| Research Article | ||
Open Vet. J.. 2024; 14(5): 1154-1160 Open Veterinary Journal, (2024), Vol. 14(5): 1154–1160 Research Article Centella asiatica extract ameliorates deoxygenation-induced neurological dysfunction in zebrafish larvaeAriani Ariani1,2*, Husnul Khotimah3, Arum Sulistyarini4, Araisa Sabrina Daniaridevi41Department of Pediatrics, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia 2Saiful Anwar General Hospital, Malang, Indonesia 3Department of Pharmacology, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia 4Bachelor of Medicine, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia *Corresponding Author: Ariani Ariani. Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia. Email: arianidr [at] ub.ac.id; ariaaani56666 [at] gmail.com Submitted: 27/01/2024 Accepted: 16/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
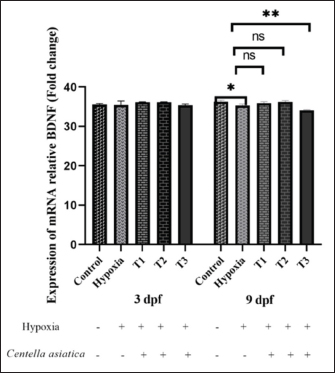
AbstractBackground: Oxygen deprivation (OD) is a critical condition that can lead to brain damage and even death. Current hypoxia management approaches are limited in effectiveness. Centella asiatica (CA), known for its neuroprotective properties, offers a potential alternative for OD treatment. Aims: This study aims to investigate the neuroprotective effects of CA on the expression of brain-derived neurotrophic factor (BDNF) and vesicular glutamate transporter 1 (VGLUT1) in zebrafish larvae under oxygen-deficient conditions. Methods: Zebrafish embryos were subjected to low oxygen levels (1.5 mg/l) 0–2 hours post-fertilization (hpf) until 3 days post-fertilization (dpf), simulating the early stages of OD. Subsequent treatment involved varying concentrations of CA (1.25–5 µg/ml) up to 9 days post-fertilization. The expression levels of BDNF and VGLUT1 were measured using PCR methods. Statistical analysis was conducted using a two-way analysis of variance to evaluate the impact of CA on the expression of BDNF and VGLUT1 in zebrafish larvae aged 3 and 9 dpf in oxygen-deprived conditions. Results: CA significantly influenced the expression of BDNF and VGLUT1 under OD (p < 0.001). An increase in BDNF expression (p < 0.001) and a decrease in VGLUT1 (p < 0.01) were observed in zebrafish larvae experiencing OD and treated with CA. There was no significant difference in BDNF and VGLUT1 expression across age variations in zebrafish larvae at 3 dpf and 9 dpf in the treatment groups (p > 0.05). CA concentration of 2.5 µg/ml effectively enhanced BDNF and reduced VGLUT1 in 3–9 dpf zebrafish larvae. Conclusion: CA demonstrates potential as a neuroprotective agent, modulating increased BDNF expression and reduced VGLUT1 under OD conditions. These findings lay a foundation for further research in developing therapies for oxygen deficiency. Keywords: Centella asiatica, Neuroprotective, Neurotoxicity, Neuroplasticity. IntroductionOxygen deprivation (OD)/hypoxic-ischemic (HI) injury has been reported in approximately 6 per 1,000 births globally, leading to chronic disabilities and high mortality rates. HI conditions result in perinatal brain damage, ultimately causing seizures, learning disorders, cerebral palsy, and epilepsy (Wang et al., 2021). OD can arise from factors such as pulmonary diseases, cardiac conditions, and high altitudes (Sydykov et al., 2021). Central and peripheral chemoreceptors respond to decreased oxygen pressure by signaling the respiratory centers in the medulla and pons. This initiates a cascade of processes that enhance pulmonary ventilation and cardiac output, which is crucial for maintaining normal human body functions (Chen et al., 2020). OD conditions are frequently used as models to study oxidative stress and neuronal damage (Lee and Jeong, 2021). Key biomarkers in neurodegeneration and neuroprotection studies include brain-derived neurotrophic factor (BDNF) and vesicular glutamate transporter 1 (VGLUT1). BDNF, a vital neurotrophic factor, plays a crucial role in neuronal survival and synaptogenesis, protecting against nerve damage and promoting the growth of new nerve cells. BDNF is expressed in various organs, notably the brain, by neurons and glial cells, especially astrocytes (Bazzari and Bazzari, 2022). VGLUT1 is responsible for transporting glutamate (an excitatory neurotransmitter in the brain) into synaptic vesicles (Wilson et al., 2005), and any malfunction in this system could contribute to a range of neurological disorders (Bathina and Das, 2015). In hypoxic conditions, the interaction between BDNF and VGLUT1 can collaborate to protect