efforts to achieve optimal levels of health and collaboration between various scientific disciplines to overcome complex health problems (Ghanbari et al., 2020). It is based on the integration of human and animal health, plants, and ecosystems. Thus, it is critical to comprehend the risks that brucellosis poses to both human and animal health. This review explains the disease brucellosis with a special focus on the etiology, history, epidemiology, pathogenesis, diagnosis, clinical symptoms, transmission, risk factors, public health importance, treatment, control, and biotherosis of brucellosis. EtiologyThe bacteria that cause brucellosis belong to the genus Brucella. Brucella bacteria are Gram-negative coccobacilli or short rods measuring 0.6 to 1.5 μm long, and 0.5 to 0.7 μm wide, do not have flagella, non-motile, do not have a capsule, do not form spores, and are aerobic (Yazdani et al., 2012). This bacterium is an intracellular facultative organism that assaults, proliferates, and survives in dendritic cells, macrophages, placental trophoblasts, and epithelial cells (López-Santiago et al., 2019). Eight species of Brucella have been described to date, as shown in Table 1. The eight classic species are B. melitensis, B. abortus, B. canis, B. suis, B. neotomae, B. ovis, B. pinnipediae, and B. cetaceae (Pfefer et al., 2018). Out of all these bacterial species, biovars include B. suis, B. abortus, and B. melitensis (Brangsch et al., 2023). The primary cause of brucellosis in goats and sheep is B. melitensis, which is also extremely pathogenic for humans and one of the most dangerous zoonoses in the world (Rossetti et al., 2022). From a socio-economic perspective, the most significant species of Brucella are those that most commonly infect animals, including B. suis, B. melitensis, B. ovis, and B. abortus (Khurana et al., 2021). Three species of Brucella, including B. melitensis, B. abortus, and B. suis, are the primary causes of brucellosis in humans in addition to decreasing animal productivity (Hull and Schumaker, 2018). Apart from B. ovis, Brucella produces urease, oxidase, catalase, nitrate reductase, and non-hemolytic urease; it is negative for indole, methyl red, and Voges-Profskauer examinations (Ilhan et al., 2008). Except for B. ovis, the majority of Brucella species use glucose as a source of energy (Occhialini et al., 2022). Due to its aerosolized mode of transmission and lack of human vaccination, Brucella species are considered potential bioterrorism agents. HistoryAn English army surgeon, George Cleghorn, documented the details of the disease in 1751 in his literature under the title “observations on the epidemical diseases in minorca from the year 1744 to 1749” (Khurana et al., 2021). Since the Crimean War on the island of Malta, the illness has been recognized as a distinct clinical entity (Wyatt, 2013). In 1886, Sir Themistocles Zammit, Hughes, and Sir David Bruce provided a detailed description of the illness (Wyatt, 2014). Bernhard Bang made the initial discovery of B. abortus, a bacterium that causes abortion in cows and high fever in humans (Senbeto, 2022). Traum and Huddleson discovered B. suis in pigs, which has also been linked to human brucellosis cases (Olsen and Tatum, 2016). Table 1. Brucellosis species and their host.
Evans disclosed that the Micrococcus melitensis, also known as B. melitensis, that was isolated from pigs and calves is of the same genus as Brucella, which was identified by Sir David Bruce (Smith and Notes, 2023). Stoenner and Lackman isolated B. neotomae from mice (Waldrop and Sriranganathan, 2019). Carmicheal and Bruner isolated B. canis from canines (Suárez-Esquivel et al., 2021). In the past ten years, B. cetaceae, a relatively novel species of Brucella, has been identified from marine mammals and has the potential to become a zoonotic hazard (Guzmán-Verri et al., 2012). Recent discoveries of various Brucella strains in humans and marine mammals highlight the significance of zoonotic transmission. EpidemiologyThe occurrence of new areas of infection and the resurgence of infection in already infected areas indicate how dynamically Brucellosis is distributed throughout different geographic regions (Lounes et al., 2021). Human brucellosis is becoming more common in Central Asian and Middle Eastern nations, while new locations have shown this trend (Nejad et al., 2020). This illness is widespread worldwide, with the exception of the United Kingdom, the Netherlands, Norway, Australia, Cyprus, Canada, Denmark, Sweden, and Finland (Jamil et al., 2022). Nonetheless, brucellosis is highly prevalent in Mediterranean Europe, Central America, Italy, Near Eastern nations, Mexico, South America, Central Asia, Africa, and India (Janowicz et al., 2020). Numerous countries have recorded cases of brucellosis; nevertheless, there is a clear issue with underreporting. The status of brucellosis in animals led the World Organization for Animal Health (OIE) to divide 156 nations into three groups in a report that examined data over 26 years (1996–2023) (Warioba et al., 2023). The three categories are as follows: brucellosis-free: nations that did not have brucellosis during the 26-year study period; non-enzootic for brucellosis: nations that did not have brucellosis during the study period, but may still have the disease; and countries infected with or free from brucellosis for a period of less than three years (Franc et al., 2018). Countries with disease-free status are located in Europe and Oceania, while countries with high or enzootic prevalence are in Central and South America, Africa, and parts of Asia (Cárdenas et al., 2019). Brucellosis is endemic in the Middle East, West Asia, Southern Europe, India, and South America (Bahmani and Bahmani, 2022). According to research conducted in Iran, biovar B. abortus 3 was the most frequently discovered biovar (Alamian et al., 2020). Low brucellosis incidence reported in endemic areas could be the result of inadequate surveillance or underreporting. B. abortus biovar 1 is the primary cause of brucellosis in buffalo in certain regions of South America, Egypt, Italy, Africa, Pakistan, and Brazil (Khurana et al., 2021). In Italy, B. abortus affects buffalo and cattle, particularly in the southern regions (Borriello et al., 2013). Brucellosis is a common issue in Egypt (Holt et al., 2011). A growing number of countries in Saudi Arabia, Kuwait, Israel, and other Southern European nations are reporting cases of B. melitensis infection in cattle, which poses a serious concern (Refai, 2002). Even though brucellosis is a disease that must be reported to local health authorities and is a nationally notifiable condition in the majority of nations, there are still very few cases of the illness, and official statistics only account for a small portion of the disease’s true incidence. Human brucellosis has resurfaced, especially in Central Asia, and the situation is getting worse in some Middle Eastern nations (Elbehiry et al., 2023). PathogenesisBrucella enters the bloodstream through lymph nodes after phagocytosis, when it causes bacteremia, a disease that is accompanied by an acute febrile phase (Yagupsky et al., 2019). Bacteria originate from the blood and are found in the reticuloendothelial system, the liver, the spleen, and numerous other locations, including the joints, kidneys, heart, and genital tract (Giambartolomei and Delpino, 2019). In humans, generalized symptoms such fever, arthralgia, malaise, headache, and sweating start to occur after the incubation period (one to four weeks) (Deng et al., 2019). Mice are frequently employed as animal models in research on the pathophysiology of brucellosis in humans and other animals (Silva et al., 2011). The bacterial ABC transporter system is linked to the intake of nutrients, the export of toxins and antibiotics, and it may be crucial for the expression of certain genes (Akhtar and Turner, 2022). During host infection, ABC transporter proteins may play a pathogenic function in Brucella (Jenner et al., 2009). Hemagglutinin is the protein that causes adhesion and has the ability to identify the type of bacteria that adheres to a host during infection (Bialer et al., 2020). There is no significant function for hemoglobulin in the pathophysiology of B. melitensis (Meena et al., 2018). Pathogenicity in human brucellosis is caused by factors such as guanine monophosphate, LPS, virB, adenine, urease enzyme, and 24 kDa protein (Ko and Splitter, 2003). The organism known as Brucella is devoid of classical virulence factors such exotoxins, pili, endotoxins, flagella, and plasmids (Głowacka et al., 2018). The capacity of Brucella to prevent lysosomes from fusing with phagosomes, which leads to degranulation and the activation of the myelo-peroxidase-halide system, as well as to prevent tumor necrosis factors and cell death in host cells, is linked to its ability to survive and multiply within host cells after evading the host’s defense mechanisms (Jiao et al., 2021). Malignant Brucella species can infect and live in both phagocytic and non-phagocytic phagocytes, such as macrophages (Huy et al., 2022). Brucella can multiply in compartments that are membrane-bound (Celli, 2019). The bactericidal actions of natural killer cells and macrophages are disrupted when bacteria inhibit TNF-α (Ahmed et al., 2016). The surface antigen for smooth lipopolysaccharide (sLPS) comes in two varieties: A and M (Bundle and McGiven, 2017). In B. abortus and B. suis, antigen A predominates, whereas M is the primary antigen in B. melitensis (Dadar et al., 2021). Numerous periplasmic, cytoplasmic, and outer membrane proteins have also been identified. sLPS from B. abortus is 100 times less potent than E. coli and Salmonella at causing macrophages to release TNFα, activate oxidative metabolism, and release lysozyme from neutrophils (Siadat et al., 2015). This sLPS characteristic helps B. abortus survive in phagocytic cells. Furthermore, polycationic molecules have no effect on Brucella sLPS, suggesting that Brucella is resistant to cationic bactericidal peptides produced by phagocytes (Barquero-Calvo et al., 2007). Additionally, sLPS inhibits Brucella’s ability to proliferate cells and does not trigger the complement cascade’s alternate pathway (Verma et al., 2018). DiagnosisBacterial isolation The gold standard test for identifying Brucella species is isolation and cultivation of the bacterium, notwithstanding the availability of other diagnostic techniques. All strains of Brucella develop somewhat slowly since specimens used for isolation are frequently contaminated; consequently, it is advised to utilize selective media, like Farrell’s medium (Tulu, 2022). Incubation usually lasts 72 hours, but a negative diagnosis can only