| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 594-603 Original Research Comparison of controlling capabilities of lactobionic acid, nisin, and K-Sorbate against dairy-borne Staphylococcus aureus, Escherichia coli, and mold in soft cheeseSalah F. Abd-Elaal1, Mohamed E.A. Alnakip1, Madiha Z. Youssef2 and Mohamed A. Bayoumi1*1Department of Food Hygiene, Safety and Technology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Department of Food Control, Faculty of Veterinary Medicine, Al`Arish University, Al`Arish, Egypt *Corresponding Author: Mohamed A. Bayoumi. Department of Food Hygiene, Safety and Technology, Faculty of Veterinary Medicine, Zagazig University, Egypt. Email: mbayoumi [at] vet.zu.edu.eg Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
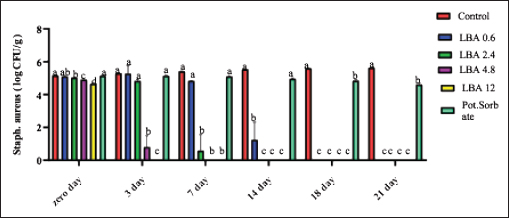
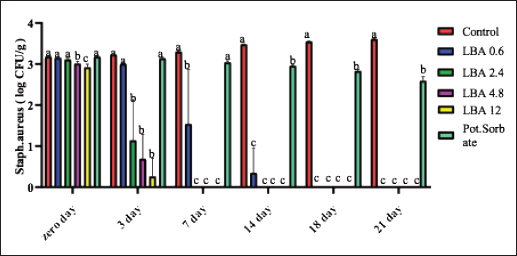
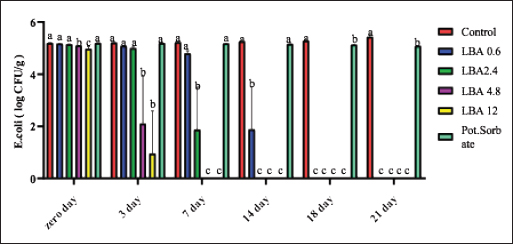
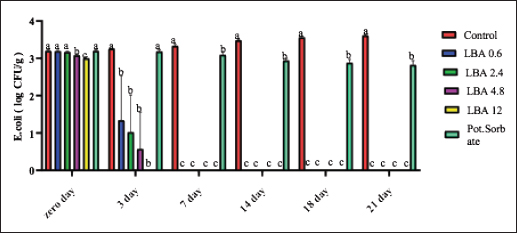
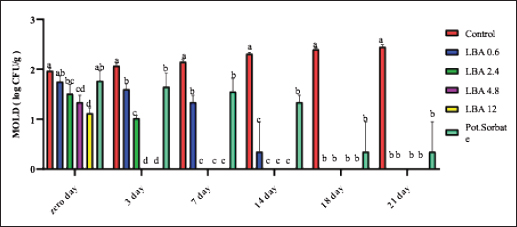
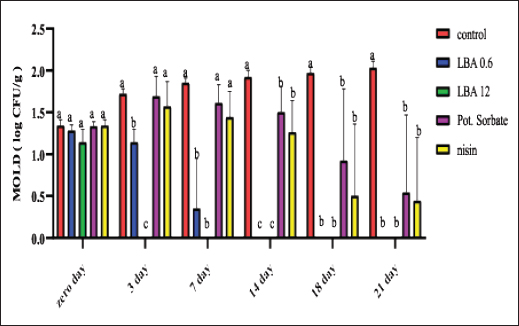
ABSTRACTBackground: The utilization of chemical preservatives holds the promise of effectively controlling microbial growth in soft cheese. Aim: The first trial aimed to compare the effectiveness of lactobionic acid (LBA) and K-Sorbate in controlling the proliferation of Staphylococcus aureus, Escherichia coli, and mold in white soft cheese. The subsequent part of the study explored the inhibitory effects of K-Sorbate, nisin, and LBA on mold populations in cheese whey. Methods: Two sets of soft cheese were produced. One set was contaminated with S. aureus, while the other was with E. coli, each at concentrations of 1 log CFU/ml and 1 log CFU/100 ml. Different concentrations of LBA were incorporated into these sets of cheese. Similar cheese samples were treated with K-Sorbate. For the subsequent part of the study, it was manufactured and divided into groups that inoculated with LBA with different concentrations, K-Sorbate, and nisin. Results: With higher S. aureus inoculation, by day 18, the positive control exhibited growth exceeding 5 log CFU/g. In contrast, the LBA treatment dropped below limit of detection (LOD) and K-Sorbate yielded 4.8 log CFU/g. While with lower S. aureus inoculation, the positive control reached log CFU/g, while LBA treatment fell below LOD by day 14, and K-Sorbate reached 2.9 log CFU/g. For E. coli inoculation, with higher concentrations, by day 18, the positive control exceeded 5 log CFU/g. Conversely, LBA treatment greatly decreased and K-Sorbate treatment measured 5.1 log CFU/g. With lower E. coli concentrations, the positive control surpassed 3 log CFU/g, yet LBA treatment dropped below LOD by day 3. Mold counts indicated some inhibition with the K-Sorbate treatment, while control groups showed growth. LBA treatments exhibit noticeable growth inhibition. About the other part of the study, the outcomes demonstrated that while growth of mold occurred in the control group, inhibitory effects were apparent in the treatment groups, and significant distinctions existed between K-Sorbate, nisin, LBA treatments, and the control group. Conclusion: Our findings suggest that LBA has the potential to effectively control the growth of E. coli, S. aureus, and mold in soft cheese. Moreover, LBA displays greater preservative efficacy compared to K-Sorbate and nisin. Keywords: LBA, K-Sorbate, Nisin, Unripened soft cheese. IntroductionEvery year, foodborne bacterial pathogens result in around 3.6 million cases of sickness, 35,000 hospitalizations, and 860 deaths in the United States (Scallan et al., 2011). Notably, dairy products were responsible for 18% of bacterial diseases that occurred between 1998 and 2008 (Painter et al., 2013). Escherichia coli and Staphylococcus aureus were among the predominant pathogens associated with dairy products outbreaks (Lu and Wang, 2017). The occurrence of these pathogens in milk products can be traced back to flawed pasteurization, contamination within the processing surroundings, equipments, and insufficient hygiene practices among individuals involved in food handling (Schon et al., 2016). Staphylococcus aureus stands as a prevalent foodborne pathogen (Aydina et al., 2011; Crago et al., 2012), frequently implicated in foodborne illness outbreaks linked to contaminated dairy products (Johler et al., 2015; Silva et al., 2020). These dairy products often contain staphylococcal enterotoxins (SEs) that were previously produced by S. aureus within the food (Hennekinne et al., 2012). Various studies have identified the presence of these SE in animal-derived foods, particularly dairy products (Abreu et al., 2021; Candido et al., 2020). Escherichia coli is responsible for causing several instances of diarrheal outbreaks in both children and adults following the consumption of milk and dairy products contaminated with this bacterium. Multiple studies have highlighted that around 1%–5% of foodborne infections are linked to the consumption of milk and dairy products, with a substantial 53% of such cases attributed to contaminated cheese (Schrade and Yager, 2001). EPEC strains have been identified as contributors to foodborne illnesses in humans, with a notable impact on causing infantile diarrhea, especially in developing nations (Silva et al., 