neurons from oxidative damage and maintain optimal neurotransmission function. These two proteins mutually support each other in preserving the balance and survival of nerve cells (Pietrancosta et al., 2020). In the last decade, research in neuroprotection has made significant strides, particularly in using natural compounds to safeguard brain function. Centella asiatica (CA) is recognized for its potent antioxidant, anti-inflammatory, and neuroprotective properties, attributed to its content of triterpenes, asiaticoside, asiatic acid, madecassoside, and madecassic acid (Sun et al., 2020). These natural compounds can aid in protecting nerves from damage caused by hypoxia (Hambali et al., 2021). This study aims to explore the effects of CA on zebrafish exposed to such conditions, focusing on the changes in BDNF and VGLUT1 expression, thereby providing a protective effect on the zebrafish brain under hypoxic conditions. Material And MethodsThe study was conducted between July 2022 and January 2023, involving the Faculty of Fisheries and Marine Science, as well as the Department of Biochemistry and Pharmacology at the Faculty of Medicine, Universitas Brawijaya, Indonesia. MaterialsOne hundred grams of CA powder underwent maceration in 900 ml of 98% ethanol for 24 hours, followed by filtration. This procedure was repeated three times, with the collected filtrates subsequently processed using rotary evaporation. The resulting extract amounted to 10 gm. The crude extract obtained from this process was transformed into a paste, subsequently diluted in normal saline, and preserved at a temperature of 0°C. AnimalsAdult zebrafish of both genders were accommodated in a 60–l glass aquarium, adhering to a sex ratio of two females for every male. The population density was maintained at 4–5 fish per square meter. Environmental conditions included a light/dark cycle of 14:10 hours and a temperature consistently controlled at 28°C ± 1°C. TetraMin Tropical Fish Flakes (USA) fed the fish twice daily. The collection of embryos began at the onset of the light period, and the egg water was renewed twice daily. The collected embryos, aged 0–2 hpf and characterized by a yolk sac, appeared transparent with a non-colored white hue, as observed by (Tran et al., 2023). Larvae at the age of 3 dpf were followed until they reached 9 dpf for subsequent measurements, in line with the methodologies of larvae aged 3 dpf and were monitored until they reached 9 dpf for measurement (Avdesh et al., 2012). Larvae aged 3 dpf were observed until they reached 9 dpf for measurement (Üstündağ et al., 2019). All protocols for handling and care of these laboratory animals were in strict compliance with the established ethical guidelines for laboratory animal welfare. Experimental designThis research is a true experimental laboratory study and uses a post-test-only control group design. Three replications were included in each group. Zebrafish embryos (Danio rerio) aged 0–2 hpf until three dpf were exposed to hypoxia where oxygen levels reached 1.5 mg/l through Nitrogen exposure, then given 3 different concentrations of CA exposure until age 9 dpf. Zebrafish embryos aged 0–2 hpf were randomly divided into five treatment groups: The control group, a normal zebrafish larvae; the Hypoxia group; T1 group (hypoxia and administered 1.25 μg/ml CA); T2 group (hypoxia and administered 2.5 μg/ml CA); and T3 group (hypoxia and administered 5 μg/ml CA). The zebrafish larvae at ages 3 dpf and nine dpf were evaluated for the relative mRNA expression of BDNF and VGLUT1. Hypoxic modelPrevious studies showed that zebrafish embryos are placed in an airtight room or a hypoxia chamber. The level of hypoxia can be adjusted by altering the oxygen concentration, achieving total hypoxia for a specific duration. In this process, zebrafish embryos are exposed to deficient levels of dissolved oxygen (less than 2 mg/l) in hypoxia chambers (Ariani et al., 2023a). In prior research, zebrafish embryos have been placed in hermetically sealed environments or hypoxia chambers, where the oxygen levels can be precisely controlled to induce complete hypoxia for specified durations. According to Ariani et al. (2023a), the embryos are exposed to low dissolved oxygen levels (below 2 mg/l) in these chambers during this procedure. In our study, nitrogen (N2) gas is introduced into the chamber to establish hypoxic conditions, achieving a partial oxygen pressure (PO2) of approximately 5 kPa, or 1.5 mg O2/l. Zebrafish