be stated after a week of incubation. Specimens that can be used for Brucella isolation include fetal gastric fluid, liver, placenta, spleen, milk (especially colostrum or milk within a week after birth), semen, lochia, supramammary (chronic and latent infections), and retropharyngeal (early infection). Lymph nodes, prescapular, iliac, and parotid lymph nodes can also be used (Mazlan et al., 2021). The colonies of the Brucella species have a glossy appearance, undamaged boundaries, raised, convex, transparent, and smooth surfaces (Mancilla et al., 2010). Bacterial colonies have a honey-like color under transmitted light. The optimal temperature for cultivating these bacteria is 37°C, with a temperature range of 20°C to 40°C, with an optimal pH ranging from 6.6 to 7.4 (Al-Afifi et al., 2022). Some species of Brucella need carbondioxide to grow. A culture can be deemed negative if no colonies form after two to three weeks of incubation, but typical colonies should appear after two to thirty days (Lobo et al., 2019). Molecular testsThe in vitro nucleic acid amplification method known as polymerase chain reaction (PCR) is frequently employed in the diagnosis of infectious diseases (Fakruddin et al., 2013). PCR is currently one of the most widely used tests for brucellosis diagnosis in both humans and animals. The most popular molecular method for diagnosing brucellosis is the PCR approach, which amplifies particular genome sequences from the genus, species, or biotype of Brucella species (Yu and Nielsen, 2013). Real-time PCR is faster and more sensitive than conventional PCR, because it does not require post-amplification PCR product handling, thereby reducing the risk of laboratory contamination and false positive results (Staggemeier et al., 2015). Brucella testing has recently become very popular using real-time PCR techniques. Modified acid-fast stainThis disease can be confirmed by finding bacteria in the smear. Utilizing modified Ziehl-Neelsen staining, smears are made from the placenta, colostrum, fetal stomach fluid, or lochia of post-abortive bovines as well as the abomasum of aborted fetuses (Chen et al., 2012). Placental cotyledons, e.g., can be used to create impression smears by forcefully pressing the slide’s surface on the tissue. Let it air dry before heating. Bacteria appear as red intracellular coccobacilli in smears stained with modified Ziehl-Neelsen, while the majority of other bacteria are blue (Mohan and Saxena, 2020). RBTSince most brucellosis control and eradication initiatives depend on these techniques, serological testing is necessary for the laboratory diagnosis of brucellosis. These tests can be broadly categorized into two categories: confirmatory testing and screening tests (Yen-Lieberman et al., 2011). Despite the fact that brucellosis has been detected in the laboratory using a number of serological tests, sensitivity, and specificity concerns prevent the use of a single test in all epidemiological studies. The Rose Bengal plate test, the enzyme-linked immunosorbent assay (ELISA), and the serum agglutination test (SAT) are the three serologic tests most frequently used to diagnose brucellosis (Díaz et al., 2011). The most used brucellosis screening test for both humans and animals is the RBT, which is simple to use and understand (Cho et al., 2010). Milk ring test (MRT)RBT has some drawbacks, including as low specificity in endemic areas, low sensitivity, particularly in chronic patients, and prozones that cause highly positive sera to look negative on RBT (Díaz et al., 2011). Another great screening test for dairy cattle is the MRT. MRT is a straightforward and efficient serological technique, although it is limited to usage with cow’s milk (Islam et al., 2023). In a glass or plastic tube, a drop of the antigen stained with hematoxylin and a little amount of milk are combined. MRT is extremely imprecise at the individual animal level and pertains to the entire herd, giving a general indication of infection status. Nevertheless, there are a number of drawbacks to this approach, including its reduced dependability in big groups and its incapacity to be applied to male animals (Novoa et al., 2022). Complement fixation test (CFT)One common confirmatory test for brucellosis is the CFT. CFT is the reference test that the Organization for Animal Health (OIE) recommends for international animal trafficking (Wilujeng et al., 2020). It is utilized as a confirmatory test for B. abortus, B. ovis, and B. melitensis infections due to its high accuracy. In the majority of the cases, CFT is performed on sera that test positive for RBT; nevertheless, much like RBT, CFT is considerably impacted by the improper use of vaccine strain 19, particularly in situations when sexually mature cattle and heifers have had new or repeated immunizations (Chisi et al., 2017). Setting stringent thresholds for infection is nearly challenging, particularly when S19 vaccine reactions are involved because of its abuse. The low number of positive reactions, the occasionally negative results in the early stages of illness, and the test’s high cost and complexity are some of the issues with CFT (Kartini et al., 2017). Additional issues include the test’s incapacity to be utilized with serum samples that have hemolyzed and the subjectivity of the interpretation of the direct complement activation by serum (anticomplementary activity) (Legesse et al., 2023). Additionally, animals infected with species antigenically similar to Brucella may produce false positive results. ELISAAs a common diagnostic procedure for brucellosis, the ELISA has gained popularity. This is a great way to identify acute from chronic disease phases and screen huge populations for Brucella antibodies. All four kinds of antibodies can be identified with remarkable ease using the ELISA approach (IgG1, IgG2, IgA, and IgM) (Faustini et al., 2021). Although ELISA is a highly effective control test in regions free of brucellosis and for survey testing in areas where vaccination has not been administered, this method is complex and cannot be used anywhere, particularly in areas where vaccination has been administered but is still lacking in standardization (Vatankhah et al., 2019). SATOne of the common serological tests used to diagnose brucellosis is the SAT. This technique is simple to use and does not call for specialized knowledge or costly equipment. Total IgM and IgG agglutination antibody levels are measured by SAT (Pabuccuoglu et al., 2011). The basis for this test is the way that antibodies react with Brucella lipopolysaccharide. The serum sample can be diluted from 1:2 to 1:64 to counteract excess antibodies that cause false negative results because of the prozone effect, which will increase the test’s specificity (Mohseni et al., 2017). The failure to diagnose B. canis infection and the development of cross-reactions between IgM immunoglobulins and Francisella tularensis, Escherichia coli O116, E. coli O157, Salmonella urbana, and Yersinia enterocolitica O:9 are drawbacks of the SAT (Perletta et al., 2023). Modifications such as the inclusion of EDTA, 2-mercaptoethanol, or antihuman globulin can help to overcome some of these inadequacies. Clinical symptomsClinical symptoms in animals Animals infected with Brucella may exhibit a variety of symptoms. The primary sign of a B. abortus infection is infertility, which can result in abortion and the birth of a frail fetus that may go on to infect other animals (Yanti et al., 2021). The reproductive system is the primary site of connection for the clinical indications, symptoms, and diverse consequences of brucellosis in numerous animal species (Jiang et al., 2019). There is a range in the incubation period from two weeks to several months. Calves can be infected in the early stages but no symptoms become visible until adulthood. The following are signs of this: endometritis, weak calves born into the world, decreased milk production, fetal membrane retention, decreased fertility, and abortion in pregnant animals (Sima et al., 2021). In underprivileged communities, abortion rates might range from 30% to 80% (Getahun et al., 2023). The newborn calf can pass away shortly after birth. Interstitial pneumonia and fibrinous pleurisy can also occur in aborted fetuses and neonatal calves (Neta et al., 2010). Male animals exhibit clinical signs of epididymitis and orchitis, and persistent infections result in hygroma (Tulu, 2022). Additionally, brucellosis has been linked to cervical bursitis in cattle (Filho et al., 2019). The acute inflammatory phase of seminal vesicles is succeeded by a chronic stage characterized by significant fibrinoid induration (Júnior et al., 2012). The testicle frequently shrinks to its normal size as a result of the fibrin tissue that finally covers the areas of dry necrosis (Nistal and Paniagua, 2008). Males typically exhibit orchitis and epididymitis, and chronic infections frequently result in hygromas (Hull and Schumaker, 2018). In bulls, the primary symptoms are epididymitis and orchitis, but in highly sensitive, unvaccinated pregnant cows, abortion happens after five months of gestation (Tulu, 2022). It is typically connected to B. abortus in equines, which causes both abortion and chronic bursal enlargement of the neck (Hussain et al., 2020). The acute signs of pig brucellosis include arthritis, orchitis, infertility, epididymitis, abortion, and the birth of feeble piglets (Hull and Schumaker, 2018). Clinical signs seen in other animal species are also present in sheep and goats (Almuzaini, 2023). Goats typically have abortions in the third or fourth month of pregnancy (Bosilkovski et al., 2020). Among dogs and cats, abortions, stillbirths, poor puppies, and infertility in both sexes are frequent occurrences (Santos et al., 2021). Clinical symptoms of infected livestock have a significant financial impact on both large and small farms and industries. In most host species, abortion or premature birth is a common but vague symptom of brucellosis (Bosilkovski et al., 2020). The majority of infected animals will miscarry just once, and others will not be affected; the disturbance to fertility is often transient (Khan and Zahoor, 2018). In sexually mature animals, the infection is restricted to the reproductive system and typically results in placentitis, which is followed by abortion in females who are pregnant, usually during the final third of the gestation period (González-Espinoza et al., 2021). Additional symptoms may include splenic abscess, arthritis in cattle and pigs, tiny intestine adhesions on post-mortem inspection in pigs, orchitis and epididymitis in sheep infected with B. melitensis and B. ovis (Godfroid et al., 2010). Brucellosis has an impact on cases of