2001). Different strains of EPEC trigger varying degrees of infection, and research has underscored the dynamic nature of these strains, as their genetic makeup gradually resembles that of hemorrhagic strains (Campos et al., 1994). Molds are frequent culprits behind the deterioration of dairy products, including cheese. In fermented dairy products, molds can flourish even in acidic conditions, leading to noticeable alterations in color, odor, and taste on the surface (Lu and Wang, 2017). The molds that invade cheese have the capacity to produce mycotoxins, which could pose health hazards. These mycotoxins hold the potential to induce severe poisoning and toxic consequences, referred to as acute and chronic mycotoxicosis (Sengun, 2008). Unripened soft cheese, a variety of cheeses formed through rennet coagulation, has inherent attributes such as elevated moisture content, decreased salt levels, and a nearly neutral pH, which renders it prone to spoilage from foodborne microbial contaminants (Flynn et al., 2021). The microbial attributes of soft cheeses can be impacted by diverse elements. These factors encompass the milk’s quality, the application of heat treatment, different technological parameters, and the degree or kinds of microbial contamination occurring during the production and storage of the cheese (Lutfiye and Ekrem, 2008). Establishing a standardized product in terms of both composition and microbial quality is still challenging due to the variety of microorganisms that might potentially contaminate the cheese during its manufacture (Temelli et al., 2006) and storage (Callon et al., 2008). K-Sorbate, a chemical preservative, has been the subject of extensive research due to its ability to inhibit the growth and survival of various microorganisms. These studies have demonstrated its effectiveness in preserving quality, preventing mold formation, and improving the visual aspects of various cheese varieties (Aworh and Egounlety, 1985; Lück, 1990; Aly, 1996). Nisin, a natural preservative, is widely used in various food products, particularly in dairy and meat items. This bacteriocin is effective against pathogenic bacteria causing foodborne illnesses and numerous other Gram-positive microorganisms responsible for food spoilage. Nisin can be applied on its own or in conjunction with other preservatives or physical treatments (Adem et al., 2016). Derived from a food-grade bacterium through fermentation, nisin’s safety and effectiveness have led to its global widespread adoption and application (Norman and Sandine, 1994). LBA is a promising food ingredient that possesses not only preservative properties but also nutritional benefits. LBA demonstrates the ability to safeguard and improve the qualities of food products through its antioxidant, chelating, acidifying, antimicrobial, texturizing, and prebiotic attributes (Ribeiro et al., 2016). The thermal characteristics of LBA, coupled with its elevated solubility, make it conducive for diverse applications in the food industry. In addition, LBA is a naturally occurring component found in milk and dairy products and exhibits antimicrobial properties (Kang et al., 2020). Several studies have explored the evaluation of both chemical and microbiological characteristics in white cheese (Nizamlioglu et al., 1989; Litopoulou and Tzanetakis, 1992; Tzanetakis et al., 1995; Volikakis et al., 2004). However, a gap exists in detailed research concerning the utilization of LBA to evaluate its impact on the chemical and microbiological attributes of unripened soft cheese. This study aims to investigate how LBA, K-Sorbate, and nisin, influence the chemical and microbiological parameters of unripened soft cheese. Materials and MethodsBacterial strainsTwo reference strains were brought from the Reference Lab for Safety Analysis of Food of Animal Origin, Animal Health Research Institute. The two strains corresponded to S. aureus (ATCC6538) and an isolate of E. coli (ATCC25922). All strains were preserved post-recovery or reactivation by freezing in Eppendorf tubes at −80°C. The tubes contained a mixture of equal proportions of brain heart infusion (BHI) broth and glycerol. Bacterial growth conditionsBefore conducting bacteriological investigations, samples were prepared and serial dilutions were performed following standard precautions. In summary, each isolate was streaked onto BHI agar (Beckton, Dickinson & Company, Franklin Lakes, NJ, USA) and then incubated at 37°C for 24 hours. An individual colony from each strain was employed to inoculate 5 ml of BHI broth. The broth cultures were then incubated at 37°C until reaching an approximate concentration with an optical density (OD) of 1.00 (~9 log CFU/mL). Preparation of wheyIn the laboratory, whey was produced using pasteurized water, NaCl 6%, CaCl2 0.05%, and citric acid salt to adjust pH to 6.1–6.2 and later, subjected to filter sterilization within a 0.20 µm surfactant-free cellulose acetate filter (Corning Inc., Corning, NY, USA). The prepared whey was poured into five containers: 1st one: control negative which contained the prepared whey without any additives, 2nd one: whey contained LBA with a concentration of 0.6 g/l, 3rd one: whey contained LBA with a concentration of 12 g/l, 4th one: whey contained