| Research Article | ||
Open Vet. J.. 2024; 14(5): 1269-1280 Open Veterinary Journal, (2024), Vol. 14(5): 1269–1280 Research Article Characterization of β-glucan obtained from Candida albicans of caprine mastitisSaddam Hussein Mahmoud* and Shaimaa Nabhan YasseinFaculty of Veterinary Medicine, University of Baghdad, Baghdad, Iraq *Corresponding Author: Saddam Hussein Mahmoud. Faculty of Veterinary Medicine, University of Baghdad, Baghdad, Iraq. Email: saddam.hussein1101f [at] covm.uobaghdad.edu.iq Submitted: 22/01/2024 Accepted: 06/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
AbstractBackground: Beta-glucan (β-glucan) is a polysaccharide containing β-glycosidic bonds that is an important structure part of different yeast cells. Aim: The purpose of the study is to characterize β-glucan obtained from Candida albicans (C. albicans) isolated from caprine mastitis. Methods: The β-glucan was extracted by using utilizing an Alkaline-acidic extraction technique. The dry weight of extracted β-glucan was 7.47/150 g with 4.98%. Results: The findings demonstrated that the extracted β-glucan had similarity in the primary peak 5.78 of liquid samples using the method of high-performance liquid chromatography when compared to the standard form of β-glucan. However, scanning electron microscopy studies revealed that the standard of β-glucan was distinct in morphology but similar to β-glucan isolated from C. albicans in terms of particle sizes in the range of 1.60–2.65 m and the lack of cell wall traces. The findings of an investigation using energy-dispersive X-ray fluorescence spectroscopy (EDS/EDX) of extracted and standard β-glucan, showed the principal elements discovered were carbon (C), oxygen (O), and nitrogen (N). Aluminum (Al), silicon (Si), nickel (Ni), and gold (Au) were also present, but in less amounts. Conclusion: The extracted β-glucan displayed a high degree of similarity and purity to the standard β-glucan, according to the findings of Fourier transform infrared spectroscopy (FT-IR) research. Keywords: Subclinical mastitis, Mycotic mastitis, HPLC, Electron microscopy, Iraq. IntroductionMycotic mastitis is less common than other agents; it has grown significantly in the last ten years and is now recognized as a major cause of mycotic mastitis in ruminants (Mohammed and Yassein, 2020). More than 26 species of fungi have been linked to mycotic mastitis, which typically develops after bacterial mastitis has been treated with antibiotics (Al-Muhna, 2014). Yeasts are regarded as an essential part of the microflora of dairy products and are typically present in high concentrations in milk due to their high protein, sugar, fat, and organic acid content. Yeasts have the potential to seriously harm public health by causing metabolic degradation in milk (Spanamberg et al., 2009). Moreover, fungi impact milk quality and shelf life (Hasan and Yassein, 2018). Candida albicans (C. albicans) is the most infective species which is responsible for more than half of the cases of candidiasis in various countries (Caggiano et al., 2017; Singh et al., 2020). Candida albicans only contains β-glucan and does not contain α-glucans; also, the cell walls of C. albicans contain both β-1,3 and β-1,6-glucans, but no intrachain combined with β-1,3/1,6 linkage (Ruiz-Herrera et al., 2006). Beta-glucan (β-glucan) is a component of cell walls in algae, bacteria, fungi, yeast, and plants (Pengkumsri et al., 2017). It belongs to a class of polysaccharides called β-glycosidic polysaccharides that are composed of glucose polymer units (Vetvicka et al., 2021), and high molecular weight found in the cell wall of many types of yeast. Yeast β-glucan is composed of a mixture of β-1, 3 and β-1, 6 glucan (Many and Vizhi, 2014). The normal yeast cells contain 30 to 45% β-1,3 glycan and 5%–10% β-1,6 glycan (Soares and Soares, 2012); while the cell wall is primarily composed of β-1,3/1,6-D-glycan (50%–60%) and mannoproteins (40%) (Klis et al., 2006). Glucans are classified as (alpha-glucan, β-glucan, and alpha-β-glucan) based on the position of glycosidic linkages (1, 3, 1, 4, and 1, 6 glucans) according to the anomeric conformation of glucose (Synytsya and Novak, 2013) and soluble or particulate (Bashir and Choi, 2017). Yeast cell wall β-glucan consists of 1→3 β-linked glucopyranosyl residues, with a small number of 1→6 β-linked branches (Upadhyayv et al., 2017). Several potential properties of β-glucans have been reported in the literature, such as being antioxidant, anti-inflammatory, anti-cholesterol, anti-aging, a blood glucose regulator, and an antitumor agent (Chamidah et al., 2017; Amer et al., 2021). Moreover, β-glucan can act as an immunomodulatory molecule, which acts via the modification