mastitis in goats and oozing skin lesions in horses (Mazlan et al., 2021). Furthermore, it might result in a considerable decrease in milk supply throughout the duration of the animal’s life; infected udders are frequently permanent, particularly in cows and goats, and the organism is continuously shed into the milk (Dadar et al., 2021). In camels, clinical symptoms of brucellosis seem to be extremely uncommon (Sprague et al., 2012). Furthermore, the diagnosis of Brucella is based on proof of the bacterium’s existence, which can be obtained in a number of ways, including the isolation of the bacterium, the identification of its antigen or genetic material, the demonstration of particular antibodies, or a reaction mediated by cells in the immune system. Clinical symptoms in humansIn humans, the main symptoms are acute febrile illness, with or without localization signs, and chronic infection (Saddique et al., 2019). A variety of non-specific clinical signs may be observed including malaise, headache, sweating, fatigue, depression, anorexia, stomach, or back pain (Hartady et al., 2014). A variable fever pattern is observed in chronic illnesses, and brucellosis fever can mimic enteric fever (Neupane et al., 2021). This condition may be absent in patients with end-stage renal disease who contract brucellosis (Turunç et al., 2008). The characteristics of the clinical and laboratory vary greatly. The well-documented case of endocarditis includes reports of isolated Brucella infections in prosthetic devices including pacemakers and implanted defibrillators as well as valves (Zhang et al., 2021). There have been isolated reports of pericarditis, myocarditis, aortitis, and venous or arterial thrombosis (Herrick et al., 2014). 10% to 20% of patients experience mild lymphadenopathy, and 20% to 30% have splenomegaly or hepatomegaly (Kawano-Dourado et al., 2015). Imaging detects hepatosplenic abscesses in 1.2% of cases, and there have been isolated reports of splenic rupture (Heller et al., 2015). Infections of the bones and joints are frequent; they include high rates of bursitis, granulomatous myositis, spinal osteomyelitis resulting from acute infection or sternotomy, and abscesses in soft tissue or muscle (Esmaeilnejad-Ganji and Esmaeilnejad-Ganji, 2019). The majority of Brucella monoarthritis cases are reactive as opposed to septic (Cerit et al., 2012). Soft tissue infections and 24 cases of infections in natural or prosthetic joints were reported in 2016 (Walsh et al., 2019). Subclinical sacroiliitis is frequently seen (Gheita et al., 2015). Reports of asymptomatic infections are also available. TransmissionAnimals most frequently contract diseases from eating grass, concentrates, hay, and water (Swai and Schoonman, 2010). Moreover, the fetus is polluted after birth; significant sources of infection include uterine fluids, aborted fetuses, and newborn calves that have high concentrations of pathogenic organisms (Khurana et al., 2021). Nonetheless, infections through the conjunctiva, respiratory mucosa, and damaged skin frequently arise (López-Santiago et al., 2019). Calves can become infected in the womb by suckling on an infected mother (Tulu, 2022). Animal brucellosis is extremely contagious, and some Brucella species can spread between species. Additionally, genital infections are possible and are more common in cases of B. suis infections (Cilia et al., 2021). The importance of venereal transmission varies depending on the species. This is how B. suis, B. ovis, and B. canis are mostly spread (Xavier et al., 2009). Although B. melitensis and B. abortus are detected in semen, there is little chance of these organisms being sexually transmitted (Prusty et al., 2016). Brucella organisms found in infected semen have the potential to spread the disease, but the risk of transmission from bulls increases significantly if the semen is utilized for artificial insemination (Li et al., 2020). Human-to-human transmission can happen through nursing, organ transplantation, transplacental transfer, and, very infrequently, sexual contact (Tuon et al., 2017). Additionally, dairy products, diseased tissue including placentas and aborted tissue, and direct contact with infected animals can all result in transmission (Franc et al., 2018). Despite the fact that pasteurization kills Brucella and prevents infections in humans, long-standing cultural customs and a lack of public awareness of the risks associated with raw milk consumption preclude some resource-constrained groups from routinely performing this process (Hull and Schumaker, 2018). Risk factorRisk factors in animal In comparison to younger cattle, older livestock were linked to higher seroprevalence. This tendency can be demonstrated since animals exposed to bacteria from different sources increase with age (Assenga et al., 2015). Similarly, compared to sexually immature heifers, sexually mature cattle had increased seropositivity (Islam et al., 2021). It is best to screen older herds of cattle for infections before testing younger herds, as this will minimize exposure to young calves. Female cattle have a greater risk of contracting Brucella disease than bulls because female cattle are kept in the herd for longer to reproduce and are, therefore, more susceptible to disease than bulls which are kept for a relatively shorter period of time (Ndazigaruye et al., 2018). Heifer immunity tends to be lowered by stress related to pregnancy and calving, which also explains the observed disparities (Ayoola et al., 2017). Programs for vaccinations should concentrate on female cattle who are housed longer and are more susceptible to infection (Bahadori et al., 2021). In the third semester, the group of animals with a history of abortion had a higher seropositivity (Tarusikirwa et al., 2023). The presence of erythritol, a growth stimulant for B. abortus, increases the susceptibility of cows to Brucella infection, especially in early pregnancy, and the condition can result in late-term abortion (Xiao et al., 2022). Following the initial abortion, the animal is capable of giving birth without any issues. Nonetheless, some sick cows might not give birth (Khan and Zahoor, 2018). Consequently, a history of abortion raises the risk of contracting Brucella. To rule out reasons for abortion, cows that undergo abortions should be kept apart from other cows and screened for brucellosis (Deresa et al., 2020). Risk factors in human Human populations at high risk of contracting brucellosis include those who care for cattle, veterinarians, slaughterhouse employees, butchers, and vendors of meat and dairy products (Madut et al., 2019). The main ways that slaughterhouse workers can become infected are via touching open wounds with their bare hands, splattering contagious liquids on their conjunctiva, and breathing in aerosols from the slaughterhouse (Pereira et al., 2020). Given that brucellosis is the most prevalent laboratory-acquired infection worldwide, laboratory workers are likewise at a significantly increased risk (Traxler et al., 2013). Thus, care should be taken when working with these bacterial cultures. People who are very susceptible to this illness should be made aware of it and urged to work while donning personal protective equipment to prevent infection (Zhang et al., 2019). Additionally, healthcare professionals need to be aware of this illness and competent in making accurate diagnoses for high-risk patients (Moriyón et al., 2023). Those who work at slaughterhouses are subjected to behaviors that raise their risk of bacterial infection, including touching contaminated tissue and breathing in droplets (Hassan et al., 2022). Annual screening of slaughterhouse employees is required to detect and treat disorders brought on by this bacterial infection as soon as possible (Madut et al., 2019). Another way to increase the chances of getting sick when handling butchered animals is to wear less closed clothing (Mburu et al., 2021). Those engaged included meat cutters and carvers who worked with blood, organs, and abortion supplies on a daily basis (Khan et al., 2020). This exposure is increased by failure to utilize personal protective equipment (boots, goggles, aprons, helmets, and gloves) and ignoring proper hygiene precautions (Golshani and Buozari, 2017). Managers of slaughterhouses are required to provide personal protection equipment and bandages to employees who have cuts on their bodies to stop the spread of infection (Acharya et al., 2018). Higher seroprevalence rates were linked to people handling aborted fetuses and helping with abortions and deliveries without donning personal protective equipment (TeshomeYimer et al., 2021). The favored carbon or energy source, erythritol, is found in tissues rich in brucella, which promotes the bacterium’s rapid growth (Petersen et al., 2013). The uterus, epididymis, breast tissue, and fetus of animals all have high quantities of erythritol (Carvalho et al., 2023). Furthermore, progesterone produced by the placenta promotes Brucella growth in vitro (Xiao et al., 2022). High quantities of germs are found in the secretions of these tissues; these concentrations are highest in the vagina soon following an abortion or childbirth (Cosford, 2018). Consequently, a major source of infection is abortion products and delivery materials. There is a significant danger of infection when these tissues are directly and frequently touched (Mehari et al., 2021). Brucellosis seropositive pregnant women who had contact with contaminated animals (Kledmanee et al., 2019). This hypothetical situation highlights how crucial it is to use personal protection equipment when working with livestock and getting rid of placentas or aborted fetuses. Raw milk consumption is a risk factor for Brucella infection for farmers and community members (Onyango et al., 2021). Brucella prefers to get its energy from breast tissue because it is high in erythritol (González-Espinoza et al., 2021). Infected cows will excrete the germs in their milk and ingestion of unpasteurized milk is a risk factor for infection in humans (Abdali et al., 2020). Some dairy farms sell their milk at marketplaces, use it for domestic consumption, or have processing facilities collect it (Mengistu and Meressa, 2023). Nevertheless, it is currently unknown how healthy this milk is. Assessing the health risks associated with milk quality is also necessary to guarantee customer safety both before and after the production process (Bacigale et al., 2023). Avoiding raw milk drinking will slow the spread of infection because there are currently no proven control measures for this disease. Public health importanceHuman brucellosis is a common disease worldwide. FAO, WHO, and OIE categorize this disease as one of the