K-Sorbate with concentration of 1 g/l and finally 5th one: whey contained nisin with concentration of 0.3 g/l. Cheese makingUnripened soft cheese was produced following the processing method outlined earlier by Barbaros (1999), with slight modifications. Raw milk was initially pasteurized at 72°C for 15 s (HTST). CaCl2 (0.02%) was added to restore the calcium balance following the heating process and NaCl (3.5%–4%) was added. Milk was cooled to 40oC in the water bath. Milk was poured into cheese vats which contained the previously prepared inoculum of each of S. aureus and E. coli separately at a final concentration of approximately 1 log CFU/ml and 1 CFU/100 ml. Milk and inoculum were thoroughly mixed. Either LBA (Syngars Technology©) or K-Sorbate were added to each inoculated treatment. Two distinct experiments were conducted, one utilizing the LBA treatment and the other the K-Sorbate treatment. For the LBA treatment, varying concentrations were introduced into the milk, specifically (0.6 g/l), (1.2 g/l), (2.4 g/l), and (12 g/l). K-Sorbate was added to the milk to attain a final concentration of 1 g/l. A positive control which contained the inoculum without LBA or K-Sorbate, and a negative control which lacked LBA or K-Sorbate were included in all experiments. Top of FormIn addition, samples with only LBA or K-Sorbate without inoculum were used for organoleptic examination and for investigation of mold and yeast. Rennet was added to each vat and mixed well, preserving unripened soft cheese samples at 0°C–4°C. For each of these LBA experiments, batches of cheese were produced in triplicate to assess the pH using a pH meter. On another level, cheese was produced by the same method and cut into similar cubes then placed in the previously prepared whey containers for investigation of the effect of LBA, K-Sorbate, and nisin on yeast and mold. Samples for microbiological analysis of samplesFor each trial, cheese samples were subjected to microbiological investigations using the same method. S. aureus, E. coli, yeast, and mold counts were enumerated on days 0, 3, 7, 14, 18, and 21 of storage. In this sense, each sample was introduced aseptically into a Whirl-Pak filter bag (Nasco, Fort Atkinson, WI), and a 1:10 (w/v) dilution was carried out using PBS. The samples underwent digestion at a regular speed for 60 seconds using a Seward Stomacher 400 Lab Blender Series (VWR International, Solon, OH). Subsequently, the digested samples were further diluted in 9 ml PBS blanks to achieve the desired dilution, followed by vortexing, followed by spreading on Baired Parker agar for detection of S. aureus, EMB for detection of E. coli, and malt extract agar for counting of yeast and mold. Each experiment was incubated at its specific temperature and time, and then enumerated. Every experiment was conducted in triplicate at a minimum. pH evaluationThe pH of each sample was assessed using a pH meter (Crison pH meter 507) both before and after the application of treatments. Sensory (organoleptic) evaluationFifty volunteers were selected based on the criteria proposed by Hashim (2002), which included an age range between 18 and 34 years and being regular consumers of unripened soft cheese. These individuals were tasked with evaluating the acceptability of the cheese in terms of color, appearance, aroma, taste, texture, and overall acceptability, utilizing a 7-point hedonic scale (1=dislike extremely, 2=dislike moderately, 3=dislike slightly, 4=neither like nor dislike, 5=like slightly, 6=like moderately, and 7=like extremely). Various cheese samples, including negative control, positive control, those treated only with LBA or K-Sorbate, and treated cheese samples, were provided to the volunteers both 24 hours and 21 days after manufacturing. Statistical analysisStatistical analyses were performed using XL-STAT version 2013.2.03 software (Addinsoft, Paris, France). The data were subjected to ANOVA to determine possible differences between treatments. Significant differences were compared using the Tukey test at the p <0.05 level. Ethical approvalNot needed for this study. ResultsThe results suggested that the LBA is enough to prevent S. aureus growth at different concentrations in unripened soft cheese and LBA treatment is more effective than K-Sorbate treatment. At S. aureus inoculation levels of 1 log CFU/ml, all LBA treatments showed more growth inhibition than K-Sorbate treatment and the control (Fig. 1). When S. aureus was inoculated into the milk at a concentration of 1log CFU/100 ml, the final cheese control concentration was approximately 3.1 CFU/g on day 0 of storage (Fig. 2). At E. coli inoculation levels of 1 log CFU/ml, all LBA treatments showed lesser growth of E. coli than K-Sorbate treatment and the control (Fig. 3). When E. coli was inoculated into the milk at a concentration of 1 log CFU/100 ml, the final cheese control concentration was approximately 3.2 CFU/g on day 0 of storage (Fig. 4). Figure 5 shows the impact of LBA and K-Sorbate Application on mold Outgrowth. Figure 6 shows the impact of LBA, K-Sorbate, and nisin applied into the whey (in which cheese was preserved) on mold outgrowth.
Fig. 1. Staphylococcus aureus counts presented as log10 CFU/g as it was inoculated at 1 log CFU/ml.