of the host immune response by the activation of innate immune cells including macrophages, neutrophils, and granulocytes (Muta, 2006). The yeast cell wall contains three forms of glucan, which differ in terms of molecular connection and branched (Saluk-Juszczak et al., 2010). The inner part contains 30 to 35% insoluble β-glucan, the intermediate layer contains 20% to 22% soluble β-glucan, and the outside part contains around 30% glucoproteins (Akramiene et al., 2007). Β-glucans are polysaccharides that display a variety of biological characteristics as a result of their diverse chemical make-up as the same as all dietary fibers (Vlassopoulou et al., 2021). This study aims to extract β-glucan from C. albicans and detect the characterization by using scanning electron microscopy (SEM), high-performance liquid chromatography (HPLC), Fourier transform infrared spectroscopy (FT-IR), and energy dispersive X-ray fluorescence spectroscopy (EDS/EDX). Materials and MethodsSource of C. albicans isolateThe isolate of C. albicans was isolated from subclinical mastitis in goats which was purified and examined macroscopically and microscopically, then exposed to the confirmatory diagnosis by implied specific tests such as germ tube production test, urease test, chlamydospore formation, and cultivation on CHROM agar according to other studies (Quinn et al., 2004; Washinton et al., 2006; Deorukhkar and Roushani, 2018). Extracting of β–glucan from C. albicans cell walls according to other studies (Mahdi and Mohsin, 2015; Bacha et al., 2017; Pengkumsri et al., 2017). The β-glucan has been extracted via a combination of bases and acids (NaOH/CH3COOH extraction). Candida albicans had been grown on sabouraud dextrose agar at 37ºC for 3–5 days. After the colony had been collected, 1.5 l of 1 M sodium hydroxide (NaOH) was added to 225 g of harvested yeast, then the mixture was heated in a magnetic stirrer at 80ºC for 2 hours. Following that, cell pellets were obtained by cold centrifuging (6,000 × g for 25 minutes) at 4ºC within three folds from water that was distilled, cells were suspended and centrifugation was repeated again. Cell pellets had been dissolved by adding 1.5 l of 1M acetic acid (CH3COOH). It was then stirred for 2 hours at 80ºC using a magnetic stirrer, centrifuged at 6,000 × g for 25 minutes, and used to collect the pellet at 4ºC. The supernatant was discarded after being washed three times with water and centrifuged (6000 ×g for 25 minutes) at 4ºC. The pellets had been mixed in 600 cc from 100% ethanol using a magnetic stirring device for 1 hour. To precipitate β-glucan, ethanol was added (Divya et al., 2020), and the solution was centrifuged (6,000 × g for 25 minutes) at 4ºC. The pellets were dried in an oven at 60ºC for 24 hours. The typical β-glucan (β-1, 3-glucan) of Euglena gracilis has been utilized through this investigation, which was provided by Sigma. β-Glucan CharacterizationSEM Standard β-glucan and β-glucan isolated from C. albicans were analyzed for morphology (shape and size) using a scanning electron microscope (SEM) (ThermoScientificTM AxiaTMChemiSEM) at various magnifications and accelerated voltages of 10 KV. The specimens were then covered by a 2-nm-thick gold layer using a magnetron sputtering system (Bacha et al., 2017). EDS/EDX analysisThe unique live quantitative energy-dispersive X-ray spectroscopy (EDS) connecting (live structural data: works EDS through scanning several signals at the same time, identifying the shape and fundamental cosmetics for an object in actual period). The β-glucans were analyzed through the EDS/EDX technique (Bacha et al., 2017). FT-IR of β-glucanThe chemical structure of C. albicans β-glucan was investigated using Fourier transformed-infrared (IR) spectrometry (Shimadzu IRPrestige-21, Japanese) in the Ministry of Science and Technology. The spectrum of FTIR (an enhanced IR spectrometer) has been used to identify the functional compounds in the glucan molecule in comparison to the standards. It was accomplished using the FTIR technique with a wavelength range of 400–4,000 cm−1 with an accuracy of 8 cm−1. This procedure required combining a comparable amount of β-glucan specimen and conventional glucan using potassium bromide (KBr), and analyzing this combination with an FTIR analyzer (Salim et al., 2017; Rasheed and Haydar, 2023). High-performance liquid chromatography (HPLC) technique of β-glucanHPLC separation was used to examine the samples and standard of β-glucan using column Lichrosphere C18 (50 × 4.6 mm), with particle size 3 mm. Mobile phase: deionized water detection: a refractive index detector and RF Shimadzu detector. The flow rate was 1.2 ml/minutes, the temperature at 30°C and the pH was 