| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 571-576 Original Research Prevalence and antibiogram of shigatoxinproducing E. coli in camel meat and offalSamah Ahmad Elkady1, Wageh Sobhy Darwish1*, Ahmad E. Tharwat1, Mahmoud A. Said2, Dalia E. ElAtriby3, Marwa Magdy Seliem4, Ahmed E. Alfifi5, Waleed R. El-Ghareeb1,5, Lamiaa M. Reda6 and Tamer M. Gad71Food Hygiene, Safety, and Technology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Medical Administration, Students' Hospital, Zagazig University, Zagazig, Egypt 3Infection Control Unit in Specialized Internal Medicine Hospital at Mansoura University, Mansoura, Egypt 4Department of Zoonoses, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 5Department of Public Health, College of Veterinary Medicine, King Faisal University, Al Hofuf, Saudi Arabia 6Educational Veterinary Hospital, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt 7Educational Veterinary Hospital, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Wageh Sobhy Darwish. Food Hygiene, Safety, and Technology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: wagehdarwish [at] gmail.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
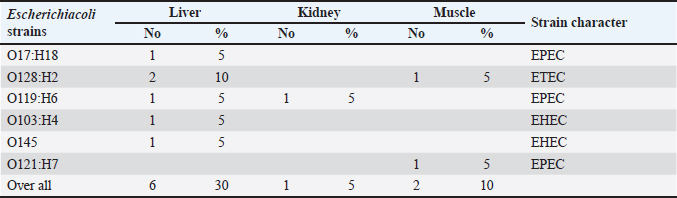
AbstractBackground: Camels are important animals in Egypt and other Arab countries on the basis of their economic value and ethnic culture. Escherichia coli is implicated in several gastrointestinal infections and outbreaks worldwide, especially in developing countries. It causes infections that might lead to death. Numerous biological activities, such as antioxidative, antibacterial, anti-diabetic, anti-mutagenic, anti-inflammatory, neuroprotective, and diuretic, are associated with coriander and coriander essential oils. Aim: This work targeted investigation of the prevalence, antibiogram, and occurrence of virulence genes of E. coli in camel meat liver and kidney. Besides, the anti-E. coli activity of coriander oil was further examined. Methods: Camel meat, liver, and kidneys were collected from local markets in Egypt. Isolation and identification of E. coli were performed. The antimicrobial susceptibility of the obtained E. coli isolates was screened using the disk diffusion assay. To detect the presence of virulence-associated genes (stx1, stx2, eaeA, and hylA gens), polymerase chain reaction was used. An experimental trial was done to investigate the anti-E. coli activity of coriander oil. Results: The obtained results revealed isolation of the following E. coli pathotypes: O17:H18, O128:H2, O119:H6, O103:H4, O145:H-, and O121:H7. The recovered E. coli isolates practiced multidrug resistance profiling with higher resistance toward Erythromycin, Nalidixic Acid, Clindamycin, and Ampicillin. However, the isolates were sensitive to Meropenem and cefoxitin. The recovered isolates had expressed different virulence attributes. Coriander oil of 2% could significantly reduce E. coli O128 count in camel meat by 65%. Conclusion: Therefore, strict hygienic measures are highly recommended during the processing of camel meat. The use of coriander oil during camel meat processing is highly recommended to reduce E. coli count. Keywords: Camel meat, Edible offal, E. coli, Antibiotic resistance, Shiga-toxin. IntroductionIn many regions of the world, camel meat, liver, and kidney are regarded as excellent sources of essential amino acids. That is also rich in protein and unsaturated fatty acids, and it may be crucial in helping to reduce food-related issues (Alao et al., 2017). Many people believe that the meat of the camel is similar to that of sheep and cattle since it has lower fat and cholesterol content while having comparable protein content (Kadim et al., 2008; Darwish et al., 2010). Without a doubt, the majority of the microbial contamination of carcasses happens during the