Fig. 2. Staphylococcus aureus counts presented as log10 CFU/g as it was inoculated at 1 log CFU/100 ml.
Fig. 3. Escherichia coli counts presented as log10 CFU/g as it was inoculated at 1 log CFU/ml.
Fig. 4. Escherichia coli counts presented as log10 CFU/g as it was inoculated at 1 log CFU/100 ml.
Fig. 5. Impact of LBA and K-Sorbate application on mold outgrowth.
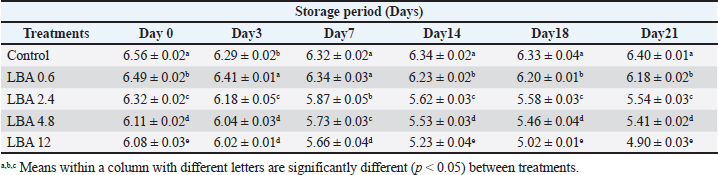
Fig. 6. Impact of LBA, K-Sorbate, and nisin applied into the whey (in which cheese was preserved) on mold outgrowth. Table 1. pH (±SD) of unripened soft cheese treated with lactobionic acid during storage at 6°C.
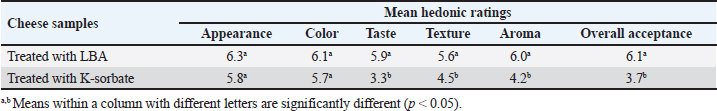
Table 2. Mean hedonic ratings for appearance, color, taste, aroma, and overall acceptance of examined samples.
The observed pH drop was attributed to the addition of LBA to milk during processing (Table 1). Table 2 showed that cheese samples treated with LBA had the highest ratings for taste (5.9), aroma (6.0), and overall acceptance (6.1) which were significantly higher compared to cheese samples treated with K-Sorbate (3.7). DiscussionImpact of LBA and K-Sorbate application during the cheese-making process on S. aureus growthDifferent concentrations of 0.6, 2.4, 4.8, and 12 g/l of LBA and 1 g/l K-Sorbate were introduced into the milk during the cheese-making process to assess their effectiveness against the growth of S. aureus in unripened soft cheese. S. aureus inoculum has been incorporated into the cheese to achieve a concentration of 1 and 1 log CFU/100 ml, representing different levels of contamination. S. aureus has the potential to contaminate bulk tank milk samples through non-hygienic practices related to cow udder handling or milking equipment (Golafrouz et al., 2020). Once introduced, it can proliferate during the cheese-making process. The objective of this experiment was to evaluate the antimicrobial impact of LBA and K-Sorbate on S. aureus contamination that might occur during cheese making. At S. aureus inoculation levels of 1 log CFU/ml, all LBA treatments showed more growth inhibition than K-Sorbate treatment and the control (Fig. 1). The final cheese control concentration was approximately 5.1 CFU/g on day 0 of storage. On day 0, there was no significant difference in S. aureus counts between the LBA 0.6 g/l and K-Sorbate treatments and the control, while there were significant differences (p < 0.05) between 2.4, 4.8, and 12 g/l LBA treatments and the control. However, a significant difference (p < 0.05) between the 0.6 g/l LBA treatment group and the control was observed starting on day 14 of storage and continued throughout the entire trial. On days 14, 18, and 21, the control grew to above 5 log CFU/g, while all LBA treatment groups fell below the limit of detection (LOD) and K-Sorbate treatment group was 4.9 log CFU/g. When S. aureus was inoculated into the milk at a concentration of 1log CFU/100 ml, the final cheese control concentration was approximately 3.1 CFU/g on day 0 of storage (Fig. 2). On day 0, there was no significant difference in S. aureus counts between the LBA 0.6, 2.4 g/l, K-Sorbate treatments, and the control, while there were significant differences (p < 0.05) between 4.8 and 12 g/l LBA treatments and the control. However, significant differences ( < 0.05) between the 0.6, 2.4, 4.8, and 12 g/l LBA treatment groups and the control were observed starting on day 7 of storage and continued throughout the entire trial. All LBA treatments inhibited S. aureus growth throughout the entirety of the trial while K-Sorbate showed some inhibition at a level less than LBA treatments. Robach and Stateler (1980) documented the antimicrobial activity of K-Sorbate against S. aureus. It is noteworthy that according to Davidson et al. (1981), the activity of sorbate is heightened at lower pH levels, a characteristic that mirrors the food model used in this study. Impact of LBA and K-Sorbate application during the cheese-making process on E. coli outgrowthAt E. coli inoculation levels of 1 log CFU/ml, all LBA treatments showed lesser growth of E. coli than the K-Sorbate treatment and the control (Fig. 3). The final cheese control E. coli count was approximately 5.2 CFU/g on day 0 of storage. On day 0, there was no significant difference in E. coli counts between the LBA 0.6, 2.4g/l, and K-Sorbate treatments and the control, while there were significant differences (p < 0.05) between 4.8 and 12 g/l LBA treatments and other groups. However, significant differences (p < 0.05) between the 2.4, 4.8, and 12 g/l LBA treatment groups and the control were observed starting at day 7 of storage and continued till the end of the trial, while significant differences between the K-Sorbate treatment and the control was observed at day 18. On day 1, the control grew to above 5 log CFU/g, while all LBA treatment groups fell below the limit of detection, and K-Sorbate treatment group was 5.1 log CFU/g. When E. coli was inoculated into the milk at a concentration of 1 log CFU/100 ml, the final cheese control concentration was approximately 3.2 CFU/g on day 0 of storage (Fig. 4). On day 0, there was no significant difference in E. coli counts between the LBA 0.6, 2.4 g/l, K-Sorbate treatments, and the control, while there was a