3.5. The samples were prepared using 10 mg had been dispersed into 250 ml to form a 40 µg/ml conventional, and then 20 µl had to be injected through an HPLC cartridge for measurement. The purification was performed utilizing liquid chromatographic Shimadzu 10AV-LC fitted by the bipolar supply pump models LC-10A Shimadzu, and the peak results were examined with a UV-Vis 10 A-SPD spectrophotometer (Salim et al., 2017; Mohamed and Al-Shamary, 2022; Rasheed and Haydar, 2023). The detection happened in UV light with a wavelength of 305 nm. As described by other studies (Salim et al., 2017; Mohamed and Al-Shamary, 2022), the sample of β-glucan isolating C. albicans and conventional β-glucan have been examined by HPLC separating at the Ministry of Science and Technology, and the concentration of samples was estimated using the equation:
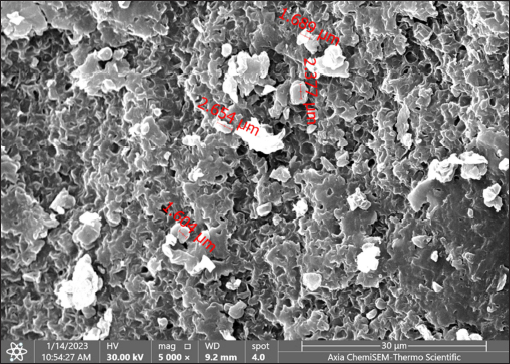
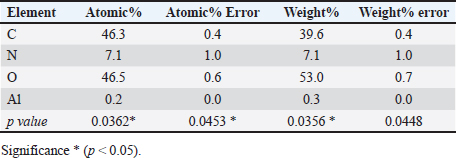
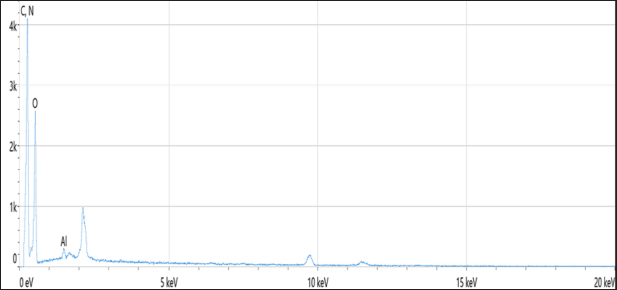
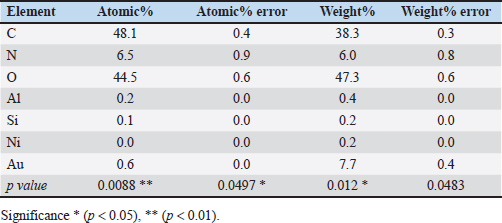
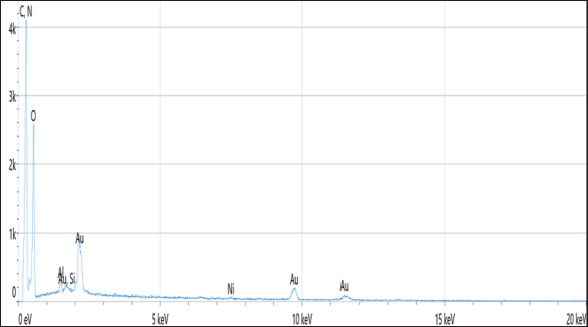
Statistical analysisThe t-test in the GraphPad Prism Software was applied to estimate significant differences at p < 0.05 (Gharban et al., 2023). Ethical approvalThis study gets a license from the Scientific Committee of the Department of Internal and Preventive Veterinary Medicine, College of Veterinary Medicine, University of Baghdad (Baghdad, Iraq). ResultsExtraction of β-glucan from C. albicansDuring the current investigation, alkaline-acidic extraction procedures were used to extract the β-glucan. The dry weight for β-glucan isolated from C. albicans using this approach was 7.47/150 gm (4.98%). The physical characteristics of β-glucan isolated out of C. albicans are characterized by the powder’s yellow color (Fig. 1). SEM image of β-glucan obtained from C. albicansThe findings for SEM evaluations, including the conventional forms of β-glucan and β-glucan derived from C. albicans, were examined at seven magnifications (5, 10, 20, 30, 50, 100, and 300 m). In general, the standard β-glucan has particles that are the same size as β-glucan isolated from C. albicans but differ in morphology. Standard β-glucan has homogenous, irregularly shaped particles that aggregate to produce irregular masses with spherical to rounded particles. The recovered C. albicans β-glucan particles resembled platelet-like aggregators, and depicts bigger, homogenous clusters of particles with a size range of 1.60–2.65 µm (Figs. 2 and 3). EDS/EDXThe EDS-EDX was one of the prospective technologies for fast element detection. It is considered alternative equipment for detecting elements in bio-materials. The results of the EDS/EDX investigation of standard β-glucan revealed that the primary element discovered were carbon, oxygen, and nitrogen with less amount of Aluminum (Al), (Table 1, Fig. 4). While the findings of this screening obtained β-glucan showed the same primary element that found in standard β-glucan with lesser quantification in aluminum, silicon (Si), nickel (Ni), and gold (AU) (Table 2, Fig. 5).
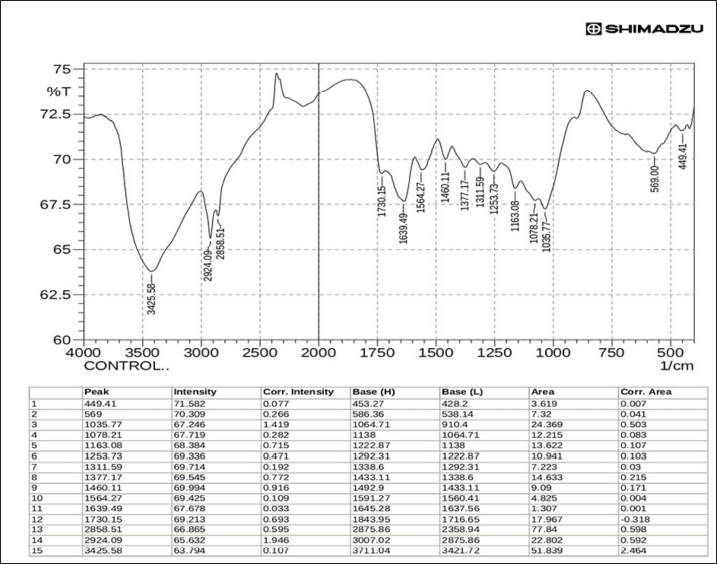
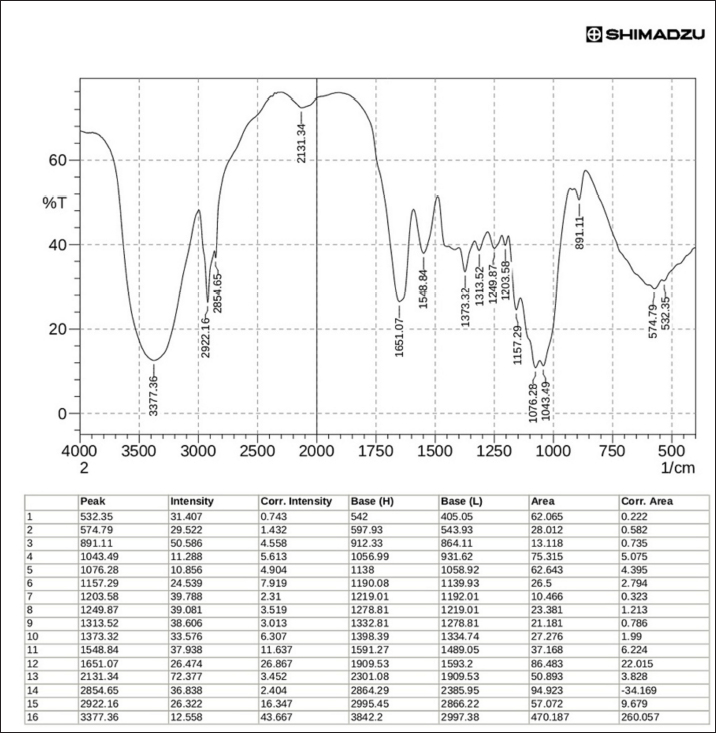
Fig. 1. β-glucan obtained from C. albicans. FT-IR of β-glucanThe characterization of Cladophora and Spirulina feed stock by FTIR suggests that the presence of functional groups of diverse elements such as the Hydroxyl unit (–OH) and amides (N–H stretch) have been accountable for adsorption on the surface activities. The current study’s FT-IR examination confirmed that the extracted β-glucan had a high degree of similarity and purity when compared to the standard (Figs. 6 and 7). FT-IR findings of standard and extracted β-glucan included the presence of proteins and other associated components. The FT-IR analyses of standard and extracted β-glucan of the fungus cells revealed 5 broad bands. The -OH bending has been seen between 3,000 and 4,000 cm−1, corresponding to a peaking of 3,377.35 cm−1 for derived β-glucan and 3,425.58 cm−1 for standard β-glucan. The 2nd band at 2,922.16 cm−1 for extracted and 2,924.09 cm−1 for standard indicated CH and CH2 stretching. The 3rd band at 1,651.07 cm−1 for derived β-glucan and the band at 1,730.15 cm−1 for standard β-glucan belongs to NH teams suggesting the existence of protein. 4th band at 1,076.28 cm-1 for derived β-glucan and the wide band at 1,078.21 cm−1 for standard β-glucan, is caused by COC bonds, which are bonds of glycosidic via cycle architecture, the peak at 10,746 cm−1 of the obtained β-glucans confirms the existence for starches. The 5th band of Carbohydrates comprised architecture may be indicated by bands within 1,043.49 cm−1 for extracted β-glucan and band at 1,035.77 cm−1 for standard β-glucan representing CO bond.