most globally distributed diseases (Laine et al., 2022). The majority of human instances of brucellosis are contracted from animals, particularly sheep, goats, and cows (Gwida et al., 2010). There are five species of Brucella that can infect humans: B. suis, B. melitensis, B. canis, B. abortus, and B. pinnipedialis (Kurmanov et al., 2022). Of these, B. melitensis is the most invasive and harmful species for humans, followed by B. abortus, B. suis, and B. canis (Dadar et al., 2019). Additionally, the US Centers for disease control and prevention have classified B. melitensis, B. suis, and B. abortus as potential biological weapons (Seleem et al., 2010). The reason for this is that the three species are very contagious due to their ease of aerosolization. Globally, humans contract brucellosis in half a million cases each year (Leong et al., 2015). The key to human infection is the frequency of infection in animal reservoirs. Typically, B. suis and B. abortus infections target livestock-related occupational groups (Hull and Schumaker, 2018). Infections with B. melitensis are more common than other kinds in the general population (Elbehiry et al., 2023). In many regions of the world, there might be anywhere from a few cases to over 500 cases of brucellosis per million people per year (Laine et al., 2023). A global report puts the annual number of human cases at 500,000 (Zhou et al., 2020). TreatmentAntibiotic treatment of brucellosis in animals is often unsuccessful due to the intracellular survival of Brucella and its adaptability in macrophages (Mode et al., 2022). Infection recurrence and low treatment success rates are prevalent in men (Alavi and Alavi, 2013). When treating brucellosis in humans, careful consideration must be given to the drug combinations used to minimize adverse effects and the development of resistance. The efficacy of treating brucellosis cases with ciprofloxacin and ceftriaxone as single medications was not encouraging (Fatani et al., 2019). Due to its lower risk of illness recurrence, combination therapy is recommended over monotherapy. For the treatment of uncomplicated brucellosis (without signs of endocarditis, spondylitis, or neurobrucellosis), multidrug therapy is suggested because monotherapy is insufficient (Yousefi-Nooraie et al., 2012). A different regimen involves taking 100 mg of doxycycline twice daily and 600–900 mg (15 mg/kg BW) of rifampicin orally once a day for six weeks (Khurana et al., 2021). Amikacin can also be taken twice a day for a week as part of this regimen to provide therapy using three different medications (Ranjbar et al., 2007). The most effective medication against experimentally produced brucellosis was azithromycin, which was followed by meropenem in in vitro tests to evaluate the sensitivity and effectiveness of pefloxacin, lomefloxacin, meropenem, and azithromycin (Maletskaia, 2002). It is also advised to follow dose regimens that include doxycycline for six weeks along with rifampicin for six weeks or with streptomycin for two to three weeks (Yousefi-Nooraie et al., 2012). The optimal treatment strategy is thought to involve a combination of doxycycline and streptomycin (Alp et al., 2006). Brucella was able to multiply and adapt inside cells even when streptomycin or doxycycline was used separately (Głowacka et al., 2018). While the doxycycline-streptomycin regimen is thought to be the most effective, it has some practical drawbacks because streptomycin needs to be given intravenously for three weeks (Solera et al., 1995). Another regimen, doxycycline for six weeks along with parental administration of gentamicin for a week is also considered suitable (Ariza et al., 2007). Comparing the effectiveness of doxycycline and rifampicin in combination with co-trimoxazole to treat patients with brucellosis revealed different rates of disease recurrence (Alavi and Alavi, 2013). In comparison to co-trimoxazole and doxycycline, the frequency of recurrence is 1.96 times higher when co-trimoxazole with rifampicin is administered (Yousefi-Nooraie et al., 2012). In addition, tauroursodeoxycholic acid or ginseng saponin fraction A have also been observed to prevent intracellular Brucella replication (Głowacka et al., 2018). Fluoroquinolones have been tried experimentally to treat brucellosis by several researchers; however, the results do not support their usage as first-line therapy (Safi and Al-Mariri, 2012). Due to the distinct characteristics of brucellosis, medical professionals and microbiologists need to collaborate closely to properly diagnose, track, and treat brucellosis in humans. Singh et al. (2015) have provided a detailed and accurate description of the necessary measures for treating dairy animals afflicted with bovine brucellosis. The course of treatment for brucellosis can have some adverse effects and typically lasts up to one month (Yousefi-Nooraie et al., 2012). Innovative anti-virulence substances that preserve essential cellular activities are presently being investigated for advanced applications. Since these antivirulence strategies do not interfere with normal processes, the risk of antibiotic resistance is greatly decreased (Martínez et al., 2019). ControlThe primary problem in endemic locations is controlling brucellosis. Controlling animal illness and preventing its spread to people is the sole method to prevent brucellosis in humans (Khan and Zahoor, 2018). In a small number of wealthy nations, brucellosis has been reduced to negligible levels through costly and time-consuming animal vaccination campaigns that were later followed by the culling of diseased animals (Bundle and McGiven, 2017). Food safety, particularly the pasteurization of milk, is crucial to preventing human infection (Owusu-Kwarteng et al., 2020). A “one health” strategy is necessary to control illnesses like brucellosis (Ghanbari et al., 2020). At-risk communities must be informed and educated by established programs, and animal and human health professionals must collaborate with livestock owners (O’Callaghan, 2020). Significant ramifications for those making political decisions are crucial. Implementing surveillance of both human and animal populations is necessary and effective vaccinations are needed for immunization programs (Franc et al., 2018). Researchers have used the RB51 and S19 vaccinations to create a novel and effective immunotherapy that protects cattle against bovine brucellosis (Saxena and Raj, 2018). Blood samples were tested negative for Brucella even after three months of vaccination with a combination of these two vaccinations administered subcutaneously at a dose of 2 ml (Simpson et al., 2018). S19 increases the humoral immune response to a greater extent than RB51, which elicits a stronger cellular immunological response (Dorneles et al., 2015). The findings of this study should promote the use of bacteriophage vaccinations for the management of brucellosis in cattle. All of the current vaccinations have the potential to induce brucellosis in humans, can spread by inoculated animals, and cannot completely produce abortion in target or non-target animals (Elbehiry et al., 2023). Additionally, RB51 is resistant to rifampicin, which is one of the recommended medications for treating human brucellosis (Negrón et al., 2019). Therefore, a novel vaccination that is both safe and efficacious for use in people and animals is required. A lot of research is being done to create new vaccines and enhance the effectiveness and safety of current ones. Presently, there is a global appeal for the creation of novel brucellosis vaccines, offering substantial rewards for the first vaccine to receive a license (Fatehi et al., 2023). BioterrorismIn addition to being a serious zoonotic disease, brucellosis is classified as category B and is associated with bioterrorism (Yagupsky and Baron, 2005). Brucella was investigated as a potential bioterrorism agent due to the severity of the disease, the unavailability of a vaccine safe for human use, and the frequent inaccuracies in the identification of isolates by clinical laboratories (Doganay and Doganay, 2013). Before 1954, when Britain focused on anthrax, brucellosis was the first microbe selected by the United States for development as a biological weapon (Bakri et al., 2018). These microorganisms can be effectively dispersed in four-pound bombs. In 1954, the U.S. military employed B. suis as a biological weapon; however, following the 1972 treaty on biological and chemical weapons, efforts to continue using this weapon were discontinued due to political shifts throughout the world (Guihot et al., 2004). Brucella is easily grown and transmitted, and when it infects humans, it can cause long-term clinical symptoms as well as persistent disease transmission (Franc et al., 2018). Food or aerosol contamination are potential sources of contamination (Noviello et al., 2004). One of this microorganism’s advantages is that it can deteriorate without becoming lethal. The infective dose of this organism is very low if infected through inhalation. An infectious aerosol dosage for humans is thought to be produced by 10–100 organisms (Silva et al., 2011). There would be $477.7 million in losses for every 100,000 individuals exposed in the event of a brucellosis bioterrorist strike (Kaufmann et al., 1997). Brucella has long been thought of as a possible bioterrorist microbe; however, no reports of its use in bioterrorist attacks have surfaced. ConclusionBrucellosis, caused by Brucella spp, is an underrecognized and neglected zoonotic disease that affects both animals and humans with unprecedented economic impact on a global scale. Brucellosis is not widely reported in some developed countries such as the United Kingdom, the Netherlands, Norway, Australia, Cyprus, Canada, Denmark, Sweden, and Finland; however, numerous countries have recorded cases of brucellosis. It is becoming a more common disease with high reports of prevalence in Mediterranean Europe, Central America, Italy, Near Eastern nations, Mexico, South America, Central Asia, Africa, and India. In animals, Brucella spp has been noted to be one of the pathogens that are responsible for orchitis, arthritis, epididymitis, and infertility in animals; thus, leading to abortion, birth of a frail fetus, reduced meat production, and low milk production. Most developing nations have an endemic form of this illness, which has a devastating financial impact on the cattle sector, particularly small-scale farmers. Among humans, the population mostly at risk are mostly those who care for cattle, veterinarians, slaughterhouse employees, and butchers via touching open wounds with their bare hands, splattering contagious liquids on their conjunctiva, and breathing in aerosols from the slaughterhouse with attendant symptoms such as fever, arthralgia, malaise, headache, sweating, anorexia, stomach or back pain, and chronic infection. An estimated 500,000 human cases due to Brucella spp infections have been recorded annually worldwide. Treatment of brucellosis is very complicated, difficult, and often unsuccessful due to the various pathogenic elements and survival strategies employed by Brucella to evade immune system cells and antimicrobials. Controlling animal illness and preventing its spread to people with a “one health” strategy is very vital in preventing brucellosis in humans. AcknowledgmentsThe authors thank the Universitas Airlangga. Author’s contributionsARK, SA, YP, and SCK drafted the manuscript. MHE, DAA, and IBM revised and edited the manuscript. AW, SCR, and KHPR took part in preparing and critical checking of the manuscript. SMY, OSMS, and AH edited the references. All authors read and approved the final version of the manuscript. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis study was supported in part with the Penelitian Unggulan Airlangga (PUA) Universitas Airlangga, Indonesia, in the fiscal year 2023, with grant number: 1710/UN3.LPPM/PT.01.03/2023. Data availabilityAll data are provided in the published article. ReferencesAbdali, F., Hosseinzadeh, S., Berizi, E. and Pourmontaseri, M. 2020. Prevalence of Brucella species in unpasteurized dairy products consumed in Shiraz province using PCR assay. Mol. Biol. Res. Commun. 9(3), 117–121. Acharya, D., Hwang, S.D. and Park, J.H. 2018. Seroreactivity and risk factors associated with human brucellosis among cattle slaughterhouse workers in South Korea. Int. J. Environ. Res. Public Health 15(11), 2396. Ahmed, W., Zheng, K. and Liu, Z.F. 2016. Establishment of chronic infection: Brucella’s stealth strategy. Front. Cell. Infect. Microbiol. 6(1), 30. Akhtar, A.A. and Turner, D.P. 2022. The role of bacterial ATP-binding cassette (ABC) transporters in pathogenesis and virulence: therapeutic and vaccine potential. Microb. Pathog. 171(1), 105734. Al-Afifi, A.H., Almashhadany, D.A., Al-Azazi, A.S.H., Khalaf, A.M., Odhah, M.N.A. and Al-Gabri, N.A. 2022. Prevalence of Brucella spp. in milk from aborted and non-aborted animals in Dhamar governorate, Yemen. Ital. J. Food Saf. 11(4), 10370. Alamian, S., Dadar, M. and Wareth, G. 2020. Role of Brucella abortus biovar 3 in the outbreak of abortion in a dairy cattle herd immunized with Brucella abortus Iriba vaccine. Arch. Razi. Inst. 75(3), 377–384. Alavi, S.M. and Alavi, L. 2013. Treatment of brucellosis: a systematic review of studies in recent twenty years. Caspian J. Intern. Med. 4(2), 636–641. Almuzaini, A.M. 2023. An epidemiological study of brucellosis in different animal species from the Al-Qassim Region, Saudi Arabia. Vaccines 11(3), 694. Alp, E., Koc, R.K., Durak, A.C., Yildiz, O., Aygen, B., Sumerkan, B. and Doganay, M. 2006. Doxycycline plus streptomycin versus ciprofloxacin plus rifampicin in spinal brucellosis [ISRCTN31053647]. BMC Infect. Dis. 6(1), 72. Ariza, J., Bosilkovski, M., Cascio, A., Colmenero, J.D., Corbel, M.J., Falagas, M.E., Memish, Z.A., Roushan, M.R., Rubinstein, E., Sipsas, N.V., Solera, J., Young, E.J. and Pappas, G. 2007. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med. 4(12), e317. Assenga, J.A., Matemba, L.E., Muller, S.K., Malakalinga, J.J. and Kazwala, R.R. 2015. Epidemiology of Brucella infection in the human, livestock and wildlife interface in the Katavi-Rukwa ecosystem, Tanzania. BMC Vet. Res. 11(1), 189. Ayoola, M.C., Akinseye, V.O., Cadmus, E., Awosanya, E., Popoola, O.A., Akinyemi, O.O., Perrett, L., Taylor, A., Stack, J., Moriyon, I. and Cadmus, S.I. 2017. Prevalence of bovine brucellosis in slaughtered cattle and barriers to better protection of abattoir workers in Ibadan, South-Western Nigeria. Pan. Afr. Med. J. 28(1), 68. Bacigale, S.B., Ayagirwe, R.B., Mutwedu, V.B., Mugumaarhahama, Y., Mugisho, J.Z., Nziku, Z., Fofana, M., Udomkun, P. and Mignouna, J. 2023. Assessing milk products quality, safety, and influencing factors along the dairy value chain in eastern Democratic Republic of the Congo. Front. Sustain. Food Syst. 7(1), 1105515. Bahadori, F., Ghofranipour, F., Zarei, F., Ziaei, R. and Ghaffarifar, S. 2021. Application of the PRECEDE-PROCEED model in prevention of brucellosis focused on livestock vaccination process. BMC Vet. Res. 17(1), 384. Bahmani, N. and Bahmani, A. 2022. A review of brucellosis in the Middle East and control of animal brucellosis in an Iranian experience. Rev. Res. Med. Microbiol. 33(1), e63–e69. Bakri, F.G., AlQadiri, H.M. and Adwan, M.H. 2018. The highest cited papers in brucellosis: identification using two databases and review of the papers’ major findings. Biomed. Res. Int. 2018(1), 9291326. Bamaiyi, P.H. 2016. Prevalence and risk factors of brucellosis in man and domestic animals: a review. Int. J. One Health 2(1), 29–34. Barquero-Calvo, E., Chaves-Olarte, E., Weiss, D.S., Guzmán-Verri, C., Chacón-Díaz, C., Rucavado, A., Moriyón, I. and Moreno, E. 2007. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS One 2(7), e631. Bialer, M.G., Sycz, G., González, F.M., Ferrero, M.C., Baldi, P.C. and Zorreguieta, A. 2020. Adhesins of Brucella: their roles in the interaction with the host. Pathogens 9(11), 942. Borriello, G., Peletto, S., Lucibelli, M.G., Acutis, P.L., Ercolini, D. and Galiero, G. 2013. Link between geographical origin and occurrence of Brucella abortus biovars in cow and water buffalo herds. Appl. Environ. Microbiol. 79(3), 1039–1043. Bosilkovski, M., Stojovski, M., Siskova, D., Ridov, A., Kostoska, E. and Krstevski, K. 2020. Brucellosis in pregnancy: case reports with different outcomes in an endemic region. Acta Clin. Croat. 59(2), 338–343. Brangsch, H., Horstkotte, M.A. and Melzer, F. 2023. Genotypic peculiarities of a human brucellosis case caused by Brucella suis biovar 5. Sci. Rep. 13(1), 16586. Branscom, L.A., Cornish, T.E. and Sondgeroth, K.S. 2019. Evaluation of serologic testing of rams in the management of Brucella ovis in a domestic sheep flock. J. Vet. Diagn. Invest. 31(1), 86–89. Bundle, D.R. and McGiven, J. 2017. Brucellosis: improved diagnostics and vaccine insights from synthetic glycans. Acc. Chem. Res. 50(12), 2958–2967. Cárdenas, L., Awada, L., Tizzani, P., Cáceres, P. and Casal, J. 2019. Characterization and evolution of countries affected by bovine brucellosis (1996–2014). Transbound. Emerg. Dis. 66(3), 1280–1290. Carvalho, T.P., Silva, L.A.D., Castanheira, T.L.L., Souza, T.D., Paixão, T.A.D., Lazaro-Anton, L., Tsolis, R.M. and Santos, R.L. 2023. Cell and tissue tropism of Brucella spp. Infect. Immun. 91(5), e0006223. Celli, J. 2019. The intracellular life cycle of Brucella spp. Microbiol. Spectr. 7(2), 10; doi: 10.1128/microbiolspec.BAI-0006-2019. Cerit, E.T., Aydın, M. and Azap, A. 2012. A case of brucellar monoarthritis and review of the literature. Rheumatol. Int. 32(5), 1465–1468. Chen, P., Shi, M., Feng, G.D., Liu, J.Y., Wang, B.J., Shi, X.D., Ma, L., Liu, X.D., Yang, Y.N., Dai, W., Liu, T.T., He, Y., Li, J.G., Hao, X.K. and Zhao, G. 2012. A highly efficient Ziehl-Neelsen stain: identifying de novo intracellular Mycobacterium tuberculosis and improving detection of extracellular M. tuberculosis in cerebrospinal fluid. J. Clin. Microbiol. 50(4), 1166–1170. Chisi, S.L., Marageni, Y., Naidoo, P., Zulu, G., Akol, G.W. and Van Heerden, H. 2017. An evaluation of serological tests in the diagnosis of bovine brucellosis in naturally infected cattle in KwaZulu-Natal province in South Africa. J. S. Afr. Vet. Assoc. 88(0), e1–e7. Cho, D., Nam, H., Kim, J., Heo, E., Cho, Y., Hwang, I., Kim, J., Kim, J., Jung, S. and More, S. 2010. Quantitative rose bengal test for diagnosis of bovine brucellosis. J. Immunoassay Immunochem. 31(2), 120–130. Cilia, G., Fratini, F., Turchi, B., Angelini, M., Cerri, D. and Bertelloni, F. 2021. Genital Brucella suis biovar 2 infection of Wild Boar (Sus scrofa) Hunted in Tuscany (Italy). Microorganisms 9(3), 582. Cosford, K.L. 2018. Brucella canis: an update on research and clinical management. Can. Vet. J. 59(1), 74–81. Dadar, M., Alamian, S., Behrozikhah, A.M., Yazdani, F., Kalantari, A., Etemadi, A. and Whatmore, A.M. 2019. Molecular identification of Brucella species and biovars associated with animal and human infection in Iran. Vet. Res. Forum 10(4), 315–321. Dadar, M., Tiwari, R., Sharun, K. and Dhama, K. 2021. Importance of brucellosis control programs of livestock on the improvement of one health. Vet. Q. 41(1), 137–151. Deng, Y., Liu, X., Duan, K. and Peng, Q. 2019. Research progress on brucellosis. Curr. Med. Chem. 26(30), 5598–5608. Deresa, B., Tulu, D. and Deressa, F.B. 2020. epidemiological investigation of cattle abortion and its association with brucellosis in Jimma Zone, Ethiopia. Vet. Med. (Auckl) 11(1), 87–98. Díaz, R., Casanova, A., Ariza, J. and Moriyón, I. 2011. The rose bengal test in human brucellosis: a neglected test for the diagnosis of a neglected disease. PLoS Negl. Trop. Dis. 5(4), e950. Doganay, G.D. and Doganay, M. 2013. Brucella as a potential agent of bioterrorism. Recent. Pat. Antiinfect. Drug Discov. 8(1), 27–33. Dorneles, E.M., Lima, G.K., Teixeira-Carvalho, A., Araújo, M.S., Martins-Filho, O.A., Sriranganathan, N., Al Qublan, H., Heinemann, M.B. and Lage, A.P. 2015. Immune response of calves vaccinated with Brucella abortus S19 or RB51 and revaccinated with RB51. PLoS One 10(9), e0136696. Elbehiry, A., Aldubaib, M., Marzouk, E., Abalkhail, A., Almuzaini, A.M., Rawway, M., Alghamdi, A., Alqarni, A., Aldawsari, M. and Draz, A. 2023. The development of diagnostic and vaccine strategies for early detection and control of human brucellosis, particularly in endemic areas. Vaccines 11(3), 654. Esmaeilnejad-Ganji, S.M. and Esmaeilnejad-Ganji, S.M.R. 2019. Osteoarticular manifestations of human brucellosis: a review. World J. Orthop. 10(2), 54–62. Fakruddin, M., Mannan, K.S., Chowdhury, A., Mazumdar, R.M., Hossain, M.N., Islam, S. and Chowdhury, M.A. 2013. Nucleic acid amplification: Alternative methods of polymerase chain reaction. J. Pharm. Bioallied. Sci. 5(4), 245–252. Fatani, D.F., Alsanoosi, W.A., Badawi, M.A. and Thabit, A.K. 2019. Ceftriaxone use in brucellosis: a case series. IDCases 18(1), e00633. Fatehi, Z., Doosti, A. and Jami, M.S. 2023. Oral vaccination with novel Lactococcus lactis mucosal live vector-secreting brucella lumazine synthase (BLS) protein induces humoral and cellular immune protection against Brucella abortus. Arch. Microbiol. 