handling and slaughter procedures, including distribution, evisceration, skinning, and preparation (Edris et al., 2013). The bacterial count of meat is increased by every procedure performed on it from the time of slaughter until it is fit for human consumption, including transportation and carcass preparation (Ali, 1992). Meat and edible offal have a higher microbial burden due in part to their high protein content and moisture content. The animal's physiological state, overall health, body temperature, and other storage circumstances all affect the microbiological quality of meat (Nychas et al., 2008). The animal itself (skin and excrement), the handler's hands and clothes, raw ingredients, cleaning water, gathering containers, and equipment are some of the many sources of microbial contamination of camel meat (Darwish et al., 2018; Morshdy et al., 2023). The potential for foodborne microbes to contaminate camel meat is one major public health issue (Ma et al. 2023). Human infections with drug-resistant bacteria can lead to serious health problems (Morshdy et al., 2021). The Gram-positive rod Escherichia coli (E. coli), most domestic and wild animals' gastrointestinal tracts are naturally home to Enterobacteriaceae; however, certain serotypes can seriously damage humans through food (Hassan et al., 2014; CDC 2018). According to Kagambèga et al. (2011), the existence of E. coli indicates the presence of fecal contamination. Less emphasis has been paid to the frequency of E. coli in edible offal and camel meat. Coriander (Coriandrum sativum L.) is mostly cultivated in the Indian subcontinent, Europe, some places of Northern Africa, and Asia for its medicinal applications. Its herbage, which is sometimes referred to as cilantro, is a basic ingredient in many dishes. For instance, ground coriander seeds are used in making the curry powders. Essential oil that can be extracted from coriander fruits, is the main flavoring agent in a wide range of dishes and candies. Coriander and coriander essential oils have a wide range of biological activities such as antioxidant, antibacterial, anti-diabetic, anxiolytic, anti-mutagenic, anti-inflammatory, neuroprotective, and diuretic properties (Silva and Domingues, 2017). The present work was set out to determine the antibiogram and prevalence of shigatoxigenic E. coli in camel meat and edible offal. Second, the anti-E. coli activity of coriander's oil was examined. Materials and MethodsSample collectionWe gathered 60 muscle, liver, and kidney samples from randomly selected camels. Samples were collected immediately following the slaughter at Zagazig's main slaughterhouse. Every animal was healthy, active, and disease-free. Samples were placed in sterile plastic bags and moved to the laboratory under aseptic conditions and assessed quickly. The collected samples were analyzed bacteriologically to isolate E. coli. Sample preparationSpecifically, 25 g of each camel meat sample was homogenized in 225 ml of sterilized buffered peptone water (0.1%) for 2 minutes at 2,500 rpm to yield a homogenate of 1/10 dilutions. The mixture was used to prepare decimal serial dilutions (ISO, 2013). The prepared samples went through the following examinations. Testing for enteropathogenic E. coliOne milliliter of the original dilution was transferred to MacConkey broth tubes having inverted Durham tubes. Inoculated tubes were left in the incubator at 37°C for 24 hours. Then, transfer 1 ml of the positive tube to MacConkey broth enrichment tubes and incubate at 44°C for a day. Loops from positively enriched broth tubes were streaked over eosin methylene blue agar media and incubated at 37°C for 24 hours. The putative colonies were green metallic chine-colored. Suspect colonies were isolated and cultivated on the slopes of nutrient agar for further identification (MacFaddin, 2000). The confirmed E. coli isolates were serologically identified using fast diagnostic E. coli antisera sets (DENKA SEIKEN Co., Japan), as described by Kok et al. 1996. Antibiotic resistance of isolated E. coliThe recovered E. coli strains were further evaluated for their antimicrobial susceptibility using the disk diffusion method described by Mary and Usha (2013). The used sensitivity discs were obtained from Oxoid Limited (Basingstoke, Hampshire, UK). Antibiogram was done in accordance with the established procedures (NCCLS, 2001). The selected antimicrobials were based on the most regularly used antimicrobials in livestock farms. Multiplex polymerase chain reaction (PCR) for the stx1, stx2, eaeA, and hylA genesGenomic DNA was isolated using the Gene JET Genomic DNA Purification Kit (Fermentas), as reported by Sambrook et al. (1989). The purified DNA was immediately used in the downstream application or kept at −20°C. Multiplex PCR was used to identify Shiga toxins (stx1 and stx2), intimin (eaeA), and haemolysin (hylA) in E. coli using specific primers (Pharmacia Biotech) and cycling conditions for stx1 and stx2 according to Dhanashree and Mallya (2008), eaeA according to Mazaheri et al. (2014), and hylaA according to Fratamico et al. (1995). The amplified DNA fragments were analyzed using 2% agarose gel electrophoresis (Applichem, Germany, GmbH) in 1× TBE buffer, stained with ethedium bromide, collected, and seen under a UV transilluminator. The fragment size was evaluated with a 100-bp plus DNA ladder (Qiagen, Germany GmbH). Anti-E. coli activity of coriander oilCamel meat free from E. coli was minced to produce meat balls (n=15 meat ball each is 50 g). The meat balls were divided into three groups each group consisted of five meat balls. Such meat balls were inoculated with E. coli O128:H2 at 6 log cfu/g. Group 1 was immersed in corn oil for 30 minutes. Group 2 was immersed in coriander oil 1% in corn oil for 30 minutes, and group 3 was immersed in coriander oil 2% in corn oil for 30 minutes. After that, the E. coli count was isolated and counted on Tryptic Soya Agar (Oxoid). Sensory characteristics and reduction rates were evaluated according to (Bourdoux et al., 2018). Statistical analysisIn the experimental reduction trial, E. coli counts were compared among treatment groups, and statistical significance was estimated using Dennett's test, (JMP, SAS Institute, Cary, NC), where p < 0.05 is considered significant. Ethical approvalNot needed for this study. ResultsThe presented results in Table 1 illustrated the serological identification of E. coli which was detected in the examined samples of muscles, liver, and kidneys of camels. The identified E. coli serotypes were O17:H18, O128:H2, O119:H6, O103:H4, O145, O121:H7. The prevalence rates in the tested tissues were 30%, 10%, and 5% in the examined liver, muscle, and kidney, respectively. Escherichia coli O128:H2 was the most prevalent serotype in the ex amined samples of camels. Table 1. Incidence and serotyping of E. coli isolated from examined samples of meat, liver, and kidney of camel (n = 20).
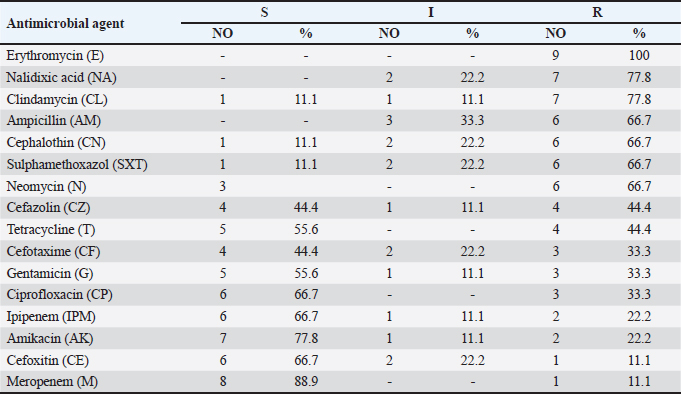
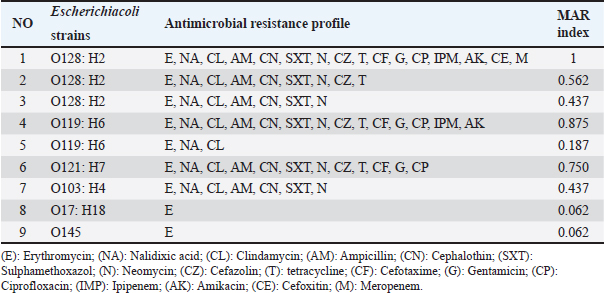
The recovered E. coli serotypes varied in their antimicrobial susceptibility as all isolates were resistant to erythromycin (100%), while showing resistance to nalidixic acid and clindamycin at 77.8% for each. The lowest resistance was recorded to cefoxitin, and meropenem at 11.1% (Table 2). Escherichia coli O28:H2 had a range for MAR index that varied from 0.437 to 1, while that of O119:H6 varied from 0.187 to 0.875, O121:H7 (0.750), O103:H4 (0.437), O17:H18 (0.062), and O145 (0.062), respectively (Table 3). Table 2. Antimicrobial susceptibility of E. coli strains.
Table 3. Antimicrobial resistance profile of E. coli strains.