Fig. 2. SEM of conventional β-glucan in magnification power: 30 μm (1.60–2.65 μm).
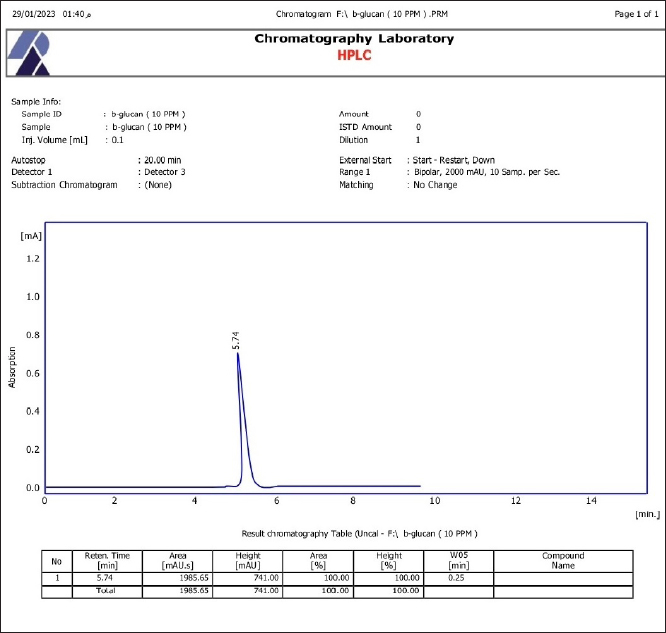
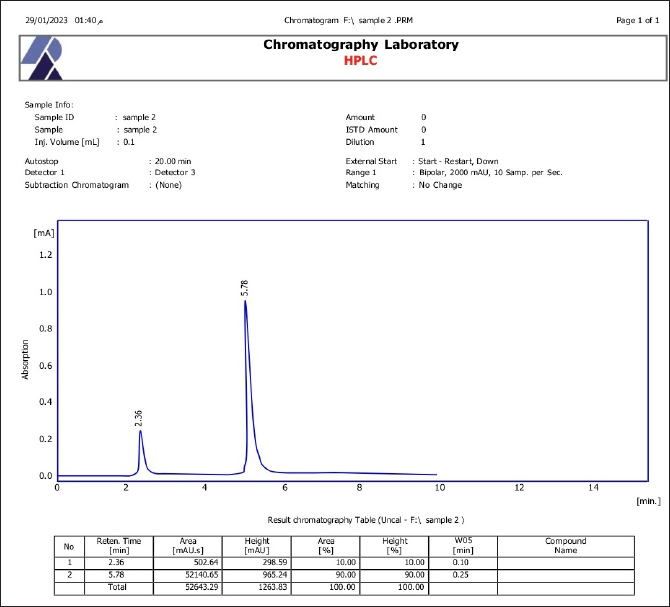
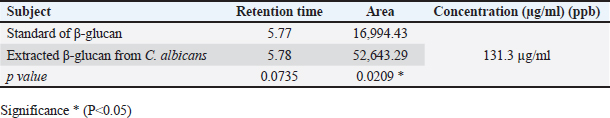
Fig. 3. SEM of β-glucan obtained from C. albicans at magnification power of 30 μm (1.60–2.65 μm). High-performance liquid chromatography analysis of β-glucansThis method was employed during this investigation to determine the purity and quality of β-glucans that were extracted from C. albicans and also to support the standard β-glucan’s structural similarity. In the present work, the extracted β-glucan’s HPLC analysis result showed its structural resemblance to the β-glucans standard. HPLC test showed that the major peak of a liquid sample of β-glucan extracted from C. albicans was 5.78, which represented the purity of the extracted β-glucan. This peak had a similar duration of retention as the conventional of β-glucans (Figs. 8 and 9), and a concentration of β-glucans (conventional and specimen of β-glucans obtained) discovered using HPLC (Table 3). Table 1. Analysis of EDS/EDX for standard β-glucan with total acquisition time: 28 seconds.
Fig. 4. Analysis of EDS/EDX for standard β-glucan with maps resolution: 768 × 512. Table 2. Analysis of EDS/EDX for extracted β-glucan with total acquisition time: 28 seconds.