larvae, ranging from 2 hours post-fertilization (hpf) to 3 dpf, undergo this hypoxia treatment before being returned to normoxic conditions (PO2 around 20 kPa or 8 mg O2/l) at 4 dpf. Biochemical assayFor the analysis of mRNA expression levels of VGLUT1 and BDNF, quantitative PCR (qPCR) was employed. This involved using a Fast RNA Tissue/Insect Kit from Zymo Research, Irvine, California, USA. The study processed twelve zebrafish larvae at 3 and 9 dpf for total RNA extraction, strictly following the manufacturer’s protocol. Subsequently, cDNA was synthesized from 100–200 ng of RNA from each sample using the LunaScript® RT Master Mix Kit from New England Biolabs, Ipswich, Massachusetts, USA, as Schweighardt et al. (Schweighardt et al., 2015) documented. Statistical analysis The data for BDNF and VGLUT are presented as mean ± standard deviation (SD) for each treatment group. Analysis was performed using GraphPad Prism 8 (La Jolla, California, USA). Statistical significance was determined using one-way analysis of variance (ANOVA). Significance levels were set at p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***), with p < 0.0001 (****) indicating significant, highly significant, and extremely significant differences, respectively. Ethical approvalAll experimental procedures involving animals in this study strictly conformed to the ethical guidelines established in the Declaration of Helsinki. Approval for the research was granted by the Health Research Ethical Committee of Universitas Brawijaya, Indonesia, as evidenced by Protocol number 179/EC/KEPK/07/2022. ResultsEffect of CA on BDNF expression in zebrafish embryos on oxygen-deficientThe research findings indicate a significant difference in the BDNF expression among the treatment groups (p < 0.001), as determined by a Two-way ANOVA (Fig. 1). According to the One-way ANOVA, there was no significant difference between treatment groups in zebrafish larvae at 3 dpf (p > 0.05); however, a trend towards increased average BDNF expression was observed in the control group (35.53) compared to the hypoxia group (34.66). The administration of CA in the hypoxia group led to a sequential increase in average BDNF expression: T1 (36.2), T2 (34.82), and T3 (36.19), compared to the group not receiving CA under OD conditions.
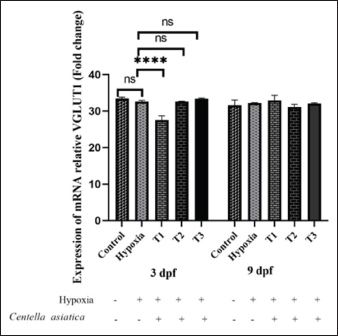
Fig. 1. BDNF expression of embryonic zebrafish at 3, and 9 dpf in the treatment groups. Data are shown as the mean ± SD. The administration of CA with varying concentrations under OD conditions showed a significant difference in BDNF expression (p < 0.001). Still, there was no difference in BDNF expression based on the duration of administration (3 dpf and 9 dpf). There was no interaction between the treatment and the duration of administration. * (p < 0.05), dan ** (p < 0.01). As per the one-way ANOVA, a significant difference was found between treatment groups in zebrafish larvae at 9 dpf ( p < 0.0001). The control group (36.27) showed a significant difference from the hypoxia group (35.33). The administration of CA under hypoxic conditions in zebrafish larvae indicated a tendency for increased average BDNF expression in groups T1 (35.92) and T2 (36.26), and a decrease in T3 (34.09), compared to the group without CA. The duration of CA administration did not affect BDNF expression (p > 0.05). There was no interaction between treatment and the age of zebrafish larvae (p > 0.05). Effect of CA on the VGLUT1 expression of zebrafish embryos under oxygen-deficientThe research findings demonstrate a significant difference in VGLUT1 expression in response to the administration of CA under OD conditions (p < 0.001), as determined by a Two-way ANOVA (Fig. 2). No significant difference was noted in VGLUT1 expression due to the variation in the ages of zebrafish larvae (p > 0.05). However, there was an interaction between the treatment and the age variation ( p < 0.0001). According to the One-way ANOVA, there were significant differences between treatment groups administering CA under OD conditions in 3 dpf zebrafish larvae (p > 0.05). There was an observed but not significant increase in VGLUT1 expression in the control group (33.49) versus the OD group (32.64). The introduction of CA in the hypoxia group decreased average VGLUT1 expression in treatment T1 (27.54), compared to the group not given CA under hypoxic conditions. Meanwhile, treatments T2 (32.65) and T3 (33.45) showed increased VGLUT1 expression after CA administration under OD conditions.