significant difference (p < 0.05) between 4.8, 12 g/l LBA treatments and the control. However, significant differences between the 0.6, 2.4, 4.8, and 12 g/l LBA treatment groups and the control were observed starting on day 3 of storage and continued till the end of the trial. All LBA treatments inhibited E. coli growth throughout the entirety of the trial while K-Sorbate showed lower inhibition. Different Previous studies investigated and proved the effects of K-Sorbate in some dairy products including white cheese, kashar cheese, and yogurt (Doğruer et al., 1996). In a study carried out on cheese, it was noted that K-Sorbate at 500 mg/kg concentration resulted in decreased coliform bacterial counts (Ozdemir and Demirci 2006). Impact of LBA and K-Sorbate application during the cheese-making process on mold outgrowthAt day 0, there was no significant difference in mold counts between the LBA 0.6 g/l, K-Sorbate treatments, and the control, while there were significant differences (p < 0.05) between 2.4, 4.8, and 12 g/l LBA treatments and the control. Significant differences between the LBA 0.6 g/l, K-Sorbate treatments, and the control started on day 3 of storage and continued till the end of the trial. Numerous studies have explored the antimicrobial properties and applications of K-Sorbate in inhibiting the growth and survival of various microorganisms. This substance has been found to extend the shelf life of different cheeses, prevent mold formation, and enhance their overall appearance. Notable research in this area includes studies conducted by Aworh and Egounlety (1985), Lück (1990), and Aly (1996). Impact of LBA, K-Sorbate, and nisin applied into the whey (in which cheese was preserved) on yeast and mold growthYeast and mold are prevalent causes of the spoilage of dairy products such as cheese and yogurt. In fermented dairy products with acidic conditions, molds can thrive, leading to noticeable alterations in surface color, odor, and flavor, as observed in studies by Lu and Wang (2017). Penicillium is frequently identified as a spoilage genus of mold, according to Garnier et al. (2017). It has been detected in refrigerated vacuum-sealed cheese, as reported by Lu and Wang (2017). Certain strains of Penicillium have demonstrated resistance to sorbate, a common preservative in cheese, and possess the ability to metabolize it. This metabolic activity results in the production of undesirable off-odors and off-flavors, as documented by Garnier et al. (2017). In our study, four containers of whey contained LBA 0.6 g/l, LBA 12 g/l, K-Sorbate, and nisin, and the 5th container contained whey without any treatment (negative control) was tested. Cheese samples were placed in five containers and examined for yeast and mold growth for several days. There was no significant difference between all treatment groups and the negative control at day 0. At day 3 LBA 0.6 g/L and LBA 12 g/l showed significant inhibition (p < 0.05) in comparison to the negative control sample and continued throughout the entire trial. K-Sorbate and nisin started to show significant differences from the negative control on day 14. These results assured that LBA treatment is more powerful and effective than other chemical preservatives such as K-Sorbate and nisin against mold growth. Impact of LBA Addition to cheese on pHThere was a drop in the pH throughout the entire trial within LBA groups. By the trial’s conclusion, the pH in the treatment groups was 0.22, 0.86, 0.99, and 1.5 pH units lower than the control for the 0.6, 2.4, 4.8, and 12 g/l treatments, respectively. Notably, in this cheese-making process, no starter cultures were introduced. The observed pH drop was attributed to the addition of LBA to milk during processing (Table 1). LBA is composed of one galactose molecule linked to one molecule of gluconic acid through an ether-like linkage. The use of LBA has been explored in the dairy industry as a key ingredient in innovative dairy technologies (Alonso et al., 2013). Previous studies have indicated that LBA exhibits antimicrobial effects against both Gram-negative and Gram-positive bacteria (Kang et al., 2020). In our study, the pH of the 12 g/L LBA treatment decreased from 5.66 to 4.90 (from day 7 to day 21), and S. aureus, E. coli, and mold counts dropped below the LOD in both challenge studies. When the pH reached 6.18 on day 21 of storage, the 0.6 g/l LBA treatment also fell below the LOD. Hence, it is plausible that the pH reduction attributed to the presence of LBA played a role in controlling the growth of S. aureus, E. coli, and mold. Top of FormImpact of LBA Addition to cheese on its sensory attributesSensory characteristics are of great importance for consumer acceptability of dairy products. Previous studies (in the Americas and Europe) on bovine cheeses obtained from milk from cows fed different dietary polyunsaturated fatty acids (PUFA) sources, such as extruded soybeans (Khanal et al., 2005), extruded linseeds (Sympoura et al., 2009), fish and sunflower oils (Jones et al., 2005), fish and soybean oils (Lynch et al., 2005), rapeseed oil (Ryhänen et al., 2005), and calcium salts of palm and fish oil in combination with soybean products (Allred et al., 2006), have reported minor effects on