DiscussionThe current study’s findings are consistent with those of Jameel and Yassein (2021) who extracted β-glucan utilizing a technique based on Alkaline-acidic extraction stages with (4.15%); also, the morphological characteristics of the C. albicans derived β-glucan powder recorded as yellow crystal particles. In addition, Al-Jumaiee et al. (2019) reported a yield of 5.95% from 4 g of the same yeast. According to Ruiz-Herrera et al. (2006) powder from β-glucan produced by C. albicans is characterized by yellow crystal particles. On the other hand, Hassan et al. (2014) mentioned that the yeasts can produce up to 12% of their weight in β-glucan when they are extracted using an alkaline acid. In this work, the amount of β-glucan produced by C. albicans had been lesser than that of Miura et al. (2003) discovered that the amount of soluble β-glucan in the identical fungus is 25.9% in (yeast forms) but 7.5% in (mycelial forms) in dry yeast cells.
Fig. 5. Analysis of EDS/EDX analysis for extracted β-glucan with maps resolution of 768 × 512.
Fig. 6. Analysis for standard β-glucans using FTIR.
Fig. 7. Analysis for extracted β-glucans using FTIR. Ibrahim (2014) discovered that the dry weight of the resulting glucan was 8.8/100 g dry yeast. AL-Rubaee (2008) claimed the ability to obtain glucan 6.25/100 g dry-weight baker’s yeast. The current study agrees with Limberger-Bayer et al. (2014) who observed that morphological characteristics for both of the samples, derived β-glucans and conventional β-glucans, were identical, exhibiting a porosity and sponge look and not any obvious signs of the creation of cell walls. The results of a study by Jameel and Yassein (2021) performed by SEM on the particles of conventional β-glucan demonstrated the existence of homogeneous, irregular aggregating generating irregular mass with cylindrical to round particles The findings of very few studies on SEM for β-glucan obtained from C. albicans showed that the particles of β-glucan obtained from C. albicans having an aggregation of booklet-like platelet having particle sizes ranging from 1.2 to 1.84 µm.
Fig. 8. HPLC analysis of standard β-glucans. SEM investigation revealed the ridge-like character of the β-glucan, as well as the shiny exterior and undulated borders. The oval to elliptical form of the granular particles indicates that the natural architecture of β-glucan particles has been preserved, as mentioned by Raikos et al. (2018). In yeast cells, the FTIR spectrum was utilized to analyze three major sections, which correspond to polysaccharides (925–1,190 cm–1), proteins (1,500–1,700 cm–1), and lipids (2,800–3,000 cm–1), (Liu et al., 2015; Pengkumsri et al., 2017; Al Dujaily and Mahmood, 2021). IR spectroscopy can monitor the molecule oscillations from covalent bonding; the IR range 4,000–500 cm−1 contains data about basic oscillations as mentioned by Limberger-Bayer et al. (2014). The FTIR technique has been utilized to identify functional groups within the structure of the chemical of β-glucan and compare it to standard groups (Rumberger et al., 2005; Ibrahim et al., 2006; Hassan et al., 2014; Salim et al., 2017). The samples FT-IR spectroscopy was utilized to examine differences in primary ingredients (carbohydrates, fat, and protein) and overall composition (Rieder et al., 2017; Salim et al., 2017; Ibrahim et al., 2020; Abd and Mohammed-Ridha, 2021). Limberger-Bayer et al. (2014) analyzed commercial and purified-isolated β-glucan. Because yeast’s outside cell walls are predominantly made of carbohydrates, cells of yeast displayed five bands, one of which was 3,409.873 cm−1. The polysaccharides include a lot of OH groups. In the case of O–H teams, the –OH stretches may be seen at 4,000–3,000 cm−1, which indicates the peak 3,434 cm−1 for derived β-glucan and 3,424 cm−1 for conventional β-glucan. The derived spectral fingerprint is 2,928 cm−1, but the commercial fingerprint is 2,919 cm−1. Moreover, Mikkelsen et al. (2010) observed that the significant absorbance in 1,651 cm−1 for obtained β-glucans and 1,631 cm−1 for conventional β-glucans was caused by the stretched on the proteins’ CN and NH categories, confirming the existence of amide linkages and protein in the sample. As a result, the peak in 1,074 cm−1 for the derived sample and 1,025 cm−1 for the conventional produced suggests the existence of glycosidic linkages and cycle monosaccharide architecture. The spectra revealed peak absorbance in 987 cm−1 for the obtained β-glucans and 899 cm−1 for the conventional β-glucans, indicating the presence of β-glycosidic anomeric linkages. Bacha et al. (2017) discovered precise spectrum data relating to conventional and extracted β-glucan from yeast cells. Conventional (3,421.678 cm−1) and extracted β-glucan (3,440.964 cm−1) have demonstrated minimal proteins. Furthermore, the highest strength of the –OH team in isolated β-glucan is larger and wider than in conventional β-glucan. The stretching vibration of –CH2 groups in isolated β-glucan has a broader range than the stretching vibration of –CH2 groups in conventional β-glucan. In addition, the proteins (–NH) band in the extracted β-glucan (1,641.109 cm−1; 1,558.14 cm−1) have been displaced to less wave lengths compared to those in conventional β-glucan. It implies the separated specimens contain less protein, confirming the pure nature of the extracted β-glucan. Further crucially, the isolated β-glucan’s spectrum absorption at 1,079.780 cm-1, 1,045.339 cm−1, and 923.879 cm−1 supports the existence of glycosidic linkages, suggesting that the β-glucan has not been disrupted.
Fig. 9. HPLC analysis of extracted β-glucans. Table 3. Concentration of β-glucans (conventional and samples of β-glucans obtained from C. albicans detected by HPLC.