205(4), 122. Faustini, S.E., Jossi, S.E., Perez-Toledo, M., Shields, A.M., Allen, J.D., Watanabe, Y., Newby, M.L., Cook, A., Willcox, C.R., Salim, M., Goodall, M., Heaney, J.L., Marcial-Juarez, E., Morley, G.L., Torlinska, B., Wraith, D.C., Veenith, T.V., Harding, S., Jolles, S., Ponsford, M.J., Plant, T., Huissoon, A., O’Shea, M.K., Willcox, B.E., Drayson, M.T., Crispin, M., Cunningham, A.F. and Richter, A.G. 2021. Development of a high-sensitivity ELISA detecting IgG, IgA and IgM antibodies to the SARS-CoV-2 spike glycoprotein in serum and saliva. Immunology 164(1), 135–147. Filho, P.M.S., Dias, A.S., Castro, I.S.P., de Souza, P.G., de Freitas Galvão, M. and Xavier, F.G. 2019. Bovine cervical bursitis co-infection caused by Brucella abortus and Onchocerca sp. J. Parasit. Dis. 43(4), 730–732. Franc, K.A., Krecek, R.C., Häsler, B.N. and Arenas-Gamboa, A.M. 2018. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary action. BMC Public Health 18(1), 125. Getahun, T., Urge, B. and Mamo, G. 2023. Seroprevalence of Bovine Brucellosis in Selected sites of Central Highland of Ethiopia. Vet. Med. (Auckl). 14(1), 11–22. Ghanbari, M.K., Gorji, H.A., Behzadifar, M., Sanee, N., Mehedi, N. and Bragazzi, N.L. 2020. One health approach to tackle brucellosis: a systematic review. Trop. Med. Health 48(1), 86. Gheita, T.A., Sayed, S., Azkalany, G.S., El Fishawy, H.S., Aboul-Ezz, M.A., Shaaban, M.H. and Bassyouni, R.H. 2015. Subclinical sacroiliitis in brucellosis. Clinical presentation and MRI findings. Z Rheumatol. 74(3), 240–245. Giambartolomei, G.H. and Delpino, M.V. 2019. Immunopathogenesis of hepatic brucellosis. Front. Cell. Infect. Microbiol. 9(1), 423. Głowacka, P., Żakowska, D., Naylor, K., Niemcewicz, M. and Bielawska-Drózd, A. 2018. Brucella—virulence factors, pathogenesis and treatment. Pol. J. Microbiol. 67(2), 151–161. Godfroid, J. 2017. Brucellosis in livestock and wildlife: zoonotic diseases without pandemic potential in need of innovative one health approaches. Arch. Public Health 75(1), 34. Godfroid, J., Nielsen, K. and Saegerman, C. 2010. Diagnosis of brucellosis in livestock and wildlife. Croat. Med. J. 51(4), 296–305. Golshani, M. and Buozari, S. 2017. A review of brucellosis in Iran: epidemiology, risk factors, diagnosis, control, and prevention. Iran Biomed. J. 21(6), 349–359. González-Espinoza, G., Arce-Gorvel, V., Mémet, S. and Gorvel, J.P. 2021. Brucella: reservoirs and niches in animals and humans. Pathogens 10(2), 186. Guihot, A., Bossi, P. and Bricaire, F. 2004. Brucellose par bioterrorisme [Bioterrorism with brucellosis]. 33(2), 119–122. Guzmán-Verri, C., González-Barrientos, R., Hernández-Mora, G., Morales, J.A., Baquero-Calvo, E., Chaves-Olarte, E. and Moreno, E. 2012. Brucella ceti and brucellosis in cetaceans. Front. Cell. Infect. Microbiol. 2(1), 3. Gwida, M., Al Dahouk, S., Melzer, F., Rösler, U., Neubauer, H. and Tomaso, H. 2010. Brucellosis—regionally emerging zoonotic disease? Croat. Med. J. 51(4), 289–295. Hartady, T., Saad, M.Z., Bejo, S.K. and Salisi, M.S. 2014. Clinical human brucellosis in Malaysia: a case report. Asian Pac. J. Trop. Dis. 4(2), 150–153. Hassan, L., Ali, S., Syed, M.A., Shah, A.A., Abbasi, S.A., Tabassum, S., Saeed, U., Melzer, F., Khan, A.U., El-Adawy, H. and Neubauer, H. 2022. Risk factors for acute brucellosis in patients on the day of admission at selected hospitals of Abbottabad, Pakistan. Front. Public Health 9(1), 669278. Heller, T., Bélard, S., Wallrauch, C., Carretto, E., Lissandrin, R., Filice, C. and Brunetti, E. 2015. Patterns of hepatosplenic brucella abscesses on cross-sectional imaging: a review of clinical and imaging features. Am. J. Trop. Med. Hyg. 93(4), 761–766. Herrick, J.A., Lederman, R.J., Sullivan, B., Powers, J.H. and Palmore, T.N. 2014. Brucella arteritis: clinical manifestations, treatment, and prognosis. Lancet Infect. Dis. 14(6), 520–526. Holt, H.R., Eltholth, M.M., Hegazy, Y.M., El-Tras, W.F., Tayel, A.A. and Guitian, J. 2011. Brucella spp. infection in large ruminants in an endemic area of Egypt: cross-sectional study investigating seroprevalence, risk factors and livestock owner’s knowledge, attitudes and practices (KAPs). BMC Public Health 11(1), 341. Hull, N.C. and Schumaker, B.A. 2018. Comparisons of brucellosis between human and veterinary medicine. Infect. Ecol. Epidemiol. 8(1), 1500846. Hussain, A., Jamil, T., Tareen, A.M., Melzer, F., Hussain, M.H., Khan, I., Saqib, M., Zohaib, A., Hussain, R., Ahmad, W., Iqbal, M. and Neubauer, H. 2020. Serological and molecular investigation of brucellosis in breeding equids in Pakistani Punjab. Pathogens 9(9), 673. Huy, T.X.N., Nguyen, T.T., Kim, H., Reyes, A.W.B. and Kim, S. 2022. Brucella phagocytosis mediated by pathogen-host interactions and their intracellular survival. Microorganisms 10(10), 2003. Ilhan, Z., Solmaz, H., Aksakal, A., Gulhan, T., Ekin, I.H. and Boynukara, B. 2008. Detection of Brucella melitensis DNA in the milk of sheep after abortion by PCR assay. Arch. Med. Vet. 40(1), 141–146. Islam, S., Barua, S.R., Moni, S.P., Islam, A., Rahman, A.K.M.A. and Chowdhury, S. 2021. Seroprevalence and risk factors for bovine brucellosis in the chittagong metropolitan area of Bangladesh. Vet. Med. Sci. 7(1), 86–98. Islam, M.S., Islam, M.A., Rahman, M.M., Islam, K., Islam, M.M., Kamal, M.M. and Islam, M.N. 2023. Presence of Brucella spp. in milk and dairy products: a comprehensive review and its perspectives. J. Food Qual. 2023(1), 2932883. Jamil, T., Akar, K., Erdenlig, S., Murugaiyan, J., Sandalakis, V., Boukouvala, E., Psaroulaki, A., Melzer, F., Neubauer, H. and Wareth, G. 2022. Spatio-temporal distribution of brucellosis in European terrestrial and marine wildlife species and its regional implications. Microorganisms 10(10), 1970. Janowicz, A., De Massis, F., Zilli, K., Ancora, M., Tittarelli, M., Sacchini, F., Di Giannatale, E., Sahl, J.W., Foster, J.T. and Garofolo, G. 2020. Evolutionary history and current distribution of the West Mediterranean lineage of Brucella melitensis in Italy. Microb. Genom. 6(11), mgen000446. Jenner, D.C., Dassa, E., Whatmore, A.M. and Atkins, H.S. 2009. ATP-binding cassette systems of brucella. Comp. Funct. Genomics 2009(1), 354649. Jiang, W., Chen, J., Li, Q., Jiang, L., Huang, Y., Lan, Y. and Li, Y. 2019. Epidemiological characteristics, clinical manifestations and laboratory findings in 850 patients with brucellosis in Heilongjiang Province, China. BMC Infect. Dis. 19(1), 439. Jiao, H., Zhou, Z., Li, B., Xiao, Y., Li, M., Zeng, H., Guo, X. and Gu, G. 2021. The mechanism of facultative intracellular parasitism of brucella. Int. J. Mol. Sci. 22(7), 3673. Júnior, C.A.C., Moustacas, V.S., Xavier, M.N., Costa, E.A., Costa, L.F., Silva, T.M.A., Paixão, T.A., Borges, A.M., Gouveia, A.M.G. and Santos, R.L. 2012. Andrological, pathologic, morphometric, and ultrasonographic findings in rams experimentally infected with Brucella ovis. Small Rumin. Res. 102(2–3), 213–222. Kartini, D., Noor, S.M. and Pasaribu, F.H. 2017. Serological and molecular detection of brucellosis in Swine at Slaughterhouse in Kapuk, Jakarta and Ciroyom, Bandung. Acta Vet. Indones. 5(2), 66–73. Kaufmann, A.F., Meltzer, M.I. and Schmid, G.P. 1997. The economic impact of a bioterrorist attack: are prevention and postattack intervention programs justifiable? Emerg. Infect. Dis. 3(2), 83–94. Kawano-Dourado, L., Peirera, D.A., Ade, M.K., Dolhnikoff, M., Silva, M.V. and Kairalla, R.A. 2015. Lymphadenopathy and fever in a chef during a stay in Europe. J. Bras. Pneumol. 41(2), 191–195. Khan, A.U., Melzer, F., Hendam, A., Sayour, A.E., Khan, I., Elschner, M.C., Younus, M., Ehtisham-Ul-Haque, S., Waheed, U., Farooq, M., Ali, S., Neubauer, H. and El-Adawy, H. 2020. Seroprevalence and molecular identification of Brucella spp. in Bovines in Pakistan-investigating association with risk factors using machine learning. Front. Vet. Sci. 7(1), 594498. Khan, M.Z. and Zahoor, M. 2018. An overview of brucellosis in cattle and humans, and its serological and molecular diagnosis in control strategies. Trop. Med. Infect. Dis. 3(2), 65. Khurana, S.K., Sehrawat, A., Tiwari, R., Prasad, M., Gulati, B., Shabbir, M.Z., Chhabra, R., Karthik, K., Patel, S.K., Pathak, M., Yatoo, M.I., Gupta, V.K., Dhama, K., Sah, R. and Chaicumpa, W. 2021. Bovine brucellosis—a comprehensive review. Vet. Q. 41(1), 61–88. Kledmanee, K., Liabsuetrakul, T. and Sretrirutchai, S. 2019. Seropositivities against brucellosis, coxiellosis, and toxoplasmosis and associated factors in pregnant women with adverse pregnancy outcomes: a cross-sectional study. PLoS One 14(5), e0216652. Ko, J. and Splitter, G.A. 2003. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin. Microbiol. Rev. 16(1), 65–78. Kurmanov, B., Zincke, D., Su, W., Hadfield, T.L., Aikimbayev, A., Karibayev, T., Berdikulov, M., Orynbayev, M., Nikolich, M.P. and Blackburn, J.K. 2022. Assays for identification and differentiation of brucella species: a review. Microorganisms 10(8), 1584. Lai, S., Chen, Q. and Li, Z. 2021. Human Brucellosis: an ongoing global health challenge. China CDC Wkly. 3(6), 120–123. Laine, C.G., Johnson, V.E., Scott, H.M. and Arenas-Gamboa, A.M. 2023. Global estimate of human brucellosis incidence. Emerg. Infect. Dis. 29(9), 1789–1797. Laine, C.G., Scott, H.M. and Arenas-Gamboa, A.M. 2022. Human brucellosis: Widespread information deficiency hinders an understanding of global disease frequency. PLoS Negl. Trop. Dis. 16(5), e0010404. Legesse, A., Mekuriaw, A., Gelaye, E., Abayneh, T., Getachew, B., Weldemedhin, W., Tesgera, T., Deresse, G. and Birhanu, K. 2023. Comparative evaluation of RBPT, I-ELISA, and CFT for the diagnosis of brucellosis and PCR detection of Brucella species from Ethiopian sheep, goats, and cattle sera. BMC Microbiol. 23(1), 216. Leong, K.N., Chow, T.S., Wong, P.S., Hamzah, S.H., Ahmad, N. and Ch’ng, C.C. 2015. Outbreak of human brucellosis from consumption of raw goats’ milk in Penang, Malaysia. Am. J. Trop. Med. Hyg. 93(3), 539–541. Li, N., Yu, F., Peng, F., Zhang, X. and Jia, B. 2020. Probable sexual transmission of brucellosis. IDCases 21(1), e00871. Lobo, J.M.G., Ortiz, Y., Gonzalez-Riancho, C., Seoane, A., Arellano-Reynoso, B. and Sangari, F.J. 2019. Polymorphisms in brucella carbonic anhydrase II mediate CO2 dependence and fitness in vivo. Front. Microbiol. 10(1), 2751. Lokamar, P.N., Kutwah, M.A., Atieli, H., Gumo, S. and Ouma, C. 2020. Socio-economic impacts of brucellosis on livestock production and reproduction performance in Koibatek and Marigat regions, Baringo County, Kenya. BMC Vet. Res. 16(1), 61. López-Santiago, R., Sánchez-Argáez, A.B., De Alba-Núñez, L.G., Baltierra-Uribe, S.L. and Moreno-Lafont, M.C. 2019. Immune response to mucosal brucella infection. Front. Immunol. 10(1), 1759. Lounes, N., Melzer, F., Sayour, A.E., Maamar, H.T., Rahal, K., Benamrouche, N., Lazri, M., Bouyoucef, A., Hendam, A., Neubauer, H. and El-Adawy, H. 2021. Identification, geographic distribution and risk factors of Brucella abortus and Brucella melitensis infection in cattle in Algeria. Vet. Microbiol. 