Fig. 2. VGLUT1 expression of embryonic zebrafish at 3, and 9 dpf in the CA treatment groups under OD conditions. There was no significant difference in VGLUT1 gene expression with the timing of CA administration (ages 3 dpf and 9 dpf), and an interaction occurred between the treatment and the timing of CA administration (p > 0.05). A significant difference in VGLUT1 gene expression was caused by the concentration of CA treatment (p < 0.05). The administration of CA at a concentration of 2.5 μg/ml. **** (p < 0.0001) dan ns (not significance). According to the One-way ANOVA, no significant difference existed between treatment groups in 9 dpf zebrafish larvae (p > 0.05). The control group (31.61) exhibited a significant difference compared to the hypoxia group (32.23). The administration of CA in OD conditions in zebrafish larvae showed a tendency for decreased average VGLUT expression in groups T2 (31.19) and T3 (32.08), and an increase in T1 (32.95) compared to the group without CA. DiscussionZebrafish have become a crucial model organism for studying hypoxic conditions due to their external fertilization, small size, rapid development, high reproductive output, and transparent larval bodies, facilitating easy observation (Üstündağ et al., 2019). Under OD conditions, oxygen diffusion from the gills to the blood is impaired, affecting respiratory efficiency (Pelster, 2021). In hypoxic conditions, zebrafish undergo a metabolic shift from aerobic to anaerobic processes, resulting in less efficient energy production and the generation of byproducts like lactic acid (Barrionuevo et al., 2010), which can lead to cellular damage. This change impacts the cells’ ability to synthesize proteins, including the BDNF. Oxygen deficiency can lead to oxidative stress, where there is an increase in the production of free radicals (Blokhina, 2003), potentially causing damage to cells and tissues, as well as affecting cellular structures and functions, including protein synthesis (Pizzino et al., 2017). CA administration was observed up to 9 dpf in this study. This is relevant considering the formation of yolk in zebrafish larvae begins between 8 and 10 hpf. The survival rate of embryos decreases drastically in the first 24 hours. It remains constant up to 72 hpf, followed by a decline at 96 hpf, suggesting that the extract causes early life-stage mortality (Tran et al., 2023). The neuroprotective role of CA can be observed in the expression of BDNF and VGLUT1. BDNF expression in the zebrafish embryo under oxygen-deficientHypoxia may cause disruptions in neuronal function, leading to a decrease in BDNF production. BDNF plays a role in various physiological processes such as neural development, synaptic plasticity, neurogenesis, nerve protection, learning, memory (Lucini et al., 2018), and mood regulation through its interaction with the tropomyosin receptor kinase B (TrkB). When BDNF binds to TrkB receptors, it activates three intracellular signaling pathways: ERK, PI3K/Akt, and phospholipase Cγ (PLCγ), which are crucial for mediating BDNF’s diverse functions (Correia et al., 2023). Research findings suggest that BDNF expression in healthy (control) zebrafish larvae is higher than in hypoxic conditions. Hypoxia in zebrafish larvae leads to decreased BDNF expression, aligning with the findings of Ariani et al., (2023b), which indicate that asiatic acid in CA can increase BDNF in intermittent OD conditions. Hypoxia can influence the expression of genes regulating BDNF production, such as the transcription factor hypoxia-inducible factor (HIF), which is activated by OD (Fuhrmann and Brüne, 2017). Mitochondrial dysfunction likely reduces the cell’s ability to synthesize BDNF, making it difficult for cells to develop, regenerate, and become more susceptible to damage (Pawlus et al., 2012). Thus, healthy conditions with adequate oxygenation support optimal neuronal function and BDNF synthesis. Administering CA at 1.25–2.5 µg/ml concentrations to zebrafish larvae increases BDNF expression. CA acts as an antioxidant against prooxidant threats entering the body. Antioxidants neutralize free radicals or oxidative stress, thereby protecting cells from damage. Oxidative stress can reduce BDNF expression, so