The results reveal that the existence of C–O–C links in the spectrum of IR light around absorption (1,028.0 cm−1) have typical characteristics of the β-glucan architecture stretched to the conventional (1,055.0 cm−1), (Hozova et al., 2007). The absorbing capacity (1,371.3 cm−1) indicates the existence of C–H aromatic bends, whereas reference absorption (1,315.4 cm−1) (Karreman et al., 2006). The current work is comparable to Ibrahim et al. (2006) who discovered similar indicating that the FT-IR spectrum of the obtained β-glucans had the look of conventional β-glucans having an elevated level of clarity. Free hydroxyl groups and carboxyl groups, on the other hand, were taken in locations (2,927.7 cm−1) and (2,922.0 cm−1) identified in carbohydrates. Boutros et al. (2022) discovered several characteristic beta glucan bands at wave numbers 890 (beta-linked polymer), 1,076 (C–O stretch), 1,372 (CHOH stretch), 2919 (C–H stretch), and 3,390 (OH stretch) in the samples. Hassan et al. (2014) showed that the IR spectrum at the absorbance of 1,041.5 cm−1 indicates the existence of C–O–C bonds which is a hallmark property of β-glucans architecture stretched to the conventional 1,051 cm−1. Another study performed by Salim et al. (2017) found this method was applied to assess the purity and quality of Pleurotus eryngii β-glucans as well as to demonstrate its similarity in structure to the conventional β-glucans. The HPLC test showed the existence of the extracted glucan as a prominent peak (2.087) of a liquid glucan. Such a peak displayed the same glucan standard retention time (2.193). Also, the HPLC examination of a liquid sample of β-glucan exhibited a single significant peak at 3.78, confirming the pure nature of the obtained β-glucans. The fact that this peak shared an identical persistence period as the β-glucans conventional indicates that the extraction process was successful as mentioned by Hassan et al. (2014). The same peak (at 3.78) was recorded by Ibrahim (2014) when analyzing the β-glucans obtained from Saccharomyces cerevisiae by HPLC. According to Jameel and Yassein (2021), the extracted β-glucan’s HPLC analysis result showed its structural resemblance to the β-glucan standard. The β-glucans obtained from S. cerevisiae and C. albicans displayed a large peak at 3.22, which represents the pureness of the β-glucans isolated from both fungi. This signal had a similar duration of retention as the conventional for β-glucan, according to the HPLC analysis. The most effective approach for identifying β-glucan was thought to be HPLC. On the other hand, Al-Aubydi and Abed (2011) demonstrated that all elements of β-glucans obtained from mushrooms were separated using the HPLC, which provided an effective approach for identifying the β-glucan. ConclusionThe results of the current study indicated that it had been beneficial in extracting β-glucans from C. albicans and demonstrated this type of extraction, which depends on an acid-base technique, may produce large amounts of extracts. In comparison to the conventional, SEM, EDS/EDX, FT-IR spectroscopy, and HPLC analysis verified the obtained β-glucans from C. albicans were β-glucans. However, the current investigation, suggests that this kind of β-glucan may be utilized in studies in science. AcknowledgmentsThe authors are thankful to all members of the laboratory in the Ministry of Science and Technology for their assistance, help, and generosity. And valuable role in HPLC, FT-IR, and SEM. Many thanks to the staff of the departments’ Internal and Preventive Veterinary Medicine, College of Veterinary Medicine, University of Baghdad—Iraq for their support and for providing the facilities during the extract processing. Authors’ contributionSHM: Isolation of C. albicans, obtaining and characterization of β-glucan, and carrying out the HPLC. SNY: Performing of SEM, quantitative EDS and FT-IR, and statistical analysis of data. The final copy of the manuscript was read and approved carefully by both authors. Conflict of interestAll authors have no conflict of interest to disclose. FundingThere is no external fund (private funding). Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAbd, I.N. and Mohammed-Ridha, M.J. 2021. Tetracycline antibiotic removal from aqueous solution using cladophora and spirulina algae biomass. Iraqi J. Agric. Sci. 52(2), 336–347. Akramiene, D., Kondrotas, A., Didziapetriene, J. and Kevelaitis E. 2007. Effects of β-glocans on the immune system. Medicina. 43(8), 597–606. Al Dujaily, A.H. and Mahmood, A.K. 2021.The effectiveness of biogenic silver nanoparticles in the treatment of caprine mastitis induced by Staphylococcus aureus. Iraqi J. Vet. Sci. 35, 73–78. Al-Aubydi, M. and Abed, R.M. 2011. The usage of β-glucan extracted from local mushroom as immunomodulator. Curr. Res. J. Biol. Sci. 3(5), 535–541. Al-Jumaiee, S.A.J., Al-Hussainy, K.S.J. and Al-Manhel, A.J.A. 2019. Effect of different level of β-glucan extracted from Baker’s Yeast Saccharomyces cerevisiae and Barley Bran in the physicochemical properties of fish patties at cooling storage periods. Basrah J. Agric. Sci. 32(1), 79–87. Al-Muhna, B.M.M. 2014. Study of the mycotic mastitis in dairy goats in Al-Diwaniya province. Available via https://www.researchgate.net/publication/316273286 Al-Rubaee, A. 2008. Extraction of glucan from yeast Saccharomyces cerevisiae. M.Sc. Thesis. College of Science—Baghdad University. Amer, M.E., Saber, S.H., Abo Markeb, A., Elkhawaga, A.A., Mekhemer, I.M.A., Zohri, A.