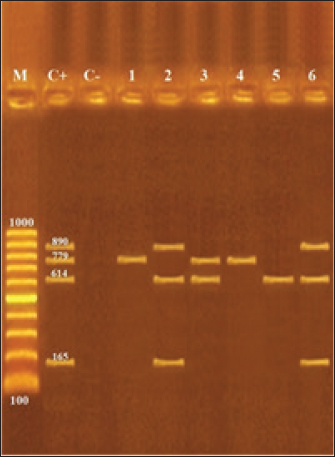
Figure 1 shows the detection of the Shiga toxin and virulence-coding genes in the recovered E. coli isolates. Where stx1 was detected in E. coli O103:H4, O119:H6, O128:H2. While stx2 was detected in O17:H18, o119:H6, and O121:H7. EaeA was detected in O103:H4 and O145. The coding gene for hylA was detected in O103:H4 and 0145.
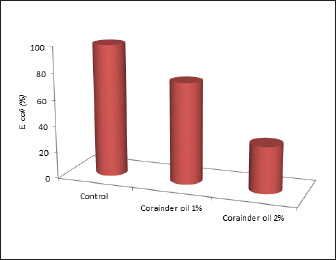
Fig. 1. Multiplex PCR of stx1 (614 bp), stx2 (779 bp), eaeA (890 bp), and hlyA (165 bp) virulence genes in the recovered E. coli isolates from camel meat, liver, and kidney. In a reduction of E. coli in the camel meat, coriander oil 1%–2% could reduce E. coli count by 24%, and 65%, respectively, with no alterations on the sensory characteristics as presented in Figure 2.
Fig. 2. Anti-E. coli effect of coriander oil. DiscussionCamel meat is an important source of animal-derived protein in several countries, particularly in the Arabian Peninsula, and Egypt. However, camel meat can be a source of foodborne pathogens. In the current study, camel meat and offal were considered as potential sources of enterotoxigenic E. coli. Likely, Tegegne et al. (2019) isolated Staphylococcus aureus and E. coli from camel meat at retail shops in Jigjiga city, Ethiopia. Other pathogens were also isolated from camel meat and offal such as Campylobacter, Enterococcus, and S. aureus as reported by Gwida et al. (2019) in Mansoura city, Egypt. Multidrug-resistant Listeria monocytogenes was also isolated from camel meat in Saudi Arabia and Egypt (Yehia et al., 2020). In Egypt, E. coli O26, O86, O111, O124, and O128 were isolated from camel meat slaughtered in Elbagour, Menouf, and Shibin Elkom slaughter houses at a prevalence rate of 30%, 20%, and 15%, respectively (Edris et al., 2013). Multidrug-resistant E. coli was also isolated from camel meat in Mansoura city (Sallam et al., 2023). Multiple drug-resistant E. coli was recovered in the current study. This could be attributed to the abuse of antimicrobials in livestock production in Egypt. Similarly, antimicrobial resistance was observed in several foodborne pathogens isolated from meat-producing animals such as camels in Egypt and other Middle East countries as reported by Rahimi et al. (2010), El-Ghareeb et al. (2019), El-Ghareeb et al. (2020), Yehia et al. (2020), Alsayeqh et al. (2021), Morshdy et al. (2023), Sallam et al. (2023). Enterotoxigenic E. coli was identified in the current study. Escherichia coli is responsible for many cases of hospitalization worldwide. The symptoms of E. coli infection include fever, abdominal pain, diarrhea, and dehydration (CDC, 2018). Coriander demonstrated clear and strong anti-E. coli action in the current study. This could be linked to its high concentration of bioactive compounds including flavonoids and carotenoids, which are renowned for their antibacterial and antioxidant properties. In keeping with the current study's findings, Scazzocchio et al. (2017) found that coriander had potent inhibitory effects against multidrug-resistant uropathogenic E. coli. Furthermore, Bourdoux et al. (2018) found that coriander, whether fresh or freeze dried, effectively inactivated E. coli O157:H7, Salmonella spp., and L. monocytogenes. ConclusionThe findings of this study revealed inadequate sanitary procedures during the handling of camel meat and edible offal at Egypt's slaughter houses and local markets, resulting in E. coli