antioxidants indirectly support the presence and function of BDNF in the brain. Hypoxia may cause disruptions in neuronal function, leading to a decrease in BDNF production. BDNF plays a role in various physiological processes such as neural development, synaptic plasticity, neurogenesis, nerve protection, learning, and memory (Shen et al., 2023). Increased BDNF in CA-treated zebrafish larvae may help maintain or enhance brain neuroplasticity and synaptic function, as well as maintain cellular integrity and BDNF function, aiding in the maintenance of plasticity (Wang et al., 2020). High BDNF expression may play a role in certain inflammatory conditions in the brain (Giacobbo et al., 2019; Porter and O’Connor, 2022). The administration of CA in zebrafish at 3 dpf did not significantly differ from that at 9 dpf in altering BDNF expression. This could be due to a stable or sufficient antioxidative capacity in zebrafish in both age groups, resulting in similar responses to antioxidants. Zebrafish at 3 and 9 dpf may have comparable levels of biological maturation (Singleman and Holtzman, 2014), enabling similar responses to antioxidants, particularly in the regulation of BDNF. VGLUT1 expression in zebrafish embryos under oxygen-deficientHypoxia influences the expression and activity of VGLUT1. Research findings indicate that oxygen-deficient increases VGLUT1 compared to healthy conditions (control group). This result is corroborated by Daniels et al., (2011), who reported that VGLUT1 levels rise in conditions of hypoxic injury, stress, methamphetamine treatment, and genetic seizure models. Oxidative stress often elevates the expression of transporters like VGLUT1 as a compensatory mechanism against damage. Hypoxia triggers the translocation of VGLUT1 to the plasma membrane, indicating an increase in VGLUT1 (Du et al., 2020). This adaptation aims to counteract oxygen deficiency and maintain glutamatergic neurotransmission function amidst hypoxic stress. Excitatory Amino Acid Transporter 2 (EAAT2/GLT-1) and VGLUT1 are primary mediators in the adult brain’s glutamate metabolism cycle. The reciprocal relationship between glutamate transporter dysfunction, impairment of the glutamatergic system, and cognitive decline has been established in neurodegenerative diseases (Du et al., 2020). Excessive VGLUT1, mainly due to hypoxic stimulation, can increase synaptic glutamate, potentially causing excitotoxicity—neuronal damage resulting from overactivation of glutamate receptors in the central nervous system. Excess extracellular glutamate can cause nerve damage (Xue et al., 2023). L-glutamate (Glu) transporters primarily transport Glu into synaptic vesicles through VGLUT1. A reduction in VGLUT1 can modulate nerve transmission efficiency and alter synaptic vesicle filling levels. This can potentially affect learning, memory, and long-term potentiation. In mouse models deficient in VGLUT1, a decrease in glutamatergic neurotransmission in cortical and hippocampal neurons has been associated with impairments in memory and learning (Lindström et al., 2020). Hypoxia triggers the activation of various transcription factors, notably HIF-1, which potentially affects the expression of genes, including VGLUT1 as an adaptive mechanism to low oxygen levels. The administration of CA in 3 dpf zebrafish under hypoxic conditions notably impacts VGLUT1 expression. In contrast, at 9 dpf, the difference in VGLUT1 expression is insignificant, though there is an overall decrease trend. This differential reduction in VGLUT1 expression could be due to various factors, including the varying sensitivities of brain cells to CA based on its concentration. Zebrafish at 3 dpf and 9 dpf exhibit different sensitivities to CA, potentially due to developmental differences in their brain cells at these ages. The immune system of zebrafish, closely resembling that of humans, starts maturing as early as 15 hpf. Blood circulation begins at 26 hpf, by which embryonic macrophages can phagocytize particles that produce reactive oxygen species and eliminate pathogens (Xie et al., 2021). Antioxidants may influence the signal transduction pathways involved in the regulation of VGLUT1 expression. Under hypoxic conditions, antioxidants