-N.A., Abujamel, T.S., Harakeh, S. and Abd-Allah, E.A. 2021. Enhancement of β-glucan biological activity using a modified acid-base extraction method from Saccharomyces cerevisiae. Molecules. 26, 21–33. Bacha, U., Nasir, M., Iqbal, S. and Anjum, A.A. 2017. Nutraceutical, anti-inflammatory and immune modulatory effects of β-glocan isolated from yeast. Bio. Med. Res. Int. 2017, 1–14. Bashir, K.M.I. and Choi, J.S. 2017. Clinical and physiological perspectives of β-glucans: the past, present, and future. Int. J. Mol. Sci. 19(9), 42–48. Boutros, J.A., Magee, A.S. and Cox, D. 2022. Comparison of structural differences between yeast β-glucan sourced from different strains of Saccharomyces cerevisiae and processed using proprietary manufacturing processes. Food Chem. 36(7), 130–138. Caggiano, G., Lovero, G., De Giglio, O., Barbuti, G., Montagna, O., Laforgia, N. and Montagna, M.T. 2017. Candidemia in the neonatal intensive care unit: a retrospective, observational survey and analysis of literature data. Bio. Med. Res. Int. 2017, 1–12. Chamidah, A., Hardoko, O. and Prihanto, A.A. 2017. Antibacterial activities of β-glucan (Laminaran) against gram-negative and gram-positive bacteria. In AIP Conference Proceedings, AIP Publishing LLC, 1844, 20–31. Deorukhkar, S.C. and Roushani, S. 2018. Identification of Candida species: conventional methods in the era of molecular diagnosis. Ann. Microbiol. Immunol. 1(1), 1002–1008. Divya, M., Gobi, N., Iswarya, A., Govindarajan, M., Alharbi, N.S., Kadaikunnan, S., Khaled, J.M., Almanaa, T.N. and Vaseeharan, B, 2020. β-glucan extracted from eukaryotic single-celled microorganism Saccharomyces cerevisiae: dietary supplementation and enhanced ammonia stress tolerance on Oreochromismossambicus, Microbial Pathog. 139, 103–117. Gharban, H.A., Al-Shaeli, S.J. and Hussen, T.J. 2023. Molecular genotyping, histopathological and immunohistochemical studies of bovine papillomatosis. Open Vet. J. 13(1), 26–41. Hasan, K.A.M. and Yassein, S.N. 2018. Prevalence and type of fungi in milk from goats with sub clinical mastitis. Online J. Vet. Res. 22(8), 669–674. Hassan, S.S., Al-Saffar, A.Z. and Ibrahim, H.K. 2014. Anti-angiogenic and immunological effect of β-Glucan extracted from Saccharomyces cerevisiae HS-1. Int. J. Biotechnol. 112, 365–373. Hozova, B., Kuniak, L., Moravckov, P. and GajdoŠov, A. 2007. Determination of water-insoluble β-d-glucan in the whole- grain cereals and pseudocereals. Czech J. Food Sci. 25, 316–324. Ibrahim, H.K. 2014. Effect of β-glucan extracted from Saccharomyces cerevisiae on angiogenesis. M.Sc. Thesis. College Board of Science/Al-Nahrain University. Ibrahim, M., Alaam, M., EI-Haes, H., Jalbout, J.F. and Leon, A. 2006. Analysis of the structure and vibrational spectra of glucose and fructose. Ecl. Quim. 31(3), 15–21. Ibrahim, O.M.S., Abed, A.M. and Yahea, N.Z. 2020. Evaluation of biological activity of GreenlySynthesized silver nanoparticles using aloe vera gel extract as antibacterial agent in-vitro and in-vivo. Iraqi J. Agric. Sci. 51(6), 1706–1715. Jameel, F.A. and Yassein, S.N. 2021. Characterization of Β-Glucan extracted from Saccharomyces cereviseae and Candida Albicans. Plant Arch. 21(1), 1722–1727. Karreman, R.J., Dague, E., Gaboriaud, F., Guiles, F., Duval J.F. and Lindsey, G.G. 2006. The stress response protein Hsp12p increase the flexibility of the yeast Sacchromyces cerevisiae. Biochim. Biophys. Acta. 1436, 239–246. Klis, F.M., Boorsma, A. and De Groot, P.W. 2006. Cell wall construction in Saccharomyces cerevisiae. Yeast. 23, 185–202. Limberger-Bayer, V.M., De Francisco, A., Chan, A., Oro, T., Ogliari, P.J. and Barreto, P.L.M. 2014. Barley β-glucans extraction and partial characterization. Food Chem. 154, 84–89. Liu, C.Y., Wang, C.J., Chung, W.C., Chu, C., Lin, W.Y. and Chung, W.H. 2015. Scanning electron microscopy-energy dispersive X-ray spectrometer (SEM-EDX) detection of arsenic and cadmium in himematsutake mushroom. African J. Biotechnol. 14(10), 878–887. Mahdi, N.R. and Mohsin, S.I. 2015. Challenged with Brucellaabortus virulent strain. Iraqi J. Vet. Med. 39(2), 79–86. Many, J.N. and Vizhi K. 2014. Analysis of different extraction methods on the yield and recovery of β-glucan from Baker’s Yeast (Saccharomyces cerevisiae). Int. J. Innov. Sci. Eng. Technol. 1(6), 268–271. Mikkelsen, M.S., Jespersen, B.M., Moller, B.L., Lerke, H.N., Larsen, F.H. and Engelsen, S.B. 2010. Comparative spectroscopic and rheological studies on crude and purified soluble barley and oat β-glucan preparations. Food Res. Int. 43(10), 2417–2424. Miura, N.N., Adachi, Y., Yadomae, T., Tamura, H., Tanaka, S. and Ohno, N. 2003. Structure and biological activities of -glucans from yeast and mycelial forms of Candida albicans. Microbiol. Immunol. 47(3), 173–182. Mohamed, A.M. and Al-Shamary, E.I. 2022. Isolation and identification of aflatoxin B1 producing fungi from stored wheat in Some Silos of Baghdad. Iraqi J. Agric. Sci. 53(6), 1427–1436. Mohammed, S.J. and Yassein, S.N. 2020. Characterization of some virulence factors of Candida albicans isolated from Subclinical Bovine Mastitis. Plant Arch. 20, 238–242. Muta, T. 2006. Molecular basis for invertebrate innate immune recognition of (1→3)-β-D-glucan as a pathogen-associated molecular pattern. Curr. Pharm. 