the sensory properties of cheese. In our study, sensory analysis was performed to determine consumer perception of treated unripened soft cheese. Table 2 showed that cheese samples treated with LBA had the highest ratings for taste (5.9), aroma (6.0), and overall acceptance (6.1) which were significantly higher compared to cheese samples which treated with K-Sorbate (3.7). Therefore, although a pH drop is present in our study, there could be a potential to use LBA as an alternative preservative method in further studies to reduce spoilage in cheese as well as its application to reduce pathogenic growth. Previous research has indicated that extended exposure to LBA leads to notable decreases in pH in fluid milk, as demonstrated by Lara-Aguilar and Alcaine (2019). However, it is worth noting that the sensory impact may be less pronounced in low-pH dairy products, topical or spray applications, or scenarios where the LBA can be removed or inactivated. In Japan, a product called Caspian Sea yogurt that includes LBA is consumed without known regulatory restrictions (Kiryu et al., 2012). In addition, there are two Japanese patents involving LBA: one as a flavor enhancer in fruit preparations and another in cheese preparations containing lactose oxidase (leading to the production of LBA). The latter was subjected to sensory evaluation, specifically regarding the attribute of hardness. Top of FormIn summary, our findings propose a range of opportunities for further exploration into the utilization of LBA for managing microbial components in dairy products, calling for additional research in this domain. ConclusionLBA was found to be an effective preservative with both a high (1 log CFU/ml) and a low (1 log CFU/100 ml) inoculum during the cheese-making process. The organoleptic evaluation was made and showed no change in taste, odor, or color for LBA treatment while there was some bitter taste with K-Sorbate. Then, we explored the efficacy of LBA, K-Sorbate, and nisin by adding them to the whey in which the cheese was preserved and examined their effect on mold outgrowth, and the results showed that LBA is a more effective antimicrobial agent than K-Sorbate and nisin. From this study, we assured that LBA is a great preservative that can be used in dairy industries without affecting the chemical or physical properties of the product. AcknowledgmentsThe authors would like to thank Eng. Esam Kamel for his technical support. Conflict of interestThe authors declare that they have no competing interests. FundingThe study did not receive any external funds. Author contributionsThe concept and design of the study: M.A.B, S.F.A. Collection and processing of material: M.A.B, M.Z.Y. Statistical processing: M.E.A, M.Z.Y. Text writing: M.A.B, M.E.A, M.Z.Y. Critical revision: M.A.B, S.F.A, M.E.A. Data availabilityAll data are provided in the manuscript. Any extra data needed can be provided on reasonable request from the corresponding author. ReferencesAbreu, A.C., Matos, L.G., Cândido, T.J., Barboza, G.R., Souza, V.V.M., Nuñez, K.V.M. and Silva, N.C.C. 2021. Antimicrobial resistance of Staphylococcus spp. isolated from organic and conventional Minas Frescal cheese producers in the state of São Paulo―Brazil. J. Dairy Sci. 104, 4012–4022. Adem, G., Nadia, O., Catherine, J. and Pascal, D. 2016. Nisin as a food preservative: part 1: physicochemical properties, antimicrobial activity, and main uses. Critical Rev. Food Sci. Nutrition 56, 1262–1274. Allred, S.L., Dhiman, T.R., Brennand, C.P., Khanal, R.C., McMahon, D.. and Luchini, N.D. 2006. Milk and cheese from cows fed calcium salts of palm and fish oil alone or in combination with soybean products. J. Dairy Sci. 89, 234–248. Alonso, S., Rendueles, M. and Díaz, M. 2013. Bio-production of lactobionic acid: current status, applications and future prospects. Biotechnol. Adv. 31, 1275–1291. Aly, M.E. 1996. Prolongation of the keeping quality of mozzarella cheese by treatment with sorbate. Nahrung Abstr. 40, 194–200. Aworh, O.C., and Egounlety, M. 1985. Preservation of West African soft cheese by chemical treatment. J. Dairy Res. 52, 187. Aydina, A., Sudagidanb, M. and Muratoglu, K. 2011. Prevalence of staphylococcal enterotoxins, toxin genes and genetic-relatedness offoodborne Staphylococcus aureus strains isolated in the Marmara Region of Turkey. Inter. J. Food Microbiol. 148, 99–106. Barbaros, H.Ö. 1999. CHEESE|microflora of white-brined cheeses. Encyclopedia Food Microbiol. 397–403. Callon, C., Gilbert, F.B., Cremoux, R.D. and Montel, M.C. 2008. Application of variable number of tandem repeat analysis to determine the origin of S. aureus contamination from milk to cheese in goat cheese farms. Food Control 19, 143–150. Campos, L.C., Whitam, T.S., Gomes, T.A.T., Andrad, J.R.C. and Trabulsi, I.R. 1994. Escherichia coli serogroup O111 includes several clones of diarrhagenic strains with different virulence properties. Infec. Immun. 62, 3282–3288. Candido, T.J., Silva, A.C., Matos, L.G., Nascimento, M., Camargo, C.H., Zanella, R.C., Rall, V.L.M., Nathália Cristina Cirone Silva, N.C.C. 2020. Enterotoxigenic potential and molecular typing of Staphylococcus sp. isolated from organic and conventional fresh minas cheese in the state of São Paulo, Brazil. Inter. Dairy J. 102, 1–8. Crago, B., Ferrato, C., Drews, S.J., Tyrrell, G. and Louie, M. 2012. Prevalence of Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) in food samples associated with foodborne illness in Alberta, Canada from 2007 to 2010. J. Food Microbiol. 