could modify the activity of certain transcription factors involved in VGLUT1 expression, such as HIF-1. This interaction suggests a complex interplay between hypoxia, antioxidant response, and the regulation of key genes in zebrafish development (Xie et al., 2021). The administration of CA under OD conditions can decrease neurotoxicity or oxidative stress. This may reflect the neurons’ efforts to maintain synaptic functionality under stress conditions. BDNF can influence both the expression and function of VGLUT1. Under normal conditions, BDNF supports synaptic plasticity and modulates VGLUT1 activity. Antioxidants can reduce the oxidative stress caused by OD, ultimately affecting the expression of VGLUT1 and BDNF. By protecting against damage caused by OD, antioxidants may help preserve neurotransmission functions and neuroplasticity regulated by BDNF and VGLUT1. Antioxidants and their influence on VGLUT1 and BDNF in hypoxic zebrafish larvaeAntioxidants can influence signal transduction pathways involved in regulating the expression of VGLUT1. In hypoxic environments, antioxidants may modify the activity of specific transcription factors crucial for expressing VGLUT1, such as HIF-1. The administration of CA under hypoxic conditions has been observed to decrease VGLUT1 and enhance BDNF expression. This could have significant implications for synaptic functionality and neuronal plasticity. It may reflect the neurons’ adaptive efforts to maintain synaptic function under stress. BDNF, a key player in neural processes, can affect both the expression and function of VGLUT1. Under typical conditions, BDNF supports synaptic plasticity and modulates VGLUT1 activity. Antioxidants can mitigate oxidative stress caused by diminished OD, ultimately impacting the expression of both VGLUT1 and BDNF. By shielding against damage from oxygen shortage, antioxidants are pivotal in preserving neurotransmission functions and neuroplasticity governed by BDNF and VGLUT1. Research by Ariani et al., (2023a), indicates that CA enhances the growth and development of hypoxia-induced zebrafish larvae, showcasing its beneficial role in neural development under hypoxic stress. ConclusionThis study reveals the significant role of CA in modulating genetic expression under oxygen-deficient conditions, particularly in the context of neurobiological expressions of BDNF and VGLUT1. Administering a concentration of 2.5 μg/ml of CA leads to increased BDNF and decreased expression of VGLUT, highlighting its potential as a therapeutic agent in managing neurobiological responses to hypoxic stress. AcknowledgmentsThe Department of Pharmacology, Medical Faculty, Universitas Brawijaya supported this study. FundingNone. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsAA, HK: concept and design of the research; AR, AS, ASD: acquisition of data, analysis, and interpretation; critical revision and final approval: AR, HK, AS, ASD. Data availabilityAll data are provided in the manuscript. ReferencesAriani, A., Ghofar, I., Khotimah, H., Nurdiana, N. and Rahayu, M. (2023a). Asiatic acid in Centella asiatica extract towards morphological development in an intermittent hypoxia intrauterine embryo model and molecular prediction pathway of insulin-like growth factor-1 (IGF-1) receptor signalling. Open Vet. J. 13(5), 629. Ariani, A., Husnul, K., Nurdiana, N. and Masruroh, R. (2023b). Asiatic acid increased locomotor and head width through inducing brain-derived neurotrophic factor in intrauterine hypoxia-exposed zebrafish. Open Vet. J. 13(10), 1326. Avdesh, A., Chen, M., Martin-Iverson, M.T., Mondal, A., Ong, D., Rainey-Smith, S., Taddei, K., Lardelli, M., Groth, D.M., Verdile, G. and Martins, R.N. (2012). Regular care and maintenance of a Zebrafish (Danio rerio) laboratory: an introduction. J. Vis. Exp. 69, 4196. Barrionuevo, W., Fernandes, M. and Rocha, O. (2010). Aerobic and anaerobic metabolism for the zebrafish, Danio rerio, reared under normoxic and hypoxic conditions and exposed to acute hypoxia during development. Braz. J. Biol. 70(2), 425–434. Bathina, S. and Das, U.N. (2015). Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 6, 1164–1178. Bazzari, A.H. and Bazzari, F.H. (2022). BDNF therapeutic mechanisms in neuropsychiatric disorders. Int. J. Mol. Sci. 23(15), 8417. Blokhina, O. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 91(2), 179–194. Chen, P.S., Chiu, W.T., Hsu, P.L., Lin, S.C., Peng, I.C., Wang, C.Y. and Tsai, S.J. (2020). Pathophysiological implications of hypoxia in human diseases. J. Biomed. Sci. 27(1), 63. Correia, A.S., Cardoso, A. and Vale, N. (2023). BDNF unveiled: exploring its role in major depression disorder serotonergic imbalance and associated stress conditions. Pharmaceutics 15(8), 2081. Daniels, R.W., Miller, B.R. and DiAntonio, A. (2011). Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol. Dis. 41(2), 415–420. Du, X., Li, J., Li, M., Yang, X., Qi, Z., Xu, B., Liu, W., Xu, Z. and Deng, Y. (2020). Research progress on the role of type I vesicular glutamate transporter (VGLUT1) in nervous system diseases. Cell Biosci. 10(1), 26. Fuhrmann, D.C. and Brüne, B. (2017). Mitochondrial composition and function under the control of hypoxia. Redox. Biol. 12, 208–215. Hambali, A., Kumar, J., Hashim, N.F.M., Maniam, S., Mehat, M.Z., Cheema, M.S., Mustapha, M., Adenan, M.I., Stanslas, J. and Hamid, H.A. (2021). Hypoxia-induced neuroinflammation in alzheimer’s disease: potential neuroprotective effects of Centella asiatica. Front. Physiol. 12, 712317. Lee, H.S. and Jeong, G.S. (2021). Protective effects of 6,7,4′-Trihydroxyflavanone on hypoxia-induced neurotoxicity by enhancement of HO-1 through Nrf2 signaling pathway. Antioxidants 10(3), 341. Lima Giacobbo, B., Doorduin, J., Klein, H.C., Dierckx, R.A.J.O., Bromberg, E. and De Vries, E.F.J. (2019). Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol. Neurobiol. 56(5), 3295–3312. Lindström, S.H., Sundberg, S.C., Larsson, M., Andersson, F.K., Broman, J. and Granseth, B. (2020). VGluT1 deficiency impairs visual attention and reduces the dynamic range of short-term plasticity at corticothalamic synapses. Cereb. Cortex. 30(3), 1813–1829. Lucini, C., D’Angelo, L., Cacialli, P., Palladino, A. and De Girolamo, P. (2018). BDNF, brain, and regeneration: insights from Zebrafish. Int. J. Mol. Sci. 19(10), 3155. Pawlus, M.R., Wang, L., Ware, K. and Hu, C.J. (2012). Upstream stimulatory factor 2 and hypoxia-inducible factor 2 cooperatively activate HIF2 target genes during hypoxia. Mol. Cell. Biol. 32(22), 4595–4610. Pelster, B. (2021). Using the swimbladder as a respiratory organ and/or a buoyancy structure—Benefits and consequences. J. Exp. Zool. Part Ecol. Integr. Physiol. 335(9–10), 831–842. Pietrancosta, N., Djibo, M., Daumas, S., El Mestikawy, S. and Erickson, J.D. (2020). Molecular, structural, functional, and pharmacological sites for vesicular glutamate transporter regulation. Mol. Neurobiol. 57(7), 3118–3142. Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., Squadrito, F., Altavilla, D. and Bitto, A. (2017). Oxidative stress: harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 1–13. Porter, G.A. and O’Connor, J.C. (2022). Brain-derived neurotrophic factor and inflammation in depression: pathogenic partners in crime? World J. Psychiatry. 12(1), 77–97. Schweighardt, A.J., Tate, C.M., Scott, K.A., Harper, K.A. and Robertson, J.M. (2015). Evaluation of commercial kits for dual extraction of DNA and RNA from human body fluids. J. Forensic Sci. 60(1), 157–165. Shen, R., Ardianto, C., Celia, C., Sidharta, V.M., Sasmita, P.K., Satriotomo, I. and Turana, Y. (2023). Brain-derived neurotrophic factor interplay with oxidative stress: neuropathology approach in potential biomarker of Alzheimer’s disease. Dement. Neuropsychol. 17, e20230012. Singleman, C. and Holtzman, N.G. (2014). Growth and maturation in the Zebrafish, Danio Rerio : a staging tool for teaching and research. Zebrafish 11(4), 396–406. Sun, B., Wu, L., Wu, Y., Zhang, C., Qin, L., Hayashi, M., Kudo, M., Gao, M. and Liu, T. (2020). Therapeutic potential of Centella asiatica and its triterpenes: a review. Front. Pharmacol. 