contamination of meat and offal. As a result, careful hygiene standards must be followed when processing camel meat and offal. Coriander oil at 2% is highly recommended for controlling E. coli infection of camel meat and offal. Conflict of interestThe authors have no conflicts of interest. FundingNot relevant. Authors contributionsAll authors contributed to this study. All authors read and approved the final manuscript. Data availabilityData will be available upon request. ReferencesAlao, B.O., Falowo, A.B., Chulayo, A. and Muchenje, V. 2017. The potential of animal by-products in food systems: production, prospects and challenges. Sustainability 9, 1089. Ali, M.H.I. 1992. Hygienic quality of mutton carcasses in Sharkia province. M.V.Sc. Faculty of Veterinary Medicine Zagazig University, Zagazig, Egypt. Alsayeqh, A.F., Baz, A.H.A. and Darwish, W.S. 2021. Antimicrobial-resistant foodborne pathogens in the Middle East: a systematic review. Environ. Sci. Pollut. Res. 28, 68111–68133. Bourdoux, S., Rajkovic, A., De Sutter, S., Vermeulen, A., Spilimbergo, S., Zambon, A., Hofland, G., Uyttendaele, M. and Devlieghere, F. 2018. Inactivation of Salmonella, Listeria monocytogenes and Escherichia coli O157: H7 inoculated on coriander by freeze-drying and supercritical CO2 drying. Innov. Food. Sci. Emerg. Technol. 47, 180–186. CDC. 2018. Escherichia coli. CDC National Center for Emerging and Zoonotic Infectious Diseases. Available via https://www.cdc.gov/ecoli/index.html (Accessed 20 February 2022). Darwish, W.S., Atia, A.S., Reda, L.M., Elhelaly, A.E., Thompson, L.A. and Saad Eldin, W.F. 2018. Chicken giblets and wastewater samples as possible sources of methicillin-resistant Staphylococcus aureus: prevalence, enterotoxin production, and antibiotic susceptibility. J. Food. Saf. 38, 12478. Darwish, W.S., Morshdy, A.E., Ikenaka, Y., Ibrahim, Z.S., Fujita, S. and Ishizuka, M. 2010. Expression and sequence of CYP1A1 in the camel. J. Vet. Med. Sci. 72, 221–224. Dhanashree, B. and Mallya, S. 2008. Detection of shiga-toxigenic Escherichia coli (STEC) in diarrhoeagenic stool and meat samples in Mangalore, India. Indian. J. Med. Res. 128, 271–277. Edris, A., Hassan, M., Shaltout, F. and El-Hosseny, S. 2013. Detection of E. coli and Salmonella organisms in cattle and camel meat. Benha. Vet. Med. J. 25, 198–204. El-Ghareeb, W.R., Abdel-Raheem, S.M., Al-Marri, T.M., Alaql, F.A. and Fayez, M.M. 2020. Isolation and identification of extended spectrum β-lactamases (ESBLs) Escherichia coli from minced camel meat in Eastern province, Saudi Arabia. Thai. J. Vet. Med. 50, 155–161. El-Ghareeb, W.R., Mulla, Z.S., Meligy, A.M.A., Darwish, W.S. and Edris, A.M. 2019. Antibiotic residue levels in camel, cattle and sheep tissues using LC-MS/MS method. J. Anim. Plant. Sci. 29, 4. Fratamico, P., Sackitey, S., Wiedmann, M. and Deng, M. 1995. Detection of Escherichia coli O157:H7 by multiplex PCR. J. Clin. Microbiol. 33, 2188–2191. Gwida, M., Zakaria, A., El-Sherbiny, H., Elkenany, R. and Elsayed, M. 2019. Prevalence of Campylobacter, Enterococcus and Staphylococcus aureus in slaughtered camels. Vet. Med. 64, 521–530. Hassan, J., Parvej, M.S., Rahman, M.B., Khan, M.S.R., Rahman, M.T., Kamal, T. and Nazir, K.H.M.N.H. 2014. Prevalence and characterization of Escherichia coli from rectal swab of apparently healthy cattle in Mymensingh, Bangladesh. Microbes. Health. 3, 12–14. ISO, 6887-1. 