32, 202–205. Davidson, P.M., Brekke, C.J. and Branen, A.L. 1981. Antimicrobial activity of butylated hydroxyanisolc, tertiary butylhydroquinone, and potassium sorbateincombination. J. Food Sci. 46, 314–316. Doğruer, Y., Gürbüz, Ü. and Nizamlhoğlu, M. 1996. Potasyum sorbatnn beyaz peynirin kimyasal ve mikrobiyolojik kalitesine etkisi. Selçuk Univ. Vet. Bil. Derg. 12, 109–116. Flynn, B., deRiancho, D., Lawton, M.R. and Alcaine, S.D. 2021. Evaluation of lactose oxidase as an enzyme-based antimicrobial for control of L. monocytogenes in fresh cheese. Foods J. 10, 1471. Garnier, L., Valence, F. and Mounier, J. 2017. Diversity and control of spoilage fungi in dairy products: an update. Microorganisms 5, 42. Golafrouz, H., Ahari, H., Anvar, A. and Shahbazzadeh, D. 2020. Detection of Staphylococcus aureus Enterotoxin A (SEA) using Dot-ELISA in milk samples. J. Med. Microbiol. Infec. Dis. 8, 132–136. Hashim, I.B. 2002. Acceptance of camel milk among elementary school students in Al Ain city, United Arab Emirates, Emir. J. Agric. Sci. 14, 54–59. Hennekinne, J.A., De Buyser, M.L. and Dragacci, S. 2012. Staphylococcus aureus and its food poisoning toxins characterization and outbreak investigation. FEMS Microbiol. Rev. 36, 815–836. Johler, S., Weder, D., Bridy, C., Huguenin, M.C., Robert, L., Hummerjohann, J. and Stephan, R. 2015. Outbreak of staphylococcal food poisoning among children and staff at a Swiss boarding school due to soft cheese made from raw milk. J. Dairy Sci. 98, 2944–2948. Jones, E.L., Shingfied, K.J., Kohen, C., Jones, A.K., Lupoli, B., Grandison, A.S., Beever, D.E., Williams, C.M., Calder, P.C. and Yaqoob, P. 2005. Chemical, physical, and sensory proper ties of dairy products enriched with conjugated linoleic acid. J. Dairy Sci. 88, 2923–2937. Kang, S., Kong, F., Shi, X., Han, H., Li, M., Guan, B., Yang, M., Cao, X., Tao, D., Zheng, Y. and Yue, X. 2020. Antibacterial activity and mechanism of lactobionic acid against Pseudomonas fluorescens and Methicillin-resistant Staphylococcus aureus and its application on whole milk. Food Control 108, 106876. Khanal, R.C., Dhiman, T.R., Ure, A.L., Brennand, C.P., Bo Man, R.L. and McMahon, D.J. 2005. Consumer acceptability of con jugated linoleic acid enriched milk and cheddar cheese from cows grazing on pasture. J. Dairy Sci. 88, 1837–1847. Kiryu, T., Yamauchi, K., Masuyama, A., Ooe, K., Kimura, T., Kiso, T. and Murakami, H. 2012. Optimization of lactobionic acid production by Acetobacter orientalis iso lated from Caucasian fermented milk, “Caspian Sea yogurt”. Biosci. Biotechnol. Biochem. 76, 361–363. Lara-Aguilar, S. and Alcaine, S.D. 2019. Short communication: screening inhibition of dairy-relevant pathogens and spoilage microorganisms by lactose oxidase. J. Dairy Sci. 102, 7807–7812. Litopoulou-Tzanetakii, E. and Tzanetakis, N. 1992. Microbiological study of white-brined cheese made from raw goat milk. Food Micro. 9, 13–19. Lu, M. and Wang, N.S. 2017. Spoilage of milk and dairy products. In The microbiological quality of food: foodborne spoilers. Eds., Beviacqua, A., Corbo, and Sinigaglia, M. Sawston, UK: Woodhead, pp: 151–178. Lück, F. 1990. Food applications of sorbic acid and its salts. Food Add. and Con. 7, 711–715. Lutfiye, Y. and Ekrem, K. 2008. Effect of sorbic acid and potassium sorbate addition to the brine on microbiological and chemical properties of Turkish white cheese during ripening, Food Sci. Technol. Res. 14, 437–444. Lynch, J.M., Lock, A.L., Dwyer, D.A., Noorbakhash, R., Bar bano, D.M. and Bauman, D.E. 2005. Flavor and stability of pasteurized milk with elevated levels of conjugated linoleic acid and vaccenic acid. J. Dairy Sci. 88, 489–498. Nizamlioglu, M., Yalcin, S. and Tekinsen, O.C. 1989. Quality of brine white cheese in Konya vicinity, J. Vete. Animal Sci. 13, 136–142. Norman, H.J. and Sandine, W.E. 1994. Nisin as a model food preservative. Crit. Rev. Food Sci. Nutrition 34, 69–93. Ozdemir, C. and Demirci, M. 2006. Selected microbiological properties of kashar cheese samples preserved with potassium sorbate. Int. J. Food Prop. 9, 515–521. Painter, J.A., Hoekstra, R.M., Ayers, T., Tauxe, R.V., Braden, C.R., Angulo, F.J. and Griffin, P.M. 2013. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. 19, 407–415. Ribeiro, J.C.B., Granato, D., Masson, M.L., Andriot, I., Mosca, A.C., Salles, C., and Guichard, E. 2016. Effect of lactobionic acid on the acidification, rheological properties and aroma release of dairy gels. Food Chem. 207, 101–106. Robach, M.C. and Stateler. C.L. 1980. Inhibition of Staphylococcus aureus by potassium sorbate in combination with sodium chloride, tertiary butylhydroquinone, butylated hydroxyanisole or ethylenediamine tetraacetic acid. J. Food Prot. 43, 208–211. Ryhänen, E.L., Tallavaara, K., Griinari, J.M., Jaakkola, S., Mantere Alhonen, S. and Shingfield, K.J. 2005. Production of conjugated linoleic acid enriched milk and dairy products from cows receiv ing grass silage supplemented with a cereal-based concentrate con taining rapeseed oil. Int. Dairy J. 15, 207–217. Scallan, E., Hoekstra, R.M., Angulo, F.J., Tauxe, R.V., Widdowson, M.A., Roy, S.L., Jones, J.L. and Griffin, P.M. 2011. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 17, 7–15. Schon, K., Schornsteiner, E., Dzieciol, M.,Wagner, M., Muller, M. and Schmitz-Esser, S. 2016. Microbial communities in dairy processing environment floor-drains are dominated by product-associated bacteria and yeasts. Food Control 70, 210–215. Schrade, J. P. and Yager, J. 2001. Implication of milk and milk products in food disease in France and in different industrialized countries. Int. J. Food Microbiol. 67, 1–17. Sengun, l., Yaman, D. and Gonul, S. 2008. Mycotoxins and mould contamination in cheese: a review. World Mycotoxin J. 1, 291–298. Silva, A.C.D.A., Rodrigues, M.X. and Silva, N.C.C. 2020. Methicillin-resistant Staphylococcus aureus in food and the prevalence in Brazil: a review. Brazilian J. Microbiol. 51, 347–356. Silva, Z.N., Cunha, A.S., Lins, M.C., Carneiro, de. A.M., Almeida, L. and Queiroz, M.L. 2001. Isolation and serological identification of enteropathogenic Escherichia coli in pasteurized milk in Brazil. Rev. Saude. Publica. 35, 375–379. Sympoura, F., Cornu, A., Tournayre, P., Massouras, T., Berdagué, J.L. and Martin, B. 2009. Odor compounds in cheese made from the milk of cows supplemented with extruded linseed and α-tocopherol. J. Dairy Sci. 92, 3040–3048. Temelli, S., Anar, S., Sen, C. and Akyuva, P. 2006. Determination of microbiological contamination sources during Turkish white cheese production. Food Control 17, 856–861. Tzanetakis, N., Vafopoulou-Mastrojiannaki, A. and LitopoulouTzanetaki, E. 1995. The quality of white-brined cheese from goat’s milk made with different starters. Food Micro. 12, 55–63. Volikakis, P., Biliaderis, C.G., Vamvakas, C., and Zerfiridis, G.K. 2004. Effects of a commercial oat-β-glucan concentrate on the chemical, physico-chemical and sensory attributes of a low-fat white-brined cheese product. Food Res. Inter. 37, 83–94. | ||
| How to Cite this Article |
| Pubmed Style Abd-elaal SF, Alnakip ME, Youssef MZ, Bayoumi MA. Comparison of Controlling Capabilities of Lactobionic acid, Nisin and K-Sorbate against Dairy-borne Staphylococcus aureus, Escherichia coli and mold in soft cheese. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 594-603. doi:10.5455/OVJ.2024.v14.i1.55 Web Style Abd-elaal SF, Alnakip ME, Youssef MZ, Bayoumi MA. Comparison of Controlling Capabilities of Lactobionic acid, Nisin and K-Sorbate against Dairy-borne Staphylococcus aureus, Escherichia coli and mold in soft cheese. https://www.openveterinaryjournal.com/?mno=190018 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i1.55 AMA (American Medical Association) Style Abd-elaal SF, Alnakip ME, Youssef MZ, Bayoumi MA. Comparison of Controlling Capabilities of Lactobionic acid, Nisin and K-Sorbate against Dairy-borne Staphylococcus aureus, Escherichia coli and mold in soft cheese. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 594-603. doi:10.5455/OVJ.2024.v14.i1.55 Vancouver/ICMJE Style Abd-elaal SF, Alnakip ME, Youssef MZ, Bayoumi MA. Comparison of Controlling Capabilities of Lactobionic acid, Nisin and K-Sorbate against Dairy-borne Staphylococcus aureus, Escherichia coli and mold in soft cheese. Open Vet. J.. (2024), [cited January 25, 2026]; 14((1) (Zagazig Veterinary Conference)): 594-603. doi:10.5455/OVJ.2024.v14.i1.55 Harvard Style Abd-elaal, S. F., Alnakip, . M. E., Youssef, . M. Z. & Bayoumi, . M. A. (2024) Comparison of Controlling Capabilities of Lactobionic acid, Nisin and K-Sorbate against Dairy-borne Staphylococcus aureus, Escherichia coli and mold in soft cheese. Open Vet. J., 14 ((1) (Zagazig Veterinary Conference)), 594-603. doi:10.5455/OVJ.2024.v14.i1.55 Turabian Style Abd-elaal, Salah F., Mohamed E.a. Alnakip, Madiha Z. Youssef, and Mohamed A. Bayoumi. 2024. Comparison of Controlling Capabilities of Lactobionic acid, Nisin and K-Sorbate against Dairy-borne Staphylococcus aureus, Escherichia coli and mold in soft cheese. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 594-603. doi:10.5455/OVJ.2024.v14.i1.55 Chicago Style Abd-elaal, Salah F., Mohamed E.a. Alnakip, Madiha Z. Youssef, and Mohamed A. Bayoumi. "Comparison of Controlling Capabilities of Lactobionic acid, Nisin and K-Sorbate against Dairy-borne Staphylococcus aureus, Escherichia coli and mold in soft cheese." Open Veterinary Journal 14 (2024), 594-603. doi:10.5455/OVJ.2024.v14.i1.55 MLA (The Modern Language Association) Style Abd-elaal, Salah F., Mohamed E.a. Alnakip, Madiha Z. Youssef, and Mohamed A. Bayoumi. "Comparison of Controlling Capabilities of Lactobionic acid, Nisin and K-Sorbate against Dairy-borne Staphylococcus aureus, Escherichia coli and mold in soft cheese." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 594-603. Print. doi:10.5455/OVJ.2024.v14.i1.55 APA (American Psychological Association) Style Abd-elaal, S. F., Alnakip, . M. E., Youssef, . M. Z. & Bayoumi, . M. A. (2024) Comparison of Controlling Capabilities of Lactobionic acid, Nisin and K-Sorbate against Dairy-borne Staphylococcus aureus, Escherichia coli and mold in soft cheese. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 594-603. doi:10.5455/OVJ.2024.v14.i1.55 |