12, 4155–4161. Pengkumsri, N., Sivamaruthi, B.S., Sirllun, S., Peerajan, S., Kesika, P., Chaiyasut, K. and Chaiyasut, C. 2017. Extraction of β-glucan from Saccharomyces cerevisiae: comparison of different extraction methods and in vivo assessment of immunomodulatory effect in mice. Food Sci. Technol. 37(1), 124–130. Quinn, P.J., Carter, M.E., Markey, B. and Carter, G.R. 2004. Clinical veterinary microbiology. London: Mosby. An Imprint of Elsevier Limited, pp: 127–136. Raikos, V., Grant, S.B., Hayes, H. and Ranawana, V. 2018. Use of β-glucan from spent brewer’s yeast as a thickener in skimmed yogurt: physicochemical, textural, and structural properties related to sensory perception. J. Dairy Sci. 101, 5821–5831. Rasheed, H.Gh. and Haydar, N.H. 2023. Purification, characterization and evaluation of biological activity of mannoprotein produced from Saccharomyces cerevisiae. Iraqi J. Agric. Sci. 54(2), 347–359. Rieder, A., Ballance, S., Bocker, U. and Knutsen, S. 2017. Quantification of 1, 3-β-D-glucan from yeast added as a functional ingredient to bread. Carbohydr. Polym. 181, 34–42. Ruiz-Herrera, J., Elorza, M.V., Valentin, E. and Sentandreu, R. 2006. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. Yeast Res. 6, 14–29. Rumberger, M.D., Lentzsch, P., Munzenberger, B. and Huttl, R.F. 2005. Nutrient amounts of ectomycorrhizaeanalysed by EDX using ESEM and ICP. Mycorrhiza. 15, 307–312. Salim, N.S., Salih, S.M. and Zedan, Z.K. 2017. ANTI-angiogenic effect of Β-glucan extracted from Pleurotus Spp. World J. Pharm. Res. 6(5), 112–127. Saluk-Juszczak, J., Krolewska, K. and Wachowicz, B. 2010. Beta-glucan from Saccharomyces cerevisiae as a blood platelet antioxidant, Platelets. 21, 451–459. Singh, D.K., Toth, R. and Gacser, A. 2020. Mechanisms of pathogenic Candida species to evade the host complement attack. Front. Cell. Infect. Microbiol. 10, 1–9. Soares, E.V. and Soares, H.M. 2012. Bioremediation of industrial effluents containing heavy metals using brewing cells of Saccharomyces cerevisiae as a green technology: a review. Environ. Sci. Pollut. Res. Int. 19(4), 1066–1083. Spanamberg, A., Ramos, J.P., Leoncini, O., Alves, S.H. and Valente, P. 2009. High frequency of potentially pathogenic yeast species in goats raw milk and creamed cheese in southern Brazil. Acta Sci. Vet. 37, 133–141. Synytsya, A. and Novak, M. 2013. Structural diversity of Fungal Mastitis (Review). Carbohydr. Polym. 92(1), 792–809. Upadhyay, T.K., Fatima, N., Sharma, D., Saravanakumar, V. and Sharma, R. 2017. Preparation and characterization of beta-glucan particles containing a payload of nanoembedded rifabutin for enhanced targeted delivery to macrophages. Excli. J. 16, 210–234. Vetvicka, V., Teplyakova, T.V., Shintyapina, A.B. and Korolenko, T.A. 2021. Effects of medicinal fungi-derived β-glucan on tumor. Progression. J. Fungi. 7, 250–263. Vlassopoulou, M., Yannakoulia, M., Pletsa, V., Zervakis, G.I. and Kyriacou, A. 2021. Effects of fungal beta-Glucans on health—a systematic review of randomized controlled trials. Food Funct. 12, 3366–3380. Washinton, W.J., Stephan, A., Willium, J., Elmer, K. and Gail, W. 2006. Konemans color atlas and textbook of diagnostic microbiology, 6th ed. Burlington, MA: Jones & Bartlett Learning, pp: 1152–1232. | ||
| How to Cite this Article |
| Pubmed Style Mahmoud SH, Yassein SN. Characterization of β-glucan obtained from Candida albicans of caprine mastitis. Open Vet. J.. 2024; 14(5): 1269-1280. doi:10.5455/OVJ.2024.v14.i5.22 Web Style Mahmoud SH, Yassein SN. Characterization of β-glucan obtained from Candida albicans of caprine mastitis. https://www.openveterinaryjournal.com/?mno=187356 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i5.22 AMA (American Medical Association) Style Mahmoud SH, Yassein SN. Characterization of β-glucan obtained from Candida albicans of caprine mastitis. Open Vet. J.. 2024; 14(5): 1269-1280. doi:10.5455/OVJ.2024.v14.i5.22 Vancouver/ICMJE Style Mahmoud SH, Yassein SN. Characterization of β-glucan obtained from Candida albicans of caprine mastitis. Open Vet. J.. (2024), [cited January 24, 2026]; 14(5): 1269-1280. doi:10.5455/OVJ.2024.v14.i5.22 Harvard Style Mahmoud, S. H. & Yassein, . S. N. (2024) Characterization of β-glucan obtained from Candida albicans of caprine mastitis. Open Vet. J., 14 (5), 1269-1280. doi:10.5455/OVJ.2024.v14.i5.22 Turabian Style Mahmoud, Saddam Hussein, and Shaimaa Nabhan Yassein. 2024. Characterization of β-glucan obtained from Candida albicans of caprine mastitis. Open Veterinary Journal, 14 (5), 1269-1280. doi:10.5455/OVJ.2024.v14.i5.22 Chicago Style Mahmoud, Saddam Hussein, and Shaimaa Nabhan Yassein. "Characterization of β-glucan obtained from Candida albicans of caprine mastitis." Open Veterinary Journal 14 (2024), 1269-1280. doi:10.5455/OVJ.2024.v14.i5.22 MLA (The Modern Language Association) Style Mahmoud, Saddam Hussein, and Shaimaa Nabhan Yassein. "Characterization of β-glucan obtained from Candida albicans of caprine mastitis." Open Veterinary Journal 14.5 (2024), 1269-1280. Print. doi:10.5455/OVJ.2024.v14.i5.22 APA (American Psychological Association) Style Mahmoud, S. H. & Yassein, . S. N. (2024) Characterization of β-glucan obtained from Candida albicans of caprine mastitis. Open Veterinary Journal, 14 (5), 1269-1280. doi:10.5455/OVJ.2024.v14.i5.22 |