2013. Microbiology of food and animal feeding stuffs: preparation of test sample, initial suspension and decimal dilutions for microbiological examination. Geneva, Switzerland: ISO. Kadim, I., Mahgoub, O. and Purchas, R. 2008. A review of the growth, and of the carcass and meat quality characteristics of the one-humped camel (Camelus dromedaries). Meat. Sci. 80, 555–569. Kagambèga, A., Martikainen, O., Lienemann, T., Siitonen, A., Traoré, A.S., Barro, N. and Haukka, K. 2011. Diarrheagenic Escherichia coli detected by 16-plex PCR in raw meat and beef intestines sold at local markets in Ouagadougou, Burkina Faso. Int. J. Food. Microbiol. 153, 154–158. Kok, T., Worswich, D. and Gowans, E. 1996. Some serological techniques for microbial and viral infections. In Practical medical microbiology, 14th ed. Eds., Collee, J., Fraser, A., Marmion, B. and Simmons, A. Edinburgh, UK: Churchill Livingstone. Ma, J.K., Alsayeqh, A.F., El-Ghareeb, W.R., Elhelaly, A.E., Seliem, M.M., Darwish, W.S. and Eissa Abdallah, K.M. 2023. Squab And Quail Meats: microbial status and prevalence of multidrug-resistant shiga toxin-producing E. Coli. Slov. Vet. Res. 60, 317–326. MacFaddin, J.F. 2000. Biochemical tests for identification medical bacteria. Baltimore, MD: Warery Press. Mary Josephine, M., Usha, R. and Maria Victorial Rani, S. 2013. Current status of seaweed diversity and their seasonal availability at Hare Island, Gulf of Mannar. Sci. Res. Rep. 3, 146–151. Mazaheri, S., Ahrabi, S. and Aslani, M., 2014. Shiga toxin-producing Escherichia coli isolated from Lettuce samples in Tehran, Iran. Jundishapur. J. Microbiol. 7(11), 1–6. Morshdy, A.E.M., Hussein, M.A., Merwad, A.M., El Lawendy, H.M., Mohamed, A.H. and Saber, T., 2021. Impact of natural and chemical agents on quality and biogenic amine formation of chilled camel minced meat. Slov. Vet. Res. 58, 389–401. Morshdy, A.E.M., Mehrez, S.M., Tharwat, A.E., Abdallah, K.M., Darwish, W., Nabawy, E.E. and Ali, E.S.M. 2023. Hygienic status of the carcass surfaces of cattle, buffalo, sheep, and camel carcasses and their contact surfaces. J. Adv. Vet. Res. 13, 1294–1298. NCCLS. 2001. Performance standards for antimicrobial susceptibility testing. Supplement M100-S11. Villanova, PA: NCCLS. Nychas, G.J.E., Skandamis, P.N., Tassou, C.C. and Koutsoumanis, K.P. 2008. Meat spoilage during distribution. J. Meat. Sci. 78, 77–89. Rahimi, E., Ameri, M. and Kazemeini, H.R. 2010. Prevalence and antimicrobial resistance of Campylobacter species isolated from raw camel, beef, lamb, and goat meat in Iran. Foodborne. Pathog. Dis. 7, 443–447. Sallam, K.I., Abd-Elrazik, Y., Raslan, M.T., Imre, K., Morar, A., Herman, V. and Zaher, H.A. 2023. Cefotaxime-, ciprofloxacin-, and extensively drug-resistant Escherichia coli O157: H7 and O55: H7 in camel meat. Foods 12, 1443. Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular cloning: laboratory manual, 2nd ed. New York, NY: Cold spring Harbor. Scazzocchio, F., Mondì, L., Ammendolia, M.G., Goldoni, P., Comanducci, A., Marazzato, M., Conte, M.P., Rinaldi, F., Crestoni, M.E., Fraschetti, C. and Longhi, C. 2017. Coriander (Coriandrum sativum) essential oil: effect on multidrug resistant uropathogenic Escherichia coli. Nat. Prod. Commun.12, 1934578X1701200438. Tegegne, H.A., Berhanu, A., Getachew, Y., Serda, B., Nölkes, D., Tilahun, S. and Sibhat, B. 2019. Microbiological safety and hygienic quality of camel meat at abattoir and retail houses in Jigjiga city, Ethiopia. J. Infect. Devel. Countr. 13, 188–194. Yehia, H.M., Elkhadragy, M.F., Aljahani, A.H. and Alarjani, K.M. 2020. Prevalence and antibiotic resistance of Listeria monocytogenes in camel meat. Biosci. Rep. 40, 20201062. | ||