254(1), 109004. Madut, N.A., Ocan, M., Muwonge, A., Muma, J.B., Nasinyama, G.W., Godfroid, J., Jubara, A.S. and Kankya, C. 2019. Sero-prevalence of brucellosis among slaughterhouse workers in Bahr el Ghazal region, South Sudan. BMC Infect. Dis. 19(1), 450. Maletskaia, O.V. 2002. Effektivnost’ nekotorykh novykh antibiotikov pri lechenii éksperimental’nogo brutselleza [efficacy of some new antibiotics in treating experimental brucellosis]. Antibio.t Khimioter. 47(11), 13–17. Mancilla, M., López-Goñi, I., Moriyón, I. and Zárraga, A.M. 2010. Genomic island 2 is an unstable genetic element contributing to Brucella lipopolysaccharide spontaneous smooth-to-rough dissociation. J. Bacteriol. 192(24), 6346–6351. Martínez, O.F., Cardoso, M.H., Ribeiro, S.M. and Franco, O.L. 2019. Recent advances in anti-virulence therapeutic strategies with a focus on dismantling bacterial membrane microdomains, toxin neutralization, quorum-sensing interference and biofilm inhibition. Front. Cell. Infect. Microbiol. 9(1), 74. Mazlan, M., Khairani-Bejo, S., Hamzah, H., Nasruddin, N.S., Salleh, A. and Zamri-Saad, M. 2021. Pathological changes, distribution and detection of Brucella melitensis in foetuses of experimentally-infected does. Vet. Q. 41(1), 36–49. Mburu, C.M., Bukachi, S.A., Tokpa, K.H., Fokou, G., Shilabukha, K., Ezekiel, M., Bonfoh, B., Kazwala, R. and Kreppel, K. 2021. Lay attitudes and misconceptions and their implications for the control of brucellosis in an agro-pastoral community in Kilombero district, Tanzania. PLoS Negl. Trop. Dis. 15(6), e0009500. Meena, D.S., Sonwal, V.S., Rohila, A.K. and Meena, V. 2018. Acute brucellosis presenting as an autoimmune hemolytic anemia. Case Rep. Infect. Dis. 2018(1), 1030382. Megid, J., Mathias, L.A. and Robles, C.A. 2010. Clinical manifestations of brucellosis in domestic animals and humans. Open Vet. Sci. J. 4(1), 119–126. Mehari, S., Zerfu, B. and Desta, K. 2021. Prevalence and risk factors of human brucellosis and malaria among patients with fever in malaria-endemic areas, attending health institutes in Awra and Gulina district, Afar Region, Ethiopia. BMC Infect. Dis. 21(1), 942. Mengistu, A.T. and Meressa, A.M. 2023. Dairy Farmers’ choice of milk market outlets: evidence from farm households in Central Ethiopia. J. Food Qual. 2023(1), 5684470. Mode, S., Ketterer, M., Québatte, M. and Dehio C. 2022. Antibiotic persistence of intracellular Brucella abortus. PLoS Negl. Trop. Dis. 16(7), e0010635. Mohan, A. and Saxena, H.M. 2020. Effect of phage targeting therapy of brucellosis on host antibody response in cattle. Phage (New Rochelle) 1(4), 223–229. Mohseni, K., Mirnejad, R., Piranfar, V. and Mirkalantari, S. 2017. A comparative evaluation of ELISA, PCR, and serum agglutination tests for diagnosis of brucella using human serum. Iran J. Pathol. 12(4), 371–376. Moriyón, I., Blasco, J.M., Letesson, J.J., De Massis, F. and Moreno, E. 2023. Brucellosis and one health: inherited and future challenges. Microorganisms 11(8), 2070. Ndazigaruye, G., Mushonga, B., Kandiwa, E., Samkange, A. and Segwagwe, B.E. 2018. Prevalence and risk factors for brucellosis seropositivity in cattle in Nyagatare District, Eastern Province, Rwanda. J. S. Afr. Vet. Assoc. 89(0), e1–e8. Negrón, M.E., Kharod, G.A., Bower, W.A. and Walke, H. 2019. Notes from the field: human Brucella abortus RB51 infections caused by consumption of unpasteurized domestic dairy products—United States, 2017–2019. MMWR Morb. Mortal. Wkly. Rep. 68(7), 185. Neupane, D.P., Dulal, H.P. and Song, J. 2021. Enteric fever diagnosis: current challenges and future directions. Pathogens 10(4), 410. Nejad, R.B., Krecek, R.C., Khalaf, O.H., Hailat, N. and Arenas-Gamboa, A.M. 2020. Brucellosis in the Middle East: current situation and a pathway forward. PLoS Negl. Trop. Dis. 14(5), e0008071. Neta, A.V.C., Mol, J.P., Xavier, M.N., Paixão, T.A., Lage, A.P. and Santos, R.L. 2010. Pathogenesis of bovine brucellosis. Vet. J. 184(2), 146–155. Nistal, M. and Paniagua, R. 2008. Non-neoplastic diseases of the testis. Urol. Surg. Pathol. 2008(1), 614–755. Noviello, S., Gallo, R., Kelly, M., Limberger, R.J., DeAngelis, K., Cain, L., Wallace, B. and Dumas, N. 2004. Laboratory-acquired brucellosis. Emerg. Infect. Dis. 10(10), 1848–1850. Novoa, M.B., Aguirre, N.P., Valentini, B., Torioni-de-Echaide, S., Signorini, M.L., Primo, M.E., Elena, S. and Vanzini, V.R. 2022. Development, validation and field evaluation of an indirect ELISA for the detection of antibodies against Brucella abortus in bulk and individual milk samples in dairy cattle. Prev. Vet. Med. 208(1), 105740. Nymo, I.H., Tryland, M. and Godfroid, J. 2011. A review of Brucella infection in marine mammals, with special emphasis on Brucella pinnipedialis in the hooded seal (Cystophora cristata). Vet. Res. 42(1), 93. O’Callaghan, D. 2020. Human brucellosis: recent advances and future challenges. Infect. Dis. Poverty. 9(1), 101. Occhialini, A., Hofreuter, D., Ufermann, C.M., Al Dahouk, S. and Köhler, S. 2022. The retrospective on atypical brucella species leads to novel definitions. Microorganisms 10(4), 813. Ohishi, K., Amano, M., Nakamatsu, K., Miyazaki, N., Tajima, Y., Yamada, T.K., Matsuda, A., Ochiai, M., Matsuishi, T.F., Taru, H., Iwao, H. and Maruyama, T. 2020. Serologic survey of brucella infection in cetaceans inhabiting along the coast of Japan. J. Vet. Med. Sci. 82(1), 43–46. Olsen, S.C. and Tatum, F.M. 2016. Swine brucellosis: current perspectives. Vet. Med. (Auckl). 8(1), 1–12. Onyango, D.L.A., Guitian, J. and Musallam, I. 2021. Brucellosis risk factors and milk hygiene handling practices in pastoral communities in Isiolo county, Kenya. Vet. Med. Sci. 7(4), 1254–1262. Owusu-Kwarteng, J., Akabanda, F., Agyei, D. and Jespersen, L. 2020. Microbial safety of milk production and fermented dairy products in Africa. Microorganisms 8(5), 752. Pabuccuoglu, O., Ecemis, T., El, S., Coskun, A., Akcali, S. and Sanlidag, T. 2011. Evaluation of serological tests for diagnosis of brucellosis. Jpn. J. Infect. Dis. 64(4), 272–276. Pereira, C.R., de Almeida, J.V.F.C., de Oliveira, I.R.C., de Oliveira, L.F., Pereira, L.J., Zangerônimo, M.G., Lage, A.P. and Dorneles, E.M.S. 2020. Occupational exposure to Brucella spp.: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 14(5), e0008164. Perletta, F., Di Pancrazio, C., Rodomonti, D., Di Febo, T., Luciani, M., Krasteva, I.M., Maggetti, M., Profeta, F., Salini, R., De Massis, F., Sacchini, F. and Tittarelli, M. 2023. Evaluation of three serological tests for diagnosis of canine brucellosis. Microorganisms 11(9), 2162. Petersen, E., Rajashekara, G., Sanakkayala, N., Eskra, L., Harms, J. and Splitter, G. 2013. Erythritol triggers expression of virulence traits in Brucella melitensis. Microbes Infect. 15(6–7), 440–449. Pfefer, T.S.L., Timme, R. and Kase, J.A. 2018. Identification of Brucella genus and eight Brucella species by Luminex bead-based suspension array. Food Microbiol. 70(1), 113–119. Prusty, B.R., Chaudhuri, P., Chaturvedi, V.K., Saini, M., Mishra, B.P. and Gupta, P.K. 2016. Visual detection of Brucella spp. in spiked bovine semen using loop-mediated isothermal amplification (LAMP) assay. Indian J. Microbiol. 56(2), 142–147. Ranjbar, M., Keramat, F., Mamani, M., Kia, A.R., Khalilian, F.O., Hashemi, S.H. and Nojomi, M. 2007. Comparison between doxycycline-rifampin-amikacin and doxycycline-rifampin regimens in the treatment of brucellosis. Int. J. Infect. Dis. 11(2), 152–156. Refai, M. 2002. Incidence and control of brucellosis in the Near East region. Vet. Microbiol. 90(1–4), 81–110. Rossetti, C.A., Maurizio, E. and Rossi, U.A. 2022. comparative review of brucellosis in small domestic ruminants. Front. Vet. Sci. 9(1), 887671. Saddique, A., Ali, S., Akhter, S., Khan, I., Neubauer, H., Melzer, F., Khan, A.U., Azam, A. and El-Adawy, H. 2019. Acute febrile illness caused by Brucella abortus infection in humans in Pakistan. Int. J. Environ. Res. Public Health 16(21), 4071. Safi, M. and Al-Mariri, A. 2012. Efficacy evaluation of some antibiotics against syrian Brucella spp isolates, in vitro. Braz. J. Microbiol. 43(4), 1269–1273. Santos, R.L., Souza, T.D., Mol, J.P.S., Eckstein, C. and Paíxão, T.A. 2021. Canine brucellosis: an update. Front. Vet. Sci. 8(1), 594291. Saxena, H.M. and Raj, S. 2018. A novel immunotherapy of Brucellosis in cows monitored non invasively through a specific biomarker. PLoS Negl. Trop. Dis. 12(4), e0006393. Seleem, M.N., Boyle, S.M. and Sriranganathan, N. 2010. Brucellosis: a re-emerging zoonosis. Vet. Microbiol. 140(3–4), 392–398. Senbeto, Y.A. 2022. Brucellosis: a review. Int. J. Adv. Res. Biol. Sci. 9(8), 146–161. Shi, C., Wang, L., Lv, D., Wang, G., Mengist, H.M., Jin, T., Wang, B., Huang, Y., Li, Y. and Xu, Y. 2021. Epidemiological, clinical and laboratory characteristics of patients with brucella infection in Anhui Province, China. Infect. Drug Resist. 14(1), 2741–2752. Siadat, S.D., Vaziri, F., Eftekhary, M., Karbasian, M., Moshiri, A., Aghasadeghi, M.R., Ardestani, M.S., Alitappeh, M.A., Arsang, A., Fateh, A., Peerayeh, S.N. and Bahrmand, A.R. 2015. Preparation and evaluation of a new lipopolysaccharide-based conjugate as a vaccine candidate for brucellosis. Osong Public Health Res. Perspect. 6(1), 9–13. Silva, T.M., Costa, E.A., Paixão, T.A., Tsolis, R.M. and Santos, R.L. 2011. Laboratory animal models for brucellosis research. J. Biomed. Biotechnol. 2011(1), 518323. Sima, D.M., Ifa, D.A., Merga, A.L. and Tola, E.H. 2021. Seroprevalence of bovine brucellosis and associated risk factors in Western Ethiopia. Vet. Med. (Auckl) 12(1), 317–324. Simpson, G.J.G., Marcotty, T., Rouille, E., Chilundo, A., Letteson, J.J. and Godfroid, J. 2018. Immunological response to Brucella abortus strain 19 vaccination of cattle in a communal area in South Africa. J. S. Afr. Vet. Assoc. 89(0), e1–e7. Singh, B.B., Dhand, N.K. and Gill, J.P.S. 2015. Economic losses occurring due to brucellosis in Indian livestock populations. Prev. Vet. Med. 119(3), 211–215. Smith, T.C. and Notes, A. 2023. Long COVID: alice evans, Brucellosis, and reflections on infectious causes of chronic disease. Clin. Infect. Dis. 77(12), 1644–1647. Solera, J., Rodríguez-Zapata, M., Geijo, P., Largo, J., Paulino, J., Sáez, L., Martínez-Alfaro, E., Sánchez, L., Sepulveda, M.A. and Ruiz-Ribó, M.D. 1995. Doxycycline-rifampin versus doxycycline-streptomycin in treatment of human brucellosis due to Brucella melitensis. The GECMEI Group. Grupo de Estudio de Castilla-la Mancha de Enfermedades Infecciosas. Antimicrob. Agents Chemother. 