11, 568032. Sydykov, A., Mamazhakypov, A., Maripov, A., Kosanovic, D., Weissmann, N., Ghofrani, H.A., Sarybaev, A.Sh. and Schermuly, R.T. (2021). Pulmonary hypertension in acute and chronic high altitude maladaptation disorders. Int. J. Environ. Res. Public Health 18(4), 1692. Tran, M.H., Nguyen, T.V.A., Do, H.G., Kieu, T.K., Nguyen, T.K.T., Le, H.D., Guerrero-Limon, G., Massoz, L., Nivelle, R., Zappia, J., Pham, H.T., Nguyen, L.T. and Muller, M. (2023). Testing biological actions of medicinal plants from northern Vietnam on zebrafish embryos and larvae: Developmental, behavioral, and putative therapeutical effects. PLoS One 18 (11), e0294048. Üstündağ, Ü.V., Çalıskan-Ak, E., Ateş, P.S., Ünal, İ., Eğilmezer, G., Yiğitbaşı, T., Ata Alturfan, A. and Emekli-Alturfan, E. (2019). White LED Light exposure inhibits the development and xanthophore pigmentation of Zebrafish embryo. Sci. Rep. 9(1), 10810. Wang, D., Li, H., Du, X., Zhou, J., Yuan, L., Ren, H., Yang, X., Zhang, G. and Chen, X. (2020). Circulating brain-derived neurotrophic factor, antioxidant enzymes activities, and mitochondrial DNA in bipolar disorder: an exploratory report. Front. Psychiatry. 11, 514658. Wang, Y., Wang, H., Zhao, P., Cheng, J., Gong, W. and Zhang, J. (2021). Asiatic acid exerts neuroprotective effect against hypoxicischemic brain injury in neonatal rats via inhibition of oxidative damage. Trop. J. Pharm. Res. 20(9), 1903–1908. Wilson, N.R., Kang, J., Hueske, E.V., Leung, T., Varoqui, H., Murnick, J.G., Erickson, J.D. and Liu, G. (2005). Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J. Neurosci. 25(26), 6221–6234. Xie, Y., Meijer, A.H. and Schaaf, M.J.M. (2021). Modeling inflammation in Zebrafish for the development of anti-inflammatory drugs. Front. Cell Dev. Biol. 8, 620984. Xue, S., Shen, T., Li, M., Leng, B., Yao, R., Gao, Y., Sun, H., Li, Z. and Zhang, J. (2023). Neuronal glutamate transporters are associated with cognitive impairment in obstructive sleep apnea patients without dementia. Neurosci. Lett. 802, 137168. | ||
| How to Cite this Article |
| Pubmed Style Ariani A, Khotimah H, Sulistyarini A, Daniaridevi AS. Centella asiatica extract ameliorates deoxygenation-induced neurological dysfunction in zebrafish larvae. Open Vet. J.. 2024; 14(5): 1154-1160. doi:10.5455/OVJ.2024.v14.i5.9 Web Style Ariani A, Khotimah H, Sulistyarini A, Daniaridevi AS. Centella asiatica extract ameliorates deoxygenation-induced neurological dysfunction in zebrafish larvae. https://www.openveterinaryjournal.com/?mno=188192 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i5.9 AMA (American Medical Association) Style Ariani A, Khotimah H, Sulistyarini A, Daniaridevi AS. Centella asiatica extract ameliorates deoxygenation-induced neurological dysfunction in zebrafish larvae. Open Vet. J.. 2024; 14(5): 1154-1160. doi:10.5455/OVJ.2024.v14.i5.9 Vancouver/ICMJE Style Ariani A, Khotimah H, Sulistyarini A, Daniaridevi AS. Centella asiatica extract ameliorates deoxygenation-induced neurological dysfunction in zebrafish larvae. Open Vet. J.. (2024), [cited January 25, 2026]; 14(5): 1154-1160. doi:10.5455/OVJ.2024.v14.i5.9 Harvard Style Ariani, A., Khotimah, . H., Sulistyarini, . A. & Daniaridevi, . A. S. (2024) Centella asiatica extract ameliorates deoxygenation-induced neurological dysfunction in zebrafish larvae. Open Vet. J., 14 (5), 1154-1160. doi:10.5455/OVJ.2024.v14.i5.9 Turabian Style Ariani, Ariani, Husnul Khotimah, Arum Sulistyarini, and Araisa Sabrina Daniaridevi. 2024. Centella asiatica extract ameliorates deoxygenation-induced neurological dysfunction in zebrafish larvae. Open Veterinary Journal, 14 (5), 1154-1160. doi:10.5455/OVJ.2024.v14.i5.9 Chicago Style Ariani, Ariani, Husnul Khotimah, Arum Sulistyarini, and Araisa Sabrina Daniaridevi. "Centella asiatica extract ameliorates deoxygenation-induced neurological dysfunction in zebrafish larvae." Open Veterinary Journal 14 (2024), 1154-1160. doi:10.5455/OVJ.2024.v14.i5.9 MLA (The Modern Language Association) Style Ariani, Ariani, Husnul Khotimah, Arum Sulistyarini, and Araisa Sabrina Daniaridevi. "Centella asiatica extract ameliorates deoxygenation-induced neurological dysfunction in zebrafish larvae." Open Veterinary Journal 14.5 (2024), 1154-1160. Print. doi:10.5455/OVJ.2024.v14.i5.9 APA (American Psychological Association) Style Ariani, A., Khotimah, . H., Sulistyarini, . A. & Daniaridevi, . A. S. (2024) Centella asiatica extract ameliorates deoxygenation-induced neurological dysfunction in zebrafish larvae. Open Veterinary Journal, 14 (5), 1154-1160. doi:10.5455/OVJ.2024.v14.i5.9 |