| How to Cite this Article |
| Pubmed Style Elkady SA, Darwish WS, Tharwat AE, Said MA, Elatriby DE, Seliem MM, Alfifi AE, El-ghareeb WR, Reda LM, Gad TM. Prevalence and antibiogram of shigatoxin-producing E. coli in camel meat and offal. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 571-576. doi:10.5455/OVJ.2024.v14.i1.52 Web Style Elkady SA, Darwish WS, Tharwat AE, Said MA, Elatriby DE, Seliem MM, Alfifi AE, El-ghareeb WR, Reda LM, Gad TM. Prevalence and antibiogram of shigatoxin-producing E. coli in camel meat and offal. https://www.openveterinaryjournal.com/?mno=188382 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i1.52 AMA (American Medical Association) Style Elkady SA, Darwish WS, Tharwat AE, Said MA, Elatriby DE, Seliem MM, Alfifi AE, El-ghareeb WR, Reda LM, Gad TM. Prevalence and antibiogram of shigatoxin-producing E. coli in camel meat and offal. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 571-576. doi:10.5455/OVJ.2024.v14.i1.52 Vancouver/ICMJE Style Elkady SA, Darwish WS, Tharwat AE, Said MA, Elatriby DE, Seliem MM, Alfifi AE, El-ghareeb WR, Reda LM, Gad TM. Prevalence and antibiogram of shigatoxin-producing E. coli in camel meat and offal. Open Vet. J.. (2024), [cited January 25, 2026]; 14((1) (Zagazig Veterinary Conference)): 571-576. doi:10.5455/OVJ.2024.v14.i1.52 Harvard Style Elkady, S. A., Darwish, . W. S., Tharwat, . A. E., Said, . M. A., Elatriby, . D. E., Seliem, . M. M., Alfifi, . A. E., El-ghareeb, . W. R., Reda, . L. M. & Gad, . T. M. (2024) Prevalence and antibiogram of shigatoxin-producing E. coli in camel meat and offal. Open Vet. J., 14 ((1) (Zagazig Veterinary Conference)), 571-576. doi:10.5455/OVJ.2024.v14.i1.52 Turabian Style Elkady, Samah A., Wageh S. Darwish, Ahmad E. Tharwat, Mahmoud A. Said, Dalia E. Elatriby, Marwa M. Seliem, Ahmed E. Alfifi, Waleed R. El-ghareeb, Lamiaa M. Reda, and Tamer M. Gad. 2024. Prevalence and antibiogram of shigatoxin-producing E. coli in camel meat and offal. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 571-576. doi:10.5455/OVJ.2024.v14.i1.52 Chicago Style Elkady, Samah A., Wageh S. Darwish, Ahmad E. Tharwat, Mahmoud A. Said, Dalia E. Elatriby, Marwa M. Seliem, Ahmed E. Alfifi, Waleed R. El-ghareeb, Lamiaa M. Reda, and Tamer M. Gad. "Prevalence and antibiogram of shigatoxin-producing E. coli in camel meat and offal." Open Veterinary Journal 14 (2024), 571-576. doi:10.5455/OVJ.2024.v14.i1.52 MLA (The Modern Language Association) Style Elkady, Samah A., Wageh S. Darwish, Ahmad E. Tharwat, Mahmoud A. Said, Dalia E. Elatriby, Marwa M. Seliem, Ahmed E. Alfifi, Waleed R. El-ghareeb, Lamiaa M. Reda, and Tamer M. Gad. "Prevalence and antibiogram of shigatoxin-producing E. coli in camel meat and offal." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 571-576. Print. doi:10.5455/OVJ.2024.v14.i1.52 APA (American Psychological Association) Style Elkady, S. A., Darwish, . W. S., Tharwat, . A. E., Said, . M. A., Elatriby, . D. E., Seliem, . M. M., Alfifi, . A. E., El-ghareeb, . W. R., Reda, . L. M. & Gad, . T. M. (2024) Prevalence and antibiogram of shigatoxin-producing E. coli in camel meat and offal. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 571-576. doi:10.5455/OVJ.2024.v14.i1.52 |