39(9), 2061–2067. Sprague, L.D., Al-Dahouk, S. and Neubauer, H. 2012. A review on camel brucellosis: a zoonosis sustained by ignorance and indifference. Pathog. Glob. Health 106(3), 144–149. Staggemeier, R., Bortoluzzi, M., Heck, T.M., Spilki, F.R. and Almeida, S.E. 2015. Quantitative vs. conventional PCR for detection of human adenoviruses in water and sediment samples. Rev. Inst. Med. Trop. Sao Paulo 57(4), 299–303. Suárez-Esquivel, M., Ruiz-Villalobos, N., Hidalgo-Jara, W., Chacón-Díaz, C., Zúñiga-Pereira, A.M., Masís-Mora, M., Fernández-Fernández, E., Hernández-Mora, G., Barquero-Calvo, E., Chaves-Olarte, E., Thomson, N.R., Foster, J.T., Moreno, E. and Guzmán-Verri, C. 2021. Canine brucellosis in Costa Rica reveals widespread Brucella canis infection and the recent introduction of foreign strains. Vet. Microbiol. 257(1), 109072. Suárez-Esquivel, M., Ruiz-Villalobos, N., Jiménez-Rojas, C., Barquero-Calvo, E., Chacón-Díaz, C., Víquez-Ruiz, E., Rojas-Campos, N., Baker, K.S., Oviedo-Sánchez, G., Amuy, E., Chaves-Olarte, E., Thomson, N.R., Moreno, E. and Guzmán-Verri, C. 2017. Brucella neotomae infection in humans, Costa Rica. Emerg. Infect. Dis. 23(6), 997–1000. Sun, X., Jiang, W., Li, Y., Li, X., Zeng, Q., Du, J., Yin, A. and Lu, Q.B. 2021. Evaluating active versus passive sources of human brucellosis in Jining City, China. PeerJ 9(1), e11637. Swai, E.S. and Schoonman, L. 2010. The use of rose bengal plate test to asses cattle exposure to Brucella infection in traditional and smallholder dairy production systems of tanga region of Tanzania. Vet. Med. Int. 2010(1), 837950. Tarusikirwa, D.F., Blacklaws, B. and Trotter, C.L. 2023. Seroprevalence and assessment of public awareness of Brucella spp., Toxoplasma gondii and Chlamydia abortus in small ruminants from selected smallholder commercial farms of Zimbabwe. PLoS One 18(6), e0287902. TeshomeYimer, B., Feleke, B.E., Bogale, K.A. and Tsegaye, G.W. 2021. Factors associated with human brucellosis among patients attending in Ayu Primary Hospital, North Showa, Ethiopia: a case control study. Ethiop. J. Health Sci. 31(4), 709–718. Traxler, R.M., Lehman, M.W., Bosserman, E.A., Guerra, M.A. and Smith, T.L. 2013. A literature review of laboratory-acquired brucellosis. J. Clin. Microbiol. 51(9), 3055–3062. Tulu, D. 2022. Bovine brucellosis: epidemiology, public health implications, and status of brucellosis in Ethiopia. Vet. Med. (Auckl) 13(1), 21–30. Tuon, F.F., Gondolfo, R.B. and Cerchiari, N. 2017. Human-to-human transmission of Brucella—a systematic review. Trop. Med. Int. Health 22(5), 539–546. Turunç, T., Demiroğlu, Y.Z., Alişkan, H., Colakoğlu, S., Timurkaynak, F., Ozdemir, N. and Arslan, H. 2008. Brucellosis in cases of end-stage renal disease. Nephrol. Dial. Transplant. 23(7), 2344–2349. Vatankhah, M., Beheshti, N., Mirkalantari, S., Khoramabadi, N., Aghababa, H. and Mahdavi, M. 2019. Recombinant Omp2b antigen-based ELISA is an efficient tool for specific serodiagnosis of animal brucellosis. Braz. J. Microbiol. 50(4), 979–984. Verma, S., Rawat, M., Kumawat, S., Qureshi, S., Mohd, G. and Tiwari, A.K. 2018. Protective role of Brucella abortus specific murine antibodies in inhibiting systemic proliferation of virulent strain 544 in mice and guinea pig. Vet. World 11(6), 794–799. Vicente, A.F., Mioni, M.S.R., Cagnini, D.Q., Ribeiro, M.G., Filho, M.F.A., Listoni, F.J.P., Ribeiro, B.L.D. and Megid, J. 2022. Phenotypic and molecular identification of Brucella suis biotype 1 in a pig from Brazil-case report. Braz. J. Microbiol. 53(1), 487–489. Waldrop, S.G. and Sriranganathan, N. 2019. Intracellular invasion and survival of Brucella neotomae, another possible zoonotic Brucella species. PLoS One 14(4), e0213601. Walsh, J., Gilleece, A., Fenelon, L., Cogley, D. and Schaffer, K. 2019. An unusual case of Brucella abortus prosthetic joint infection. J. Bone Jt. Infect. 4(6), 277–279. Warioba, J.P., Karimuribo, E.D., Komba, E.V.G., Kabululu, M.L., Minga, G.A. and Nonga, H.E. 2023. Occurrence and risk factors of brucellosis in commercial cattle farms from selected districts of the Eastern Coast Zone, Tanzania. Vet. Med. Int. 2023, 4904931. Wilujeng, E., Suwarno, S., Praja, R.N., Hamid, I.S., Yunita, M.N. and Wibawati, P.A. 2020. Serodetection of brucellosis using rose bengal test and complement fixation test method in dairy cattle in Banyuwangi. J. Vet. Med. 3(2), 188–195. Wyatt, H.V. 2013. Lessons from the history of brucellosis. Rev. Sci. Tech. 32(1), 17–25. Wyatt, H.V. 2014. How did sir David Bruce forget zammit and his Goats? J. Malt. Hist. 4(1), 39–44. Xavier, M.N., Costa, É.A., Paixão, T.A. and Santos, R.L. 2009. The genus Brucella and clinical manifestations of brucellosis. Cienc. Rural 39(7), 2252–2260. Xiao, Y., Li, M., Guo, X., Zeng, H., Shuai, X., Guo, J., Huang, Q., Chu, Y., Zhou, B., Wen, J., Liu, J. and Jiao, H. 2022. Inflammatory mechanism of brucella infection in placental trophoblast cells. Int. J. Mol. Sci. 23(21), 13417. Yagupsky, P. and Baron, E.J. 2005. Laboratory exposures to brucellae and implications for bioterrorism. Emerg. Infect. Dis. 11(8), 1180–1185. Yagupsky, P., Morata, P. and Colmenero, J.D. 2019. Laboratory diagnosis of human brucellosis. Clin. Microbiol. Rev. 33(1), e00073–19. Yanti, Y., Sumiarto, B., Kusumastuti, T.A., Panus, A. and Sodirun, S. 2021. Seroprevalence and risk factors of brucellosis and the brucellosis model at the individual level of dairy cattle in the West Bandung District, Indonesia. Vet. World 14(1), 1–10. Yazdani, R., Doosti, A. and Dehkordi, P.G. 2012. Construction of a novel recombinant vector as Brucella melitensis vacB gene knockout candidate. Afr. J. Microbiol. Res. 6(4), 802–808. Yen-Lieberman, B., Daniel, J., Means, C., Waletzky, J. and Daly, T.M. 2011. Identification of false-positive syphilis antibody results using a semiquantitative algorithm. Clin. Vaccine Immunol. 18(6), 1038–1040. Yousefi-Nooraie, R., Mortaz-Hejri, S., Mehrani, M. and Sadeghipour, P. 2012. Antibiotics for treating human brucellosis. Cochrane Database Syst. Rev. 10(10), CD007179. Yu, W.L. and Nielsen, K. 2010. Review of detection of Brucella spp. by polymerase chain reaction. Croat. Med. J. 51(4), 306–313. Yuan, H.T., Wang, C.L., Liu, L.N., Wang, D., Li, D., Li, Z.J. and Liu, Z.G. 2020. Epidemiologically characteristics of human brucellosis and antimicrobial susceptibility pattern of Brucella melitensis in Hinggan League of the Inner Mongolia Autonomous Region, China. Infect. Dis. Poverty 9(1), 79. Zhang, H., Xie, S., Wang, Y., Zhao, X., Yi, J., Wang, Z., Liu, Q., Deng, X., Li, B., Cui, B., Wang, Y. and Chen, C. 2021. A case report of endocarditis and spondylitis caused by Brucella melitensis biovar 3. BMC Infect. Dis. 21(1), 460. Zhang, N., Zhou, H., Huang, D.S. and Guan, P. 2019. Brucellosis awareness and knowledge in communities worldwide: a systematic review and meta-analysis of 79 observational studies. PLoS Negl. Trop. Dis. 13(5), e0007366. Zhou, K., Wu, B., Pan, H., Paudyal, N., Jiang, J., Zhang, L., Li, Y. and Yue, M. 2020. ONE health approach to address zoonotic brucellosis: a spatiotemporal associations study between animals and humans. Front. Vet. Sci. 7(1), 521. | ||
| How to Cite this Article |
| Pubmed Style Khairullah AR, Kurniawan SC, Puspitasari Y, Aryaloka S, Silaen OSM, Yanestria SM, Widodo A, Moses IB, Effendi MH, Afnani DA, Ramandinianto SC, Hasib A, Riwu KHP. Brucellosis: Unveiling the complexities of a pervasive zoonotic disease and its global impacts. Open Vet. J.. 2024; 14(5): 1081-1097. doi:10.5455/OVJ.2024.v14.i5.1 Web Style Khairullah AR, Kurniawan SC, Puspitasari Y, Aryaloka S, Silaen OSM, Yanestria SM, Widodo A, Moses IB, Effendi MH, Afnani DA, Ramandinianto SC, Hasib A, Riwu KHP. Brucellosis: Unveiling the complexities of a pervasive zoonotic disease and its global impacts. https://www.openveterinaryjournal.com/?mno=185226 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i5.1 AMA (American Medical Association) Style Khairullah AR, Kurniawan SC, Puspitasari Y, Aryaloka S, Silaen OSM, Yanestria SM, Widodo A, Moses IB, Effendi MH, Afnani DA, Ramandinianto SC, Hasib A, Riwu KHP. Brucellosis: Unveiling the complexities of a pervasive zoonotic disease and its global impacts. Open Vet. J.. 2024; 14(5): 1081-1097. doi:10.5455/OVJ.2024.v14.i5.1 Vancouver/ICMJE Style Khairullah AR, Kurniawan SC, Puspitasari Y, Aryaloka S, Silaen OSM, Yanestria SM, Widodo A, Moses IB, Effendi MH, Afnani DA, Ramandinianto SC, Hasib A, Riwu KHP. Brucellosis: Unveiling the complexities of a pervasive zoonotic disease and its global impacts. Open Vet. J.. (2024), [cited January 25, 2026]; 14(5): 1081-1097. doi:10.5455/OVJ.2024.v14.i5.1 Harvard Style Khairullah, A. R., Kurniawan, . S. C., Puspitasari, . Y., Aryaloka, . S., Silaen, . O. S. M., Yanestria, . S. M., Widodo, . A., Moses, . I. B., Effendi, . M. H., Afnani, . D. A., Ramandinianto, . S. C., Hasib, . A. & Riwu, . K. H. P. (2024) Brucellosis: Unveiling the complexities of a pervasive zoonotic disease and its global impacts. Open Vet. J., 14 (5), 1081-1097. doi:10.5455/OVJ.2024.v14.i5.1 Turabian Style Khairullah, Aswin Rafif, Shendy Canadya Kurniawan, Yulianna Puspitasari, Suhita Aryaloka, Otto Sahat Martua Silaen, Sheila Marty Yanestria, Agus Widodo, Ikechukwu Benjamin Moses, Mustofa Helmi Effendi, Daniah Ashri Afnani, Sancaka Cashyer Ramandinianto, Abdullah Hasib, and Katty Hendriana Priscilia Riwu. 2024. Brucellosis: Unveiling the complexities of a pervasive zoonotic disease and its global impacts. Open Veterinary Journal, 14 (5), 1081-1097. doi:10.5455/OVJ.2024.v14.i5.1 Chicago Style Khairullah, Aswin Rafif, Shendy Canadya Kurniawan, Yulianna Puspitasari, Suhita Aryaloka, Otto Sahat Martua Silaen, Sheila Marty Yanestria, Agus Widodo, Ikechukwu Benjamin Moses, Mustofa Helmi Effendi, Daniah Ashri Afnani, Sancaka Cashyer Ramandinianto, Abdullah Hasib, and Katty Hendriana Priscilia Riwu. "Brucellosis: Unveiling the complexities of a pervasive zoonotic disease and its global impacts." Open Veterinary Journal 14 (2024), 1081-1097. doi:10.5455/OVJ.2024.v14.i5.1 MLA (The Modern Language Association) Style Khairullah, Aswin Rafif, Shendy Canadya Kurniawan, Yulianna Puspitasari, Suhita Aryaloka, Otto Sahat Martua Silaen, Sheila Marty Yanestria, Agus Widodo, Ikechukwu Benjamin Moses, Mustofa Helmi Effendi, Daniah Ashri Afnani, Sancaka Cashyer Ramandinianto, Abdullah Hasib, and Katty Hendriana Priscilia Riwu. "Brucellosis: Unveiling the complexities of a pervasive zoonotic disease and its global impacts." Open Veterinary Journal 14.5 (2024), 1081-1097. Print. doi:10.5455/OVJ.2024.v14.i5.1 APA (American Psychological Association) Style Khairullah, A. R., Kurniawan, . S. C., Puspitasari, . Y., Aryaloka, . S., Silaen, . O. S. M., Yanestria, . S. M., Widodo, . A., Moses, . I. B., Effendi, . M. H., Afnani, . D. A., Ramandinianto, . S. C., Hasib, . A. & Riwu, . K. H. P. (2024) Brucellosis: Unveiling the complexities of a pervasive zoonotic disease and its global impacts. Open Veterinary Journal, 14 (5), 1081-1097. doi:10.5455/OVJ.2024.v14.i5.1 |